Abstract
Background
Bulky or multiple lymph node (LN) metastases are associated with poor prognosis in cervical cancer, and the size or number of LN metastases is not yet reflected in the staging system and therapeutic strategy. Although the therapeutic effects of surgical resection of bulky LNs before standard treatment have been reported in several retrospective studies, well-planned randomized clinical studies are lacking. Therefore, the aim of the Korean Gynecologic Oncology Group (KGOG) 1047/DEBULK trial is to investigate whether the debulking surgery of bulky or multiple LNs prior to concurrent chemoradiation therapy (CCRT) improves the survival rate of patients with cervical cancer IIICr diagnosed by imaging tests.
Methods
The KGOG 1047/DEBULK trial is a phase III, multicenter, randomized clinical trial involving patients with bulky or multiple LN metastases in cervical cancer IIICr. This study will include patients with a short-axis diameter of a pelvic or para-aortic LN ≥2 cm or ≥3 LNs with a short-axis diameter ≥1 cm and for whom CCRT is planned. The treatment arms will be randomly allocated in a 1:1 ratio to either receive CCRT (control arm) or undergo surgical debulking of bulky or multiple LNs before CCRT (experimental arm). CCRT consists of extended-field external beam radiotherapy/pelvic radiotherapy, brachytherapy and LN boost, and weekly chemotherapy with cisplatin (40 mg/m2), 4–6 times administered intravenously. The primary endpoint will be 3-year progression-free survival rate. The secondary endpoints will be 3-year overall survival rate, treatment-related complications, and accuracy of radiological diagnosis of bulky or multiple LNs.
Trial Registration
ClinicalTrials.gov Identifier: NCT05421650; Clinical Research Information Service Identifier: KCT0007137
Keywords: Cervical Cancer, Lymph Node Metastasis, Bulky Lymph Node, Lymph Node Excision, Concurrent Chemoradiotherapy, Progression-Free Survival
INTRODUCTION
Cervical cancer is one of the top 10 malignant tumors worldwide in terms of both incidence and mortality. It is the fourth most common cancer among women after breast, colorectal, and lung cancers [1]. Lymph node (LN) metastasis is one of the most important prognostic factors of cervical cancer. The recurrence rate increases by ≥40% in patients with LN metastasis compared with those without LN metastasis. The 5-year survival rate for patients without LN metastasis is approximately 88%–96%, whereas the 5-year survival rate for those with LN metastasis decreases to 50%–74% depending on the number, location, and size of the LNs [2,3,4]. The revised 2018 International Federation of Obstetrics and Gynecology (FIGO) staging system for cervical cancer includes imaging and/or pathological findings to assess retroperitoneal LNs. Patients with pelvic and/or para-aortic LN metastases are designated as stage IIIC with notations of r (imaging) and p (pathology) [5].
Standard radiotherapy for cervical cancer is effective in eradicating most subclinical metastatic pelvic LNs. However, bulky LNs pose a substantial risk of pelvic failure [6,7]. The size or number of LN metastases is a poor prognostic factor for cervical cancer. The larger the size of the LNs and the greater the number of LNs, the worse the prognosis [8,9,10,11,12,13]. Inoue et al. [10] reported that there was a significant increase in recurrence within 1 year after treatment in cases with node sizes of ≥ 20 mm compared with <20 mm among 152 patients with stage IB to IIB cervical carcinoma. Regarding multiple LN metastases, Zhou et al. [13] reported that in a large cohort study of 2,222 patients with cervical cancer, the survival rate was lower when ≥3 LN metastases were present compared with 1–2 LN metastases. Because it is difficult to increase the radiation dose and anticancer agents in consideration of the adverse effects, a more efficient selective treatment is required for LN metastasis with poor prognosis.
Pretreatment resection of bulky LN has been studied as a strategy to overcome the limitations of radiotherapy. Several retrospective studies have suggested that surgical resection of macroscopic LNs prior to radiation therapy (RT) or concurrent chemoradiation therapy (CCRT) improves survival rate [14,15,16,17,18,19,20]. Most studies have shown that the survival rate of macroscopic LN resection is similar to that of microscopic LN resection prior to RT or CCRT. Cosin et al. [17] evaluated 266 patients with cervical cancer who underwent pelvic and aortic lymphadenectomies before receiving radiation. The 5-year survival rate was comparable at 50% and 46% for patients with microscopic metastases and bulky metastasis-positive LNs who achieved complete LN resection. However, negative results of surgical LN resection have been reported. Yang et al. [21] retrospectively compared the survival rate between 148 women with cervical cancer IB2–IIIB who underwent surgical versus radiographic assessment of pelvic and para-aortic LNs prior to CCRT from 2000 to 2017. They reported no difference in 5-year progression-free survival (PFS) and 5-year overall survival (OS) rates between these 2 groups of women.
As such, the effects of pretreatment surgical resection of bulky or multiple LNs cannot be confirmed as only retrospective studies have shown controversial results. Thus, a well-planned prospective study is required. Therefore, this study aims to investigate whether debulking surgery of bulky or multiple LNs prior to CCRT improves the survival rate of patients with cervical cancer IIICr diagnosed by imaging through a phase III, global multicenter, randomized controlled trial (RCT).
MATERIALS AND METHODS
1. Objectives
This study aims to investigate whether debulking surgery for bulky or multiple LNs prior to CCRT improves the survival rate of patients with stage IIICr cervical cancer diagnosed using imaging tests.
2. Trial design
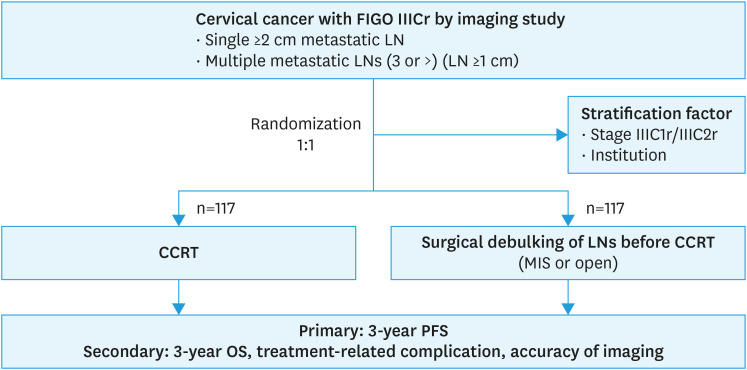
The Korean Gynecologic Oncology Group (KGOG) 1047/DEBULK trial is a phase III, global multicenter, RCT. This study will comprise patients newly diagnosed with cervical cancer stage IIICr (FIGO 2018) with pelvic or para-aortic LN with a short-axis diameter ≥2 cm or ≥3 LNs with a short-axis diameter ≥1 cm by computed tomography (CT) or magnetic resonance imaging (MRI) whether auxiliary use of positron emission tomography (PET)-CT. Eligible patients will be randomized 1:1 to either receive CCRT (control group) or undergo surgical debulking of LNs before CCRT (experimental group) (Fig. 1). All patients will receive weekly platinum-based chemotherapy with cisplatin (40 mg/m2) and definitive radiotherapy concurrently. In the experimental group, surgical debulking of the LNs will be additionally performed via either minimally invasive surgery (traditional laparoscopy or robotic-assisted laparoscopy) or open surgery before CCRT. The leading research institute is CHA Ilsan Medical Center in Korea, and 26 global institutions (23 institutions in Korea, 1 in India, 1 in Vietnam, and 1 in Malaysia) are participating. The participant recruitment and follow-up periods are 4 and 3 years, respectively.
Fig. 1. Trial schema.
CCRT, concurrent chemoradiation therapy; FIGO, International Federation of Obstetrics and Gynecology; LN, lymph node; MIS, minimal invasive surgery; OS, overall survival; PFS, progression-free survival.
3. Endpoints
The primary endpoint of the study will be 3-year PFS rate. The secondary endpoints will include 3-year OS rate, treatment-related complications, and accuracy of the radiological diagnosis of bulky or multiple LNs.
PFS will be defined as the time from the start of treatment to the first documented sign of disease progression or death from any cause. OS will be defined as the time from the first treatment to death from any cause. Remission from the disease is defined using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Complete remission is the disappearance of all primary and metastatic lesions, and the evaluation period is implemented 3 months after the completion of CCRT. Treatment-related complications will be assessed at every visit according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. In particular, lymphatic complications will be evaluated through the measurement of lymphedema grade based on physical examination, the Gynecologic Cancer Lymphedema Questionnaire [22], and the evaluation of lymphoceles using CT at screening, within 30 days of LN dissection (applicable only to the experimental group), 3 months after the end of CCRT treatment, and annually thereafter. Additionally, quality of life (QOL) will be evaluated using the European Organization for Research and Treatment of Cancer QOL questionnaire (QLQ) C30 and QLQ-CX24 3 months after CCRT completion and annually thereafter. The accuracy of radiological diagnosis of bulky or multiple LNs will be estimated only in the treatment group.
4. Eligibility criteria
Inclusion criteria
1) Women aged ≥20 years and ≤70 years.
2) Patients newly diagnosed with squamous cell carcinoma (SCC), adenocarcinoma, or adenosquamous carcinoma.
3) Patients with a short-axis diameter of the pelvic LN or para-aortic LN under the renal vein of ≥2 cm or ≥3 LNs with a short-axis diameter ≥1 cm in CT or MRI (PET-CT can be used for auxiliary tool).
4) Patients with CCRT planned as a treatment for cervical cancer.
5) Patients whose competency is Eastern Cooperative Oncology Group performance score 0–1.
6) Patients who have signed the approved informed consent form for study participants.
7) Patients in whom surgical debulking for LN metastasis was possible, as confirmed by radiological examination.
Exclusion criteria
1) Patients who have been diagnosed with cancer of any organ other than thyroid cancer (excluding stage 0 cancer) within the previous 5 years.
2) Patients who are pregnant or plans to conceive during the clinical study period.
3) Patients with any active infectious disease or incurable severe inflammation.
4) Patients who cannot undergo surgery due to internal or surgical disease.
5) Patients who cannot receive chemotherapy due to internal or surgical disease.
6) Patients with a history of pelvic RT (PRT).
7) Patients with a history of subtotal hysterectomy.
8) Patients with remote metastasis other than a pelvic or para-aortic LN (e.g., lung, subclavian, and inguinal LNs).
5. Sample size
The comparative indicator in this phase III randomized study will be the 3-year PFS rate. The predictive value of the 3-year PFS rate is 40%–75% when CCRT alone (control group) is performed in patients with cervical cancer IIIC (LN metastasis). According to the outcomes reported by the Korean cancer centers, considering that the 3-year PFS rate of IIIC1 (pelvic LN metastasis) is approximately 70%–75% and the 3-year PFS rate of IIIC2 (para-aortic LN metastasis) is approximately 40%–50% [23], the 3-year PFS rate of the comparative indicator to be used in this study can be approximately 60%. However, no studies have investigated the 3-year PFS rate in patients receiving CCRT after LN debulking surgery. Lim et al. [24] only reported a 3-year PFS rate of approximately 75%–80% after surgical staging of localized cervical cancer in a Korean study. Based on this, the expected difference in the 3-year PFS rates between the control and experimental groups was estimated to be 15%. The participant recruitment and follow-up periods are 4 and 3 years, respectively; when the significance level is 0.05 and the test power is 80%, the sample size required is 204 patients. Considering a dropout rate of 13%, the study requires 234 cases. The sample size is 117 participants in both the control and experimental groups.
6. Participant recruitment and informed consent
If the participant is suitable for the eligibility criteria, researchers should explain that the current condition of bulky or multiple LN metastasis has the limitations of treatment performance, and that standard treatment is difficult to expect satisfactory results from CCRT in the current literature. They should explain that the purpose of this study is to investigate whether the cure rate can be increased by selecting and removing only bulky or multiple LNs. In addition, it should be explained that the complications arising from the addition of surgical treatment may increase. Since then, they should explain the need for this phase III, RCT because there have been good research results retrospectively so far, but there are no prospective results yet. After the participant agrees to participate in the study and informed consent is obtained, randomization is performed.
7. Randomization
After confirming compliance with the eligibility criteria, randomization will be performed in a 1:1 ratio between the control and experimental groups. The study participants will be assigned using the stratified block randomization method with the following stratification factors: 1) cervical cancer stage (IIICr1 vs. IIICr2) and 2) participating institutions. Randomization numbers will be generated by an independent statistical expert using Microsoft Windows-based SAS version 9.4 or higher (SAS Institute Inc., Cary, NC, USA), and the randomization number will be assigned using the Interactive Web Based Response System. Because this is an open-label trial, no blinding will be performed.
8. Treatment
Treatment should be performed within 2 weeks of randomization.
Control arm (CCRT only)
CCRT consists of extended-field external beam radiotherapy (EF-EBRT), brachytherapy and LN boost (LNB), and weekly chemotherapy with cisplatin 40 mg/m2 administered intravenously. Chemotherapy will be administered 4–6 times.
Three-dimensional conformal RT (3D-CRT) and intensity-modulated RT (IMRT) can be used as radiation therapies. The entire area, including the pelvis and para-aortic LNs, should be treated 5 times a week at a dose of 1.8 Gy, with a total dose of 45–50.4 Gy/25-28 fractions (Fx). After 3D-CRT or IMRT, low-dose-rate (LDR) or high-dose-rate (HDR) brachytherapy is allowed. The dose prescriptions for brachytherapy are 27.5–30 Gy/4–6 Fx for HDR and 35–40 Gy/1–2 Fx for LDR according to the site’s protocol for brachytherapy using the image-guided brachytherapy technique or Point A-based 2-dimensional technique. 3D brachytherapy using CT or MRI is strongly recommended. LNB should be performed for gross LN metastasis using 3D-CRT or IMRT technique. Sequential and simultaneous integrated boosting techniques can also be used. The dose prescription for LNB should be calculated by adding the abdominal and pelvic doses. For a LN with a minor diameter of ≤1 cm but suspected of metastasis on imaging tests (CT, MRI, or PET-CT), a radiation equivalent to a total dose of 55–60 Gy should be prescribed. Radiation equivalent to 60–64 Gy should be prescribed for a short-axis diameter of 1–2 cm and 64–68 Gy for a diameter >2 cm. Ideally, all radiation therapies should be completed within 56 days (with a maximum of 63 days to maintain adherence) after therapy initiation.
Experimental arm (surgical resection before CCRT)
All laparoscopic, robotic, and open surgeries are permitted. In laparoscopic or robotic surgery, LN access can be intraperitoneal or retroperitoneal. On imaging examination, LNs with a short-axis diameter of ≥1 cm are excised. Notably, the entire LN is not dissected because this study aims to debulk bulky or multiple LNs.
The LN area is anatomically bound by arteries, and the LN is divided into 4 levels based on the border. Level 1 includes the external iliac, internal iliac, and obturator areas; level 2 includes the common iliac area; level 3 includes the infra-mesenteric area below the inferior mesenteric artery among the para-aortic areas; and level 4 includes the infrarenal area below the renal vein among the para-aortic areas [25]. The size, number, and location of the resected LNs will be recorded according to the above levels. If all of the resected LNs are found negative as a result of the frozen or final biopsy, a decision for a treatment policy for the primary tumor is to be made on whether CCRT or surgical treatment including hysterectomy is to be performed depending on the treatment policy of each institution. If hysterectomy is performed instead of CCRT to treat the primary tumor, the subject will be dropped from the clinical trial.
Postoperative LN assessment by imaging (CT or MRI) will be performed before CCRT within 30 days of surgery to evaluate whether the applicable LN has been successfully resected.
CCRT should be performed within 30 days of surgery. CCRT consists of RT, including EF-EBRT or PRT, brachytherapy, and LNB, and weekly chemotherapy with cisplatin 40 mg/m2, 4–6 times administered intravenously. Extended-field RT is the principle for EBRT. However, PRT is permitted only if the postoperative imaging examination is negative for LNs, and histopathological results show 0, 1, or 2 LN metastases <2 cm present only in the pelvic LN (level 1).
9. Follow-up
Follow-up will be performed every 3 months for 2 years and thereafter every 6 months. The total follow-up period is 3 years after the completion of treatment, and in the case of death, it is extended to the time of death. Physical status evaluation, physical examination, Papanicolaou smear, tumor markers (SCC antigen and carcinoembryonic antigen), and treatment-related complications will be performed at every visit. Palpation of superficial LNs, such as the inguinal and left subclavian LNs, and rectal examination will be performed, if required. Lymphatic complications and the QLQ will be administered 3 months after CCRT and annually thereafter. CT and MRI should be performed within the following follow-up time frames: 1) CT will be performed 3, 6, 12, 24, and 36 months after CCRT completion; 2) MRI will be performed 18 and 30 months after CCRT completion; and 3) PET-CT can be performed additionally if necessary. If recurrence is suspected, imaging and pathological examinations should be immediately performed.
10. Statistical analyses
We plan to form a Data Safety Monitoring Board (DSMB) for the professional review of safety data for this study. The DSMB will evaluate the safety-related matters of registered study patients during the entire study period, provide advice on study progress, and operate through the KGOG. An interim analysis is conducted 2 years after the start of the clinical trial (i.e., after the first participant is enrolled) or when 50% of the events (relapse) have occurred, whichever occurs first. No adjustments are made for multiple tests.
Demographic and baseline characteristic data will be analyzed using descriptive statistics (mean, standard deviation, median, minimum, and maximum) for continuous data. Independent t-tests or Wilcoxon’s rank-sum tests will be used to compare the 2 groups after testing for normality. For categorical data, the number and percentage of participants will be presented, and χ2 or Fisher’s exact tests will be used to compare the 2 groups. The significance level for the final analysis will be set at 5%, and if multiple comparisons are necessary, Bonferroni correction will be used.
For survival, the time to death or recurrence will be estimated and presented using Kaplan-Meier analysis and median survival time. A log-rank test will be conducted to compare the control and experimental groups. PFS and OS will be analyzed using a stratified log-rank test stratified by disease stage (IIIC1r and IIIC2r). Cox proportional hazards regression will be used to model PFS as a function of the treatment group and other well-known and potential prognostic factors, such as age and histologic features. We will estimate the hazard ratios for treatment and other potential prognostic factors using 95% confidence intervals. We will perform analyses for OS similar to those for PFS.
To evaluate treatment-related and lymphatic complications, the incidence will be compared between the 2 groups using the χ2 or Fisher’s exact test. For the QOL, independent t-tests or Wilcoxon rank-sum tests will be used to compare the 2 groups after testing for normality.
Sensitivity, specificity, positive predictive value, negative predictive value, and area under the curve will be used to determine the accuracy of the diagnosis based on imaging tests (CT/MRI/PET-CT) for the presence of bulky or multiple LN metastases and the results of surgical histopathological metastasis in the experimental group.
11. Ethics
This trial will be conducted in accordance with the Pharmaceutical Clinical Trial Management Standards (KFDA Notice No. 2008-39) and the Declaration of Helsinki, 59th World Medical Association General Assembly, Seoul, October 2008, and International Council for Harmonisation Good Clinical Practice standards. The study protocol has been approved by the ethics committees of each participating institution, and informed consent was obtained from each enrolled patient.
12. Trial registration ID
ClinicalTrials.gov Identifier: NCT05421650; Clinical Research Information Service (CRIS): KCT0007137.
DISCUSSION
CCRT is a standard treatment for IIICr cervical cancer. However, the standard irradiation dose of 45–50.4 Gy eradicates only 60% of the LNs ≥2 cm [26]. Bulky LNs are difficult to shrink using standard treatments. Most studies have pointed out that the prognosis is poor depending on the size of the LNs [8,9,10]. The definition of bulky LNs is relatively ambiguous, but generally a 2 cm diameter of LNs, which we chose as the standard in our study, is often accepted [10,27]. The risk factors for nodal metastasis in cervical cancer are the size and number of affected LNs, which are directly related to survival [11,12,13]. Therefore, we attempted to confirm not only the size but also the efficacy of surgical resection of multiple metastatic LNs.
Advanced strategies, such as nodal boosting or surgical LN debulking, are recommended to overcome the limitations of bulky LN management. Recently, a retrospective study showed no survival benefit from either nodal boosting or debulking strategy in locally advanced cervical cancer with bulky LNs (≥1.5 cm) [28]. However, the median bulky LNs size differed between the groups, with 18 mm in the nodal boosting group and 22 mm in the surgical debulking group. In addition, radiotherapy was not standardized between institutions. Therefore, a prospective RCT is required.
We will perform RT-quality assurance/quality control (QA/QC) to reduce errors among patients, institutions, and countries. In multicenter studies involving RT, uniform and accurate dosimetry and treatment delivery are important to minimize interinstitutional errors. Therefore, in our study, the RT-QA process will be developed, and the RT-QA/QC committee (comprising radiation oncologists from the KROG) will be formed to ensure uniform and robust RT protocol adherence. It ensures that the radiotherapy specified in the protocol meets the standard of care, answers any questions the institution may have, and reviews the plans during the trial. The institutions participating in the study will undergo a radiotherapy credentialing process that includes machine QA and end-to-end testing. All cases will be reviewed by the RT-QA/QC committee at appropriate times.
To minimize the surgical complications caused by lymphadenectomy, the protocol of this study only allows dissection of LNs ≥1 cm in size, not full lymphadenectomy. Lymphatic complications between the control and experimental groups will be compared objectively and subjectively [22] and analyzed as a secondary endpoint. In addition, the accuracy of imaging methods (CT, MRI, or PET-CT) for the diagnosis of bulky LNs metastases will be evaluated.
Currently, a similar randomized trial, CQGOG103, is being conducted in China [29]. This is also a national, prospective, multicenter, randomized clinical study evaluating the efficacy of LN dissection in stage IIICr cervical cancer. However, there are 3 differences between these 2 studies. The first, the participants are those with short diameter of image-positive pelvic and/or para-aortic LNs ≥15 mm in CQGOG103, whereas our trial include LNs ≥20 mm. Second, full lymphadenectomy is performed in the Chinese study, whereas surgical debulking of only bulky LN or multiple LNs is performed in our study to remove the poor prognostic factors for RT failure and minimize the complications of LN dissection. Finally, the primary endpoint in CQGOG103 is 2-year PFS, whereas our study will evaluate 3-year PFS rate as the primary endpoint. Nevertheless, the common purpose of these 2 studies is to determine a way to overcome poor prognostic factors, such as the variability of LN metastases. Therefore, these results will be helpful in developing the best strategy for treating cervical cancer with poor prognostic factors.
CCRT has been the standard care for patients with locally advanced cervical cancer for over 20 years. However, regional LN metastases with bulky or multiple statuses have shown treatment failure and poor prognosis for CCRT. To overcome the limitations of the current standard of care, strategic treatment is required, such as for breast cancer, in which genetic markers and LN metastatic status are considered in the selection of the treatment modality. Therefore, this study will be helpful for personalizing treatment strategies based on the size or number of LN metastases in cervical cancer.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Kyung Hee Han, Dr. Seong Min Kim, Dr. Min-Jeong Kim, Eunsun Lee and Seulae Yun for supporting this trial.
Footnotes
Funding: This work was supported by the National R&D Program for Cancer Control through the National Cancer Center (NCC) funded by the Ministry of Health & Welfare, Republic of Korea (HA22C0083).
Presentation: This study is presented 38th Annual Meeting Korean Society of Gynecologic Oncology, 2023 and Annual Global Meeting of the International Gynecologic Cancer Society, 2023.
Conflict of Interest: Yong Bae Kim received research funding from the National R&D Program for Cancer Control through the National Cancer Center (NCC) funded by the Ministry of Health & Welfare, Republic of Korea (HA22C0021) and 2023 Bio Industry Technology Development Project funded by Ministry of Trade, Industry and Energy (20024089). Asima Mukhopadhyay received honoraria from the National R&D Program for Cancer Control through the National Cancer Center (NCC) funded by the Ministry of Health & Welfare, Republic of Korea (HA22C0083) for her lecture presented at the 2022 Asian Society of Gynecologic Oncology (ASGO) webinar. Nirmala Chandralega Kampan received support for attending the meeting from Roche.
- Conceptualization: Y.B.S., K.J.W., K.J.H., E.K.Y., R.J.W.
- Funding acquisition: Y.B.S., K.J.H., E.K.Y., R.J.W.
- Investigation: Y.B.S., L.K.B., L.K.H., C.H.K., K.J.Y., L.M.C., C.C.H., C.H., K.D.Y., K.Y.H., C.J.S., L.C.H., K.J.W., K.S.W., K.Y.B., C.C.H., H.D.G., S.Y.J., J.S., K.M.K., J.D.H., P.H., K.S.M., P.S.I., S.J.Y., M.A., T.D.H.Q., K.N.C., K.J.H., E.K.Y., R.J.W.
- Methodology: Y.B.S., L.K.B., L.K.H., C.H.K., K.J.Y., L.M.C., C.C.H., C.H., K.D.Y., K.Y.H., C.J.S., L.C.H., K.J.W., K.S.W., K.Y.B., C.C.H., H.D.G., S.Y.J., J.S., K.M.K., J.D.H., P.H., K.S.M., P.S.I., S.J.Y., M.A., T.D.H.Q., K.N.C., K.J.H., E.K.Y., R.J.W.
- Project administration: Y.B.S., K.J.H., E.K.Y., R.J.W.
- Supervision: L.K.B., L.K.H., C.H.K., K.J.Y., L.M.C., C.C.H., C.H., K.D.Y., K.Y.H., C.J.S., L.C.H., K.J.W., K.S.W., K.Y.B., C.C.H., H.D.G., S.Y.J., J.S., K.M.K., J.D.H., P.H., K.S.M., P.S.I., S.J.Y., M.A., T.D.H.Q., K.N.C., K.J.H., E.K.Y., R.J.W.
- Writing - original draft: Y.B.S., L.G.J., K.J.H., E.K.Y., R.J.W.
- Writing - review & editing: L.K.B., L.K.H., C.H.K., K.J.Y., L.M.C., C.C.H., C.H., K.D.Y., K.Y.H., C.J.S., L.C.H., K.J.W., K.S.W., K.Y.B., C.C.H., H.D.G., S.Y.J., J.S., K.M.K., J.D.H., P.H., K.S.M., P.S.I., S.J.Y., M.A., T.D.H.Q., K.N.C., L.G.J., K.J.H., E.K.Y., R.J.W.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Soisson AP, Soper JT, Clarke-Pearson DL, Berchuck A, Montana G, Creasman WT. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990;37:390–395. doi: 10.1016/0090-8258(90)90374-t. [DOI] [PubMed] [Google Scholar]
- 3.Tinga DJ, Timmer PR, Bouma J, Aalders JG. Prognostic significance of single versus multiple lymph node metastases in cervical carcinoma stage IB. Gynecol Oncol. 1990;39:175–180. doi: 10.1016/0090-8258(90)90428-n. [DOI] [PubMed] [Google Scholar]
- 4.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 5.Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 6.Wharton JT, Jones HW, 3rd, Day TG, Jr, Rutledge FN, Fletcher GH. Preirradiation celiotomy and extended field irradiation for invasive carcinoma of the cervix. Obstet Gynecol. 1977;49:333–338. [PubMed] [Google Scholar]
- 7.Withers HR, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys. 1995;31:353–359. doi: 10.1016/0360-3016(94)00354-N. [DOI] [PubMed] [Google Scholar]
- 8.Song S, Kim JY, Kim YJ, Yoo HJ, Kim SH, Kim SK, et al. The size of the metastatic lymph node is an independent prognostic factor for the patients with cervical cancer treated by definitive radiotherapy. Radiother Oncol. 2013;108:168–173. doi: 10.1016/j.radonc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Oh J, Seol KH, Choi YS, Lee JW, Bae JY. Clinical significance of lymph node size in locally advanced cervical cancer treated with concurrent chemoradiotherapy. Yeungnam Univ J Med. 2019;36:115–123. doi: 10.12701/yujm.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue T, Chihara T, Morita K. The prognostic significance of the size of the largest nodes in metastatic carcinoma from the uterine cervix. Gynecol Oncol. 1984;19:187–193. doi: 10.1016/0090-8258(84)90179-3. [DOI] [PubMed] [Google Scholar]
- 11.Pedone Anchora L, Carbone V, Gallotta V, Fanfani F, Cosentino F, Turco LC, et al. Should the number of metastatic pelvic lymph nodes be integrated into the 2018 FIGO staging classification of early stage cervical cancer? Cancers (Basel) 2020;12:1552. doi: 10.3390/cancers12061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SC, Lin LC, Kuo YT, Lin YW. Radiographic number of positive pelvic lymph nodes as a prognostic factor in cervical cancer treated with definitive concurrent chemoradiotherapy or intensity-modulated radiotherapy. Front Oncol. 2018;8:546. doi: 10.3389/fonc.2018.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Wu SG, Sun JY, Liao XL, Li FY, Lin HX, et al. Incorporation of the number of positive lymph nodes leads to better prognostic discrimination of node-positive early stage cervical cancer. Oncotarget. 2017;8:26057–26065. doi: 10.18632/oncotarget.15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downey GO, Potish RA, Adcock LL, Prem KA, Twiggs LB. Pretreatment surgical staging in cervical carcinoma: therapeutic efficacy of pelvic lymph node resection. Am J Obstet Gynecol. 1989;160:1055–1061. doi: 10.1016/0002-9378(89)90160-9. [DOI] [PubMed] [Google Scholar]
- 15.Potish RA, Downey GO, Adcock LL, Prem KA, Twiggs LB. The role of surgical debulking in cancer of the uterine cervix. Int J Radiat Oncol Biol Phys. 1989;17:979–984. doi: 10.1016/0360-3016(89)90145-4. [DOI] [PubMed] [Google Scholar]
- 16.Hacker NF, Wain GV, Nicklin JL. Resection of bulky positive lymph nodes in patients with cervical carcinoma. Int J Gynecol Cancer. 1995;5:250–256. doi: 10.1046/j.1525-1438.1995.05040250.x. [DOI] [PubMed] [Google Scholar]
- 17.Cosin JA, Fowler JM, Chen MD, Paley PJ, Carson LF, Twiggs LB. Pretreatment surgical staging of patients with cervical carcinoma: the case for lymph node debulking. Cancer. 1998;82:2241–2248. doi: 10.1002/(sici)1097-0142(19980601)82:11<2241::aid-cncr20>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Suprasert P, Srisomboon J, Charoenkwan K, Siriaungul S, Khunamornpong S, Siriaree S, et al. Outcomes of abandoned radical hysterectomy in patients with stages IB-IIA cervical cancer found to have positive nodes during the operation. Int J Gynecol Cancer. 2005;15:498–502. doi: 10.1111/j.1525-1438.2005.15315.x. [DOI] [PubMed] [Google Scholar]
- 19.Richard SD, Krivak TC, Castleberry A, Beriwal S, Kelley JL, 3rd, Edwards RP, et al. Survival for stage IB cervical cancer with positive lymph node involvement: a comparison of completed vs. abandoned radical hysterectomy. Gynecol Oncol. 2008;109:43–48. doi: 10.1016/j.ygyno.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Cheung TH, Lo KW, Yim SF, Yau SH, Yu MM, Yeung WK. Debulking metastatic pelvic nodes before radiotherapy in cervical cancer patients: a long-term follow-up result. Int J Clin Oncol. 2011;16:546–552. doi: 10.1007/s10147-011-0225-3. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Delara R, Magrina J, Magtibay P, Yi J, Langstraat C, et al. Comparing survival outcomes between surgical and radiographic lymph node assessment in locally advanced cervical cancer: a propensity score-matched analysis. Gynecol Oncol. 2020;156:320–327. doi: 10.1016/j.ygyno.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical care tool to identify lower extremity lymphedema in gynecologic cancer survivors. Gynecol Oncol. 2010;117:317–323. doi: 10.1016/j.ygyno.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin W, Ham TY, Park YR, Lim MC, Won YJ. Comparing survival outcomes for cervical cancer based on the 2014 and 2018 International Federation of Gynecology and Obstetrics staging systems. Sci Rep. 2021;11:6988. doi: 10.1038/s41598-021-86283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim MC, Bae J, Park JY, Lim S, Kang S, Seo SS, et al. Experiences of pretreatment laparoscopic surgical staging in patients with locally advanced cervical cancer: results of a prospective study. J Gynecol Oncol. 2008;19:123–128. doi: 10.3802/jgo.2008.19.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M, Choi CH, Chun YK, Kim YH, Lee KB, Lee SW, et al. Surgical manual of the Korean Gynecologic Oncology Group: classification of hysterectomy and lymphadenectomy. J Gynecol Oncol. 2017;28:e5. doi: 10.3802/jgo.2017.28.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gogineni E, Bloom B, Diaz Molina F, Villella J, Goenka A. Radiotherapy dose escalation on pelvic lymph node control in patients with cervical cancer. Int J Gynecol Cancer. 2021;31:524–529. doi: 10.1136/ijgc-2020-001342. [DOI] [PubMed] [Google Scholar]
- 27.Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Prognostic factors in node-positive patients with stage IB-IIB cervical cancer treated by radical hysterectomy and pelvic lymphadenectomy. Int J Gynaecol Obstet. 2006;93:130–135. doi: 10.1016/j.ijgo.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Olthof EP, Wenzel H, van der Velden J, Spijkerboer AM, Bekkers R, Beltman JJ, et al. Treatment of bulky lymph nodes in locally advanced cervical cancer: boosting versus debulking. Int J Gynecol Cancer. 2022;32:861–868. doi: 10.1136/ijgc-2022-003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He M, Guo M, Zhou Q, Tang Y, Zhong L, Liu Q, et al. Efficacy of lymph node dissection on stage IIICr of cervical cancer before CCRT: study protocol for a phase III, randomized controlled clinical trial (CQGOG0103) J Gynecol Oncol. 2023;34:e55. doi: 10.3802/jgo.2023.34.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]