Abstract
Background
The role of routine intravascular imaging in percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) remains unclear. This study evaluated the clinical outcomes of PCI guided by different imaging modalities in AMI patients.
Materials and methods
Data from AMI patients who had undergone PCI between 2012 and 2022 were analyzed. The mean follow-up was 12.9 ± 1.73 months. The imaging modality-either intravascular ultrasound (IVUS), optical coherence tomography (OCT), or angiography alone-was selected at the operator's discretion. The primary endpoint was major adverse cardiac events (MACEs), including cardiovascular (CV) death, myocardial infarction (MI), target vessel revascularization.
Results
Of the 1,304 PCIs performed, 47.5% (n = 620) were guided by angiography alone, 37.0% (n = 483) by IVUS, and 15.4% (n = 201) by OCT. PCI guided by intravascular imaging modalities was associated with lower 1-year rates of MI (1.3%, P = 0.001) and MACE (5.2%, P = 0.036). OCT-guided PCI was linked to lower rates of 1-year CV death (IVUS vs. OCT: 6.2% vs. 1.5%, P = 0.016) and MACE (IVUS vs. OCT: 6.4% vs. 2.5%, P = 0.032). Intravascular imaging modalities and diabetes were identified as predictors of better and worse 1-year MACE outcomes, respectively.
Conclusion
PCI guided by intravascular imaging modalities resulted in improved 1-year clinical outcomes compared to angiography-guided PCI alone in AMI patients. OCT-guided PCI was associated with lower 1-year MACE rates compared to IVUS-guided PCI. Therefore, intravascular imaging should be recommended for PCI in AMI, with OCT being particularly considered when appropriate.
Keywords: acute myocardial infarction (AMI), percutaneous coronary intervention (PCI), optical coherence tomography (OCT), intravascular ultrasound (IVUS), intravascular image, MACE
Introduction
Emergent reperfusion of the ischemic myocardium represents the most significant advancement in treating acute myocardial infarction (AMI) over the past three decades. Studies have demonstrated that adequate reperfusion in AMI patients leads to reduced infarct size, lower early death rates, preserved left ventricular function, and improved survival (1, 2). The culprit lesion in AMI often arises from acute occlusion of a major coronary artery due to a large thrombus, vessel spasm, or acute coronary dissection. Identifying the “true” culprit lesion using angiography alone is challenging, as it may be located proximal or distal to the angiographically identified site (3). This difficulty poses a challenge for interventional cardiologists in selecting the appropriate stent placement zone and in choosing the correct stent size and length for AMI patients undergoing PCI. Both Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) imaging provide tomographic cross-sectional imaging of the vessel wall, offering valuable morphological information regarding the coronary lesion, aiding in stent size selection, optimizing stent expansion based on vessel diameter, and detecting incomplete apposition, longitudinal stent deformation and/or edge dissection (4–10). A large observational cohort study from the Pan-London PCI registry documented that OCT-guided PCI in patients with stable coronary artery disease was associated with improved procedural outcomes, in-hospital events, and long-term survival compared to those associated with standard angiography-guided PCI alone (11). Additionally, a few non-inferiority trials have shown no difference in cardiovascular outcomes between OCT-guided PCI and IVUS-guided PCI in elective PCI patients (12–14). Recently in a 2024 meta-analysis comparing intravascular imaging-guided and angiography-guided PCI, it was found that using intravascular imaging (OCT or intravascular ultrasound) improves PCI safety and effectiveness (15). This includes reduced risks of death, myocardial infarction, stent thrombosis, and repeat revascularization compared to angiography alone. Notably, outcomes were similar between OCT-guided and intravascular ultrasound-guided PCI. In another multicenter randomized controlled trial, IVUS-guided PCI was found to significantly reduce 1-year rates of target vessel failure in ACS patients compared to angiography-guided PCI, due to fewer MIs and revascularizations (16). However, the benefits of routine incorporating invasive imaging modalities (IVUS or OCT) into AMI PCI and the comparison between these modalities remain controversial in real-world clinical practice (17–19). Therefore, this study aimed to compare clinical outcomes between angiography alone and invasive imaging modalities (IVUS and OCT)-guided PCI in AMI patients.
Methods
Study population and enrollment
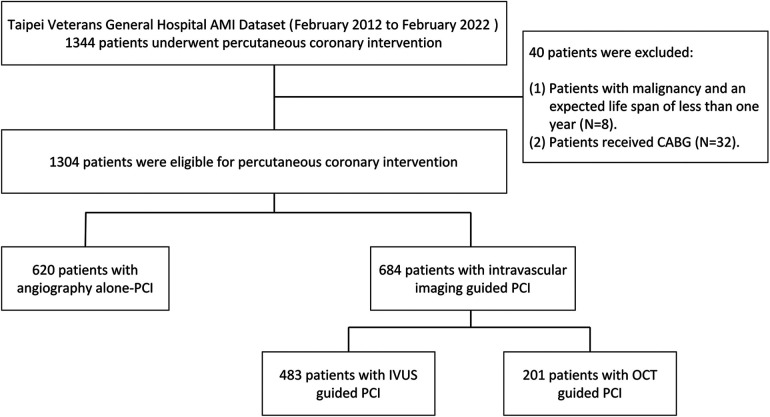
This retrospective registry comprised consecutive AMI patients who had undergone PCI from February 2012 to February 2022 (Figure 1). Chronic kidney disease (CKD) was defined as eGFR < 60 ml/min/1.73 m2. AMI was characterized by the presence of significant new Q waves in at least two electrocardiography leads or symptoms compatible with MI, accompanied by an increase in cTn above the 99th percentile upper reference limit (20). Data on baseline and procedural characteristics, medical history, clinical examination, operation records, and clinical outcomes were collected through a medical chart review. AMI PCI was conducted following the standard practices of our hospital. The procedures were performed using either the radial or femoral approach. All patients received dual antiplatelet therapy before the procedure, and the activated clotting time was maintained between 250 and 300 s throughout the procedure. The administration of glycoprotein IIb/IIIa receptor antagonists and/or manual thrombus aspiration was at the operator's discretion. Patients were categorized based on the imaging modalities used to evaluate lesion characteristics, which included angiography alone, angiography plus IVUS, and angiography plus OCT. The selection of the invasive imaging modality was at the operator's discretion. Although there are no strict written rules at our hospital, the indications and systematic criteria were based on the global current consensus for pre- and post-stenting goals: (1) selecting suitable patients, (2) pre-stenting balloon sizing, (3) stent sizing, (4) post-stenting balloon sizing, (5) ensuring complete apposition and adequate expansion (no underexpansion or malapposition), and (6) avoiding edge dissection (21). The study protocol received approval from the institutional review board at Taipei Veterans General Hospital.
Figure 1.
The flow of study. CABG: Coronary artery bypass graft surgery.
Imaging modalities (IVUS and OCT)
Our hospital is a medical center with a high volume of intravascular procedures. Intravascular imaging is routinely performed in nearly all PCIs, with an 80% penetration rate in stable coronary artery disease. In AMI PCI, invasive imaging (IVUS and OCT) may be performed after the restoration of TIMI flow in the target vessel, either after thrombectomy or the use of small balloon inflation. In this study, operators carefully reviewed the IVUS and OCT imaging results and chose the landing zone and the stent size and length. After stent implantation, IVUS or OCT pullback was performed again to identify any suboptimal results, such as stent under-expansion or malposition.
Clinical follow-up and outcomes
In-hospital complications were documented at the time of discharge. Clinical outcomes, including death, myocardial infarction (MI), and target vessel revascularization (TVR), were recorded 12 months post-discharge. MACE, a composite endpoint, encompasses cardiovascular (CV) death, MI, and TVR. CV death is classified as any death definitively caused by CV issues or any death not explicitly attributed to non-CV causes. Non-fatal MI is characterized by significant new Q waves in at least two electrocardiography leads or symptoms indicative of MI, accompanied by an elevation in cTn above the 99th percentile upper reference limit (20). TVR is the clinically driven repeat revascularization during follow-up due to restenosis, either within the target lesion or the same epicardial coronary artery. Confirmation of all cardiac events was obtained through a review of patient medical records via a dedicated electronic system, which recorded patient events, hospitalizations, and outpatient clinic follow-up details.
Statistical analysis
Continuous and categorical variables were represented as means ± standard deviation and numbers with percentages, respectively. We used Chi-square or Fisher's exact test for categorical variables and Student's t-test, one-way ANOVA, or Kruskal–Wallis test for continuous variables to compare baseline characteristics between groups. We conducted PSM at a 1:1 ratio to ensure robust matching of patients. The matching criteria included age, gender, diabetes mellitus (DM), chronic kidney disease (CKD)/end-stage renal disease (ESRD), and congestive heart failure (CHF). These variables were chosen to reduce bias from confounding variables by indication. To evaluate the effectiveness of the matching process, we calculated standardized mean differences (SMDs) before and after matching, and the SMDs below the threshold of 0.1 for all included variables. Cox regression model is performed to examine the association of clinical outcomes within 1 year follow-up period. The hazard ratio (HR) and 95% CI are calculated and p value of <0.05 is considered to be statistically significant. The Kaplan–Meier (KM) method was used to calculate the cumulative survival rate for different imaging modalities. All tests were two-tailed, and P values ≤0.05 were considered statistically significant.
Results
Demographic and procedural characteristics
During the study period, 1,304 consecutive AMI patients who had undergone PCI were enrolled. Of these, 47.5% (n = 620) underwent angiography-guided PCI, 37.0% (n = 483) underwent IVUS-guided PCI, and 15.4% (n = 201) underwent OCT-guided PCI. We classified the patients into two groups: those who received angiography-guided PCI alone and those who received intravascular imaging-guided PCI. The baseline and procedural characteristics of the enrolled patients are discussed in Table 1. Patients in the intravascular imaging-guided PCI group had more stents used (2.9 ± 1.4, P < 0.001) and greater stent lengths (23.2 ± 9.0, P < 0.001). The propensity score matching (PSM) for assessing the impact of imaging modalities is discussed in Table 2. The patients in the intravascular imaging-guided PCI group still had greater stent lengths (22.1 ± 8.3, P < 0.001).
Table 1.
Demographic & procedural data of patients with acute myocardial infarction.
| Variables | Angiography alone | Intra-vascular imaging | P-value |
|---|---|---|---|
| N = 620 | N = 684 | ||
| Baseline characteristics | |||
| Male | 514 (82.9%) | 564 (82.5%) | 0.961 |
| Age | 62.3 ± 11.8 | 62.5 ± 10.8 | 0.971 |
| Hypertension | 316 (51.0%) | 338 (49.4%) | 0.810 |
| Diabetes | 143 (23.1%) | 156 (22.8%) | 0.890 |
| Hyperlipidemia | 248 (40.0%) | 207 (30.3%) | 0.531 |
| CKD/ESRD | 176 (28.4%) | 157 (23.0%) | 0.734 |
| Smoker | 221 (35.6%) | 241 (35.2%) | 0.946 |
| Clinical presentation | |||
| NSTEMI | 385 (62.1%) | 402 (58.8%) | 0.892 |
| STEMI | 235 (37.9%) | 282 (41.2%) | 0.811 |
| Cardiac history | |||
| History of MI (>3M) | 41 (6.6%) | 39 (5.7%) | 0.767 |
| History of CHF | 46 (7.4%) | 38 (5.6%) | 0.453 |
| Prior PCI | 40 (6.5%) | 38 (5.7%) | 0.763 |
| LVEF (%) | 56.3 ± 10.3 | 51.6 ± 11.3 | 0.246 |
| Number of vessel disease | 0.716 | ||
| Single | 310 (50.0%) | 327 (47.8%) | |
| Double | 217 (35.0%) | 250 (36.5%) | |
| Triple | 93 (15.0%) | 107 (15.6%) | |
| Targeted vessel | |||
| LM | 10 (1.6%) | 38 (5.6%) | 0.001 |
| LAD | 370 (59.7%) | 428 (62.6%) | 0.484 |
| LCX | 143 (23.1%) | 128 (18.7%) | 0.186 |
| RCA | 270 (43.5%) | 306 (44.7%) | 0.889 |
| Procedural characteristics | |||
| Number of stents | 1.6 ± 0.9 | 2.9 ± 1.4 | <0.001 |
| Stent length | 18.8 ± 7.2 | 23.2 ± 9.0 | <0.001 |
| DES | 467 (75.3%) | 536 (78.4%) | 0.832 |
| BMS | 153 (24.7%) | 148 (21.6%) | 0.732 |
| Laboratory data | |||
| T. Cholesterol (mg/dl) | 153.6 ± 37.5 | 158.3 ± 44.2 | 0.366 |
| LDL-C (mg/dl) | 90.4 ± 34.0 | 93.9 ± 35.7 | 0.437 |
| eGFR | 62.4 ± 24.3 | 58.4 ± 26.6 | 0.768 |
| HbA1c | 6.71 ± 1.58 | 6.87 ± 1.27 | 0.761 |
BMS, bare metal stent; CHF, congestive heart failure; CKD/ESRD, chronic kidney disease/end stage renal disease; DES, drug eluting stent; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1C; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LDL-C, low density lipoprotein cholesterol; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST segment elevation myocardial infarction.
Table 2.
Propensity score matching of the impact of imaging modalities .
| Variables | Angiography alone | Intra-vascular imaging | P-value |
|---|---|---|---|
| N = 598 | N = 598 | ||
| Characteristics | |||
| Male | 493 (82.4%) | 486 (81.3%) | 0.971 |
| Age | 62.8 ± 11.4 | 62.1 ± 9.7 | 0.579 |
| Hypertension | 287 (48.0%) | 257 (43.0%) | 0.442 |
| Diabetes | 102 (17.1%) | 116 (19.4%) | 0.621 |
| Hyperlipidaemia | 245 (40.9%) | 180 (30.1%) | 0.421 |
| CKD/ESRD | 163 (27.3%) | 148 (24.7%) | 0.784 |
| Smoker | 198 (33.1%) | 204 (34.1%) | 0.836 |
| Clinical presentation | |||
| NSTEMI | 365 (61.0%) | 346 (57.9%) | 0.862 |
| STEMI | 233 (39.0%) | 252 (42.1%) | 0.801 |
| Cardiac history | |||
| History of MI (>3M) | 30 (5.0%) | 35 (5.9%) | 0.701 |
| History of CHF | 42 (7.0%) | 37 (6.2%) | 0.643 |
| Prior PCI | 32 (5.4%) | 33 (5.5%) | 0.863 |
| LVEF (%) | 56.1 ± 9.5 | 55.6 ± 10.4 | 0.946 |
| No. vessel disease | 0.702 | ||
| Single | 299 (50.0%) | 279 (46.7%) | |
| Double | 208 (34.8%) | 235 (39.2%) | |
| Triple | 91 (15.2%) | 84 (14.0%) | |
| Target vessel | |||
| LM | 7 (1.2%) | 14 (2.3%) | 0.413 |
| LAD | 349 (58.4%) | 364 (60.9%) | 0.697 |
| LCX | 135 (22.6%) | 103 (17.2%) | 0.216 |
| RCA | 267 (44.6%) | 301 (50.3%) | 0.371 |
| No. of stents used | 1.6 ± 1.0 | 2.6 ± 1.1 | 0.261 |
| Stent length | 18.2 ± 6.1 | 22.1 ± 8.3 | <0.001 |
| DES | 450 (75.2%) | 458 (76.6%) | 0.912 |
| BMS | 148 (24.7%) | 140 (23.4%) | 0.892 |
| Laboratory data | |||
| T. Cholesterol (mg/dl) | 155.1 ± 32.5 | 158.1 ± 41.2 | 0.322 |
| LDL-C (mg/dl) | 90.1 ± 34.1 | 93.2 ± 35.2 | 0.431 |
| eGFR | 62.3 ± 24.1 | 58.1 ± 26.3 | 0.760 |
| HbA1c | 6.70 ± 1.49 | 6.84 ± 1.22 | 0.760 |
BMS, bare metal stent; CHF, congestive heart failure; CKD/ESRD, chronic kidney disease/end stage renal disease; DES, drug eluting stent; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1C; LAD, left anterior descending coronary artery; LCX, Left circumflex coronary artery; LDL-C, low density lipoprotein cholesterol; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST segment elevation myocardial infarction.
In the subgroup analysis, patients undergoing intravascular imaging-guided PCI were categorized into OCT-guided and IVUS-guided PCI groups (Table 3). Those in the OCT-guided PCI group were older (63.5 ± 11.3, P = 0.001) and exhibited a greater incidence of multi-vessel disease (P = 0.001). The PSM for the subgroup analysis is shown in Table 4. After matching, no significant differences were observed between the two groups, except for a higher prevalence of LM disease in the OCT-guided PCI group (P = 0.032).
Table 3.
Characteristics of enrolled acute myocardial infarction patients in IVUS guided and OCT guided group.
| Variables | IVUS N = 483 | OCT N = 201 | P-value |
|---|---|---|---|
| Baseline Characteristics | |||
| Male | 396 (82.0%) | 168 (83.5%) | 0.866 |
| Age | 60.5 ± 10.5 | 63.5 ± 11.3 | 0.001 |
| Hypertension | 245 (50.7%) | 93 (46.3%) | 0.782 |
| Diabetes | 103 (21.3%) | 53 (26.3%) | 0.573 |
| Hyperlipidemia | 135 (28.0%) | 72 (35.8%) | 0.239 |
| CKD/ESRD | 110 (22.7%) | 47 (23.4%) | 0.722 |
| Smoker | 177 (36.6%) | 64 (31.8%) | 0.261 |
| Clinical presentation | |||
| NSTEMI | 284 (58.8%) | 118 (58.7%) | 0.922 |
| STEMI | 200 (41.4%) | 82 (40.7%) | 0.902 |
| Cardiac History | |||
| History of MI (>3M) | 24 (5.0%) | 15 (7.5%) | 0.207 |
| History of CHF | 24 (5.0%) | 14 (7.0%) | 0.424 |
| Prior PCI | 25 (5.2%) | 13 (6.5%) | 0.653 |
| LVEF (%) | 52.5 ± 10.1 | 50.5 ± 9.3 | 0.592 |
| Number of Vessel disease | 0.001 | ||
| Single | 251 (52.0%) | 76 (37.8%) | |
| Double | 168 (34.8%) | 82 (40.8%) | |
| Triple | 64 (13.3%) | 43 (21.4%) | |
| Targeted Vessel | |||
| LM | 18 (3.7%) | 20 (10.0%) | 0.013 |
| LAD | 308 (63.8%) | 120 (59.7%) | 0.373 |
| LCX | 71 (14.7%) | 57 (28.4%) | <0.001 |
| RCA | 215 (44.5%) | 91 (45.3%) | 0.872 |
| Procedural characteristics | |||
| Number of stents | 2.7 ± 1.1 | 3.1 ± 1.2 | 0.382 |
| Stent length | 22.6 ± 6.2 | 24.2 ± 7.1 | 0.362 |
| DES | 385 (79.7%) | 151 (75.1%) | 0.862 |
| BMS | 98 (20.3%) | 50 (24.9%) | |
| Laboratory data | |||
| T. Cholesterol (mg/dl) | 157.1 ± 42.5 | 159.1 ± 41.2 | 0.322 |
| LDL-C (mg/dl) | 92.1 ± 32.1 | 95.2 ± 32.2 | 0.431 |
| eGFR | 59.1 ± 22.1 | 58.1 ± 23.3 | 0.760 |
| HbA1c | 6.80 ± 1.61 | 6.88 ± 1.28 | 0.769 |
BMS, bare metal stent; CHF, congestive heart failure; CKD/ESRD, chronic kidney disease/end stage renal disease; DES, drug eluting stent; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1C; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LDL-C, low density lipoprotein cholesterol; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST segment elevation myocardial infarction.
Table 4.
Propensity score matching of the impact of different imaging modalities.
| Variables | IVUS | OCT | P-value |
|---|---|---|---|
| N = 190 | N = 190 | ||
| Characteristics | |||
| Male | 157 (82.6%) | 156 (82.1%) | 0.864 |
| Age | 61.9 ± 11.4 | 62.6 ± 10.5 | 0.631 |
| Hypertension | 80 (42.1%) | 81 (42.6%) | 0.999 |
| Diabetes | 45 (23.7%) | 42 (22.1%) | 0.757 |
| Hyperlipidaemia | 61 (32.2%) | 56 (29.5%) | 0.673 |
| CKD/ESRD | 40 (21.1%) | 44 (23.2%) | 0.763 |
| Smoker | 72 (37.9%) | 60 (31.6%) | 0.489 |
| Clinical presentation | |||
| NSTEMI | 109 (57.4%) | 111 (58.4%) | 0.911 |
| STEMI | 81 (42.6%) | 79 (41.6%) | 0.908 |
| Cardiac History | |||
| History of MI (>3M) | 7 (3.7%) | 10 (5.3%) | 0.451 |
| History of CHF | 11 (5.8%) | 12 (6.3%) | 0.824 |
| Prior PCI | 11 (5.8%) | 9 (4.7%) | 0.683 |
| LVEF (%) | 51.9 ± 10.4 | 52.6 ± 12.5 | 0.566 |
| No. Vessel disease | 0.379 | ||
| Single | 83 (43.7%) | 70 (36.8%) | |
| Double | 70 (36.8%) | 75 (39.9%) | |
| Triple | 37 (19.5%) | 45 (23.7%) | |
| Target Vessel | |||
| LM | 6 (3.2%) | 16 (8.4%) | 0.032 |
| LAD | 115 (60.5%) | 111 (58.4%) | 0.511 |
| LCX | 27 (14.2%) | 49 (25.8%) | 0.332 |
| RCA | 76 (40.0%) | 74 (38.9%) | 0.894 |
| Procedural characteristics | |||
| No. of stents used | 2.4 ± 0.9 | 2.8 ± 1.2 | 0.312 |
| Stent length | 22.0 ± 8.8 | 23.7 ± 9.3 | 0.370 |
| DES | 145 (76.3%) | 139 (73.2%) | 0.769 |
| BMS | 45 (23.7%) | 51 (26.8%) | |
| Laboratory data | |||
| T. Cholesterol (mg/dl) | 157.3 ± 40.5 | 159.0 ± 40.1 | 0.302 |
| LDL-C (mg/dl) | 91.1 ± 31.1 | 96.2 ± 31.2 | 0.231 |
| eGFR | 58.8 ± 21.1 | 58.3 ± 22.3 | 0.802 |
| HbA1c | 6.72 ± 1.6 | 6.87 ± 1.3 | 0.779 |
BMS, bare metal stent; CHF, congestive heart failure; CKD/ESRD, chronic kidney disease/end stage renal disease; DES, drug eluting stent; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1C; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LDL-C, low density lipoprotein cholesterol; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST segment elevation myocardial infarction.
Twelve-months clinical outcomes
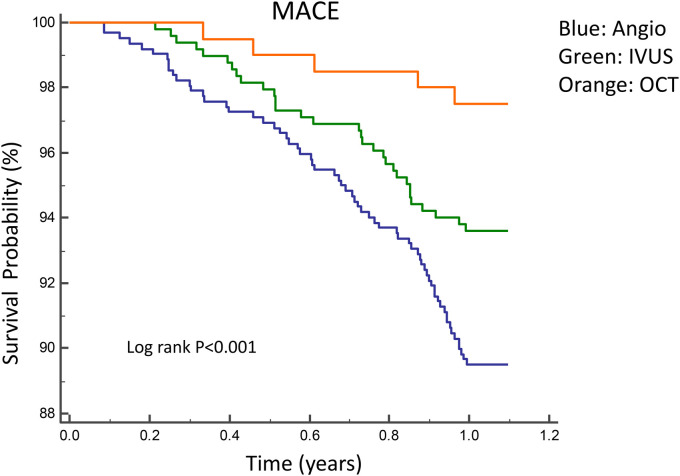
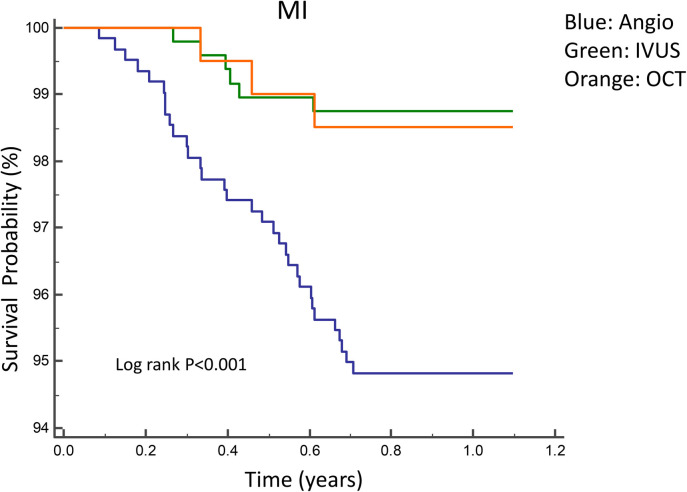
Figures 2, 3 illustrate the comparisons of clinical outcomes among angiography-guided, IVUS-guided, and OCT-guided PCI groups. Kaplan–Meier curves revealed significant differences in the risk of MI and MACE across the three groups, with the OCT-guided group exhibiting superior clinical outcomes. At 12 months, MI and MACE rates were significantly lower in the intravascular imaging-guided PCI group (MI: 1.3%, P = 0.001; MACE: 5.2%, P = 0.036, respectively; Table 5). The PSM analysis confirmed the continued significance of lower MACE rates (MACE: 1.7%, P = 0.028; Table 6).
Figure 2.
Kaplan–Meier curves for 1-year MACE among angiography-guided, IVUS-guided and OCT-guided PCI. MACE, Major adverse cardiac events.
Figure 3.
Kaplan–Meier curves for 1-year MI among angiography-guided, IVUS-guided and OCT-guided PCI.
Table 5.
1-year clinical outcomes between 2 groups of patients with acute myocardial infarction.
| Variables | Angiography Alone | Intra-Vascular Imaging | P-value |
|---|---|---|---|
| N = 620 | N = 684 | ||
| CV death | 28 (4.5%) | 33 (4.8%) | 0.762 |
| Non fatal MI | 32 (5.2%) | 9 (1.3%) | 0.001 |
| TVR | 12 (1.9%) | 10 (1.5%) | 0.422 |
| MACE | 65 (10.5%) | 36 (5.2%) | 0.036 |
CV, cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction; TVR, target vessel revascularization.
Table 6.
Propensity score matching of the impact of imaging modalities guided PCI on 1-year clinical outcomes.
| Variables | Angiography alone | Intra-Vascular Imaging | P-value |
|---|---|---|---|
| N = 598 | N = 598 | ||
| CV death | 15 (2.5%) | 20 (3.3%) | 0.561 |
| Non fatal MI | 14 (2.3%) | 3 (0.5%) | 0.081 |
| TVR | 10 (1.7%) | 2 (0.3%) | 0.156 |
| MACE | 25 (4.2%) | 10 (1.7%) | 0.028 |
CV, cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction; TVR, target vessel revascularization.
Subgroup analysis revealed that the 12-month CV death and MACE rates were significantly lower in the OCT-guided PCI group (MACE: 2.5%, P = 0.032; CV death: 1.5%, P = 0.016, respectively; Table 7). The PSM analysis confirmed the significance of the reduced 12-month MACE and CV death rate (MACE: 2.6%, P = 0.014; CV death: 1.6%, P = 0.032, respectively; Table 8).
Table 7.
One-year clinical outcomes of IVUS-guided vs. OCT-guided PCI.
| Variables | IVUS | OCT | P-value |
|---|---|---|---|
| N = 483 | N = 201 | ||
| CV death | 30 (6.2%) | 3 (1.5%) | 0.016 |
| Non fatal MI | 6 (1.2%) | 3 (1.5%) | 0.872 |
| TVR | 8 (1.7%) | 2 (0.9%) | 0.255 |
| MACE | 31 (6.4%) | 5 (2.5%) | 0.032 |
CV, cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction; TVR, target vessel revascularization.
Table 8.
Propensity score matching of the impact of different imaging modalities guided PCI on 1-year clinical outcomes.
| Variables | IVUS | OCT | P-value |
|---|---|---|---|
| N = 190 | N = 190 | ||
| CV death | 10 (5.3%) | 3 (1.6%) | 0.032 |
| Non fatal MI | 4 (2.1%) | 2 (1.1%) | 0.561 |
| TVR | 6 (3.2%) | 1 (0.5%) | 0.081 |
| MACE | 20 (10.5%) | 5 (2.6%) | 0.014 |
CV, cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction; TVR, target vessel revascularization.
Predictors of 1-year MACE
Cox regression analysis revealed the use of intravascular imaging as a strong predictor of improved 1-year MACE outcomes [Hazard Ratio (HR) = 0.38, P < 0.001; Table 9]. When adjusted for age, sex, diabetes, and hypertension in multivariate analysis, intravascular imaging use remained a robust predictor of favorable 1-year MACE results (HR: 0.29, P = 0.001; Table 9). Additionally, DM significantly predicted poorer 1-year MACE outcomes (HR = 3.28, P = 0.001; Table 9).
Table 9.
COX regression analysis for 1-year MACE in two groups (angiography-alone vs. intravascular guided).
| Variables | Uni-variate analysis hazard ratio (95% CI) | P-value | Multi-variate analysis hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Age | 1.12 (0.96, 1.21) | 0.111 | 1.14 (0.96, 1.28) | 0.138 |
| Sex | 0.76 (0.04, 2.16) | 0.211 | 0.59 (0.21, 1.89) | 0.261 |
| Diabetes | 4.16 (2.12, 8.41) | <0.001 | 3.28 (1.63, 6.55) | 0.001 |
| Hypertension | 1.66 (1.02, 2.58) | 0.026 | 1.42 (0.88, 2.37) | 0.138 |
| Intra-vascular imaging guided PCI | 0.38 (0.15, 0.66) | <0.001 | 0.29 (0.12, 0.56) | 0.001 |
PCI, percutaneous coronary intervention.
Discussion
Main findings and clinical relevance
This study demonstrated that intravascular-guided PCI is associated with improved 1-year non fatal MI and MACE outcomes compared to that associated with angiography-guided PCI alone. These findings are particularly relevant in the context of acute myocardial infarction (AMI) where optimizing stent deployment is critical. Furthermore, intravascular imaging serves as a predictor of enhanced 1-year MACE outcomes after adjusting for various variables. Moreover, subgroup analysis revealed that OCT use is linked with reduced 1-year CV death and MACE in AMI PCI.
Role of IVUS guidance in AMI patients
Theoretically, the advantage of IVUS guidance in AMI PCI may relate to improved stent implantation guidance, a known predictor of reduced restenosis or ST in elective PCIs. However, in AMI patients, determining the appropriate stent size, length, and optimization of stent deployment can be challenging due to the presence of a large thrombus burden, which may result in under- or oversized stent selection, smaller minimal stent CSA from under-expansion, or perforation from overexpansion. Large observational cohort studies, randomized trials, and meta-analyses have demonstrated a lower incidence of TVR and fewer MACEs, MI, and ST in IVUS-guided elective PCI compared to those associated with angiography-guided intervention (22–28). The HORIZONS-AMI trial's IVUS sub-study indicated that the final post-procedure minimal stent cross-sectional area (CSA) was a strong predictor of early ST and in-stent restenosis in AMI patients. A well-expanded stent with a final stent CSA ≥5 mm2 by IVUS was an independent predictor of freedom from ST and restenosis in AMI PCI (29). Furthermore, the ADAPT-DES study observed that IVUS-guided AMI PCI was associated with lower 1-year rates of ST, MI, and MACE (17). However, the benefit of routine IVUS guidance in AMI PCI remains controversial. A Korean study reported no prevention of ST events with IVUS-guided STEMI PCI, which was associated with a higher adverse event rate. This study noted greater stent lengths and a higher number of stents used in the IVUS-guided arm, potentially negating the benefits of IVUS for patients with stable CAD undergoing elective PCI (18). The CREDO-Kyoto AMI registry sub-analysis (19) reported no benefit of routine IVUS guidance in reducing TVR, ST, and mortality in AMI patients undergoing PCI. Conversely, a 2022 meta-analysis found that IVUS-guided AMI PCI was associated with a lower risk of all-cause mortality, MACE, and TVR compared to those associated with angio-guided PCI (30). This analysis included nine studies with a total of 838,902 patients and a maximum follow-up of five years, potentially supporting the use of IVUS in AMI PCI. However, data comparing IVUS-guided and OCT-guided AMI PCI remain limited. Our study observed that intravascular image-guided PCI was associated with lower event rates compared to that associated with angiography-guided PCI. Moreover, OCT-guided PCI was linked to even lower event rates than that associated with IVUS-guided PCI.
Role of OCT guidance in AMI patients
IVUS has fundamental limitations, such as slower catheter pullback, poor axial resolution (100–200 µm), and limited discrimination of plaque subtypes compared to those associated with OCT. OCT provides superior resolution (10 µm) images, capable of more accurately identifying lesion characteristics, dissection, plaque prolapse, stent mal-apposition, and strut coverage compared to that associated with IVUS (6–8). However, OCT is unsuitable for investigating large and totally occluded vessels, coronary arteries with massive dissection, left main or RCA ostial lesion and periprocedural stent-related complications. The CLI-OPCI registry results suggested that OCT use in patients undergoing PCI could improve clinical outcomes (31). In the CLI-OPCI study, OCT guidance was associated with a significantly lower risk of cardiac death and MI, even after multivariable analysis (OR = 0.49, P = 0.037) or propensity-score-adjusted analyses. The CLI-OPCI II study demonstrated that an in-stent minimum lumen area >4.5 mm2 [hazards ratio (HR): 1.64, P = 0.040] was an independent predictor of better clinical outcomes in non-left main lesion (32). A multicenter RCT, the OCTOBER trial, published in 2023, reported that OCT-guided PCI is associated with significantly lower MACE at 2 years compared to that associated with angio-guided PCI in complex bifurcation lesions (HR = 0.7, P = 0.035) (33). The cardiovascular outcomes were not affected by the increased contrast use and longer procedure time (33). Another meta-analysis supported this finding and further suggested that OCT use may improve outcomes in AMI patients (34). In OCT-guided AMI PCI, OCT imaging can identify the location of the culprit lesion, the site of thrombosis, and the longitudinal extent of disease in the culprit vessel. OCT imaging in AMI culprit lesions can provide useful information to distinguish between stent under-expansion and/or tissue prolapse. Due to its high resolution, OCT is more sensitive to the detection of stent mal-apposition, dissection, thrombus, and tissue protrusion, which may not be identified on angiography alone or even with IVUS. The DOCTORS trial (35) was the first randomized, prospective, multicenter trial to investigate the use of OCT in NSTEMI patients, revealing that OCT findings led to a change in procedural strategy in 50% of the patients, mainly driven by the optimization of stent expansion, and were associated with higher fractional flow reserve (FFR) values at the end of the procedure than those associated with angiography-guided PCI alone. In the present study, OCT-guided AMI PCI was associated with better clinical outcomes. After adjusting for differences in baseline characteristics across groups with different imaging modalities, 1-year MACE remained lowest with OCT guidance in AMI PCI compared to that associated with angiography-guided alone PCI and IVUS-guided PCI. The possible mechanism by which OCT-guided AMI PCI achieves better long-term outcomes may be due to several factors. First, OCT can detect plaque characteristics in more detail. Second, OCT offers superior resolution for thrombus recognition, making it more sensitive in identifying culprit vessels even after spontaneous thrombolysis and reperfusion, particularly in patients with multivessel NSTEMI. Third, the high resolution of OCT allows for more precise detection of stent malapposition, resulting in better stent apposition rates and larger post-stent minimal lumen areas.
Limitations
This study had several limitations. First, as a single-center study with high-volume OCT and IVUS usage, the results may not be generalizable to centers that do not routinely use invasive coronary imaging. Second, the decision to use IVUS or OCT imaging for PCI guidance, as well as the responses to IVUS and OCT findings, was at the operator's discretion. This could potentially introduce significant selection bias due to factors such as angiographic complexity, diagnostic uncertainty, the patient's hemodynamic instability and time pressure, or the operator's preference for one imaging modality over the other. However, despite some degree of selection bias, the study results enhance the potential that using intravascular imaging, especially OCT, may offer benefits for clinical outcomes. Third, our results may not be applicable to bioresorbable scaffold (BVS) implantation in AMI PCI, as only DES and BMS were used in this study. Fourth, with only 1-year follow-up data available, a larger-scale study with long-term outcomes (beyond one year) is necessary to confirm the superior benefits of OCT guidance observed in this study. Fifth, OCT's inability to image ostial and left main lesions represents another limitation.
While we adjusted for known variables such as age, gender, diabetes mellitus (DM), chronic kidney disease (CKD)/end-stage renal disease (ESRD), and congestive heart failure (CHF) using propensity score matching, unmeasured confounders may still have influenced our results. Factors such as operator experience, patient adherence to medication, and lifestyle changes post-PCI could not be fully accounted for and may have impacted clinical outcomes. As a retrospective study, our findings are subject to inherent biases associated with this study design. These include recall bias, selection bias, and the potential for incomplete or inaccurate data recording. While we utilized robust statistical methods to mitigate these biases, prospective, randomized controlled trials are necessary to validate our findings. In light of these limitations, our study should be interpreted with caution. We emphasize the need for further research, including large-scale, multicenter, prospective studies with long-term follow-up and economic evaluations, to confirm the clinical benefits and cost-effectiveness of OCT and IVUS-guided PCI in AMI patients.
Conclusion
Angiography remains the standard for guiding procedural decisions during primary PCI; however, it has well-known limitations in providing detailed information on vessel walls and atherosclerotic plaque characteristics. Our study suggests that imaging-guided PCI, particularly with OCT, is associated with improved 1-year clinical outcomes compared to angiography-guided PCI alone. Specifically, OCT-guided PCI in AMI appears to be associated with lower 1-year MACE rates compared to IVUS-guided PCI.
Given the study's limitations, including its single-center design and the focus on short-term outcomes, it is important to recommend OCT as a potential option rather than a universal standard for all PCI in AMI. OCT's superior resolution allows for better characterization of unstable plaques, assessment of thrombus burden, and improved stent apposition, which may be particularly beneficial in complex cases. However, the choice of imaging modality should be individualized based on patient characteristics, procedural context, and available resources. Further research and validation are needed to fully establish the role of OCT in routine clinical practice and to guide more nuanced recommendations.
Acknowledgments
The authors are indebted to for her excellent technical assistance.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board, Taipei Veterans General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
T-YL: Writing – review & editing, Writing – original draft. Y-YC: Writing – review & editing, Data curation. S-SH: Data curation, Writing – review & editing. C-HW: Data curation, Writing – review & editing. L-WC: Writing – review & editing, Data curation. Y-LC: Writing – review & editing, Data curation. WH: Writing – review & editing, Data curation. C-HH: Writing – review & editing, Data curation. M-JC: Data curation, Writing – review & editing. W-CH: Writing – review & editing, Supervision, Methodology. T-ML: Writing – review & editing, Supervision, Resources, Methodology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Brodie B, Pokharel Y, Garg A, Kissling G, Hansen C, Milks S, et al. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. (2012) 5:1043–51. 10.1016/j.jcin.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR J, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, et al. Stent thrombosis. J Am Coll Cardiol. (2010) 56:1357–65. 10.1016/j.jacc.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 3.Bhindi R, Kajander OA, Jolly SS, Kassam S, Lavi S, Niemela K, et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: the optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur Heart J. (2015) 36:1892–900. 10.1093/eurheartj/ehv176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mintz GS, Painter JA, Pichard AD, Kent KM, Satler LF, Popma JJ, et al. Atherosclerosis in angiographically "normal" coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. (1995) 25:1479–85. 10.1016/0735-1097(95)00088-l [DOI] [PubMed] [Google Scholar]
- 5.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. (2001) 37:1478–92. 10.1016/s0735-1097(01)01175-5 [DOI] [PubMed] [Google Scholar]
- 6.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. (2012) 59:1058–72. 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Zhou YJ, Liu YY, Shi DM. Is this spontaneous coronary intramural hematoma or fibrotic plaque?: an inconsistent finding between optical coherent tomography and intravascular ultrasound. JACC Cardiovasc Interv. (2013) 6:983–4. 10.1016/j.jcin.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 8.Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet. (2017) 390:793–809. 10.1016/S0140-6736(17)31957-8 [DOI] [PubMed] [Google Scholar]
- 9.Toth GG, Achim A, Kafka M, Wu X, Lunardi M, Biswas S, et al. Bench test and in vivo evaluation of longitudinal stent deformation during proximal optimisation. EuroIntervention. (2022) 18:83–90. 10.4244/EIJ-D-21-00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Q, Fan Y, Xu Z, Zhang J. Evaluation of post-dilatation on longitudinal stent deformation and postprocedural stent malapposition in the left main artery by optical coherence tomography (OCT): an in vitro study. BMC Med Imaging. (2024) 24:53. 10.1186/s12880-024-01223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DA, Rathod KS, Koganti S, Hamshere S, Astroulakis Z, Lim P, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the pan-London PCI cohort. JACC Cardiovasc Interv. (2018) 11:1313–21. 10.1016/j.jcin.2018.01.274 [DOI] [PubMed] [Google Scholar]
- 12.Otake H, Kubo T, Takahashi H, Shinke T, Okamura T, Hibi K, et al. Optical frequency domain imaging versus intravascular ultrasound in percutaneous coronary intervention (OPINION trial): results from the OPINION imaging study. JACC Cardiovasc Imaging. (2018) 11:111–23. 10.1016/j.jcmg.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Ali ZA, Karimi Galougahi K, Maehara A, Shlofmitz RA, Fabbiocchi F, Guagliumi G, et al. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN III: OPTIMIZE PCI trial. EuroIntervention. (2021) 16:1085–91. 10.4244/EIJ-D-20-00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. (2017) 38:3139–47. 10.1093/eurheartj/ehx351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone GW, Christiansen EH, Ali ZA, Andreasen LN, Maehara A, Ahmad Y, et al. Intravascular imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis. Lancet. (2024) 403:824–37. 10.1016/S0140-6736(23)02454-6 [DOI] [PubMed] [Google Scholar]
- 16.Li X, Ge Z, Kan J, Anjum M, Xie P, Chen X, et al. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet. (2024) 403:1855–65. 10.1016/S0140-6736(24)00282-4 [DOI] [PubMed] [Google Scholar]
- 17.Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. (2014) 129:463–70. 10.1161/CIRCULATIONAHA.113.003942 [DOI] [PubMed] [Google Scholar]
- 18.Youn YJ, Yoon J, Lee JW, Ahn SG, Ahn MS, Kim JY, et al. Intravascular ultrasound-guided primary percutaneous coronary intervention with drug-eluting stent implantation in patients with ST-segment elevation myocardial infarction. Clin Cardiol. (2011) 34:706–13. 10.1002/clc.20966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakatsuma K, Shiomi H, Morimoto T, Ando K, Kadota K, Watanabe H, et al. Intravascular ultrasound guidance vs. angiographic guidance in primary percutaneous coronary intervention for ST-segment elevation myocardial infarction—long-term clinical outcomes from the CREDO-Kyoto AMI registry. Circ J. (2016) 80:477–84. 10.1253/circj.CJ-15-0870 [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 21.Raber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European association of percutaneous cardiovascular interventions. EuroIntervention. (2018) 14:656–77. 10.4244/EIJY18M06_01 [DOI] [PubMed] [Google Scholar]
- 22.Steinvil A, Zhang YJ, Lee SY, Pang S, Waksman R, Chen SL, et al. Intravascular ultrasound-guided drug-eluting stent implantation: an updated meta-analysis of randomized control trials and observational studies. Int J Cardiol. (2016) 216:133–9. 10.1016/j.ijcard.2016.04.154 [DOI] [PubMed] [Google Scholar]
- 23.Bavishi C, Sardar P, Chatterjee S, Khan AR, Shah A, Ather S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. (2017) 185:26–34. 10.1016/j.ahj.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. (2015) 314:2155–63. 10.1001/jama.2015.15454 [DOI] [PubMed] [Google Scholar]
- 25.Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes with intravascular ultrasound-guided stent implantation: a meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv. (2016) 9:e003700. 10.1161/CIRCINTERVENTIONS.116.003700 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Farooq V, Garcia-Garcia HM, Bourantas CV, Tian N, Dong S, et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. (2012) 8:855–65. 10.4244/EIJV8I7A129 [DOI] [PubMed] [Google Scholar]
- 27.Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. (2015) 8:e002592. 10.1161/CIRCINTERVENTIONS.115.002592 [DOI] [PubMed] [Google Scholar]
- 28.Khan SU, Agarwal S, Arshad HB, Akbar UA, Mamas MA, Arora S, et al. Intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention: systematic review and meta-analysis. Br Med J. (2023) 383:e077848. 10.1136/bmj-2023-077848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SY, Maehara A, Cristea E, Witzenbichler B, Guagliumi G, Brodie B, et al. Usefulness of minimum stent cross sectional area as a predictor of angiographic restenosis after primary percutaneous coronary intervention in acute myocardial infarction (from the HORIZONS-AMI trial IVUS substudy). Am J Cardiol. (2012) 109:455–60. 10.1016/j.amjcard.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Groenland FTW, Neleman T, Kakar H, Scoccia A, des Plantes AC Z, Clephas PRD, et al. Intravascular ultrasound-guided versus coronary angiography-guided percutaneous coronary intervention in patients with acute myocardial infarction: a systematic review and meta-analysis. Int J Cardiol. (2022) 353:35–42. 10.1016/j.ijcard.2022.01.021 [DOI] [PubMed] [Google Scholar]
- 31.Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the centro per la Lotta contro l'Infarto-optimisation of percutaneous coronary intervention (CLI-OPCI) study. EuroIntervention. (2012) 8:823–9. 10.4244/EIJV8I7A125 [DOI] [PubMed] [Google Scholar]
- 32.Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. (2015) 8:1297–305. 10.1016/j.jcmg.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 33.Holm NR, Andreasen LN, Neghabat O, Laanmets P, Kumsars I, Bennett J, et al. OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med. (2023) 389:1477–87. 10.1056/NEJMoa2307770 [DOI] [PubMed] [Google Scholar]
- 34.Kuku KO, Ekanem E, Azizi V, Melaku G, Bui A, Meirovich YF, et al. Optical coherence tomography-guided percutaneous coronary intervention compared with other imaging guidance: a meta-analysis. Int J Cardiovasc Imaging. (2018) 34:503–13. 10.1007/s10554-017-1272-2 [DOI] [PubMed] [Google Scholar]
- 35.Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting). Circulation. (2016) 134:906–17. 10.1161/CIRCULATIONAHA.116.024393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.