Abstract
Entry of duck hepatitis B virus (DHBV) is initiated by specific interaction of its large envelope protein (L) with a cellular entry receptor, recently identified as carboxypeptidase D (CPD; historically gp180). In this report, we present evidence demonstrating that this receptor is down-regulated as a result of DHBV infection: (i) receptor levels determined by Western blot were much reduced in DHBV-infected duck livers and undetectable by immunostaining in infected cultured hepatocytes; (ii) results from metabolic labeling experiments indicate enhanced receptor protein turnover; (iii) the kinetics of receptor loss from newly infected cells correlated with the accumulation of newly synthesized viral protein; (iv) expression of DHBV L protein, transduced from a recombinant adenovirus, was sufficient to eliminate gp180/CPD from the Golgi compartment, its normal predominant location; (v) gp180/CPD remained absent from the Golgi compartment in infected hepatocytes, even after overexpression from a recombinant adenovirus, while residual amounts subsequently became detectable in a perinuclear compartment, containing DHBV L protein; (vi) expression of DHBV L protein in a HepG2 cell line, stably expressing gp180/CPD, leads to incomplete receptor maturation and induces its degradation. Taken together, these data are consistent with a model in which the virus receptor interacts early in the biosynthetic pathway with the viral L protein, leading to its retention in a pre-Golgi compartment and to subsequent degradation, thus preventing receptor interference with the export of DHBV via the secretory pathway which it shares with its receptor. Accordingly, and analogously with receptor down-regulation in retroviral systems, DHBV receptor down-modulation may account for the much-reduced efficiency of DHBV superinfection of preinfected hepatocytes.
Viruses enter their host cells after specific interactions with cell surface receptors. While these molecules allow attachment and entry of the virus, they have been evolutionarily selected for not interfering with virus production during the later stages in the replication cycle. Furthermore, infected cells should be largely protected from superinfection, allowing progeny virus to efficiently spread to other, yet-uninfected cells. Some viruses, such as measles virus (26), influenza virus, classical retroviruses (17), and lentiviruses (11, 23), have evolved mechanisms to circumvent these problems by down-regulating their cellular attachment receptors upon infection. Influenza virus, for example, encodes an enzyme, neuraminidase, to clip sialic acid residues off glycoproteins present along the secretory pathway and on the surface of infected cells. On the other hand, simple retroviruses, which do not possess accessory proteins, use their envelope glycoproteins to form a complex with their receptor in the endoplasmic reticulum (ER), leading to the retention of the receptor and subsequent degradation (6).
Hepatitis B viruses (HBVs) (hepadnaviruses) are small, enveloped DNA viruses, causing acute or chronic hepatitis in infected animals (10). These viruses have a narrow host range and show distinct liver tropism. Since the medically relevant human HBV entry studies are hampered by the lack of an appropriate infection system (7), the duck HBV (DHBV) model has been widely used to experimentally investigate hepadnaviral entry. With this system, a block in superinfecting DHBV-infected cells or test animals has previously been noticed (J. Pugh and J. Summers, personal communication), and more recently this effect was quantified as an approximately 20-fold reduction in infectibility in DHBV-expressing primary duck hepatocytes (24). However, the mechanism for the observed infection interference and the fate of the entry receptor in infected cells have not been addressed.
Studies from several groups have led to the identification and characterization of the primary receptor molecule, the duck carboxypeptidase D (CPD; historically termed gp180), which is used by avian hepadnaviruses to attach to and to enter the host hepatocytes (3, 4, 18, 21, 22, 28–31). Unlike most virus receptors, gp180/CPD is not enriched at the cell surface but is located in the trans-Golgi, from where it functions by cycling to the plasma membrane and back (3, 4, 9, 32). At the plasma membrane, gp180/CPD binds with high affinity to a distinct region within the pre-S ectodomain of the DHBV large (L) envelope protein (4, 29–31). The virus is subsequently coendocytosed together with its receptor and fuses with an internal membrane, presumably as a consequence of interaction with a (yet unknown) species-specific fusion receptor (3, 4, 29).
In the study reported here, we have examined the fate of the DHBV entry receptor upon virus infection and found that gp180/CPD is down-regulated upon virus infection and that this process is mediated by synthesis of the large viral envelope protein. This receptor modulation most likely contributes to the superinfection interference observed for hepadnavirus infection.
MATERIALS AND METHODS
Viruses and antisera.
DHBV-containing sera from ducks, infected 1 day posthatching with clonal DHBV-16, were collected to yield a virus pool with a titer of about 1010/ml. Construction and production of recombinant adenoviruses (lacking the E1 and E3 regions) encoding gp180/CPD were described previously (3, 15). An adenovirus expressing the DHBV L protein was generated using a shuttle plasmid, pAD-CMV-DL, containing the following relevant sequences: the 5′ inverted terminal repeat and the encapsidation signal of adenovirus type 5 (Ad5) spanning nucleotides 1 to 461, an expression cassette of the DHBV large envelope gene (DHBV positions 727 to 815) (27) under the direction of the cytomegalovirus (CMV) immediate-early promoter, and nucleotides 3322 to 5778 of Ad5. Recombinant adenovirus Ad-CMV-L was produced by homologous recombination in 293 cells after cotransfection with plasmid pJM17, which contains a complete Ad5 genome with the E1 gene replaced by BR322 DNA. Adenovirus isolates were plaque purified, expanded on 293 cells, and characterized by restriction enzyme digestion.
Rabbit anti-gp180/CPD antisera were each generated by three consecutive intramuscular and subcutaneous injections of 0.5 mg of the respective antigen mixed with Freund's complete (first injection) or incomplete (further injections) adjuvant. The antiserum D886, used for immunostaining and as purified immunoglobulin G (IgG) for immunoprecipitations, was raised against the extracellular domain of gp180/CPD in the native state (30). For the generation of antiserum D887 for Western blotting, we used a denatured fragment of gp180/CPD (amino acids Val847 to Val1221), which was expressed and purified as a hexahistidine fusion protein from Escherichia coli (2). TGN46 antiserum was purchased from Biozol/Serotec; antiserum D084 recognizing the DHBV L protein (25) was kindly provided by Christa Kuhn.
Cell culture and infection experiments.
Primary duck hepatocytes (PDHs) were prepared and cultured as described elsewhere (16). The HepG2.18 cell line, stably expressing gp180/CPD under control of the CMV promoter, was generated by Hüseyin Sirma by cotransfection of HepG2 cells with plasmid pC-myc-gp180 (4) and a plasmid expressing neomycin resistance. After clonal selection with G418, cells were maintained in Dulbecco modified Eagle medium (DMEM) (4.5 g of glucose per liter) containing G418 (80 μg/ml), 10% fetal calf serum, and nonessential amino acids. Cells to be analyzed with the confocal microscope were plated on collagen-coated, chambered cover glass (Nunc). For in vitro DHBV infection, 8 × 105 cells, cultivated for 3 to 8 days in 12-well plates, were infected with 4 × 107 to 8 × 107 DNA-containing DHBV particles (as determined by DNA dot blot) and incubated overnight. After removal of the inoculum, cells were washed twice. At various time points cells were harvested and the proteins were analyzed by Western blotting. For transduction with recombinant adenoviruses encoding DHBV L protein (Ad-L) or green fluorescent protein (Ad-GFP), the hepatocytes were incubated overnight with about 5 × 106 or 1 × 108 expression-forming units (determined on 293 cells) per 106 cells for immunostaining and biochemical analysis, respectively. These cells were analyzed 6 days postinfection as described below. For adenovirus infections in Fig. 6, DHBV-infected and uninfected cells were incubated overnight with about 107 GFP-expressing units of the recombinant gp180/CPD-expressing virus per 106 cells. Cells were analyzed 4 days postinfection using indirect immunofluorescence.
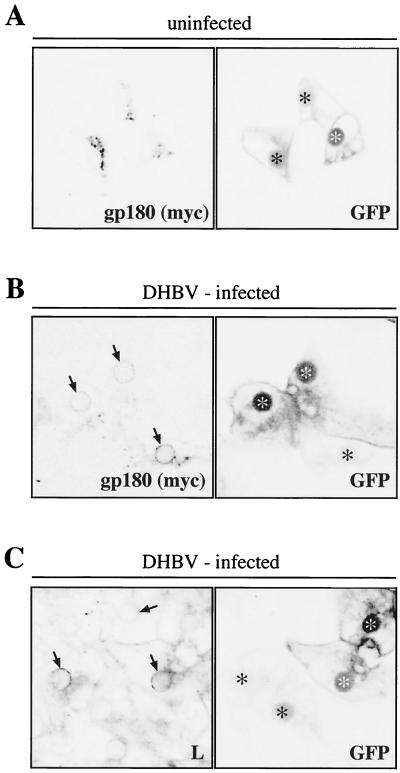
FIG. 6.
In DHBV-infected hepatocytes, overexpressed-gp180/CPD staining is detected in a perinuclear, DHBV L-containing compartment. DHBV-infected (A) or uninfected (B and C) PDHs were infected with a recombinant adenovirus encoding a myc-tagged gp180/CPD and GFP. At day 4 postinfection, the cells were fixed and immunostained with an antibody against the myc tag (A and B) or an anti-DHBV L antibody (C). Immunofluorescence and GFP fluorescence were analyzed by confocal microscopy. Arrows indicate the gp180/CPD and DHBV L-containing compartment (B and C). Adenovirus-infected cells within the confluent hepatocyte monolayers are indicated by asterisks. As noticed before (3), GFP was preferentially preserved in the cell nuclei after formaldehyde fixation and subsequent permeabilization with Triton X-100; however, such treatment did not affect the detection of gp180/CPD.
Metabolic labeling experiments.
Two to 5 days postplating, HepG2.18 cells (60 to 70% confluency) were washed twice with phosphate-buffered saline (PBS) and once with methionine- and cysteine-free DMEM (Gibco). After starvation for 1 h at 37°C, cells were metabolically labeled with Pro-Mix [35S] (Amersham) at 50 or 300 μCi/six-well plate for 1 h at 37°C. Cells were washed three times with PBS at 4°C, supplemented with either hepatocyte maintenance medium (16) or DMEM (4.5 g of glucose per liter), containing 10% fetal calf serum and nonessential amino acids (HepG2.18), and further incubated at 37°C for different periods. After the chase, the medium was removed and the cells were harvested in 750 μl of lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% Na-deoxycholate [pH 6.8]), supplemented with a protease inhibitor cocktail tablet (Boehringer). After sonication to complete membrane protein solubilization, the lysate was cleared by centrifugation (20 min, 15,000 × g, 4°C) and preincubated for 1 h with either Staphylococcus aureus protein A (Pansorbin S; Calbiochem) or protein A-Sepharose (Amersham/Pharmacia). After centrifugation, anti-gp180/CPD IgG was added to the lysate and incubation was continued for 2 h. Immune complexes were collected by incubation with Pansorbin or protein A-Sepharose (30 min) and centrifugation. Immunoprecipitates were thoroughly washed with lysis buffer and subsequently solubilized in sodium dodecyl sulfate (SDS) sample buffer (including 5 mM dithiothreitol) for protein analysis.
Protein analysis.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by standard procedures in 8 or 15% polyacrylamide gels. Prior to loading, proteins were dissolved in a reducing SDS sample buffer and boiled for 5 min. After electrophoresis, gels were blotted on a nitrocellulose or polyvinylidene difluoride membrane using a semidry blotting apparatus (Bio-Rad) or dried and subjected to autoradiography using a Molecular Dynamics PhosphorImager. Proteins on Western blot membranes were probed with antibodies and detected by enhanced chemiluminescence (ECL; Amersham) or quantified by enhanced chemifluorescence (ECF; Amersham) as specified in the manufacturer's manuals.
Immunofluorescence analysis.
For indirect immunofluorescence, the cells were washed with PBS and fixed with 3% paraformaldehyde for about 20 min at room temperature. The fixed cells were permeabilized with 0.25% Triton X-100 in PBS and immunostained with monoclonal antibody 9E10 (recognizing the myc epitope tag), anti-DHBV L-protein antiserum (D084), or anti-gp180/CPD antiserum (D886) and fluorescent secondary antibodies (goat anti-rabbit and anti-mouse; Dianova). GFP fluorescence was basically preserved during all procedures. For fluorescence analysis we used the Leica TCS NT confocal laser-scanning microscope (63× NA; 1.2 lens; water immersion). Sequential excitation and scanning of the two fluorescent channels (separate excitations at 488 and 568 nm) were used to avoid cross-bleeding of the fluorochromes between channels.
RESULTS
Cellular DHBV receptor levels are reduced upon DHBV infection in duck livers and in cultured hepatocytes.
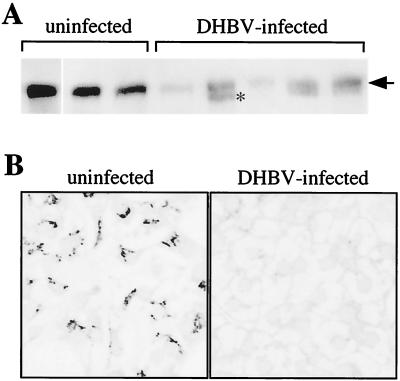
To investigate the influence of DHBV production on DHBV's primary cellular entry receptor, gp180/CPD, we compared receptor levels in livers of DHBV-infected and uninfected animals by Western blotting. Liver samples from uninfected and infected ducklings were homogenized, and equal amounts of protein were loaded on an SDS gel, blotted, and probed with an anti-gp180/CPD antibody (Fig. 1A). All DHBV-infected animals showed a marked reduction of gp180/CPD in the liver, albeit with some variation among the individual animals. In some cases, a second, faster-migrating species was observed (gp170; asterisk in Fig. 1A) and was shown to be gp180/CPD related, as it reacted with several different anti-gp180/CPD antisera (not shown). In infected animals, receptor levels were found to be reduced only in liver tissue and not in other organs such as lung or kidney (data not shown).
FIG. 1.
The DHBV receptor is down-regulated in infected liver cells. (A) Western blot of duck liver biopsies probed with an anti-gp180/CPD antiserum. Samples were derived from DHBV-infected and uninfected ducklings (equivalent amounts of total protein were loaded). The arrow indicates the position of gp180/CPD. The position of gp170, occurring in some of the infected liver samples, is indicated by an asterisks. (B) Primary hepatocytes from DHBV-infected and uninfected test animals were fixed and immunostained with an anti-gp180/CPD antiserum (fluorescein-conjugated secondary antibody). The fluorescence was analyzed with a confocal laser-scanning microscope (63 × 1.2 lens), showing a single focal plane within the hepatocyte monolayer (not detecting nonhepatocytes sitting on top).
This initial biochemical analysis was complemented on the cellular level with liver cell cultures from congenitally DHBV-infected ducklings in which all (>99%) hepatocytes are known to stain positive for DHBV-specific antigens (24), as well as from uninfected animals, using indirect immunofluorescence and confocal laser scanning microscopy. As shown in Fig. 1B, gp180/CPD was no longer detectable in DHBV-positive hepatocytes, while uninfected control cells showed the typical Golgi distribution of the receptor protein, shown previously to costain with a Golgi marker protein (4). Nonhepatocytes, always present in up to 10% of the primary liver cell cultures (20), did not display reduced receptor levels (2), an observation probably explaining the residual gp180/CPD levels detected in Western blot analysis of total liver protein. Taken together, these findings indicate that ongoing DHBV replication results in a strong down-regulation of the cellular entry receptor gp180/CPD.
DHBV receptor down-regulation parallels viral protein expression in newly infected primary hepatocyte cultures.
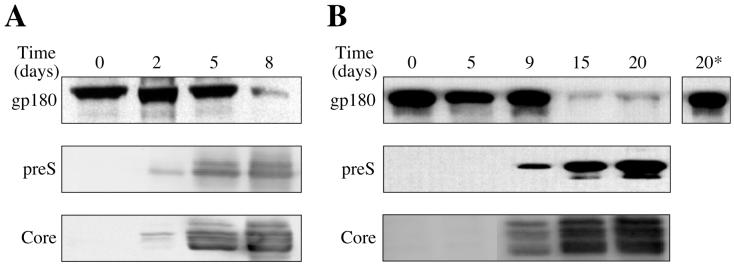
To study the mechanism of receptor down-modulation during DHBV infection, we assayed first whether this cellular reaction could be reconstituted in newly infected cultured PDHs. PDHs were infected by incubation with DHBV-containing duck serum, harvested at different times postinfection, and analyzed by Western blotting for gp180/CPD and for DHBV large envelope protein and core protein as markers of viral protein synthesis (Fig. 2). While parallel, uninfected control cultures did not show significant alterations with time, gp180/CPD levels in the infected cultures remained only initially unchanged and dropped (5- to 10-fold as determined in a parallel ECF Western analysis) rather abruptly about 1 week postinfection. As exemplified in two kinetically diverse experiments, this reduction of gp180/CPD did not depend on the time passed postinfection but correlated with the two viral proteins reaching high intracellular levels (i.e., between days 5 and 8 in Fig. 2A or between days 9 and 12 in Fig. 2B). This correlation suggests a mechanism involving titration of newly synthesized gp180/CPD by a viral protein that is produced in saturating amounts once virus replication has reached steady-state levels followed by a slow decay of the protein preexisting in the Golgi.
FIG. 2.
Receptor down-modulation correlates with viral protein expression upon DHBV infection of cultured cells. Western blots of cultured duck hepatocytes, harvested at the indicated time points postinfection, probed with an antiserum against gp180/CPD (upper panels), DHBV L protein (middle panels), and DHBV core protein (lower panels). 20*, anti-gp180/CPD immunoblot of the respective uninfected control cells 20 days post-mock infection. Panels A and B show two kinetically diverse experiments.
Correlation of virus spread and gp180/CPD loss in hepatocytes was confirmed by indirect immunofluorescence, detecting a loss of gp180/CPD staining in cells expressing DHBV proteins (not shown). As mentioned above, nonparenchymal cells within the culture, not supporting virus production, maintained normal levels of gp180/CPD.
DHBV large envelope protein expression is sufficient to mediate receptor down-regulation.
As receptor down-regulation in DHBV-infected cultures correlated with viral protein synthesis, we next assessed which of the viral proteins was causing this effect. An obvious candidate was the large viral envelope protein, the binding partner of the virus receptor during the entry process. To test this possibility, we induced in PDHs the expression of DHBV L only, through infection with a recombinant adenovirus encoding the large envelope protein under CMV promoter control (Ad-L). Although the DHBV DNA insert in this adenovirus also contains the promoter for expression of the small (S) DHBV envelope protein, S expression appeared to be suppressed at least 10-fold (probably because of the dominance of the strong upstream CMV promoter), as judged by Western blotting of lysates from transduced cells with an anti-S antibody (not shown). In parallel, another set of cells was infected with authentic DHBV. After a further 6 days of culture, the cells were fixed, coimmunostained with an antibody against the DHBV L protein and an anti-gp180/CPD antiserum, and analyzed by confocal microscopy. As shown in Fig. 3A, gp180/CPD was absent from cells expressing the viral envelope protein, in contrast to neighboring uninfected cells in the hepatocyte monolayer.
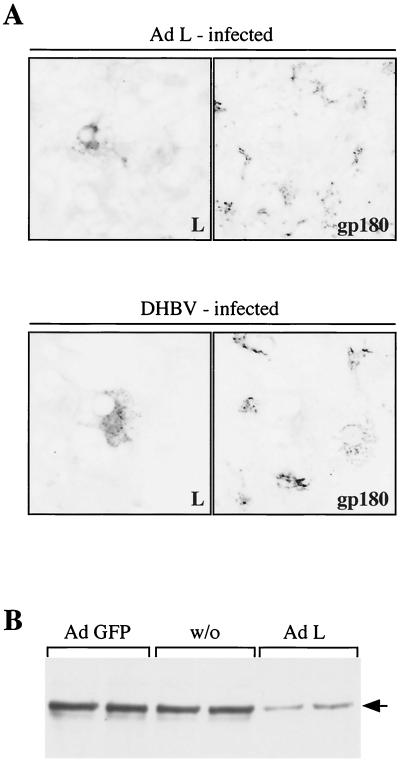
FIG. 3.
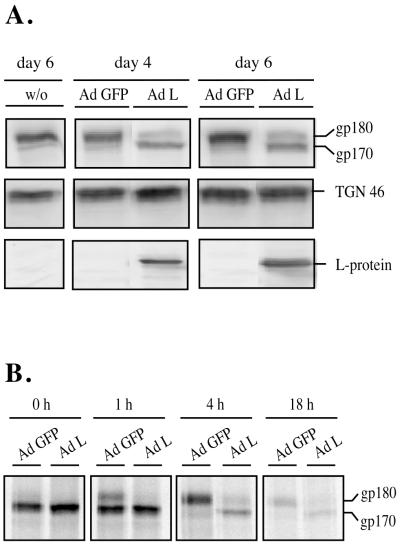
DHBV L-protein expression is sufficient for receptor down-regulation. (A) Indirect immunofluorescence of PDHs infected for 4 h with Ad-L (upper panels) or DHBV (lower panels). At day 6 postinfection, the cells were fixed and stained with an anti-L antibody and an antiserum against gp180/CPD (secondary antibodies were rhodamine and fluorescein conjugated for the right and left panels, respectively). Double fluorescence was analyzed by confocal microscopy (63× lens; sequential scans of the two fluorescent channels). (B) Anti-gp180/CPD Western blot of lysates of PDHs, infected on day 4 postplating with Ad-GFP or Ad-L. w/o, control cells without adenovirus infection. The position of gp180/CPD is indicated (arrow).
This observation was complemented by a biochemical analysis. Ad-L-infected PDH cultures showed reduced gp180/CPD levels in Western blot analysis, while control cells, infected with an adenovirus encoding the GFP, showed no change in receptor levels (Fig. 3B). From these results, we conclude that expression of the DHBV large envelope protein is sufficient to cause receptor down-modulation.
Accelerated gp180/CPD turnover in DHBV-infected hepatocytes.
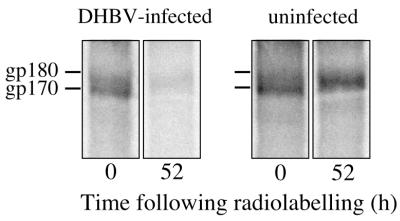
To examine the mode of receptor down-regulation by synthesis of its viral ligand, we followed the fate of gp180/CPD after metabolic labeling in pulse-chase experiments. DHBV-infected and uninfected duck hepatocyte cultures were incubated for 1 h with a [35S]Met-Cys-containing medium and then, or after a chase period of 52 h, lysed by addition of a detergent-containing buffer. gp180/CPD was recovered from cell lysates by immunoprecipitation and subsequently analyzed by SDS-PAGE and autoradiography (Fig. 4).
FIG. 4.
DHBV receptor turnover differs in DHBV-infected hepatocytes. DHBV-infected and uninfected duck hepatocytes were labeled for 1 h with 35S-amino acids and then either lysed directly or after a chase for 52 h. Labeled proteins immunoprecipitated with anti-gp180/CPD antibodies were analyzed by SDS-PAGE and autoradiography. The positions of gp180/CPD and a gp180/CPD-precursor (gp170 [33]) are indicated on the left.
In these experiments, precipitates from infected and uninfected cells contained similar amounts of radiolabeled gp180/CPD species after the pulse period (Fig. 4, 0 h), indicating comparable synthesis rates in infected and uninfected hepatocytes. At later time points, gp180/CPD signals were similarly reduced in infected cells compared to uninfected cells (Fig. 4, 52 h). Like in the livers of some of the DHBV-infected ducklings, a faster-migrating 170-kDa species was observed after the pulse period, in addition to the mature gp180/CPD. This species (gp170) has been previously identified as a biosynthetic CPD precursor differing from gp180 in its glycosylation residue(s) (33); notably, in some experiments, gp170 was found to persist over longer periods in infected PDH cultures only (up to several hours [not shown]), a phenomenon that became very evident in experiments with a gp180/CPD-expressing hepatoma cell line (see below).
Since the DHBV receptor was synthesized at similar rates in infected and uninfected cells, the low receptor levels in the infected cells must be the result of accelerated receptor turnover. Accordingly, we found in infected cultures significantly reduced signals of gp180/CPD after various chase periods following pulse-labeling. However, varying concentrations of nonhepatocytes in our cultures precluded the determination of the fraction of gp180/CPD that was derived from DHBV-expressing hepatocytes.
DHBV L transduction results in enhanced degradation of duck gp180/CPD in a stably transfected human hepatoma cell line.
As noted above, biochemical analysis of gp180/CPD in DHBV-infected hepatocyte cultures suffers from the presence of significant amounts of nonhepatocytes, mainly sinusoidal endothelial cells, that are infectible neither by DHBV nor by transducing adenoviruses but very active in abortive uptake of DHBV particles (2). To circumvent these problems, we made use of a human hepatoma cell line, HepG2.18, which stably expresses gp180/CPD under CMV promoter control. As in the experiments with PDHs in Fig. 3B, expression of L protein from a transducting adenovirus (Ad-L) resulted in a strong decrease in gp180/CPD steady-state levels, whereas uninfected HepG2.18 cells or cells infected with a GFP-expressing adenovirus (Ad-GFP) did not show significant alterations in receptor levels (Fig. 5A). Furthermore, TGN46, a TGN-resident protein with patterns of expression similar to those of gp180/CPD, remained unchanged after L-protein expression (Fig. 5A), indicating that gp180/CPD down-regulation was not caused by unspecific effects of L-protein synthesis, such as ER stress. As observed in infected duck liver (Fig. 1A), L-protein expression resulted in the appearance of a gp180/CPD-related 170-kDa species, the incompletely glycosylated precursor of gp180/CPD (28).
FIG. 5.
DHBV L-protein expression leads to incomplete processing and enhances degradation of gp180/CPD in HepG2.18 cells. (A) HepG2.18 cells, constitutively synthesizing gp180/CPD, were infected with Ad-L or Ad-GFP to more than 95%, as judged by GFP fluorescence or by anti-L immunostaining performed with a parallel culture dish. On days 4 and 6 postinfection, cellular lysates were analyzed by Western blotting using antibodies against gp180/CPD (upper panel), the trans-Golgi network protein TGN46 (middle panel) or the DHBV L protein (lower panel). Lysates of uninfected cells (w/o) are shown as a control. The immature form of gp180/CPD is indicated (gp170). (B) HepG2.18 cells were infected with Ad-L or Ad-GFP as above, and 65 h postinfection cells were metabolically labeled for 1 h with 35S-amino acids and gp180/CPD was immunoprecipitated at 0, 1, 4, and 18 h after the pulse. Immature gp170 and the fully processed gp180 are indicated.
A pulse-chase labeling experiment with gp180/CPD in the HepG2.18 cell line under comparable conditions confirmed these results (Fig. 5B). Immediately after the pulse (0 h) equal amounts of immature gp170 were detected in the Ad-L- and the Ad-GFP-transduced cells, indicating that the rate of receptor synthesis was not influenced by L-protein expression. While at this time point mature gp180 was barely detectable, 1 h later about one-third, and 4 h later virtually all, of the precursor molecules had matured in the Ad-GFP-infected cells. In contrast, only very minor amounts of completely glycosylated gp180 were detectable in the Ad-L-transduced control. In addition, 4 h after the pulse, more than 70% of total gp180/CPD had been degraded in the L-protein-expressing cells while 90% of newly synthesized receptor was still present in the GFP control. After 18 h of chase, the gp180 level had decreased to about 10%, whereas degradation of the p170 precursor upon L expression was nearly complete in the control cells. We therefore conclude that L-protein expression prevents the complete maturation of gp180/CPD, thereby inducing an accelerated degradation of the protein.
DHBV receptor, overexpressed in DHBV-infected cells, is detected at low levels in a pre-Golgi compartment.
The above data suggest that DHBV uses its receptor-binding envelope protein to achieve receptor modulation in productively infected cells, similarly to what has been described for some retroviruses (5, 6). We therefore wanted to assess whether DHBV uses a similar mechanism. However, as immunofluorescence with our anti-gp180/CPD antiserum did not show detectable receptor levels in DHBV-positive hepatocytes (Fig. 1B), we were not able to localize gp180/CPD in DHBV-infected cells.
To overcome this limitation of detection, we increased the rate of receptor synthesis (at least 10-fold [3]) by superinfection with a recombinant adenovirus encoding a myc-tagged gp180/CPD and additionally GFP as a marker identifying transduced cells (3). After 4 days, allowing for receptor synthesis from the recombinant adenovirus, cells were fixed and stained with an antibody against the myc tag (recognizing selectively the transduced recombinant gp180/CPD) or an anti-DHBV L antibody and analyzed by confocal microscopy. As shown in Fig. 6A, gp180/CPD transduction in DHBV-negative PDHs led to a normal Golgi-like distribution of the overexpressed myc-tagged receptor, indistinguishable from that of endogenous gp180/CPD (Fig. 1A). As expected, much less gp180/CPD (myc tag)-specific staining was observed in DHBV-infected cells (Fig. 6B). In most cells, residual gp180/CPD staining was concentrated in a compartment surrounding the cell nuclei (Fig. 6B, arrows); additional gp180/CPD was detected occasionally in small vesicles. L protein, known to accumulate in the ER and/or ER-to-Golgi intermediate compartment (10), showed a similar perinuclear distribution in a parallel set of gp180/CPD-transduced, DHBV-infected hepatocytes (Fig. 6C), suggesting an intimate association of L and gp180/CPD in a common compartment. However, the distribution of DHBV L seemed not to be influenced by gp180/CPD overexpression, as it did not differ between cells transduced or not transduced with Ad-gp180 (Fig. 6C). These results furthermore suggest that L-protein production is sufficiently strong to override even enhanced gp180/CPD expression.
DISCUSSION
In this study, we have investigated the fate of a hepadnavirus entry receptor upon experimental infection. Taking advantage of the DHBV animal model where a cellular carboxypeptidase (gp180/CPD) has been identified as an essential part of the receptor system (3, 4, 21, 22, 28, 29, 31), we obtained a set of biochemical and cell biological data demonstrating that the levels of this uptake receptor are drastically reduced in DHBV-infected hepatocytes or upon expression of the receptor-interacting large viral envelope protein. In immunofluorescence analysis, the receptor protein virtually disappeared from the trans-Golgi network, the compartment that it predominantly occupies in the uninfected cell; the receptor is therefore expected to be essentially absent from the cell surface, where receptor density is already very low even in the uninfected hepatocyte (4). These data thus suggest that hepadnavirus should be added to the still-limited number of well-documented examples of viral pathogens that down-modulate their cellular receptors in the productively infected host cell.
Generally, receptor down-modulation may be envisaged to serve two purposes: (i) to prevent receptor molecules from interfering with virus morphogenesis by interacting intracellularly with the viral ligand that it is supposed to encounter only on the surface of the target cell and (ii) to promote virus spread to yet-uninfected cells by preventing loss of newly synthesized virus particles to cells with an already established infection. Nonenveloped viruses avoid the former problem by producing progeny particles separated from the cellular secretory compartment, the site where the membrane-anchored cell surface receptor molecules mature and are exported. In the morphogenesis of enveloped viruses, receptor interference represents a major problem, however, as the viral envelope (glyco)proteins use the same secretory pathway for synthesis, maturation, and export as do the cellular receptors. Conceptually, receptor interference may be even more relevant in the case of hepadnaviruses, such as DHBV, where cytoplasmic capsids bud not from the plasma membrane but intracellularly into a post-ER, pre-Golgi compartment (10). Therefore, not only are the viral envelope proteins cosynthesized with the receptor at the ER membrane, but the outgoing virus particles are also destined to pass through the trans-Golgi network, where the bulk of the gp180/CPD molecules are normally localized. Interaction with the receptor is expected to lead either to capsid reimport into the cytoplasm (i.e., intracellular reinfection) or, more likely, to virus inactivation (3). Efficient receptor elimination from the cellular secretory system thus appears to be an essential element in the hepadnavirus replication strategy, a prediction that is consistent with the much-reduced efficiency of DHBV superinfection of preinfected hepatocytes (24).
The results obtained in this study demonstrate that the total cellular pool of the DHBV uptake receptor is indeed strongly down-modulated by interaction with a viral ligand in the infected hepatocyte as well as in a stably gp180/CPD transfected hepatoma cell line (HepG2.18). They furthermore suggest that this down-modulation occurs posttranslationally through degradation of the newly synthesized receptor polypeptide. Accordingly, DHBV infection (or DHBV L transduction) did not affect the rate of receptor synthesis in metabolic labeling experiments but resulted in enhanced gp180/CPD turnover (Fig. 4). Similarly, no change in synthesis of the gp170 precursor protein and enhanced turnover of CPD polypeptides was observed in HepG2.18 cells upon DHBV L expression from a transducing recombinant adenovirus (Fig. 5). Moreover, we observed an increased proportion of the faster-migrating gp170, a species characterized in a detailed study by others as an immature (incompletely glycosylated) gp180/CPD precursor (33), in Western blots of infected duck liver tissue (Fig. 1A) or L-expressing HepG2.18 cells (Fig. 5A), and in metabolic labeling of proteins in DHBV-infected hepatocytes or in HepG2.18 cells (Fig. 5B). In agreement with these biochemical data, gp180/CPD was no longer detectable by immunostaining in the Golgi in hepatocytes that were either persistently DHBV infected (Fig. 1B) or transduced with an L-protein-expressing construct (Fig. 3A). Importantly, gp180/CPD was also absent from the Golgi, even if gp180/CPD was overexpressed from a transduced, CMV promoter-driven gp180/CPD construct, with residual immunostaining now being reliably detectable in a perinuclear, L-containing, ER-like compartment (Fig. 6B). Taken together, these findings support the hypothesis that the DHBV receptor is arrested in a pre-Golgi compartment by forming a complex with its ligand, the viral large envelope protein. Correspondingly, it has been shown for human immunodeficiency virus (HIV) that the newly synthesized envelope protein precursor gp160 causes retention of the CD4 receptor in the ER, thus leading to its premature degradation (5).
While we can only speculate about the subsequent steps leading eventually to receptor degradation, our present data indicate that the newly synthesized receptor molecules are complexed and degraded soon after synthesis, and they therefore suggest that L was present in saturating levels once viral protein synthesis had reached steady-state levels (Fig. 1B and 2). This assumption is further supported by several lines of evidence. (i) Receptor overexpression from a transducing adenovirus did not detectably affect intracellular L-protein levels and localization as visualized by immunostaining (Fig. 6C). (ii) In analogous experiments described elsewhere, receptor overexpression also did not interfere with the production and release of virus particles from DHBV-infected cultured hepatocytes (3). (iii) In keeping with these experimental observations, we estimate the receptor level in the uninfected liver to be about 105 molecules per cell (S. Reuter and S. Urban, unpublished results). This is about 50-fold below the amount of L protein produced per cell per day as calculated from a value of 500 virus particles produced in cell culture (20) and assuming a 1,000-fold excess of SVPs, each containing 10 fully translocated L-protein molecules. The massive overproduction of nucleocapsid-free subviral particles, a hallmark of hepadnavirus replication, thus may also serve, among other functions, in clearing the cellular secretory pathway from components that otherwise would most likely interfere with virus export.
The mechanism outlined above predicts that DHBV receptor down-modulation is tightly controlled by the vast excess of L protein produced in the ER and exported as part of SVPs. However, the highly variable degree of viral gene expression among individual producer cells in the infected liver (12) may also be reflected by a fluctuating potential for superinfection, thereby creating a replication space allowing replication of variant virus species (34). Given that receptor down-regulation is conserved between hepadnaviruses, as it is among retroviruses, our findings may also have important implications for medical virology as the generation and spread within individual patients of viable and defective HBV sequence variants (with potential variations in pathogenicity) is a major issue in medical HBV research (13).
Several other enveloped viruses are known to efficiently down-modulate cellular receptor levels in productively infected cells. Viruses with genomes more complex than the DHBV genome often encode accessory proteins to cope with the task of receptor down-regulation (e.g., HIV vpu and nef [8]). DHBV, and probably other HBVs, uses for this purpose its receptor-interacting envelope protein, a strategy that is also employed by the classical, simple retroviruses, as demonstrated for reticuloendotheliosis virus (6). Hence, this similar mechanism of receptor down-regulation adds to the list of conserved features relating the hepadnaviruses to the classical retroviruses (1).
ACKNOWLEDGMENTS
K.M.B. and S.U. contributed equally to this work.
We are grateful to Claudia Kruse for contributing to the initial phase of this work, to Beate Zachmann-Brand for providing recombinant adenovirus encoding DHBV L, and to Huseyin Sirma for providing the cell line HepG2.18 as well as for performing an initial DHBV L transduction experiment. We also thank Christa Kuhn for antisera, Elizabeth Grgacic and Ulrike Protzer for comments on the manuscript, and Karin Coutinho for expert editorial assistance.
This work was supported by a Boehringer Ingelheim Fonds predoctoral fellowship to K.M.B. and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Boeke J D, Stoye J P. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 343–435. [PubMed] [Google Scholar]
- 2.Breiner K M. Carboxypeptidase D (gp180): Rezeptor, Transzeptor und Signalmolekül für Vogelhepatitis B Viren. Ph.D thesis. Heidelberg, Germany: University of Heidelberg; 1998. [Google Scholar]
- 3.Breiner K M, Schaller H. Cellular receptor traffic is essential for productive duck hepatitis B virus infection. J Virol. 2000;74:2203–2209. doi: 10.1128/jvi.74.5.2203-2209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiner K M, Urban S, Schaller H. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J Virol. 1998;72:8098–8104. doi: 10.1128/jvi.72.10.8098-8104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crise B, Buonocore L, Rose J K. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus type 1 glycoprotein precursor. J Virol. 1990;64:5585–5593. doi: 10.1128/jvi.64.11.5585-5593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delwart E L, Panganiban A T. Role of reticuloendotheliosis virus envelope glycoprotein in superinfection interference. J Virol. 1989;63:273–280. doi: 10.1128/jvi.63.1.273-280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMeyer S, Gong J Z, Suwandhi W, van Pelt J, Soumillon A, Yap S H. Organ and species specificity of hepatitis B virus (HBV) infection: a review of literature with a special reference to preferential attachment of HBV to human hepatocytes. J Viral Hepat. 1997;4:145–153. doi: 10.1046/j.1365-2893.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 8.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 9.Eng F J, Varlamov O, Fricker L D. Sequences within the cytoplasmic domain of gp180/carboxypeptidase D mediate localization to the trans-Golgi network. Mol Biol Cell. 1999;10:35–46. doi: 10.1091/mbc.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganem D. Hepadnaviridae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2703–2737. [Google Scholar]
- 11.Geleziunas R, Bour S, Wainberg M A. Cell surface down-modulation of CD4 after infection by HIV-1. FASEB J. 1994;8:593–600. doi: 10.1096/fasebj.8.9.8005387. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti L G, Matzke B, Schaller H, Chisari F V. High level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunther S, Fischer L, Pult I, Sterneck M, Will H. Naturally occurring variants of hepatitis B virus. Adv Virus Res. 1999;52:25–137. doi: 10.1016/s0065-3527(08)60298-5. [DOI] [PubMed] [Google Scholar]
- 14.Guo J T, Pugh J C. Topology of the large envelope protein of duck hepatitis B virus suggests a mechanism for membrane translocation during particle morphogenesis. J Virol. 1997;71:1107–1114. doi: 10.1128/jvi.71.2.1107-1114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hild M, Weber O, Schaller H. Glucagon treatment interferes with an early step of duck hepatitis B virus infection. J Virol. 1998;72:2600–2606. doi: 10.1128/jvi.72.4.2600-2606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–120. [PubMed] [Google Scholar]
- 18.Ishikawa T, Kuroki K, Lenhoff R, Summers J, Ganem D. Analysis of the binding of a host cell surface glycoprotein to the pre-S protein of duck hepatitis B virus. Virology. 1994;202:1061–1064. doi: 10.1006/viro.1994.1440. [DOI] [PubMed] [Google Scholar]
- 19.Klingmüller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol. 1993;67:7414–7422. doi: 10.1128/jvi.67.12.7414-7422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klöcker U, Schultz U, Schaller H, Protzer U. Endotoxin stimulates liver macrophages to release mediators that inhibit an early step in hepadnavirus replication. J Virol. 2000;74:5525–5533. doi: 10.1128/jvi.74.12.5525-5533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroki K, Cheung R, Marion P L, Ganem D. A cell surface protein that binds avian hepatitis B virus particles. J Virol. 1994;68:2091–2096. doi: 10.1128/jvi.68.4.2091-2096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuroki K, Eng F, Ishikawa T, Turck C, Harada F, Ganem D. gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J Biol Chem. 1995;270:15022–15028. doi: 10.1074/jbc.270.25.15022. [DOI] [PubMed] [Google Scholar]
- 23.Piguet V, Schwartz O, Le Gall S, Trono D. The down-regulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 24.Protzer U, Nassal M, Chiang P W, Kirschfink M, Schaller H. Interferon gene transfer by a hepatitis B virus vector efficiently suppresses wild-type virus infection. Proc Natl Acad Sci USA. 1999;96:10818–10823. doi: 10.1073/pnas.96.19.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlicht H J, Kuhn C, Guhr B, Mattagliano R J, Schaller H. Biochemical and immunological characterization of the duck hepatitis B virus envelope proteins. J Virol. 1987;61:2280–2285. doi: 10.1128/jvi.61.7.2280-2285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider-Schaulies J, Schnorr J J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential down-regulation of CD46 by measles virus wild-type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swameye I, Schaller H. Dual topology of the large envelope protein of duck hepatitis B virus: determinants preventing pre-S translocation and glycosylation. J Virol. 1997;71:9434–9441. doi: 10.1128/jvi.71.12.9434-9441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong S, Li J, Wands J R. Interaction between duck hepatitis B virus and a 170-kilodalton cellular protein is mediated through a neutralizing epitope of the pre-S region and occurs during viral infection. J Virol. 1995;69:7106–7112. doi: 10.1128/jvi.69.11.7106-7112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban S, Breiner K M, Fehler F, Klingmüller U, Schaller H. Avian hepatitis B virus infection is initiated by the interaction of a distinct pre-S subdomain with the cellular receptor gp180. J Virol. 1998;72:8089–8097. doi: 10.1128/jvi.72.10.8089-8097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban S, Kruse C, Multhaup G. A soluble form of the avian hepatitis B virus receptor. Biochemical characterization and functional analysis of the receptor ligand complex. J Biol Chem. 1999;274:5707–5715. doi: 10.1074/jbc.274.9.5707. [DOI] [PubMed] [Google Scholar]
- 31.Urban S, Schwarz C, Marx U C, Zentgraf H, Schaller H, Multhaup G. Receptor recognition by a hepatitis B virus reveals a novel mode of high affinity virus-receptor interaction. EMBO J. 2000;19:1217–1227. doi: 10.1093/emboj/19.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varlamov O, Fricker L. Intracellular trafficking of metallocarboxypeptidase D in AtT-20 cells: localization to the trans-Golgi network and recycling from the cell surface. J Cell Sci. 1998;111:877–885. doi: 10.1242/jcs.111.7.877. [DOI] [PubMed] [Google Scholar]
- 33.Varlamov O, Wu F, Shields D, Fricker L D. Biosynthesis and packaging of carboxypeptidase D into nascent secretory vesicles in pituitary cell lines. J Biol Chem. 1999;274:14040–14045. doi: 10.1074/jbc.274.20.14040. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y Y, Summers J. Low dynamic state of viral competition in a chronic avian hepadnavirus infection. J Virol. 2000;74:5257–5265. doi: 10.1128/jvi.74.11.5257-5265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]