Abstract
Background
Blood supply problems in remote areas are well known. To overcome this shortage, many countries have developed innovative walking blood bank (WBB) protocols. However, no common standards have yet been set for their use and common actions. Given that these procedures involve a certain risk, it would be interesting to analyse the activating criteria that lead to using this unusual protocol. Thus, this review aimed to identify indications for a WBB and the common risk mitigation measures.
Material and methods
This PRISMA-compliant review only included studies published from 1985 to 25th of January 2023 that describe adult male military casualties requiring blood transfused locally using a walking blood transfusion protocol. All relevant data (i.e., activation and contextual factors and risk mitigation measures) were tabulated to retrieve information from the selected military studies.
Results
Our results indicated that activation criteria were homogeneous across the 12 reviewed studies. Whole blood was collected from a WBB when there was a shortage of blood products and when platelets were needed. In the literature reviewed, the main risks associated with such a protocol, namely hemolytic adverse events and transfusion transmitted diseases, are mitigated by the use of typing and screening measures if they are reported. However, there is less consistency in the implementation of those risk mitigation measures.
Discussion
This unusual protocol needs to be integrated into the medical support plan until conventional transfusion support can take over, and should include on-site blood collection from a donor, whether a WBB or an emergency donor panel. The benefits of such a protocol outweigh the risks in a life-threatening situation, especially since these risks can be anticipated and minimised by planning to pre-screen all potential donors before their deployment. Finally, educating and training the staff who must implement this unusual procedure can also improve the safety and survival rate of future patients.
Keywords: walking blood bank, whole blood, emergency donor panel, indications, risk mitigation measures
INTRODUCTION
Over the last decade, transfusion medicine has evolved towards fractionated whole blood components such as red blood cells, platelets, or plasma, to improve the efficiency of storage and use in a standard hospital environment1. However, in austere environment (e.g., combat zones), military medical support must also provide the most appropriate product for the treatment of shock and coagulopathies, as hemorrhage remains a major cause of death among combat casualties2. Nevertheless, logistical constraints limit access and/or storage of these blood products3. The medical support system has been forced to adapt by developing innovative solutions that improve combat casualty care (e.g., DCR)4. They have therefore developed techniques, such as walking blood bank (WBB) protocol, to sufficiently access blood anywhere to support combat casualties until their evacuation5 and thereby increase their survival rate6. A WBB is a pool of donors available “on call” to donate whole blood (WB) in the event of an emergency7. These donors are among those deployed and consent to be registered as prospective donors prior to deployment8.
In addition to its essential role in increasing the survival rate of hemorrhagic patients, WB also offers biological advantages by providing all the blood components in a single transfusion to counteract the lethal triad observed in hemorrhage patients9,10. Essential blood components are often in short supply on the battlefield, especially platelets. Due to their short shelf life −between 5 and 7 days depending on the country–platelets are usually unavailable. This is why the use of WB, which contains platelets, can be essential for the treatment of certain hemorrhagic patients in extreme environments. Whole blood transfusion seems to be the only accessible solution in logistically challenging situations. This solution would address the need for platelets and logistical issues5. Any disadvantages that may arise seems far outweighed by the benefits of such a transfusion11. While risks will always exist, we can control and mitigate them. The literature shows that if the donor is pre-screened and a clear protocol is followed11, WB transfusion from a WBB is safe and effective. WBB implementation currently appears to rely on several different protocol-driven techniques11.
There is no existing interoperable protocol for the use of WBB even within the NATO coalition based on different national regulations.
The aim of this review is to identify situations where the benefits exceed the risks of resorting to a military WBB by focusing on these two questions:
What military context leads to the activation of a WBB (when/where)? and
What measures can be taken to minimize the inherent risk of such an implementation on the battlefield?
MATERIALS AND METHODS
This systematic review was conducted according to Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature search and screening criteria
PubMed and Scopus databases were searched using the following keywords: “walking blood bank”; “walking AND blood AND bank”; “Emergency whole blood”; “Buddy transfusion”; “Blood far forward”; “walking donor”; “Emergency donor panel” and “warm fresh whole blood”. All articles published from 1985 (after HIV appearance in blood transfusion) until 25th of January 2023 were considered.
Selection of studies (exclusion and inclusion criteria)
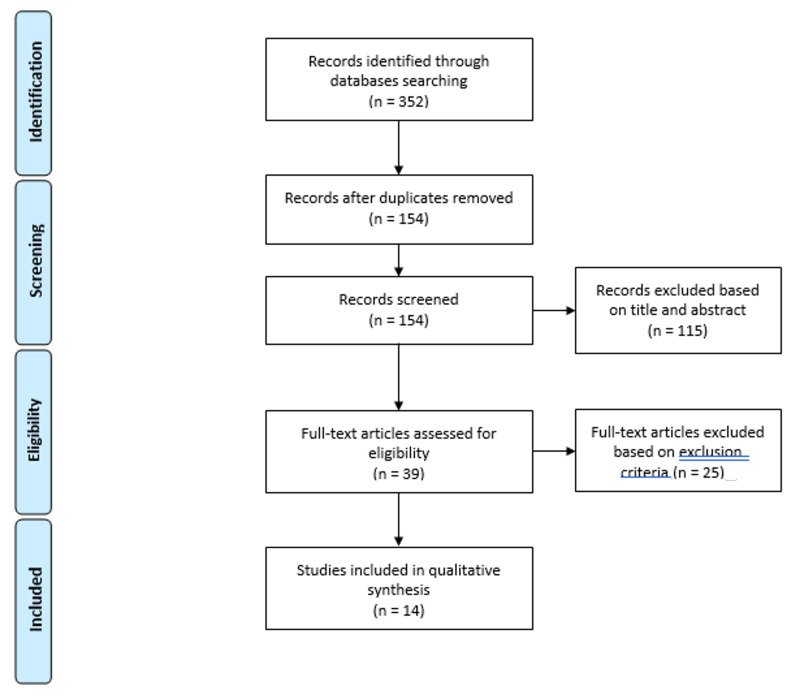
First, the lead investigator identified relevant studies by reviewing the abstracts according to the inclusion and exclusion criteria. In addition, two authors independently assessed all the full texts, and then the full list of eligible studies was agreed by all the authors. The exclusion and inclusion criteria for study selection are described in Table I. Studies were included if they described male military adults who were injured and required transfusion of blood collected in the field according to a WBB protocol. Our research focused specifically on adult male military patients, who make up over 95% of our deployable at-risk-population. Furthermore, studies in women tend to reflect transfusion in a perinatal setting, which is not representative of managing bleeding patients in the military. Moreover, studies reporting field-tested protocols and information on at least two of the three outcomes of interest (see Table I) may be considered even if they did not include patients. A flowchart illustrating the selection procedure is presented in Figure 1.
Table I.
Exclusion and inclusion criteria for military study selection
| Inclusion criteria | Exclusion criteria | ||
| Screening | Language | Papers written in English | Papers written in all other languages |
| Study design | Prospective (including feasibility studies) or retrospective studies in international peer-reviewed journal | Unpublished material, communication, letter to the editor, reviews, and conference abstract | |
| Publication year | Papers published from 1986 onwards | Papers published up to 1985 to include the ITT related risk | |
| Participants | Military males adults if patients are involved | Females and children | |
| Eligibility | Outcomes | At least 2 of 3:
|
Analysis of donor, patient, or use of the Walking Blood Bank apart |
| Setting | Military setting | Civilian setting |
Figure 1.
PRISMA flowchart illustrating the entire selection process, from the literature search to the selection of studies of interest based on the inclusion and exclusion criteria
The screening process allows the rejection of duplicates and papers that do not meet the inclusion criteria based on titles and abstracts (i.e., language, year of publication, study design and participants). The eligibility process involves full-text analysis of the remaining papers based on specific outcome criteria, namely the setting and reporting of at least 2 of the following 3 aspects: activation criteria, donor safety or/and patient safety.
Data extraction and analysis
The data were extracted by the lead author and checked by a second author to ensure accuracy. Disagreements were discussed and decision was taken by a third author. The literature review was divided into two steps: activation indicators and risk mitigation measures.
To retrieve information from the selected studies, several tables were created. All relevant data regarding the activation factors of a WBB are compiled in Table II. The following contextual factors were determined:
Table II.
Summary of the indications of activation of a walking Blood Bank
| Authors | Basic situation | Walking blood bank activation | |||
|---|---|---|---|---|---|
| Blood bank product available? | Type of Injuries | Situation | Activation indicator | WB used | |
| Lewis et al ., 2020 | Yes (CSWB + Full CT) | Blast injury, hemorrhage | Mass casualties, massive transfusion | Depletion of CSWB/evacuation impossible or delayed | FWB |
| Miller et al ., 2018 | Yes (frozen pRBC + FFP) | No specific injury described: Helicopter crash | Mass casualties, massive transfusion | Platelets needed/severe coagulopathy | FWB |
| Bassett et al ., 2016 | Yes (Full CT) | Traumatic amputations, blast injury, shrapnel injury | Massive transfusion | Combat injured patients likely to require massive transfusion (benefits from early activation) | FWB |
| Strandenes et al ., 2015 | No (no blood bank available) | No specific injury described: Feasibility study for Norwegian frigate conducting antipiracy operations | Remote situation | Planning | CSWB for banking |
| Garcia Hejl et al ., 2015 | Yes (pRBC, FDP) | No specific injury described: Feasibility study | Mass casualties, massive transfusion | Platelets needed/severe coagulopathy | FWB |
| Hrezo and Clark, 2003 | No | Rectal bleeding | Remote situation | Shortage of blood products | FWB |
| Gaspary et al ., 2020 | Yes (CSWB + Full CT) | No specific injury described: feasibility study | Mass casualties, massive transfusion | Shortage of blood products (CS LTOWB serve to start massive transfusion until FWB become available from the WBB) | FWB |
| Hakre et al ., 2013 | Not reported | IED Blast | Mass Casualties, massive transfusion + remote situation | Shortage of blood products | FWB |
| Malsby et al ., 2005 | Not reported | Gunshot wound | Massive transfusion + remote situation | Shortage of blood products | FWB |
| Liu et al ., 2014 | Yes (RBC, FFP, PLT) | No specific injury described: Hit by a ship cable | Massive transfusion | To correct coagulopathy when all other blood products failed | FWB |
| Gaddy et al ., 2021 | No (any products available at POI) | Gunshot wound | Remote situation | Absence of blood products (transfusion after extraction before evacuation, POI) | FWB |
| Song et al ., 2021 | Yes (CSWB) | Blast injury | Remote situation | No access to stored blood product at the POI: Delay for evacuation | FWB |
CSWB: cold stored whole blood; pRBC: packed red blood cells; FDP: freeze-dried plasma; CT: components therapy; FFP: fresh frozen plasma; RBC: red blood cells; PLT: platelets; POI: point of injury; IED: improvised explosive device; CS LTOWB: cold stored low titer O whole blood; FWB: fresh whole blood; WB: whole blood.
availability of a blood bank and type of product in stock,
type of patient injury,
type of emergency situation (i.e., massive transfusion, mass casualty, remote, or combinations of the above).
In addition, this table also included the activation criteria of the WBB as well as information on the type of WB used (i.e.: cold-stored WB or fresh warm WB).
All the mitigating and protective measures implemented in each study to minimize the risk associated with the use of a WBB were summarized in Tables III to V. These countermeasures were grouped into two categories: donor-related and patient-related. The latter were likely to occur at two different times, before deployment and on-site during blood collection. Information on donor-related activities is provided as follows:
Table III.
Summary of the “typing” risk mitigation measure
| Authors | Type of WB | Pre-deployment | At collection |
|---|---|---|---|
| Lewis et al ., 2020 | Type sp. & LTOWB | Not detailed | Not reported |
| Miller et al ., 2018 | Type sp. | Only a 10% sample of on board personal | Confirmation |
| Bassett et al ., 2016 | Not reported | Refer to CPG | Refer to CPG |
| Strandenes et al ., 2015 | LTOWB + AWB | National standard procedure for regular donor in civilian health care: Grouping + titer | Confirmation (rapid test) |
| Garcia Hejl et al ., 2015 | Type sp. | No reported | Type |
| Hrezo and Clark, 2003 | Type sp. | Only a 10% sample of population | Type + Crossmatch |
| Gaspary et al ., 2020 | LTOWB | Not reported | Samples collected on site and send back to homeland for titer analysis |
| Hakre et al ., 2013 | OWB + AWB | Not reported | Not reported |
| Malsby et al ., 2005 | OWB | Not detailed | Not reported |
| Liu et al ., 2014 | Not reported | Not reported | Not reported |
| Gaddy et al ., 2021 | Type sp. LTOWB prehospital |
Yes: blood ID card | Confirmation by rapid test required but not executed due to tactical limitations - use of blood ID card |
| Song et al ., 2021 | LTOWB | Not reported | Not reported |
WB: whole blood; Type sp.: ABO type specific; LTOWB: low titer O whole blood; OWB: O whole blood; AWB: A whole blood; LTOWB: low titer O whole blood; CPG: clinical practice guidelines; ID: identification.
donor screening before deployment and
donor screening at blood collection.
This distinction was made because fully equipped laboratories and remotely accessible laboratories differ greatly in terms of resources, procedures, availability, as well as the sensitivity and specificity of the tests used. The blood grouping, the type of screening (i.e., infectious disease screening using questionnaire, nucleic acid testing, serology, or rapid test) and the virus tested were reported if mentioned. Donor screening included questionnaires and/or tests, and we considered both as one. The tests might differ depending on national requirements. Regarding the risk associated with the product, a distinction was made between the studies using only O WB and using type-specific blood or both depending on the situations. The tables also listed if the authors did consider the titer of hemolysins (low or not) in the product. All medical and related laboratory parameters helping to assess the patient’s status were reported in the tables. Finally, the data concerning the patient’s follow-up after transfusion were also included when available.
Assessment of the quality of evidence
The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to rate the reliability of evidence from each included study. This was assessed by the lead author and independently verified by two others.
RESULTS
Search results
The literature search identified 352 records, of which 154 were assessed for eligibility after removing 198 duplicates. Based on title, abstract and article type, 115 studies were also excluded according to the inclusion and exclusion criteria defined in Table I. There was also one study exclusion on language grounds. The lead investigator identified 39 relevant studies through a review of abstracts against the exclusion criteria. Twelve papers agreed by all authors were included in the review12–23. A summary of the results of the literature search is shown in Figure 1.
Quality of evidence
Nine of the 12 included articles were case reports and series12,13,16–22. Therefore, they were all graded “very low” according to the GRADE system. There were also three prospective observational studies14,15,23. They were all graded as “low” quality according to the GRADE system. Clustering by repeat authors did not appear to be an area of potential bias. These low-quality gradings were mainly due to the observational design of all studies, putting them at risk of bias, imprecision, inconsistency and indirectness. There was no disagreement between the reviewers with regard to the risk of bias and the GRADE rating.
Analysing results
Activation indicators of a WBB
Based on the situations considered (see Table II), the literature review identified four studies that only referred to a remote environment to support the use of a WBB17,21–23. Another reported having the WBB protocol ready to provide blood during an event combining remote situations, mass casualty and massive transfusion18. The remaining studies supported the activation of the WBB, either for massive transfusions16–20, or for a combination of mass casualties and massive transfusions12–15, or for a combination of massive transfusions and remote situations19.
Accordingly, apart from the study by Strandenes and colleagues23, all studies justified the use of a WBB as a response to shortages of blood products, and/or delays in evacuation (see Table II)12,13,22,14–21. Shortages were either contextual or caused by the depletion of available supplies due to acute point-in-time demand12,17–19,22. Some of the authors also pointed out the shortage of a specific blood component: blood platelets13,14,20. As platelets were often scarce on the battlefield, they could only be obtained from WB. Whole blood has made the difference in the stabilisation and recovery of coagulopathic patients with certain types of injuries resulting in bleeding casualties13,14,20.
All 9 retrospective studies described hemorrhagic patients with either uncontrollable bleeding or coagulopathy due to various traumatic injuries as the cause of injury leading to activation of a WBB (see Table II)12,13,16–22. Among the remaining three prospective studies, two studies evaluated the feasibility of setting up a WBB and the supply potential generated by implementing the protocol14,15, while the third one described the protocol they used to collect and bank WB from a pool of identified donors to anticipate potential needs on board23. It was also the only study to specify the use of cold-stored WB as a means of accessing and maintaining a “blood bank” without having home blood23.
Risk mitigation measures of a WBB
Linked to the donor
Two measures are reported to be used to limit donor-related risks, namely blood typing (Table III) and donor screening (Table IV). Both can be performed in early pre-deployment planning and/or on-site at the time of collection.
Table IV.
Summary of the “screening” risk mitigation measure
| Authors | Pre-deployment | At collection |
|---|---|---|
| Lewis et al ., 2020 | Not detailed | Not reported |
| Miller et al ., 2018 | Only a 10% sample of on board personal HBV - HCV - Syphilis - malaria |
Rapid tests |
| Bassett et al ., 2016 | Refer to CPG | Refer to CPG |
| Strandenes et al ., 2015 | National standard procedure for regular donor in civilian health care | Combined rapid test |
| Garcia Hejl et al ., 2015 | No reported | Questionnaire Rapid tests HIV, HCV + complete HBV vaccination |
| Hrezo and Clark, 2003 | Only a 10% sample of population. Questionnaire Serologic tests: HIV, HCV, HBV, HTLV |
Rapid testing |
| Gaspary et al ., 2020 | Recommanded JTS CPG but not executed | Rapid testing |
| Hakre et al ., 2013 | Questionnaire Screening (90 days): HIV, HCV, HBV, Syphilis, HTLV, West Nile virus (sample back to the US). Complete HBV vaccination. |
Rapid tests: HIV, HCV, HBV |
| Malsby et al ., 2005 | Not detailed | Not reported |
| Liu et al ., 2014 | Not reported | Not reported |
| Gaddy et al ., 2021 | Not reported | Not reported |
| Song et al ., 2021 | Not reported | Not reported |
HBV: hepatitis B virus; HCV: hepatitis C virus; CPG: clinical practice guidelines; HIV: human immunodeficiency virus; HTLV: human T-lymphotropic virus; JTS: Joint Trauma system; TTD: transfusion transmitted diseases.
Across studies, blood typing prior to deployment and its confirmation at the time of collection were often combined with the aim of establishing a registry of potential donors and their blood groups that could be confirmed at the time of collection13,16,17,21,23. Three studies focusing on patient or donor screening failed to report pre-deployment or on-site blood group typing18,20,22. The study by Song and colleagues reported on the use of a donor registry, but did not provide any details on the potential risk reduction measures that were taken either prior to deployment or at the time of collection22. Nevertheless, it seemed to be a relatively important measure as most authors reported it, even though the protocols were quite different, and the lack of reporting did not mean that it was not done. The use of WB from only O donor, rather than type-specific or compatible blood, was reported in only 3 studies15,19,22. Furthermore, two studies did not even address this issue and did not specify the product used16,20.
For donor screening risk mitigation measures (i.e., tests or questionnaires), all details provided by authors are shown in Table IV. Eight studies reported pre-deployment screening as part of the donor registry planning in the preparedness phase12,13,15–19,23 and seven at the time of collection13–18,23. Six studies performed pre-deployment and on-site screening13,15–18,23. Two studies did not report on-site testing but did report pre-deployment testing12,13,16–19, and one reported on-site testing but did not report pre-deployment testing14. Despite this, only two studies reported no screening at least once during the process20,21. In their study, Song and colleagues did not report any screening before or at the time of collection, but specified that the protocol was to “call” donors from a registry22.
Linked to the patients
It was not possible to identify only one or even a few important parameters for patient follow-up, as all authors used different parameters (see Table V), except for the prospective study by Strandenes et al, which used no parameters for follow-up23. From a transfusion perspective, the parameters reported in these studies can be divided into two main types:
Table V.
Summary of the patients’ follow-up parameters
| Authors | Patient follow-up/Measured indicators |
|---|---|
| Lewis et al ., 2020 | TACO - Surgery - Recovery |
| Miller et al ., 2018 | HR - Blood Pressure - pH - Lactate - Hb - PLT count |
| Bassett et al ., 2016 | pH - BE - Hb. 30 days follow-up: survival + transfusion reaction/blood borne pathogens transfer - OR time - time to transfer |
| Strandenes et al ., 2015 | Not reported |
| Garcia Hejl et al ., 2015 | Sample for immunoassays infectious agents: HTLV, HIV, HBV, Syphilis + Nucleic Acid Testing: HIV, HCV, HBV |
| Hrezo and Clark, 2003 | Blood count - PT/PTT - Hb - HR - BP - sO2 . Sample for future serologic testing. 48 h follow-up - Surgery |
| Gaspary et al ., 2020 | Sample back for pre-screening to add donor to register |
| Hakre et al ., 2013 | Transfusion associated adverse events. TTD’s: HTLV - WBC - Temperature |
| Malsby et al ., 2005 | Pulse - BP - Surgery. Follow-up 4 weeks |
| Liu et al ., 2014 | Temperature - HR - Respiratory rate - BP - Hb - PT- INR - PTT - PLT count - Calcium level - Surgery - Acute lung injury - Respiratory distresses |
| Gaddy et al ., 2021 | sO2 - BP - HR - Respiration - Pulse - Glasgow score - Surgery |
| Song et al ., 2021 | Survival - Surgery |
TACO: transfusion-associated circulatory overload; HR: heart rate; Hb: hemoglobin; PLT: platelets; BE: base excess; OR: operating room; HTLV: human T lymphotropic virus; HIV: human immunodeficiency virus; HBV: hepatitis B virus; PT/PTT: prothrombin time/partial thromboplastin time; BP: blood pressure; sO2: oxygen saturation; TTD: transfusion transmitted disease; WBC: white blood cells; INR: international normalized ratio.
the medical parameters, where the most commonly reported were blood pressure, heart rate, survival rate, surgery, transfusion reactions and laboratory parameters reflecting the status of the patient (e.g.: hemoglobin or pH, lactate)12,13,16,17,19–22 and
adverse events related to TTDs or screening on sample return to the home country14–18.
Patient follow-up for potential TTDs was reported in five studies14–18. Hakre and colleagues focused their analysis on one patient’s seroconversion following an on-site walking blood transfusion18.
DISCUSSION
This review aimed to identify activation criteria for military WBB as well as the risk mitigation measures associated with their use. Our first research question investigated the rationale for its application. Two main trends have been identified in the literature to justify the use of WBB protocols:
access to blood products in case of shortage (i.e., logistical indication of activation)12,15–23 and
access to blood products for the treatment of a hemorrhagic patient when a required specific component is not available (i.e., clinical indication of activation)13,14,20.
All but two of the studies21,23 reported on the use of fresh WB to overcome the shortage of blood products12–22. Gaddy et al. reported collecting blood for a casualty during a combat assault and withdrawing it at the site of injury. There was no shortage of blood, but blood was not immediately available on site21. Strandenes and colleagues, however, chose a different strategy, collecting blood to build up an emergency bank23. These two different strategies are equally acceptable and can be chosen according to the initial situation: collecting to meet a specific need based on a shortage or creating a bank based on an absence. Yet, both strategies are named differently: one is called a “walking blood bank” while the other is called an “emergency donor panel” (EDP). The NATO Blood Panel recently discussed this difference24. It was decided that the WBB refers to WB collected for banking. In contrast, the emergency donor panel refers to a pool of pre-screened donors who are ready to give blood for immediate use without banking24. One may notice that this distinction is not yet clear in the literature. Therefore, to ensure that all studies were included, we decided to extend our search to the most used terms in the literature. Furthermore, all authors reported using this protocol to avoid overwhelming their designated transfusion system for highly demanding patients presenting with uncontrolled bleeding leading to massive transfusion or hemorrhagic shock. As previously reported in the literature, WB is an essential resource for DCR, e.g., at sea, it offers operational flexibility as the use of component therapy, the ratio “1:1 RBC”: FFP” is not always and everywhere sustainable23. Our analysis led us to the same conclusion. The use of FWB collected on site could become, in exceptional situations, the only solution to access blood and save lives. While this review focuses only on the military setting, it was also used in isolated and large geographical areas presenting blood supply challenges comparable to military theatres (e.g.,25–28). The Norwegian Preparedness Plan is the more developed and published model for using WBB/EDP in the civil world when geography or supply is difficult to secure27.
Finally, some authors reported choosing to use FWB in order to obtain a clinical advantage20, as FWB offers a better survival rate in hemorrhagic shock29. However, it is still a highly controversial topic as the purported benefits of FWB are still not clearly evidence based30,31.
Concerning our second research question, while the awareness of risk is common to all articles, the protocols differ in their implementation regarding the use of risk mitigation measures, both in terms of the type of test and the timing of its implementation. Our review showed that risks related to both donors and products need to be considered. It is well established in the literature that FWBs should come from pre-screened donors to reduce the higher risk of TTD29. However, in our review, even if both TTD screening and blood typing are considered to reduce the risk, the techniques used, and the timing of the interventions varied widely and did not allow standardization of practice. There are two main explanations for this. The first one would be the national regulation, which is quite specific to each country. Therefore, because all requirements and protocols are different (Germany, USA, UK, Canada)32, interoperability in the use of WBB cannot be adopted by all NATO members. As it also depends on the prevalence in the home country, there are no standards for TTD screening29. The second one relates to the bias inherent to the design. Most of the reviewed studies were case studies. This implies that the data used are those that are available a posteriori and some of the data may be missing without necessarily indicating that the procedure such as testing was not carried out. Furthermore, it is also possible that some information is missing because the authors choose to omit reporting some data and not because the full test was not carried out. Not reporting did not mean that it did not happen. Regarding the product used, it would be more convenient in terms of the risk of transfusion reactions to use only O donors. However, our results do not reflect this. Most authors reported using ABO type specific WB, but unfortunately did not rationalise their choice12–14,17,18,21,23. Indeed, O donors represent approximately 45% of the Caucasian population, whereas A donors represent approximately 45% of the same population. By limiting the sample to O donors, an important part of the donor pool is excluded. This may be important for obtaining sufficient resources. Nonetheless, this presupposes that the typing has been determined pre-deployment or at the time of donation. In addition, some authors report also considering the hemolysin titer in O WB12,15,21–23. However, there is no consensus on titer determination, either from a technical point of view or from a cut-off point of view. Therefore, not every nation would consider a donor as a low titre donor using the same levels. This is part of the limitation of the use of low titers in an international setting33. This would lead to complications in communication, monitoring and interoperability decisions. Finally, patient outcomes were also considered in the studies reviewed, but there was no evidence of a consensus on these and their reporting was inconsistent. Nevertheless, all efforts should be made to assess patients’ stability according to the resources available.
CONCLUSIONS
A blood collection protocol, whether a WBB or an emergency donor panel, must be part of the transfusion support concept because it provides access to resources that are otherwise inaccessible. Obviously, this will only be implemented in exceptional situations due to the associated risk. Most stakeholders are aware of these risks, which, if mitigated, are outweighed by benefits. Therefore, measures are taken to prevent, monitor and minimize the risks entailed by such protocol. To ensure a comprehensive selection of donors for the registry, it is essential to include this comprehensive protocol in the medical support planning process of operation. The key to success are donors, their education and regular follow-up. Based on this review, there is a clear need for such a protocol in the military operational setting, but it can also be applied in the civilian world, particularly in remote locations. However, it must be part of the country’s preparedness plan to ensure the best possible care for patients.
Footnotes
AUTHORS’ CONTRIBUTIONS: JD proposed the research question, performed the literature search, the data extraction and analysis, assessed the quality and drafted the manuscript. ED checked the accuracy of the literature search, participation to the analysis, independently verified the quality assessment, contributed to the writing of the manuscript. FT checked the accuracy of the literature search, participation to the analysis, independently verified the quality assessment, resolved discrepancies between JD and ED during data extraction and revised the manuscript. VD is responsible for the supervision and revised the manuscript. All Authors contributed to the final manuscript and approved its final version. JD is guarantor.
FUNDING: This study was funded by grant HFM19-06 allocated by the Belgian Royal Higher Institute for Defense.
The Authors declare no conflicts of interest.
REFERENCES
- 1.McCoy CC, Brenner M, Duchesne J, Roberts D, Ferrada P, Horer T, et al. Back to the future: whole blood resuscitation of the severely injured trauma patient. Shock. 2021;56(Suppl 1):9–15. doi: 10.1097/SHK.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):431–437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 3.Beckett A, Callum J, da Luz LT, Schmid J, Funk C, Glassberg E, et al. Fresh whole blood transfusion capability for Special Operations Forces. Can J Surg. 2015;58(3 Suppl 3):S153–6. doi: 10.1503/CJS.012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball CG. Damage control resuscitation: history, theory and technique. Can J Surg. 2014;57:55–60. doi: 10.1503/cjs.020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinaud C, Travers S, Pasquier P, Sailliol A, Ausset S. Blood far forward program: Update on French armed forces policy. Transfusion. 2021;61(Suppl 1):S354–S355. doi: 10.1111/trf.16457. [DOI] [PubMed] [Google Scholar]
- 6.Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, et al. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318:1581–1591. doi: 10.1001/jama.2017.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goforth CW, Tranberg JW, Boyer P, Silvestri PJ. Fresh whole blood transfusion: military and civilian implications. Crit Care Nurse. 2016;36:50–57. doi: 10.4037/ccn2016780. [DOI] [PubMed] [Google Scholar]
- 8.Strandenes G, De Pasquale M, Cap AP, Hervig TA, Kristoffersen EK, Hickey M, et al. Emergency whole-blood use in the field: a simplified protocol for collection and transfusion. Shock. 2014;41(Suppl 1):76–83. doi: 10.1097/SHK.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 9.Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: U.s. military and potential civilian applications. Crit Care Med. 2008;36(Suppl 7):S340–S345. doi: 10.1097/CCM.0B013E31817E2EF9. [DOI] [PubMed] [Google Scholar]
- 10.Crowe E, DeSantis SM, Bonnette A, Jansen JO, Yamal JM, Holcomb JB, et al. Whole blood transfusion versus component therapy in trauma resuscitation: a systematic review and meta-analysis. J Am Coll Emerg Physicians Open. 2020;1:633–641. doi: 10.1002/emp2.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahr M, Cap AP, Dishong D, Yazer MH. Practical Considerations for a Military Whole Blood Program. Mil Med. 2020;185:e1032–e1038. doi: 10.1093/milmed/usz466. [DOI] [PubMed] [Google Scholar]
- 12.Lewis C, Nilan M, Srivilasa C, Knight RM, Shevchik J, Bowen B, et al. Fresh whole blood collection and transfusion at point of injury, prolonged permissive hypotension, and intermittent REBOA: extreme measures led to survival in a severely injured soldier-A case report. J Spec Oper Med. 2020;20:123–126. doi: 10.55460/03EF-6LU6. [DOI] [PubMed] [Google Scholar]
- 13.Miller BT, Lin AH, Clark SC, Cap AP, Dubose JJ. Red tides: Mass casualty and whole blood at sea. J Trauma Acute Care Surg. 2018;85(1S Suppl 2):S134–S139. doi: 10.1097/TA.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Hejl C, Martinaud C, Macarez R, Le Golvan A, Dulou R, Longin Roche C, et al. The implementation of a multinational “walking blood bank” in a combat zone: the experience of a health service team deployed to a medical treatment facility in Afghanistan. J Trauma Acute Care Surg. 2015;78:949–954. doi: 10.1097/TA.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 15.Gaspary MJ, Kyle AI, Lawson SM, Birkla J, Bolton ED, Bergeron KP, et al. Obstacles to an effective low-titer O walking blood bank: a deployed unit’s experience. Mil Med. 2021;186:e137–e142. doi: 10.1093/milmed/usaa236. [DOI] [PubMed] [Google Scholar]
- 16.Bassett AK, Auten JD, Zieber TJ, Lunceford NL. Early, prehospital activation of the walking blood bank based on mechanism of injury improves time to fresh whole blood transfusion. [Accessed July 6, 2017];J Spec Oper Med. 2016 16:5–8. http://www.ncbi.nlm.nih.gov/pubmed/27450595 . [PubMed] [Google Scholar]
- 17.Hrezo RJ, Clark J. The walking blood bank: an alternative blood supply in military mass casualties. Disaster Manag Response. 2003;1:19–22. doi: 10.1016/s1540-2487(03)70005-4. [DOI] [PubMed] [Google Scholar]
- 18.Hakre S, Manak MM, Murray CK, Davis KW, Bose M, Harding AJ, et al. Transfusion-transmitted human T-lymphotropic virus Type i infection in a United States military emergency whole blood transfusion recipient in Afghanistan, 2010. Transfusion. 2013;53:2176–2182. doi: 10.1111/trf.12101. [DOI] [PubMed] [Google Scholar]
- 19.Malsby R, Frizzi J, Ray P, Raff J. Walking donor transfusion in a far forward environment. South Med J. 2005;98:809–810. doi: 10.1097/01.SMJ.0000154313.53641.6C. [DOI] [PubMed] [Google Scholar]
- 20.Liu YH, Chao CS, Chang YP, Chin HK. Hemostatic resuscitation for massive hemorrhage with warm fresh whole blood in a patient with severe blunt trauma. Asian J Surg. 2014;37:205–207. doi: 10.1016/j.asjsur.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Gaddy M, Fickling A, Hannick VC, Shackelford SA. Use of walking blood bank at point of injury during combat operations: a case report. J Spec Oper Med. 2021;21:94–98. doi: 10.55460/V05K-FKXN. [DOI] [PubMed] [Google Scholar]
- 22.Song KH, Winebrenner HM, Able TE, Bowen CB, Dunn NA, Shevchik JD. Ranger O Low Titer (ROLO): whole blood transfusion for forward Ddeployed units. Mil Med. 2021:usab473. doi: 10.1093/milmed/usab473. [DOI] [PubMed] [Google Scholar]
- 23.Strandenes G, Austlid I, Apelseth TO, Hervig TA, Sommerfelt-Pettersen J, Herzig MC, et al. Coagulation function of stored whole blood is preserved for 14 days in austere conditions: A ROTEM feasibility study during a Norwegian antipiracy mission and comparison to equal ratio reconstituted blood. J Trauma Acute Care Surg. 2015;78:S31–S38. doi: 10.1097/TA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 24.NATO. Blood Panel Meeting Record from the Summer Meeting 30 Jun – 01 Jul 2022. [Accessed on 01/10/2022];NSO Public Website (nato.int) [Restricted access]. [Google Scholar]
- 25.Kaada SH, Apelseth TO, Hagen KG, Kristoffersen EK, Gjerde S, Sønstabø K, et al. How do I get an emergency civilian walking blood bank running? Transfusion. 2019;59:1446–1452. doi: 10.1111/trf.15184. [DOI] [PubMed] [Google Scholar]
- 26.Katsura M, Matsushima K, Kitamura R, Kawasaki K, Takaesu R, Fukuma S, et al. The use of warm fresh whole blood transfusion in the austere setting: a civilian trauma experience. J Trauma Acute Care Surg. 2020;89:e28–e33. doi: 10.1097/TA.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 27.Apelseth TO, Arsenovic M, Strandenes G. The Norwegian blood preparedness project: a whole blood program including civilian walking blood banks for early treatment of patients with life-threatening bleeding in municipal health care services, ambulance services, and rural hospitals. Transfusion. 2022;62(Suppl 1):S22–S29. doi: 10.1111/trf.16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakaiya R, Morrison F, Halbrook J, Rawson J, Lotz L. Problems with a walking donor transfusion program. Transfusion. 1979;19:577–580. doi: 10.1046/j.1537-2995.1979.19580059813.x. [DOI] [PubMed] [Google Scholar]
- 29.Cap AP, Pidcoke HF, DePasquale M, Rappold JF, Glassberg E, Eliassen HS, et al. Blood far forward: Time to get moving! J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S2–S6. doi: 10.1097/TA.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 30.Malkin M, Nevo A, Brundage SI, Schreiber M. Effectiveness and safety of whole blood compared to balanced blood components in resuscitation of hemorrhaging trauma patients-A systematic review. Injury. 2021;52:182–188. doi: 10.1016/j.injury.2020.10.095. [DOI] [PubMed] [Google Scholar]
- 31.Naumann DN, Boulton AJ, Sandhu A, Campbell K, Charlton W, Gurney JM, et al. Fresh whole blood from walking blood banks for patients with traumatic hemorrhagic shock: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2020;89:792–800. doi: 10.1097/TA.0000000000002840. [DOI] [PubMed] [Google Scholar]
- 32.DaCambra MP, Kao RL, Berger C, McAlister VC. Utilization profile of the Canadian-led coalition role 2 medical treatment facility in Iraq: the growing requirement for multinational interoperability. Can J Surg. 2018;61:S195–S202. doi: 10.1503/cjs.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant SWJ, Heil KM. Practical limitations of emergency donor panels as a risk mitigation in small-scale short-term training team operations. BMJ Mil Heal. 2023;169:e97–e99. doi: 10.1136/bmjmilitary-2020-001529. [DOI] [PubMed] [Google Scholar]