Abstract
Background
Platelet-rich plasma (PRP) as a non-surgical therapy for facial rejuvenation is increasingly adopted. This article aims to review the literature and critically appraise the available evidence regarding the efficacy and safety of PRP for facial rejuvenation.
Material and methods
An overview of systematic reviews (SRs) of PRP use for facial rejuvenation. The methodological quality of the SRs was assessed using the AMSTAR-2 checklist; quality of the evidence from the trials included in each SR was appraised following the GRADE approach.
Results
Thirteen SRs published between 2015 and 2023, reporting data from 114 overlapping reports, based on 28 individual primary studies (18 uncontrolled reports), were included in this umbrella review. Eight primary studies evaluated PRP in combination with other treatments (laser therapy, fat grafting, hyaluronic acid, basic fibroblast growth factor), and 20 PRP monotherapy. Most of the included primary studies were uncontrolled, and meta-analysis for outcomes related to facial rejuvenation was conducted in only 1 of the 13 SRs, showing that patients treated with PRP as an adjunct treatment have increased satisfaction over controls without PRP (mean difference, 0.63; 95% confidence intervals (CIs) 0.25/1; p=0–001; low certainty of evidence due to risk of bias (ROB) and inconsistency). No other quantitative data were available from the SRs, although 4 SRs concluded in a descriptive way reveal that PRP combined with laser therapy increased subject satisfaction and skin elasticity, and decreased the erythema index (very low certainty of evidence due to imprecision, unsystematic clinical observations, and ROB). The occurrence of adverse events was a predefined outcome in only 2 SRs (15%). Almost all the SRs demonstrated poor compliance with the AMSTAR 2 items, and the confidence in the results of SRs was graded as low or critically low in 12 of the 13 SRs.
Discussion
The available evidence is insufficient to suggest firm conclusions about the use of PRP, alone or in combination with other treatments, in promoting facial rejuvenation.
Keywords: platelet-rich plasma, facial rejuvenation, umbrella review, systematic review, meta-analysis
INTRODUCTION
A significant advance that has emerged in the last two decades in the field of transfusion medicine regards the development of blood components for non-transfusion use, in particular, platelet-rich plasma (PRP)-based technologies. Platelet-rich plasma (PRP) has been used in different non-transfusion indications due to its role in tissue regeneration and healing1–5. Besides platelets, PRP contains some inflammatory cells (i.e., monocytes and polymorphonuclear neutrophils) and large amounts of proteins, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF) and adhesion molecules (i.e., fibrin, fibronectin and vitronectin).
Such growth factors and cells have been shown to promote cell recruitment, proliferation and angiogenesis, which may be implicated in tissue regeneration and healing, and have been extensively studied in humans in a wide range of clinical situations in areas such as orthopedics, sport medicine and dentistry6–10. An area, which has received increasing attention in recent years, is that of PRP use in dermatology. Several trials and SRs evaluated the use of PRP for the treatment of alopecia, acne scars, chronic wounds and vitiligo11–14. Moreover, the use of PRP in cosmetics and skin care is receiving increasing attention. PRP has been evaluated in the field of aesthetic dermatology, and several clinical studies and systematic reviews (SRs) on the use of PRP as non-invasive skin and facial rejuvenation method have been published in the last years15–21. However, their conclusions show the extensive heterogeneity among studies in terms of design, conduct, lack of standardization in outcome measures, and reporting. The current study is an overview of systematic reviews, also called umbrella review, review of (systematic) reviews, and “meta-review”. Umbrella reviews provide an overview of multiple systematic reviews on a given research question, taking in consideration the SR as the object of the analysis rather than the primary study22,23.
The current overview is aimed to reappraise the validity of the conclusions of the SRs and meta-analyses related to PRP use for non-surgical treatment of skin aging and facial rejuvenation. The decision to perform this overview is because PRP is increasingly adopted as non-surgical treatment of the signs of skin aging, and for this reason new data from recently published clinical trials, SRs and meta-analyses are available. Increasing the number of studies can improve precision of effect estimates, allowing additional comparisons or subgroup analyses to be performed. In this umbrella review, we have also applied new review methods such as the AMSTAR-2 tool, and a GRADE assessment, with the aim of enhancing the existing results in terms of the certainty of the review’s findings24,25.
MATERIALS AND METHODS
The protocol of this overview of reviews has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42023486477. The results are reported according to the PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions26.
Review question/objective
The aim of this umbrella review is to evaluate the efficacy and safety of PRP injection as facial rejuvenation treatment, either as monotherapy or in combination with other treatment modalities.
Inclusion and exclusion criteria
We considered for inclusion in this overview SRs that comprised randomized controlled trials (RCTs) and non-RCTs (i.e., prospective and retrospective comparative cohort studies, and non-comparative studies such as case-control studies and case-series) assessing the safety and efficacy of PRP for facial rejuvenation. Traditional reviews with no clear methodological approach were excluded from this umbrella review. SRs evaluating other use of PRP were excluded unless they also reported data on PRP use for facial rejuvenation that could be evaluated separately.
Intervention and outcomes
Treatment with PRP for facial rejuvenation, either as monotherapy or in combination was compared to any control. In all primary studies, PRP is used by injection; only one study evaluated topical PRP (with the addition of fractional laser technology). We included the following outcomes: patients, satisfaction scores, physician assessed outcomes, and adverse reactions.
Search strategy
The search was conducted from inception to November 2023 in the following databases: MEDLINE (through PubMed), medRxiv and bioRxiv, Embase, Epistemonikos, and Cochrane library. The searches were carried-out without languages restriction using Medical Subjects Heading: (“Platelet rich plasma/PRP”) AND (“systematic review” OR “meta-analysis”) AND (“treatment” OR “therapy”) AND (“Facial rejuvenation” OR “Skin rejuvenation”). Furthermore, we checked the reference lists of the most relevant manuscripts (original studies and reviews) to identify potentially eligible studies not captured by the electronic literature search.
Study selection and data extraction
All titles were screened by two assessors (MC and IP). Eligibility assessment was based on the title or abstract and on the full text if required. Full texts of possibly eligible articles were obtained and assessed independently by two reviewers (MC and FM). Both reviewers compared the identified articles. The two assessors also independently extracted quantitative and qualitative data from each selected study, with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (IP). Findings are presented in tabular format with supporting text. Tabulation of results include: first author name and year of publication, clinical setting (e.g., outpatients and hospitalized patients, number of RCTs and non-RCTs included in the SR, intervention and control group, the outcomes assessed, and the main conclusion of the review as reported by authors.
Assessment of methodological quality and overlap in systematic reviews
We used the AMSTAR-2 critical appraisal checklist for SRs, a tool that evaluates both quantitative and qualitative reviews24. The tool is suitable for reviews including randomised and non-randomised studies. It includes 16 domains (7 considered critical) relating to the research question, review design, search strategy, study selection, data extraction, justification for excluded studies, description of included studies, risk of bias, sources of funding, meta-analysis, heterogeneity, publication bias, and conflicts of interest (see footnote of Table II for details of each question). Two review authors (MC, FM) independently assessed the quality of evidence in the included reviews and the methodological quality of the SRs. We resolved discrepancies through discussion or, if needed, through a third review author (IP). We did not exclude reviews based on AMSTAR 2 ratings, but considered the ratings in interpretation of our results. We rated overall confidence in the results of the review according to Shea et al.24, as follows:
Table II.
Assessment of methodological quality with AMSTAR 2 tool for each comparison of the efficacy and safety outcomes
| Author, year reference | AMSTAR-2 DOMAIN | Overall confidence in the results* | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Leo, 2015 28 | Critically low | ||||||||||||||||
| Lynch, 2015 29 | Critically low | ||||||||||||||||
| Sclafani, 2015 30 | Critically low | ||||||||||||||||
| Frautschi, 2017 31 | Low | ||||||||||||||||
| Lei, 2019 32 | Low | ||||||||||||||||
| Gupta, 2019 33 | Low | ||||||||||||||||
| Kaushik, 2019 34 | Critically low | ||||||||||||||||
| Maisel-Campbell, 2020 35 | Low | ||||||||||||||||
| Nanda, 2021 36 | Critically low | ||||||||||||||||
| Xiao, 2021 37 | Low | ||||||||||||||||
| Evans, 2021 38 | High | ||||||||||||||||
| Buzalaf, 2022 39 | Critically low | ||||||||||||||||
| Gentile, 2023 40 | Low | ||||||||||||||||
| Methodological requirement met | Methodological requirement partly met, or not specified | Methodological requirement unmet |
| Amstar-2 domains. Although AMSTAR 2 consists of 16 items, critical domains include items 2, 4, 7, 9, 11, 13, and 15 |
|
*We rated overall confidence in the results of the review according to Shea et al.24, as follows:
|
high, no or one non-critical weakness;
moderate, more than one non-critical weakness but no critical flaws;
low, one critical flaw with or without non-critical weaknesses;
critically low, more than one critical flaw with or without non-critical weaknesses.
Methods to describe and quantify the overlap in overviews of reviews have been described, and for the current overview, we have narratively discussed it and applied the corrected covered area (CCA) index, calculated as follows27: CCA = k – r / r (c – r) where k is the number of reports in reviews (sum of ticked boxes), r is the number of rows (index publications), and c is the number of columns (SRs included). Criteria for interpreting the overlap index are: slight (0–5%), moderate (6–10%), high (11–15%) or very high (>15%) overlap. The CCA was calculated both across all reports included and for specific outcomes.
Summary of the evidence and appraisal of the quality of evidence
For the quantitative synthesis, we report the effect size [odds ratio (OR), risk ratio (RR), risk difference (RD), or standardized mean difference (SMD) with the 95% confidence intervals (CIs)] as reported in individual reviews, and their main conclusions.
The quality of evidence was appraised following the GRADE approach (Grades of Recommendation, Assessment, Development, and Evaluation)25. Whenever available, the grading of the quality of evidence reported in each SR was considered to define the quality of evidence. When the authors of the study did not report grading of evidence, the GRADE approach was applied based on the information available from the individual review. Studies can be downgraded for concerns over risk of bias, indirectness (applicability of the results to the question), inconsistency (heterogeneity between study results), imprecision (low number of studies and/or participants), and publication bias. The GRADE approach has four levels of certainty; very low (the true effect is probably markedly different from the estimated effect), low (the true effect might be markedly different from the estimated effect), moderate (the true effect is probably close to the estimated effect), and high (the true effect is similar to the estimated effect).
RESULTS
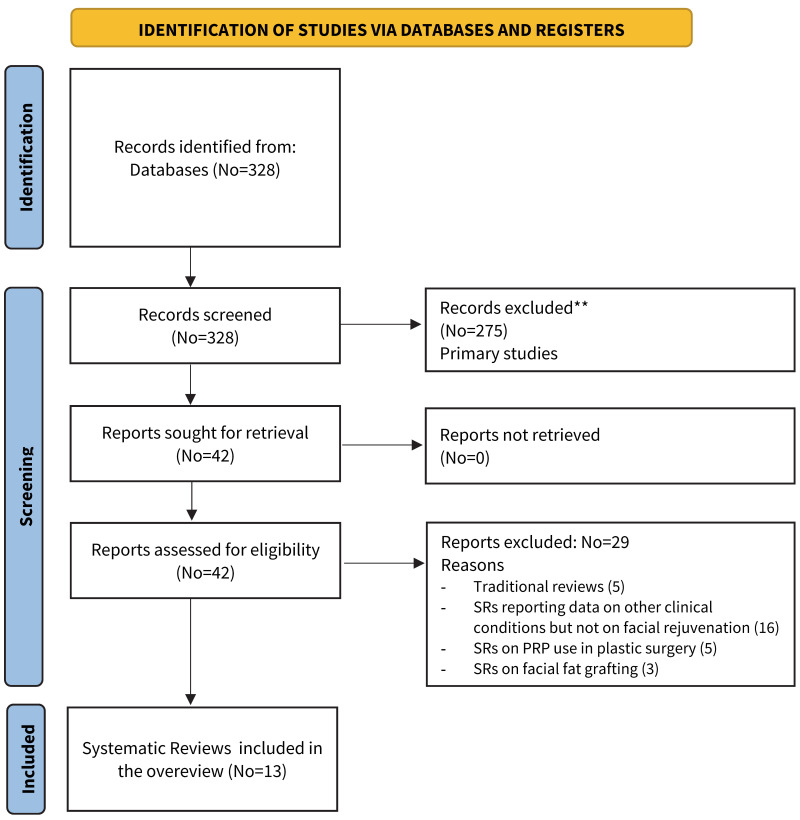
The electronic and manual search retrieved 328 references The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is reported in Figure 1. At the first stage of screening titles and abstracts, 42 references were selected for eligibility, and the full text examined. After the full texts were examined with regards to inclusion and exclusion criteria, 29 records were excluded (traditional reviews, SRs on other clinical conditions, SRs on PRP for plastic surgery, SRs on facial fat grafting). Finally, 13 SRs were included in the umbrella review28–40.
Figure 1.
PRISMA Flow chart of study selection process
PRP: platelet-rich plasma; SR: systematic review.
Description of the studies
The 13 SRs included 114 overlapping reports based on 28 individual primary studies. All the studies in the SRs included in this overview used autologous PRP, often in combination with fractional laser therapy or fat grafting; primary studies always report the type of preparation and where available the anticoagulant used for activation but the final number of platelets obtained is never reported. The primary studies included 8 RCTs (3 parallel groups, 5 split-face), 3 non-randomized split-face studies, 1 controlled cohort study, and 18 uncontrolled studies (case-report or case-series). Eight primary studies (5 RCTs, 1 cohort study, and 2 case reports) evaluated PRP in combination, and with other treatments (laser therapy, fat grafting, hyaluronic acid, basic fibroblast growth factor), while 20 (3 RCTs, 1 non-randomized split-face study, and 16 case report/series) PRP monotherapy. Therefore, 16/20 (80%) of the PRP monotherapy studies were uncontrolled, compared to 2/8 (25%) of the studies with PRP in combination. The main characteristics of the SRs included are summarized in Table I. Two SRs included only controlled or uncontrolled studies with PRP monotherapy34,40, while the remaining SRs included both studies with PRP monotherapy or in combination with other treatment. All primary studies reported PRP used by injection and only one study evaluated topical PRP (with the addition of fractional laser technology).
Table I.
Main characteristics of SRs included in the overview update
| First author, year ref | Clinical setting | Studies included in quantitative analysis for facial rejuvenation | Interventions | Main outcomes | Main results as reported in the SR | |||
|---|---|---|---|---|---|---|---|---|
| Overall (patients) | RCT | Other | Experimental | Control | ||||
| Leo, 2015 28 | PRP in various dermatologic indications including hair restoration, scar revision, striae distensae, skin rejuvenation and dermal augmentation | 4 (75) | 2 (1 split face) | 2 case series | PRP (1–3 injections); PRP plus Fractional Laser therapy (3 topical applications) | Saline; platelet poor plasma; Fractional laser therapy only | Patient’s satisfaction, clinical assessment by dermatologists | Future studies should utilize control treatments, preferably split-side treatments, so that the efficacy of PRP treatments can be better defined |
| Lynch, 2015 29 | PRP for a range of dermatological indications including wound healing, fat grafting, alopecia, scar revision, striae distensae e and dermal volume augmentation | 2 (45)ve | 1 | 1 case serie | PRP (3 injections); PRP plus Fractional Laser therapy (3 topical applications) | Fractional laser therapy only | Patient’s satisfaction, clinical assessment by dermatologists | Current evidence is not sufficiently robust to recommend routine use of PRP for any dermatologic indication Further study may be justified in the context of well-designed trials |
| Sclafani, 2015 30 | PRP in facial rejuvenation and wound healing | 7 (197) | 3 (2 split face) | 3 case series, 1 cohort study (retrospective) | PRP (1–3 injections); PRP plus Fractional Laser therapy (3 topical applications); PRP plus carbon dioxide laser | Fractional laser therapy only; saline | Patient’s satisfaction, clinical assessment by dermatologists | PRP represent an as-of-yet untapped adjunct in facial rejuvenation, but the level of evidence from the available published data is low |
| Frautschi, 2017 31 | PRP for esthaetic aesthetic surgery (aging skin, scalp alopecia, lipofilling, fractional laser and facial surgery) | 5 (147) | 1 | 3 case series, 1 cohort study | PRP (1–3 injections); PRP plus Fractional Laser therapy (3 topical applications); PRP + fat grafting; PRP + MACS-lift-fat grafting (1 injection) | Fractional laser therapy only; fat grafting only; MACS-lift-fat grafting only | Patient’s satisfaction, clinical assessment by dermatologists | The evidence for PRP clinical effectiveness in aesthetic practice remains largely speculative |
| Lei, 2019 32 | PRP in animal models, and for facial rejuvenation, and alopecia | 8 (282) | 2 | 5 case series, 1 cohort study | PRP (1–3 injections); PRP plus Fractional Laser therapy (3 topical applications); PRP + Fat grafting; PRP + MACS-lift-fat grafting (1 injection); PRP plus HA | Fractional laser therapy only; Fat grafting only; MACS-lift-fat grafting only | Patient’s satisfaction, clinical assessment by dermatologists | PRP may play a role in promoting tissue regeneration, oxidative stress and revascularization, which form the theoretical basis for the use of PRP in the clinical treatment of facial rejuvenation |
| Gupta, 2019 33 | PRP in various dermatologic indications including hair restoration, scar revision, aging skin | 9 (168) | 2 (1 split face) | 6 case series, 1 cohort study | PRP (1–6 injections); PRP plus Fractional Laser therapy (3 topical applications) | Saline; growth factors; Fractional laser therapy only | Patient’s satisfaction, clinical assessment by dermatologists | Only upon completion of well-designed clinical trials can standardized protocols for PRP to treat dermatologic conditions be defined |
| Kaushik, 2019 34 | PRP in various dermatologic indications including androgenetic alopecia and facial/ skin rejuvenation | 4 (50) | 1 (split face) | 3 case series | PRP (1–3 injections) | Saline | Patient’s satisfaction, clinical assessment by dermatologist | PRP is a potentially interesting modality in facial rejuvenation, but further well-designed evidence is needed before it can be considered an established therapy in this setting |
| Maisel-Campbell, 2020 35 | PRP for treatment of the visible signs of skin aging | 18 (374) | 7 (5 split face) | 10 case series, 1 cohort study | PRP (1–3 injections), PRP plus Fractional Laser therapy (3 topical applications); PRP + Fat grafting; PRP + MACS-lift-fat grafting (1 injection); PRP plus carbon dioxide laser | Saline; platelet poor plasma; growth factors; Fractional Laser therapy only; fat grafting only; MACS-lift-fat grafting only | Patient’s satisfaction, clinical assessment by dermatologist, adverse events | PRP injections are safe and may be modestly beneficial for aging skin |
| Nanda, 2021 36 | PRP for skin rejuvenation and acne scars. | 7 (574) | 4 (2 split face) | 2 case series, 1 cohort study | PRP (1–3 injections), PRP plus Fractional Laser therapy (3 topical applications); PRP + Fat grafting; PRP + MACS-lift-fat grafting (1 injection) | Saline; platelet poor plasma; Fractional Laser therapy only; fat grafting only; MACS-lift-fat grafting only | Patient’s satisfaction, clinical assessment by dermatologist, adverse effects | Further well-designed trials are required |
| Xiao, 2021 37 | PRP for facial rejuvenation | 13 (376) | 4 (2 split face) | 8 case series, 1 cohort study | PRP (1–3 injections); PRP plus Fractional Laser therapy (3 topical applications); PRP + Fat grafting; PRP + MACS-lift-fat grafting (1 injection); PRP + HA | Saline; platelet poor plasma; Fractional Laser therapy only; fat grafting only; MACS-lift-fat grafting only | Patient’s satisfaction, clinical assessment by dermatologist | There is very limited clinical evidence to establish the effectiveness of PRP in facial rejuvenation |
| Evans, 2021 38 | PRP for facial rejuvenation | 14 (365) | 4 (2 split face) | 10 case series | PRP (1–6 injections); PRP plus Fractional Laser therapy (3 topical applications); PRP + HA | Saline; platelet poor plasma; Topical TCA + LA; growth factors; Fractional Laser therapy only | Patient’s satisfaction, clinical assessment by dermatologist, adverse effects | Meta-analysis of 3 RCTs shows increased satisfaction of PRP treated pts. over controls of saline, platelet-poor plasma, mesotherapy, and as an adjunct to laser therapy Further studies are required to address limitations of the current literature |
| Buzalaf, 2022 39 | PRP for facial rejuvenation | 17 (443) | 4 (3 split face) | 13 case series | PRP (1–6 injections), PRP plus CO2 laser, PRP + HA | Saline; platelet poor plasma; growth factors; plasma gel | Patient’s satisfaction, clinical assessment by dermatologist | The quality of the available evidence is low, and further studies are needed |
| Gentile, 2023 40 | PRP for facial rejuvenation | 8 (125) | 3 (3 split face) | 5 case series | PRP (1–6 injections) | Saline; platelet poor plasma; growth factors; | Patient’s satisfaction, clinical assessment by dermatologist, Adverse effects | Further well-designed studies are needed to confirm the efficacy of PRP for facial rejuvenation and to define standard protocols |
HA: hyaluronic acid; LA: lactic acid; MACS: Minimal Access Cranial Suspension; PRP: platelet rich plasma; RCT: randomized controlled trial; TCA: trichloroacetic acid.
Methodological quality of SRs (Table II)
Of the included SRs, one had only two methodological requirements partially met38, 5 had several methodological requirements partly met31,33,35,37,40, and 7 had several requirements unmet/partially met28–30,32,34,36,39. All the reviews did not report details of the funding source that had supported the work, and did not assess publication of bias. Only 2 SRs reported a list of excluded studies and reasons for exclusion37,38; meta-analysis was performed with appropriate statistical methods in 2 SRs33,38, but only one did it for outcomes related to facial rejuvenation38. Other commonly unmet or partially met requirements included evaluation of ROB and heterogeneity assessment. Overall, almost all of the included SRs demonstrated poor compliance with the AMSTAR 2 items; as a consequence, confidence in the results was graded as low in 6 SRs31–33,35,37,40, critically-low in 628–30,34,36,39, and moderate in one38. Concerning the overlap across all reports included in the overview, the CCA index shows a very high rate of overlapping across the SRs.
Summary of the effect of PRP on the main outcomes
The most commonly reported outcomes were patient’s satisfaction and clinical assessment by dermatologists. Various clinical evaluator tools (e.g., Skin Homogeneity and Texture Scale; Wrinkle Severity Rating Scale, Global Aesthetic Improvement Scale), collagen mean optical density, and skin measures of homogeneity were also reported. Due to the fact that most of the included primary studies were uncontrolled, meta-analysis (the quantitative synthesis) for outcome related to facial rejuvenation was conducted in only 1 of the 13 SRs, and relates to patient satisfaction score following treatment with PRP as an adjunct treatment over controls (including saline, mesotherapy, platelet-poor plasma and laser alone) from 3 RCTs (Mean Difference, 0.63; 95% CIs, 0.25/1; p=0–001; low certainty of evidence due to ROB and inconsistency)38. No other quantitative synthesis is available from the SRs, although 4 of the SRs concluded in a descriptive way that PRP combined with laser therapy increased subject satisfaction and skin elasticity, and decreased the erythema index29–31,36.
The occurrence of adverse events was reported in detail in only 2 of the 13 SRs (15%)35,38. Five SRs did not mention the occurrence of adverse events at all28,30–33, while other 4 SRs reported only general statements on PRP safety29,34,37,39. Two SRs stated that PRP is safe, the most commonly reported side effects being pain at the injection site, erythema, and edema36,40. The SR by Maisel-Campell et al.35 reported only mild and transient adverse events with PRP monotherapy in 320 subjects from 16 studies; there were no reports of infection, scarring or post-inflammatory hyperpigmentation, while transient post-injection pain or burning was observed in approximately two-thirds of subjects, lasting minutes to an hour. Erythema resolving within days was also commonly reported, while edema and tenderness lasting less than 1 week were less commonly reported. No serious adverse events were reported. Likewise, the SR by Evans et al. shows that PRP injections provide a minimal risk to the patient, without risk of infection, allergy, or post-inflammatory hyperpigmentation38. Mild side effects attributable to any dermal injection are to be expected, and include erythema, pain, a burning sensation, ecchymosis, swelling, a feeling of pressure, and tenderness. The addition of calcium chloride without use of topical anesthetics may produce significant pain, but is preventable with the addition of topical anesthetic to PRP38.
The results of the analyses for the main outcomes and the GRADE assessment are summarized in Table III.
Table III.
Main conclusions of SRs with PRP for facial rejuvenation
| Review, yearref | Main outcome/s | Meta-analysis results | GRADE assessment of primary studies: certainty of evidence (reason/s for downgrading) | Comment |
|---|---|---|---|---|
| Leo, 2015 28 | Effect of PRP on wrinkles; augmentation in dermal collagen in pts receiving PRP in conjuction with laser therapy | Quantitative synthesis not feasible | Very low (imprecision, unsystematic clinical observations, ROB) | No firm conclusions can be drawn |
| Lynch, 2015 29 | Patient satisfaction and blind assessment of dermatologist | Quantitative synthesis not feasible | Very-low (serious imprecision, ROB) | PRP combined with fractional laser increased subject satisfaction and skin elasticity and decreased the erythema index |
| Sclafani, 2015 30 | Patients satisfaction, assessment of dermatologist | Quantitative synthesis for facial rejuvenation not feasible | Very-low (serious imprecision, ROB) | PRP combined with fractional laser increased subject satisfaction and skin elasticity and decreased the erythema index |
| Frautschi, 2017 31 | Patients satisfaction, assessment of dermatologists | Quantitative synthesis for facial rejuvenation not feasible | Very-low (serious imprecision, ROB) | PRP combined with fractional laser increased subject satisfaction and skin elasticity and decreased the erythema index |
| Lei, 2019 32 | Patient’s satisfaction, clinic assessment by dermatologists | Quantitative synthesis for facial rejuvenation not feasible | Very low (imprecision, unsystematic clinical observations, ROB) | The available evidence about PRP in promoting facial rejuvenation is inadequate |
| Gupta, 2019 33 | Patient’s satisfaction, clinic assessment by dermatologists, | Quantitative synthesis for facial rejuvenation not yet feasible | Very low (imprecision, unsystematic clinical observations, ROB) | Inconsistent outcomes |
| Kaushik, 2019 34 | Patient’s satisfaction, clinic assessment by dermatologists | Quantitative synthesis for facial rejuvenation not yet feasible | Very low (imprecision, unsystematic clinical observations, ROB) | No firm conclusions can be drawn |
| MaiselCampbell, 2020 35 | Patient’s satisfaction, clinic assessment by dermatologists, Adverse events | Quantitative synthesis for facial rejuvenation not feasible | Very low (imprecision, unsystematic clinical observations, ROB) | PRP injections are safe and may be modestly beneficial for aging skin |
| Nanda, 2021 36 | Patient’s satisfaction, clinic assessment by dermatologists | Quantitative synthesis not available | Very low (imprecision, unsystematic clinical observations, ROB) | PRP combined with fractional laser increased subject satisfaction and skin elasticity and decreased the erythema index |
| Xiao, 2021 37 | Patient’s satisfaction, clinic assessment by dermatologists | Quantitative synthesis not available | Very low (imprecision, unsystematic clinical observations, ROB) | No firm conclusions can be drawn |
| Evans, 2021 38 | Patient’s satisfaction, clinic assessment by dermatologists; adverse events | Mean Difference in pts. satisfaction score: 0.63 (from 0.25 to 1; p=0–001) | Low (ROB, inconsistency due to heterogeneity) | PRP produces increased pts. satisfaction scores over controls. Mild side effects related to PRP injections are to be expected |
| Buzalaf, 2022 39 | Patient’s satisfaction, clinic assessment by dermatologists. | Quantitative synthesis not available | Very low (imprecision, unsystematic clinical observations, ROB) | No firm conclusions can be drawn |
| Gentile, 2023 40 | Patient’s satisfaction, clinic assessment by dermatologists, Adverse events | The principal summary measures were reported as p-value, percentage and ratio | Very low (imprecision, unsystematic clinical observations, ROB | No firm conclusions can be drawn |
PRP: platelet-rich plasma; ROB: risk of bias.
DISCUSSION
Overviews of reviews (umbrella reviews) assemble several SRs on the same condition and permit to consider for inclusion the highest level of evidence available, such as SRs and meta-analyses22,23,41. Indeed, in this umbrella review we have reappraised the results of 13 SRs, published between 2015 and 2023, on the clinical use of PRP, as monotherapy or in combination with other treatments, as a non-surgical therapy for facial rejuvenation. The SRs included in this overview present data from 114 overlapping reports, based on 28 individual primary studies, mostly non randomized. The studies evaluated more commonly PRP monotherapy, but also PRP in combination with other treatments (laser therapy, fat grafting, hyaluronic acid, basic fibroblast growth factor). Since most of the studies (71%) included in the SRs were uncontrolled, on average the certainty of evidence from primary studies with GRADE assessment ranged from very-low to low, and this represents the main limit of the analysis, both in the SRs evaluated and in the current overview. The quality of the evidence for the outcomes analysed in primary studies was downgraded due to risk of bias, imprecision, unsystematic clinical observations, inconsistency between studies, and imprecision25.
Further limits of the overview are related to the high rate of overlap of primary studies, as indicated by the CCA index (22%). Overlap in overviews of reviews comes from the use of multiple identical primary studies in similar reviews, usually when the reviews are updated frequently, as the authors will often add new studies in addition to the original studies, or with reviews that cover similar topics but may have a different focus42.
Beside the limits of primary studies, we have also to consider the confidence we can have on the results of the SRs included in the umbrella reviews basing on the AMSTAR-2 evaluation24. The majority of evaluated SRs had many critical requirement (for example absence of a registered protocol, ROB, publication bias and heterogeneity assessment) unmet or only partially met, and meta-analysis was performed with appropriate statistical methods only in one SR. For these reasons, 12 of the 13 SRs were graded critically low or at best low for the methodological quality.
The quantitative synthesis for outcomes related to facial rejuvenation was conducted in only one SR and involved “patient satisfaction score” following treatment with PRP as an adjunct treatment over controls not receiving PRP38. Data from 3 RCTs in this SR show a significant increase in patients’ satisfaction in PRP recipients compared to control, but the quality of the evidence was rated as low due to ROB and inconsistency. Patient satisfaction is an important and necessary consideration for cosmetic treatments, such as facial rejuvenation. However, this outcome as well as other subjective outcomes (e.g., clinicians satisfaction scores) in the absence of a control group or blindness in RCTs, are susceptible to bias, particularly performance and detection biases.
Due to the fact that most of the included primary studies were uncontrolled, no other quantitative synthesis is available from the SRs, although 4 of the SRs concluded in a descriptive way that PRP combined with laser therapy increased subject satisfaction and skin elasticity, and decreased the erythema index (very-low certainty of evidence due to imprecision, unsystematic clinical observations, and ROB).
The large majority of the SRs did not mention the occurrence of adverse events at all, or reported only general statements on PRP safety. Only 2 SRs included the occurrence of adverse events among the predefined outcomes. Only mild and transient adverse events with PRP injection were reported.
Another important limit for interpreting PRP research, in this field as well as for other clinical conditions, is lack of standardization of PRP preparation protocols, administration schedules, and follow-up duration. There was a large variability in the number of PRP administration (from 1 to 6); moreover, PRP was applied as a topical administration in one study and as an injection in all other primary studies.
In conclusion, this overview of reviews summarizes the existing evidence about the efficacy and safety of PRP, either alone or in combination with other treatment modalities, for facial rejuvenation. The results suggest very limited clinical evidence of PRP in this setting, mostly for uncontrolled studies. Further well-designed randomized controlled trials need to be performed to evaluate the efficacy of PRP in facial rejuvenation, ideally with a blind design in order to prevent the risk of bias related to subjective outcomes in open-label trials.
Footnotes
AUTHORS’ CONTRIBUTIONS: Conceptualization: MC. Methodology: MC. Data extraction: FM, IP, MC. Writing-preparation original draft: MC. Writing-review and editing: FM, IP, SP, VDA. All Authors have read and agreed to the published version of the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Piccin A, Di Pierro AM, Canzian L, Primerano M, Corvetta D, Negri G, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15:333–340. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Martinez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Fron Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pachito DV, Latorraca COC, Riera R. Efficacy of platelet-rich plasma for non-transfusion use: overview of systematic reviews. Int J Clin Pract. 2019:e13402. doi: 10.1111/ijcp.13402. [DOI] [PubMed] [Google Scholar]
- 5.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Plateletrich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 6.Franchini M, Cruciani M, Mengoli C, Marano G, Pupella S, Veropalumbo E, et al. Efficacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. 2018;16:502–513. doi: 10.2450/2018.0111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchini M, Cruciani M, Mengoli C, Masiello F, Marano G, D’Aloja E, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfus. 2019;17:357–367. doi: 10.2450/2019.0177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasterlain AS, Braun HJ, Harris AH, Kim HJ, Dragoo JL. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41:186–193. doi: 10.1177/0363546512466383. [DOI] [PubMed] [Google Scholar]
- 9.Masiello F, Pati I, Veropalumbo E, Pupella S, Cruciani M, De Angelis V. Ultrasound-guided injection of platelet-rich plasma for tendinopathies: a systematic review and meta-analysis. Blood Transfus. 2023;21:119–136. doi: 10.2450/2022.0087-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruciani M, Franchini M, Mengoli C, Marano G, Pati I, Masiello F, et al. Platelet-rich plasma for sports-related muscle, tendon and ligament injuries: an umbrella review. Blood Transfus. 2019;17:465–478. doi: 10.2450/2019.0274-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pixley JN, Cook MK, Singh R, Larrondo J, McMichael AJ. A comprehensive review of platelet-rich plasma for the treatment of dermatologic disorders. J Dermatolog Treat. 2023;34:2142035. doi: 10.1080/09546634.2022.2142035. [DOI] [PubMed] [Google Scholar]
- 12.Cruciani M, Masiello F, Pati I, Marano G, Pupella S, De Angelis V. Plateletrich plasma for the treatment of alopecia: a systematic review and meta-analysis. Blood Transfus. 2023;21:24–36. doi: 10.2450/2021.0216-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruciani M, Masiello F, Pati I, Pupella S, De Angelis V. Platelet rich plasma use for treatment of acne scars: an overview of systematic reviews. Blood Transfus. 2023 doi: 10.2450/BloodTransfus.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: a systematic review. J Am Acad Dermatol. 2019;81:834–846. doi: 10.1016/j.jaad.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Gao J, Zheng H, Zou C, Yu Z, Wu Z, et al. Study of platelet-rich plasma application for skin and plastic surgery in recent 20 years: a bibliometric analysis. J Cosmet Dermatol. 2023;22:1852–1862. doi: 10.1111/jocd.15653. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj S, Orbuch D, Wang JV, Geronemus RG. preparation and utility of platelet-rich plasma (PRP) for facial aging: a comprehensive review. Adv Ther. 2022;39:4021–4036. doi: 10.1007/s12325-022-02239-6. [DOI] [PubMed] [Google Scholar]
- 17.Willemsen JC, van der Lei B, Vermeulen KM, Stevens HP. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014;38:1057–1063. doi: 10.1007/s00266-014-0361-z. [DOI] [PubMed] [Google Scholar]
- 18.Mehryan P, Zartab H, Rajabi A, Pazhoohi N, Firooz A. Assessment of efficacy of platelet-rich plasma (PRP) on infraorbital dark circles and crow’s feet wrinkles. J Cosmet Dermatol. 2014;13:72–78. doi: 10.1111/jocd.12072. [DOI] [PubMed] [Google Scholar]
- 19.Yuksel EP, Sahin G, Aydin F, Senturk N, Turanli AY. Evaluation of effects of platelet-rich plasma on human facial skin. J Cosmet Laser Ther. 2014;16:206–208. doi: 10.3109/14764172.2014.949274. [DOI] [PubMed] [Google Scholar]
- 20.Kang BK, Shin MK, Lee JH, Kim NI. Effects of platelet-rich plasma on wrinkles and skin tone in Asian lower eyelid skin: preliminary results from a prospective, randomised, split-face trial. Eur J Dermatol. 2014;24:100–101. doi: 10.1684/ejd.2014.2267. [DOI] [PubMed] [Google Scholar]
- 21.Peng GL. Platelet-rich plasma for skin rejuvenation: facts, fiction, and pearls for practice. Facial Plast Surg Clin North Am. 2019;27:405–411. doi: 10.1016/j.fsc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkorm P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Health. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 23.Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L, Chapter V. Overviews of reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022) Cochrane; 2022. [Accessed on 01/06/2023]. Available at: www.training.cochrane.org/handbook. [Google Scholar]
- 24.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013 GRADE Working Group. 2013. 2013. [Accessed on 01/06/2023.]. Available at: gdt.guidelinedevelopment.org/app/handbook/handbook.html.
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennessy EA, Johnson BT, Keenan C. Best practice guidelines and essential methodological steps to conduct rigorous and systematic meta-reviews. Appl Psychol Health Well Being. 2019;11:353–381. doi: 10.1111/aphw.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leo MS, Kumar AS, Kirit R, Konathan R, Sivamani RK. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14:315–323. doi: 10.1111/jocd.12167. [DOI] [PubMed] [Google Scholar]
- 29.Lynch MD, Bashir S. Applications of platelet-rich plasma in dermatology: a critical appraisal of the literature. J Dermatolog Treat. 2016;27:285–289. doi: 10.3109/09546634.2015.1094178. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani AP, Azzi J. Platelet preparations for use in facial rejuvenation and wound healing: a critical review of current literature. Aesthetic Plast Surg. 2015;39:495–505. doi: 10.1007/s00266-015-0504-x. [DOI] [PubMed] [Google Scholar]
- 31.Frautschi RS, Hashem AM, Halasa B, Cakmakoglu C, Zins JE. current evidence for clinical efficacy of platelet rich plasma in aesthetic surgery: a systematic review. Aesthet Surg J. 2017;37:353–362. doi: 10.1093/asj/sjw178. [DOI] [PubMed] [Google Scholar]
- 32.Lei X, Xu P, Cheng B. problems and solutions for platelet-rich plasma in facial rejuvenation: a systematic review. Aesthetic Plast Surg. 2019;43:457–469. doi: 10.1007/s00266-018-1256-1. [DOI] [PubMed] [Google Scholar]
- 33.Gupta AK, Versteeg SG, Rapaport J, Hausauer AK, Shear NH, Piguet V. The efficacy of platelet-rich plasma in the field of hair restoration and facial aesthetics - A systematic review and meta-analysis. J Cutan Med Surg. 2019;23:185–203. doi: 10.1177/1203475418818073. [DOI] [PubMed] [Google Scholar]
- 34.Kaushik A, Kumaran MS. Platelet-rich plasma: the journey so far! Indian Dermatol Online J. 2020;11:685–692. doi: 10.4103/idoj.IDOJ_369_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maisel-Campbell AL, Ismail A, Reynolds KA, Poon E, Serrano L, Grushchak S, et al. A systematic review of the safety and effectiveness of plateletrich plasma (PRP) for skin aging. Arch Dermatol Res. 2020;312:301–315. doi: 10.1007/s00403-019-01999-6. [DOI] [PubMed] [Google Scholar]
- 36.Nanda S, Chauhan K, Shetty V, Dashore S, Bhatia S. Platelet-rich plasma in aesthetics. Indian Dermatol Online J. 2021;12(Suppl 1):S41–S54. doi: 10.4103/idoj.idoj_290_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao H, Xu D, Mao R, Xiao M, Fang Y, Liu Y. Platelet-rich plasma in facial rejuvenation: a systematic appraisal of the available clinical evidence. Clin Cosmet Investig Dermatol. 2021;14:1697–1724. doi: 10.2147/CCID.S340434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans AG, Ivanic MG, Botros MA, Pope RW, Halle BR, Glassman GE, et al. Rejuvenating the periorbital area using platelet-rich plasma: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:711–727. doi: 10.1007/s00403-020-02173-z. [DOI] [PubMed] [Google Scholar]
- 39.Buzalaf MAR, Levy FM. Autologous platelet concentrates for facial rejuvenation. J Appl Oral Sci. 2022;30:e20220020. doi: 10.1590/1678-7757-2022-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gentile P, Garcovich S. Systematic Review: Platelet-rich plasma use in facial rejuvenation. Plast Reconstr Surg. 2023;152:72e–82e. doi: 10.1097/PRS.0000000000010150. [DOI] [PubMed] [Google Scholar]
- 41.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181:488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hennessy EA, Johnson BT. Examining overlap of included studies in meta-reviews: guidance for using the corrected covered area index. Res Synth Methods. 2020;11:134–145. doi: 10.1002/jrsm. [DOI] [PMC free article] [PubMed] [Google Scholar]