Abstract
Purpose
In high-risk non-muscle-invasive bladder cancer (NMIBC), intravesical Bacillus Calmette-Guérin (BCG) is the standard adjuvant therapy post-transurethral resection of bladder tumor (TURBT). Intravesical gemcitabine, used as an alternative or second-line therapy amid BCG shortages, lacks outcome studies in the Korean population.
Materials and Methods
Patients who received weekly intravesical gemcitabine for 6 weeks after TURBT from 2019 to 2022 were retrospectively investigated. Based on the American Urological Association risk classification, patients with high- or very high-risk NMIBC who refused cystectomy were included. Maintenance treatment was performed depending on their risk. Recurrence was defined as histologic confirmation on subsequent cystoscopic biopsies or TURBT. Disease free survival (DFS) was evaluated by the Kaplan–Meier method.
Results
The study included 60 patients, comprising 45 high-risk (group 1) patients with a median age of 76 years and 15 very high-risk (group 2) patients with a median age of 68 years. Among them, 28 patients had previously received intravesical BCG. Over a median follow-up of 22 months, recurrence occurred in 31 patients in group 1 and 11 in group 2. The DFS rates of the high-risk group and the very high-risk group were 57.8% versus 40% at 1 year, 20.7% versus 21.3% at 2 years and 20.7% versus 21.3% at 3 years, respectively (p=0.831). Tis stage (p=0.042) and prostatic urethra invasion (p=0.028) were significant predictors of DFS. Cancer-specific mortality rates were 2.2% in group 1 and 6.7% in group 2 (p=0.441).
Conclusions
Similar DFS outcome between high-risk and very high-risk patients were observed based on short-term results in Korea. This finding is crucial for clinical practice; however, studies analyzing more patients and long-term outcomes are needed.
Keywords: Bladder cancer, Gemcitabine, Intravesical instillation
Graphical Abstract
INTRODUCTION
In South Korea, bladder cancer is the 8th most common type of cancer among men. The incidence rate of bladder cancer is 9.3 per 100,000 population per year, and the number of new cases of bladder cancer in 2020 was 4,753 [1]. Non-muscle-invasive bladder cancer (NMIBC) constitutes 70%–80% of primary diagnoses and is characterized by frequent recurrence, and 20%–25% patients progress to muscle-invasive bladder cancer [2]. International guidelines recommend treatment methods based on the classification of NMIBC into low-, intermediate-, and high-risk groups, according to the probability of recurrence and progression [3,4]. According to the American Urological Association (AUA) risk stratification, high-risk bladder cancer encompasses tumors exhibiting characteristics such as high-grade Ta or T1 tumors, carcinoma in situ (CIS), and T1 tumors with high-grade features. Very high-risk bladder cancer includes Bacillus Calmette-Guérin (BCG) unresponsive, variant histology, lymphovascular invasion, and involvement of the prostatic urethra. Patients with high-risk NMIBC are reported to have especially high recurrence rates of up to 80%, and progression rates of up to 50% in 5 years [5]. In these patients, intravesical BCG instillation of after transurethral resection of bladder tumor (TURBT) is recommended, which includes a 6-week initiation phase and subsequent maintenance sessions.
However, intravesical BCG therapy shows local or systemic unfavorable reactions in approximately 70% of cases, and 5%–9% of patients discontinue the treatment due to adverse effects [6]. In addition, a global shortage of BCG has occurred due to various factors, including the complexity of mass production. The BCG vaccine has a lengthy incubation period and requires specialized equipment, making it susceptible to bacterial contamination. These factors ultimately led to the closure of the BCG vaccine production facility at Sanofi in 2012. Consequently, extensive research has been conducted worldwide to explore alternatives to replace BCG.
Meanwhile, a Cochrane review in 2012 found intravesical gemcitabine had similar efficacy to BCG, at least in the intermediate risk group, and was superior in BCG-refractory patients, while inferior in high-risk patients [7]. In Korea, the use of intravesical gemcitabine was approved as off-label therapy in July 2018, licensed in May 2019, and covered by insurance benefits in February 2022. Because it was approved for use recently, there is a lack of studies about the outcomes of intravesical gemcitabine in the Korean population. In this study, we analyzed the short-term outcomes of intravesical gemcitabine for NMIBC patients in Korea.
MATERIALS AND METHODS
The patient records and information were anonymized prior to analysis. Data were retrospectively collected from patients who received weekly intravesical gemcitabine therapy for 6 weeks for NMIBC at our institution from August 2019 to March 2022. This retrospective study received approval from the Institutional Review Board of Yonsei University Severance Hospital (approval number: 4-2023-0220). The written informed consent was waived due to the study’s retrospective design. Patients with high-risk or very high-risk NMIBC, based on the AUA risk classification, who refused cystectomy were included. There were 3 intermediate-risk patients who were excluded because the group affected by the BCG shortage was limited to those with high risk or above [4]. Patients who had incomplete data, terminated intravesical gemcitabine therapy before 6 weeks for any reason, had muscle-invasive bladder cancer, or had coexistence of upper tract urothelial carcinoma were excluded.
All patients had undergone TURBT, and gemcitabine was initiated approximately 4–6 weeks after TURBT. Patients received 2,000 mg intravesical gemcitabine in 50 mL saline for 1 hour once a week for 6 consecutive weeks for induction therapy. Four weeks after induction therapy, maintenance treatment was recommended and if patient agree we entered the maintenance therapy. When patients entered the maintenance therapy period, gemcitabine was further administered once a month for 12 months. The side effects of intravesical gemcitabine (i.e., severe lower urinary tract symptoms, hematuria, or fever) were monitored at each visit. The severity grading of side effects was assessed according to the Common Terminology Criteria for Adverse Events, version 5.0 [8].
Cystoscopy was performed 4 weeks after the end of induction therapy, and subsequent cystoscopies were performed every 3 months for 2 years and at 6-month intervals thereafter. Recurrence was defined as histological confirmation on subsequent cystoscopic biopsy or TURBT. Disease-free survival (DFS) was defined as the time from the start of induction therapy to recurrence and evaluated by the Kaplan–Meier method. Cox regression analysis was performed to identify predictive factors for DFS. The level of significance was set at 0.05 in all analysis. All statistical analysis was performed using IBM SPSS version 25.0 (IBM Corp.).
RESULTS
Table 1 presents the clinical characteristics of the patients according to risk group. A total of 60 patients were included, with 45 patients in the high-risk group and 15 patients in the very high-risk group. The patients who showed variant histology (n=1), prostatic urethra invasion (n=5), and BCG failure (n=9) were classified as the very high-risk group. The median age was 76 years (interquartile range [IQR] 68–79) for the high-risk group and 68 years (IQR 62–77) for the very high-risk group (p=0.099). In group 1 and 2, 39 patients (86.7%) and 13 patients (86.7%) were male, respectively (p>0.999). The presence of CIS was observed in 17 patients (37.8%) in the high-risk group and 5 patients (33.3%) in the very high-risk group (p=0.757). Regarding the T stage, there was a significant difference between the two risk groups (p=0.048). In the high-risk group, 6 patients (13.3%) had Ta tumors, 31 patients (68.9%) had T1 tumors, and 8 patients (17.8%) had Tis tumors. In the very high-risk group, all 15 patients had T1 tumors. Thirty-eight patients (84.4%) in the high-risk group and 12 patients (80.0%) in the very high-risk group had multifocal tumor lesions (p=0.700). There was no significant difference in tumors larger than 3 cm, with 16 patients (35.6%) in the high-risk group and 4 patients (26.7%) in the very high-risk group (p=0.527).
Table 1. Patient characteristics according to risk group.
| Characteristic | High (n=45) | Very high (n=15) | p-value | |
|---|---|---|---|---|
| Age (y) | 76 (68–79) | 68 (62–77) | 0.099 | |
| Sex, male | 39 (86.7) | 13 (86.7) | <0.999 | |
| Carcinoma in situ | 17 (37.8) | 5 (33.3) | 0.757 | |
| T stage | 0.048 | |||
| Ta | 6 (13.3) | 0 (0.0) | ||
| T1 | 31 (68.9) | 15 (100.0) | ||
| Tis | 8 (17.8) | 0 (0.0) | ||
| Number of lesions | 0.700 | |||
| Solitary | 7 (15.6) | 3 (20.0) | ||
| Multifocal | 38 (84.4) | 12 (80.0) | ||
| Size | 0.527 | |||
| ≤3 cm | 29 (64.4) | 11 (73.3) | ||
| <3 cm | 16 (35.6) | 4 (26.7) | ||
| Variant histology | 0 (0.0) | 1 (6.7) | 0.250 | |
| Prostatic urethra invasion | 0 (0.0) | 5 (33.3) | 0.001 | |
| Prior BCG | 0.003 | |||
| No | 29 (64.4) | 3 (20.0) | ||
| Yes | 16 (35.6) | 12 (80.0) | ||
| BCG failure | 0 (0.0) | 9 (60.0) | <0.001 | |
| Gemcitabine maintenance | 6 (13.3) | 3 (20.0) | 0.678 | |
Values are presented as median (interquartile range) or number (%).
BCG, Bacillus Calmette–Guérin.
One patient (6.7%) in the very high-risk group had variant histology, which was urothelial carcinoma with glandular differentiation, while none were observed in the high-risk group (p=0.250). Prostatic urethra invasion was found in 5 patients (33.3%) in the very high-risk group, compared to none in the high-risk group, showing a significant difference (p=0.001). Sixteen patients (35.6%) in the high-risk group and 12 patients (80.0%) in the very high-risk group had received prior BCG therapy (p=0.003). They did not undergo BCG maintenance. BCG failure was observed in 9 patients (60.0%) in the very high-risk group, while none were observed in the high-risk group (p<0.001). Six patients (13.3%) in the high-risk group and 3 patients (20.0%) in the very high-risk group received gemcitabine maintenance therapy (p=0.678).
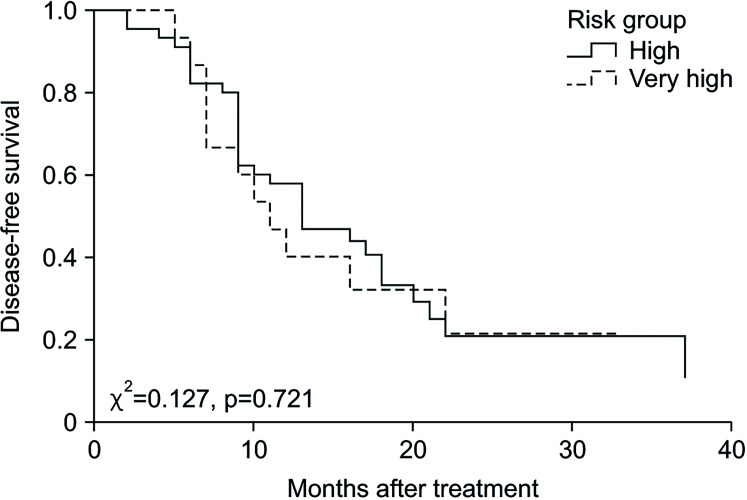
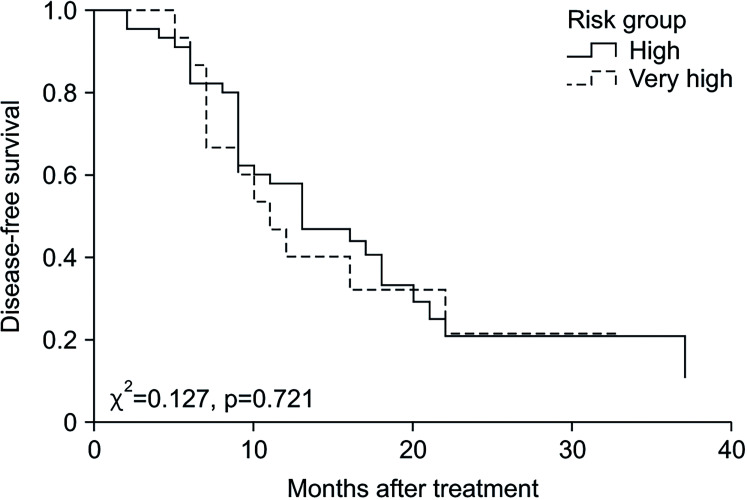
Outcomes of the intravesical gemcitabine therapy according to risk group are shown in Table 2. The median value of follow-up duration was 22 months (IQR 16–30) in total, 19 months (IQR 16–29) in the high-risk group, and 26 months (IQR 21–38) in the very high-risk group (p=0.313). The recurrence rate did not significantly differ between the two groups, with 31 patients (68.9%) in the high-risk group and 11 patients (73.3%) in the very high-risk group (p=0.509). The median duration of DFS was 13 months (IQR 9–17) in the high-risk group and 11 months (IQR 7–19) in the very high-risk group (p=0.831). The Kaplan–Meier curve for DFS is demonstrated in Fig. 1 and there was no difference between the two groups (p=0.721). The DFS rates of the high-risk group and the very high-risk group were 57.8% vs. 40% at 1 year, 20.7% vs. 21.3% at 2 years, and 20.7% vs. 21.3% at 3 years, respectively (p=0.831). Univariate analysis indicated Tis stage (HR 3.963, p=0.048) and prostatic urethra invasion (HR 2.718, p=0.044) were significant predictors of short DFS. Risk group (HR 1.054, p=0.881) was not associated with DFS. Multivariate analysis revealed that Tis stage (HR 4.162, p=0.042) and prostatic urethra invasion (HR 3.084; p=0.028) remained significant predictors (Table 3). Cancer specific mortality showed no significant difference between the high-risk and very high-risk group (2.2% and 6.7%, p=0.441).
Table 2. Outcomes according to risk group.
| High (n=45) | Very high (n=15) | p-value | |
|---|---|---|---|
| Follow-up duration (mo) | 19 (16–29) | 26 (21–38) | 0.313 |
| Recurrence | 31 (68.9) | 11 (73.3) | 0.509 |
| Disease-free survival (mo) | 13 (9–17) | 11 (7–19) | 0.831 |
| Cancer-specific mortality | 1 (2.2) | 1 (6.7) | 0.441 |
Values are presented as median (interquartile range) or number (%).
Fig. 1. Kaplan–Meier curve of disease-free survival.
Table 3. Cox regression analysis for disease-free survival.
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age | 1.010 | (0.975–1.046) | 0.580 | - | - | - | |
| Sex | |||||||
| Male | 1 | (reference) | - | - | - | - | |
| Female | 1.209 | (0.468–1.120) | 0.695 | - | - | - | |
| Carcinoma in situ | 1.453 | (0.783–1.696) | 0.236 | - | - | - | |
| T stage | |||||||
| Ta | 1 | (reference) | - | 1 | (reference) | - | |
| T1 | 1.816 | (0.554–1.950) | 0.325 | 1.628 | (0.492–5.386) | 0.425 | |
| Tis | 3.963 | (1.009–1.562) | 0.048 | 4.162 | (1.056–16.402) | 0.042 | |
| Number of lesions | |||||||
| Solitary | 1 | (reference) | - | - | - | - | |
| Multifocal | 1.501 | (0.630–1.575) | 0.359 | - | - | - | |
| Size | |||||||
| ≤3 cm | 1 | (reference) | - | - | - | - | |
| >3 cm | 1.497 | (0.788–1.844) | 0.218 | - | - | - | |
| Variant histology | 0.045 | (0.000–1.497) | 0.392 | - | - | - | |
| Prostatic urethra invasion | 2.718 | (1.029–1.182) | 0.044 | 3.084 | (1.128–8.430) | 0.028 | |
| Prior BCG | 0.934 | (0.509–1.717) | 0.827 | - | - | - | |
| BCG failure | 0.844 | (0.354–1.015) | 0.703 | - | - | - | |
| Gemcitabine maintenance | 0.510 | (0.200–1.305) | 0.160 | - | - | - | |
| Risk group | 1.054 | (0.527–1.108) | 0.881 | - | - | - | |
HR, hazard ratio; CI, confidence interval; BCG, Bacillus Calmette-Guérin.
Side effects were observed in 7 patients (15.6%) in the high-risk group and 2 patients (13.3%) in the very high-risk group (p=0.601). Urinary symptoms were reported by 4 patients (8.9%) in the high-risk group and 1 patient (6.7%) in the very high-risk group. Hematuria was observed in 1 patient (2.2%) in the high-risk group and 1 patient (6.7%) in the very high-risk group, while hematuria requiring hospitalization (grade 3 in Common Terminology Criteria for Adverse Events, version 5.0) was reported in 1 patient (2.2%) in the high-risk group. One patient (2.2%) in the high-risk group experienced fatigue as a side effect. These adverse events were deemed to be manageable in their severity.
DISCUSSION
For the treatment of high-risk NMIBC, the recommended options include TURBT, followed by intravesical BCG therapy. Intravesical BCG therapy has been proven to reduce the progression to muscle-invasive disease and is commonly used as a first-line treatment for high-risk NMIBC patients [9]. However, BCG is derived from Mycobacterium bovis, face challenges is mass production due to factors like quality control and susceptibility to contamination. Sanofi closed its BCG production facility in 2012, and Merck continues to struggle with supply shortages. And these leads worldwide BCG shortage for intravesical BCG [10,11].
Moreover, the use of intravesical BCG can result in both local and systemic effects. Local complications, which are more common, occur in approximately 62.8%–75.2% of patients and are usually not severe. These complications, such as chemical cystitis, bacterial cystitis, hematuria, and increased urination frequency, show up shortly after BCG treatment and typically resolve within 48–72 hours. Each of these conditions affects approximately 20%–50% of patients, based on extensive studies. On the other hand, systemic side effects are less frequent but still impact approximately 30%–40% of patients. They can occasionally be life-threatening. These symptoms include general discomfort (15.5%–24.8% of patients); skin rash (2.2%–2.7%); fever (7.5%–17.1%); and infections, such as sepsis (1%–9% of patients). Some data suggest that only 29.4%–66.7% of patients initially suspected of having BCG-related sepsis actually have a confirmed BCG infection, as opposed to bacterial infection or a hypersensitivity reaction. BCG infections have been found in various organs, including the prostate, epididymis, testicles, glans penis, liver, kidneys, and lungs [12].
In light of the ongoing BCG shortage and the side effects, there is a need for the evaluation of alternative regimens that can demonstrate similar effectiveness for high-risk or very high-risk NMIBC patients who require early radical cystectomy but decline this. According to the National Comprehensive Cancer Network guidelines, in cases of BCG failure among patients categorized as very high risk, cystectomy is considered the primary recommendation. However, as an alternative, intravesical chemotherapy is also advised. The available chemotherapy agents include epirubicin, gemcitabine, mitomycin, interferon alpha, thiotepa, and immune checkpoint inhibitors. Furthermore, intravesical maintenance therapy with mitomycin C (MMC) has been reported to reduce the risk of recurrence in intermediate- and high-risk NMIBC, although it is acknowledged to be less effective than BCG [13]. A meta-analysis of 2,820 NMIBC patients revealed that BCG maintenance therapy lowered the risk of recurrence by 32% compared to that of MMC maintenance therapy. However, in situations where BCG maintenance therapy was not employed, the MMC maintenance group exhibited a 28% higher risk of recurrence. Notably, there were no discernible differences in overall survival, progression-free survival, and cancer-specific survival rates [14].
Lamm et al. [15] showed that BCG treatment led to a notably greater percentage of complete responses compared to doxorubicin. Of the 67 patients undergoing doxorubicin, 23 achieved a complete response, while 45 of the 64 patients undergoing BCG instillation achieved the same. The median time for a complete response was 3.4 months for those treated with doxorubicin and 2.9 months for those treated with BCG [15].
Intravesical gemcitabine is used as an alternative therapy or secondary therapy [16]. Prasanna et al. [9] showed that intravesical BCG continues to be the established first-line adjuvant therapy; however, gemcitabine may be a viable alternative for patients who are unsuitable for BCG treatment or those who have experienced BCG relapse. Gemcitabine has demonstrated comparable DFS rates and, in certain instances, potential superiority, along with a significantly improved safety profile. In their study, the 2-year DFS rate of gemcitabine was 55%, whereas it was 48% in the BCG group [9]. Based on the existing evidence, it appears that intravesical gemcitabine could play a role in treating intermediate-risk patients, serving as an alternative to MMC for individuals with recurrent disease after prior treatment and for high-risk patients with NMIBC who have not responded to BCG therapy [17].
Shelley et al. [17] found that in high-risk patients, gemcitabine demonstrated a significantly higher recurrence rate compared to that of BCG (53.1% vs. 28.1%), with a shorter time to recurrence for gemcitabine (25.5 months vs. 39.4 months). Additionally, in a separate trial involving high-risk patients who had previously failed intravesical BCG therapy, gemcitabine was associated with significantly fewer recurrences (52.5% vs. 87.5%) and a longer time to recurrence (3.9 months vs. 3.1 months) compared to those of BCG [7].
In Korea, intravesical gemcitabine was initially employed as an off-label therapy until it gained recent recognition for medical insurance coverage in 2022. Consequently, there is a scarcity of research on intravesical gemcitabine in Korea. While diverse international studies have substantiated the efficacy of intravesical gemcitabine in NMIBC, the duration of insurance coverage in Korea is relatively short, contributing to a limited body of studies on the Korean population. With a 22-month follow-up period, the authors of this study aimed to evaluate the efficacy of intravesical gemcitabine on the Korean population. In the present study, gemcitabine instillation demonstrated a median DFS duration of 13 months for the high-risk group and 11 months for the very high-risk group. The recurrence rate was 68.9% (31 patients) in the high-risk group and 73.3% (11 patients) in the very high-risk group. The outcomes did not exhibit a notable distinction between the high-risk group and the very high-risk group, the latter of which requires early cystectomy. However, these findings revealed higher recurrence rates compared to the findings of the Cochrane review. Moreover, we identified Tis stage and prostatic urethra invasion as predictors of DFS. Risk group, prior BCG therapy or gemcitabine maintenance therapy were not significantly associated with survival. However, prostatic urethra invasion was considered a very high-risk factor. Therefore, analysis through future studies targeting larger cohorts of very high-risk patients will be necessary.
In contrast to the above-mentioned research, Sternberg et al. [18] showed that in high risk NMIBC after BCG failure, intravesical gemcitabine instillation resulted in a cumulative 5-year disease progression observed in 19% of patients with BCG-refractory disease and 22% of those experiencing other forms of BCG failure. Out of the 69 patients assessed, 25 attained a complete response, 19 achieved a partial response, and 20 exhibited no response. In a prospective, randomized phase 2 trial involving patients with high-risk NMIBC and 1 failed BCG course, participants were randomly assigned to receive either intravesical gemcitabine or intravesical BCG treatment. The trial enrolled 80 participants, with 40 in each group. The results demonstrated a lower disease recurrence rate in the intravesical gemcitabine group (52.5%) compared to that in the intravesical BCG group (87.5%) (p=0.002). Additionally, the intravesical gemcitabine group exhibited a significantly higher 2-year recurrence-free survival rate (19%) compared to the intravesical BCG group (3%) (p<0.008) [19]. This establishes that gemcitabine instillation could potentially serve as an effective therapy, either in cases of BCG shortage for high-risk NMIBC or for patients with very high-risk NMIBC who refuse radical cystectomy.
In this regard, intravesical gemcitabine appears to be more effective than other intravesical instillation agents in the context of BCG shortage. However, rather than replacing BCG, it seems more appropriate to compare it with a larger patient population and long-term outcomes, particularly in high-risk BCG-failure patients who refuse cystectomy.
While our study had certain limitations. Notably its retrospective design and the small number of patients confined to high-risk and very high-risk groups, it also boasted strengths. We investigated the efficacy of intravesical gemcitabine for both high-risk patients in the context of the BCG shortage and very high-risk patients who refused radical cystectomy, with a total follow-up duration of 22 months. In a recent multicenter study from Korea comparing intravesical gemcitabine and BCG in intermediate and high-risk patients, the authors also noted a limitation of small sample size of 63 patients treated with intravesical gemcitabine, with a follow-up period of 9.2 months [20]. Given its relatively recent inclusion in insurance coverage in Korea, further research on intravesical gemcitabine for high-risk and very high-risk bladder cancer among Koreans appears to be necessary. However, further investigation with larger patient cohorts and longer follow-up period is warranted.
CONCLUSIONS
Our study demonstrates that real-world efficacy of treatment for high-risk and very high-risk patients based on short-term results in Korea. Similar DFS outcome between two risk groups were observed. This finding is crucial for clinical practice as it provides evidence that the existing treatment protocols are equally effective for both high-risk and very high-risk patient populations. The results of this preliminary study are promising; however, studies analyzing more patients and long-term outcomes are needed.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: None.
- Research conception and design: Gang Kyu Kim and Ji Eun Heo.
- Data acquisition: Gang Kyu Kim, Young Heun Jo, Jongsoo Lee, Hyun Ho Han, Won Sik Ham, Won Sik Jang, and Ji Eun Heo.
- Statistical analysis: Gang Kyu Kim and Ji Eun Heo.
- Data analysis and interpretation: Gang Kyu Kim and Ji Eun Heo.
- Drafting of the manuscript: Gang Kyu Kim.
- Critical revision of the manuscript: Ji Eun Heo.
- Administrative, technical, or material support: Gang Kyu Kim and Ji Eun Heo.
- Supervision: Ji Eun Heo.
- Approval of the final manuscript: all authors.
References
- 1.Kang MJ, Jung KW, Bang SH, Choi SH, Park EH, Yun EH, et al. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. 2023;55:385–399. doi: 10.4143/crt.2023.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ) Eur Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–475. doi: 10.1016/j.eururo.2005.12.031. discussion 475-7. [DOI] [PubMed] [Google Scholar]
- 6.Hurle R, Contieri R, Casale P, Morenghi E, Saita A, Buffi N, et al. Midterm follow-up (3 years) confirms and extends short-term results of intravesical gemcitabine as bladder-preserving treatment for non-muscle-invasive bladder cancer after BCG failure. Urol Oncol. 2021;39:195.e7–195.e13. doi: 10.1016/j.urolonc.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Jones G, Cleves A, Wilt TJ, Mason M, Kynaston HG, Shelley M. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev. 2012;1:CD009294. doi: 10.1002/14651858.CD009294.pub2. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [internet] National Institutes of Health; 2017. Nov 27, [cited 2023 Aug 21]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. [Google Scholar]
- 9.Prasanna T, Craft P, Balasingam G, Haxhimolla H, Pranavan G. Intravesical gemcitabine versus intravesical Bacillus Calmette-Guérin for the treatment of non-muscle invasive bladder canceR: an evaluation of efficacy and toxicity. Front Oncol. 2017;7:260. doi: 10.3389/fonc.2017.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dal Moro F. BCG shortage in Europe. Prev Med. 2013;57:146. doi: 10.1016/j.ypmed.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Yoo J, Kim YJ, Tae BS, Park JY. Therapeutic options in high-risk nonmuscle invasive bladder cancer during the shortage of Bacillus Calmette-Guérin. J Urol Oncol. 2020;18:73–77. [Google Scholar]
- 12.Koch GE, Smelser WW, Chang SS. Side effects of intravesical BCG and chemotherapy for bladder cancer: what they are and how to manage them. Urology. 2021;149:11–20. doi: 10.1016/j.urology.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Sung HH. Optimal management of Bacillus Calmette-Guérin-refractory non-muscle-invasive bladder cancer in 2023. J Urol Oncol. 2023;21:228–240. [Google Scholar]
- 14.Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Lamm DL, Blumenstein BA, Crawford ED, Montie JE, Scardino P, Grossman HB, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–1209. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 16.Han MA, Maisch P, Jung JH, Hwang JE, Narayan V, Cleves A, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev. 2021;6:CD009294. doi: 10.1002/14651858.CD009294.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelley MD, Jones G, Cleves A, Wilt TJ, Mason MD, Kynaston HG. Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review. BJU Int. 2012;109:496–505. doi: 10.1111/j.1464-410X.2011.10880.x. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg IA, Dalbagni G, Chen LY, Donat SM, Bochner BH, Herr HW. Intravesical gemcitabine for high risk, nonmuscle invasive bladder cancer after bacillus Calmette-Guérin treatment failure. J Urol. 2013;190:1686–1691. doi: 10.1016/j.juro.2013.04.120. [DOI] [PubMed] [Google Scholar]
- 19.Di Lorenzo G, Perdonà S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116:1893–1900. doi: 10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Kim KH, Kim HS, Yoon HS, Kim JH, Kim JW, et al. Comparative analysis of recurrence rates between intravesical gemcitabine and bacillus Calmette-Guérin induction therapy following transurethral resection of bladder tumors in patients with intermediate- and high-risk bladder cancer: A retrospective multicenter study. Investig Clin Urol. 2024;65:248–255. doi: 10.4111/icu.20230313. [DOI] [PMC free article] [PubMed] [Google Scholar]