Abstract
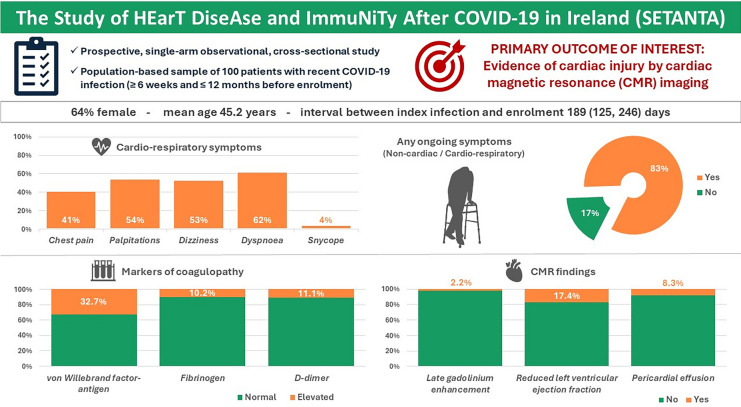
SETANTA (Study of HEarT DiseAse and ImmuNiTy After COVID-19 in Ireland) study aimed to investigate symptom burden and incidence of cardiac abnormalities after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 and to correlate these results with biomarkers of immunological response and coagulation. SETANTA was a prospective, single-arm observational cross-sectional study condcuted in a primary practice setting, and prospectively registered with ClinicalTrials.gov (identifier: NCT04823182). Patients with recent COVID-19 infection (≥ 6 weeks and ≤ 12 months) were prospectively enrolled. Primary outcomes of interest were markers of cardiac injury detected by cardiac magnetic resonance imaging (CMR), which included left ventricular ejection fraction, late gadolinium enhancement and pericardial abnormalities, as well as relevant biomarkers testing immunological response and coagulopathy. 100 patients (n = 129 approached) were included, amongst which 64% were female. Mean age of the total cohort was 45.2 years. The median (interquartile range) time interval between COVID-19 infection and enrolment was 189 [125, 246] days. 83% of participants had at least one persistent symptom, while 96% had positive serology for prior SARS-CoV-2 infection. Late gadolinium enhancement, pericardial effusion, was present in 2.2% and 8.3% respectively, while left ventricular ejection fraction was below the normal reference limit in 17.4% of patients. Von Willebrand factor antigen was elevated in 32.7% of patients and Fibrinogen and D-Dimer levels were found to be elevated in 10.2% and 11.1% of patients, respectively. In a cohort of primary practice patients recently recovered from SARS-CoV-2 infection, prevalence of persistent symptoms and markers of abnormal coagulation were high, despite a lower frequency of abnormalities on CMR compared with prior reports of patients assessed in a hospital setting.
Trial Registration: Clinicaltrials.gov, NCT04823182 (prospectively registered on 30th March 2021).
Keywords: COVID-19, Inflammation, Biomarkers, Magnetic resonance imaging
Subject terms: Respiratory signs and symptoms, Biomarkers, Cardiology
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 first emerged in Wuhan, China in December 2019. With its rapid spread across every continent and its significant mortality and morbidity, there has been considerable interest in the cardiovascular complications of infection, with myocardial injury having been noted to be widely prevalent1,2. Mechanisms of myocardial injury include acute coronary syndrome, exacerbation of pre-existing cardiovascular disease, arrhythmia, and myocarditis3–5. Microvascular angiopathy, endothelial dysfunction as well as coagulopathy have been suggested as mediators of the more severe cardiovascular sequelae of the disease6. While short term complications are best described in recent times, more information has also emerged about the long-term cardiovascular complications of COVID-197,8.
Several studies assessing the effect of COVID-19 on myocardial tissue through the medium of cardiac magnetic resonance (CMR) imaging have been published. In 2020, Puntmann et al. reported results from a prospective observational cohort study that included 100 patients recently recovered from COVID-199. CMR revealed cardiac involvement in 78% of patients with ongoing myocardial inflammation in 60%, as well as a substantial number with scarring evident on native longitudinal relaxation time (T1) and late gadolinium enhancement (LGE) assessment. Similar data has been reproduced in several studies with varying designs, which have been generally small10,11 or have focused on specific populations such as recovering athletes12.
There has also been significant interest in terms of immunity and coagulopathy post COVID-19 infection. The immunological response to the disease, although investigated in detail at this point, is still not fully understood. Severe cases of COVID-19 tend to have a more vigorous IgG response against SARS-CoV-2 compared with mild cases13. A prior study found that anti-SARS-CoV-2 antibodies to the spike protein decreased over 60 days in health care personnel, with 58% of seropositive individuals becoming seronegative14. Moreover, it has been shown that patients with SARS-CoV-2 are at higher risk of arterial and venous thrombotic events with reports of venous thromboembolism prevalence of up to 69% in critically ill patients15. Elevations in Von-Willebrand factor (VWF) or D-dimer many months post recovery, even in a non-ICU cohort, potentially point towards continued coagulopathy and likely indicate ongoing endothelial dysfunction weeks or months post initial illness16.
Thus, the aims of this study were to investigate the (i) incidence of cardiac abnormalities as assessed by CMR imaging in unselected patients after acute COVID-19 infection in Ireland, and (ii) to examine the correlation with immunological response and biomarkers of coagulation.
Materials and methods
Study design and participants
SETANTA was a prospective, community-based, observational cross-sectional study. All consecutive patients with recent COVID-19 infection were identified at three primary care centres in Ireland and invited to participate in the study. Key study inclusion criteria were (i) ≥ 18 years, (ii) SARS-CoV-2 infection ≥ 6 weeks and ≤ 12 months before enrolment, as evidenced by positive reverse-transcriptase polymerase chain reaction (RT-PCR) SARS-CoV-2 nasopharyngeal swab, and (iii) written informed consent. Key exclusion criteria were (i) prior history of myocarditis or ischemic heart disease, (ii) contraindication to gadolinium (eGFR < 30 ml/min) or regadenoson (including cardiac conduction disease, asthma, seizures, pregnancy or breast-feeding), (iii) inability to provide written informed consent, fill out the safety questionnaires or cooperate with the scan and breath holds, (iv) insufficient CMR image quality. This study was approved by local hospital Institutional Review Board (Ref. No. 1/378/2199) and conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all participants prospectively during their screening visit. The study was also prospectively registered on http://www.cliniclatrials.gov platform (ClinicalTrials.gov identifier: NCT04823182).
Pre-specified outcomes of interest
The primary outcomes of interest were markers of cardiac injury detected by CMR, including, left ventricular ejection fraction (LVEF), left ventricular end diastolic volume (LVEDV), right ventricular ejection fraction (RVEF), native T1, native transverse relaxation time (T2), late gadolinium enhancement, and pericardial abnormalities. Secondary outcomes included anti-SARS-CoV-2 antibody testing and markers of coagulation and immunity.
Data collection
Eligible patients, who provided written informed consent, were invited to attend a baseline visit over two consecutive days at a hospital clinic. The first visit comprised of consent and collation of baseline demographics, medical history, current medications, and self-reported SARS-CoV-2 symptoms. Symptoms were self-defined by patients as mild, moderate, severe or none. A modified version of the SAQ7 (Seattle Angina Questionnaire 7) was used to assess anginal-type symptoms. Assessments included vital signs, electrocardiogram (ECG), 24-h ambulatory ECG recording and blood sampling by a dedicated study nurse. The patient returned to the hospital clinic after 24 h and underwent CMR imaging. Incidence of major adverse cardiac events (MACE) were followed up via telephone at 1, 6 and 12 months following the baseline visit, with changes in QOL indices reviewed at months 6 and 12.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance (CMR) imaging was performed on a 1.5 T whole body scanner (Magnetom Sola, Siemens Healthcare Sector, Erlangen, Germany). Full details with regard to baseline sequences are provided in the Supplementary appendix. Perfusion imaging was carried out after the administration of 0.4 mg of regadenoson as a rapid bolus over 10 s. This was followed by 0.1 mmol/kg of the gadolinium-based contrast agent gadobutrol (Gadovist; Bayer Phama, Berlin, Germany) infused 45 to 60 s after. Late gadolinium enhancement (LGE) imaging was carried out in HLA, VLA, LVOT and short axis views with cross-cut imaging over any apparent LGE, 10 min after administration of gadolinium.
Electrocardiogram assessment
Standard published definitions were used for ambulatory ECG parameters17. For further details, please see the Supplementary appendix.
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Results
A cohort of 100 patients was included between February and September 2021, of which 96 underwent CMR imaging (Supplementary Fig. 1). The median (IQR) time interval between SARS-CoV-2 infection and enrolment was 189 (125, 246) days. Mean age was 45.2 ± 12.8 years. The majority of subjects were females (64%), with a mean BMI of 27.1 ± 5.2 kg/m2. Patients had a high prevalence of hypercholesterolemia and smoking history (20% and 41%, respectively). Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics.
| Participants | 100 |
| Age, y | 45.2 ± 12.8 |
| Female, sex | 64 (64) |
| Ethnicity, Caucasian | 96 (96) |
| Blood pressure (systolic), mmHg | 133.9 ± 16.4 |
| Blood pressure (diastolic), mmHg | 82.2 ± 10.6 |
| Heart rate, bpm | 73.0 ± 12.0 |
| Height, m | 1.7 ± 0.1 |
| Weight, kg | 78.4 ± 17.8 |
| BMI, kg/m2 | 27.1 ± 5.2 |
| Oxygen saturation, % | 97.7 ± 1.2 |
| Temperature, °C | 36.5 ± 0.4 |
| Duration since index SARS-CoV-2 diagnosis (days) | 189 (125, 246) |
| History of hypertension | 12 (12) |
| History of diabetes mellitus | 1 (1) |
| History of Hypercholesterolemia | 20 (20) |
| History of Heart failure | 0 (0) |
| History of chronic obstructive pulmonary disease/asthma | 7 (7) |
| History of stroke | 1 (1) |
| History of deep vein thrombosis/pedal oedema | 5 (5) |
| New onset deep vein thrombosis/pedal oedema | 3/5 (60) |
| History of renal disease | 1 (1) |
| History of liver disease | 1 (1) |
| History of smoking | 41 (41) |
| Current | 9/41 (22) |
| Ex-smoker | 32/41 (78) |
| Average alcohol intake (units/week) | 8.3 ± 7.9 |
| Family history of premature coronary artery disease | 17 (17) |
| Long-term medications | |
| Aspirin | 3 (3) |
| Anticoagulants | 3 (3) |
| Angiotensin II receptor blockers | 5 (5) |
| Angiotensin-converting enzyme inhibitor | 1 (1) |
| Calcium channel blocker | 3 (3) |
| Lipid-lowering therapy | 7 (7) |
| Colchicine | 2 (2) |
| Antiarrhythmics | 2 (2) |
| SARS-CoV-2 infection | |
| Hospitalized for index SARS-CoV-2 infection | 14 (14) |
| Ventilatory support | 3/14 (21) |
| Invasive | 1/3 (33) |
| Hospitalization length (days) | 3 (1, 6.75) |
| Non-cardiac symptoms since infection | |
| Fever, % (persistent, resolved, never) | 3, 50, 47 |
| Sore throat, % (persistent, resolved, never) | 2, 29, 69 |
| Cough, % (persistent, resolved, never) | 4, 49, 47 |
| Myalgia, % (persistent, resolved, never) | 11, 63, 26 |
| Loss of sense of smell, % (persistent, resolved, never) | 17, 32, 51 |
| Loss of sense of taste, % (persistent, resolved, never) | 15, 31, 54 |
| Fatigue/Malaise, % (persistent, resolved, never) | 43, 43, 14 |
| Headache, % (persistent, resolved, never) | 22, 44, 34 |
| Rash, % (persistent, resolved, never) | 1, 9, 90 |
| GI disturbance, % (persistent, resolved, never) | 6, 33, 61 |
| Symptom severity during infection | |
| Severe | 18 (18) |
| Moderate | 35 (35) |
| Mild | 43 (43) |
| None | 4 (4) |
| Vaccination status | |
| Vaccinated for SARS-CoV-2 at index visit (at least one dose) | 60 (60) |
| Only one dose | 18/60 (30) |
| Fully vaccinated | 44/60 (73.3) |
| Type of vaccine | |
| Pfizer | 34/60 (56.7) |
| AstraZeneca | 21/60 (35) |
| Moderna | 3/60 (5) |
| Janssen | 2/60 (3.3) |
Data expressed as number (%), mean ± SD or median (interquartile range).
Clinical status at index infection and at baseline
Nearly half of patients reported mild symptoms (43%), with 18% reporting severe symptoms during the index infection. A total of 14 patients (14%) required hospitalization, with 3 patients in this group receiving ventilatory support. Median hospital stay was 3 days (1, 6.75) (Table 1). The most common non-cardiac symptoms since infection included fatigue/malaise in 86% (persistent in 43%), myalgia in 74% (persistent in 11%), cough in 53% (persistent in 4%), fever in 53% (persistent in 3%), loss of sense of taste in 46% (persistent in 15%), loss of sense of smell in 45% (persistent in 17%) and sore throat in 31% (persistent in 2%) (Table 1).
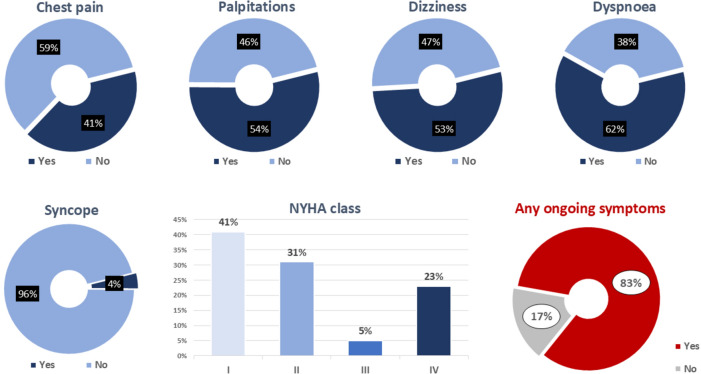
In terms of cardio-respiratory symptoms following the SARS-CoV-2 diagnosis, a significant number of patients reported chest pain (41%), palpitations (54%), dizziness/lightheadedness (53%), and dyspnoea (62%). The majority of patient symptoms were classified as NYHA Class I (41%), with 23% reporting NYHA Class IV dyspnoea (Table 1, Fig. 1B). With regard to chest pain, 23% of patients were deemed to have severe anginal equivalent symptoms. Overall, 83% of patients had any ongoing symptom. The mean self-reported health score on EQ-5D-5L questionnaire at inclusion was 78.1 ± 16.2.
Fig. 1.
Cardio-respiratory symptoms following SARS-CoV-2 diagnosis. This figure highlights the percentages of patients with cardiorespiratory symptoms.
Results of biochemical and serological testing
At inclusion, 60% of patients had received at least one dose of a vaccine (Table 1). 86% of patients had a positive antibody test against the SARS-CoV-2 nucleocapsid (NCP), while 96% had a positive antibody test against the SARS-CoV-2 spike protein at inclusion. Von Willebrand factor antigen was elevated in 32.7% of patients, while Fibrinogen and D-Dimer levels were raised in 10.2% and 11.1% of patients, respectively. Inflammatory markers were elevated in a considerable proportion of patents (ferritin in 32.7%, LDH in 33.7%) (Table 2 and Supplementary Figs. 2 and 3).
Table 2.
Biochemical and serological parameters.
| Reference | Results | Above WNL | |
|---|---|---|---|
| Roche Elecsys Spike Protein Total Antibody Assay | |||
| Positive | – | 86 (86) | – |
| Equivocal | – | 1 (1) | – |
| Negative | – | 13 (13) | – |
| Roche Elecsys NCP total antibody assay | – | ||
| Positive | – | 96 (96) | – |
| Negative | – | 4 (4) | – |
| White blood cells, × 109/L | 4.00–11.00 | 6.2 ± 1.8 | 2 (2) |
| Haemoglobin, g/dL | 11.5–16.5 | 13.9 ± 1.5 | 3 (3) |
| Platelets, × 109/L | 150–400 | 231.8 ± 50.1 | 0 (0) |
| Neutrophils, × 109/L | 2–7.5 | 3.5 ± 1.2 | 1 (1) |
| Lymphocytes, × 109/L | 1.50–4.00 | 1.9 ± 0.5 | 0 (0) |
| D-dimer, µg/mL | < 0.50 | 0.4 ± 0.9 | 11 (11.1) |
| Fibrinogen, g/L | 2.0–4.0 | 3.2 ± 0.6 | 10 (10.2) |
| Prothrombin time, s | 11.4–15.0 | 13.4 ± 2.5 | 4 (4) |
| International normalized ratio | ≤ 1.0 | 1.0 ± 0.2 | 11 (11) |
| Activated partial thromboplastin clotting time, s | 25.1–32.9 | 28.7 ± 2.8 | 5 (5) |
| Creatinine, μmol/L | 45–84 | 71.9 ± 14.2 | 26 (26) |
| C-reactive protein, mg/L | < 5.0 | 2.9 ± 3.5 | 10 (10) |
| Troponin T- HS, ng/L | Males < 22.0, females < 14.0 | 7.9 ± 2.1 | 0 (0) |
| NT-proB-type, ng/L | < 125 | 81.1 ± 136.7 | 12 (12) |
| Ferritin, µg/L | 13–150 | 116.8 ± 87.3 | 32 (32.7) |
| Lactate dehydrogenase, U/L | 135–214 | 201.7 ± 32.1 | 32 (33.7) |
| von Willebrand factor-antigen, IU/mL | 0.5–1.5 | 1.3 ± 0.8 | 32 (32.7) |
| Soluble ST2, pg/mL | 6.74–20.4 | 30.0 ± 9.8 | 31 (40.3) |
| Galectin-3, ng/mL | < 17.8 | 15.4 ± 4.3 | 15 (19.5) |
Data expressed as number (%) or mean ± SD. NCP, nucleocapsid; WNL, within normal limits. D-dimer was available for 99, fibrinogen, ferritin and von Willebrand factor-antigen for 98, lactate dehydrogenase for 95, and ST-2 and galectin-3 for 77 cases. The remaining parameters were available for all 100 cases.
Cardiac imaging findings
CMR parameters are summarized in Table 3. Stress perfusion imaging was normal for all included patients. Late gadolinium enhancement was present in 2 patients (Supplementary Fig. 4A). A total of 8 patients recently recovered from COVID-19 had a pericardial effusion (Supplementary Fig. 4B). Mean LVEF (short axis) was 60.8 ± 5.7%, with a LVEF below 55% in 17.4% of patients (Supplementary Fig. 4C). Although a majority of our cohort were female (64%), 62.5% (10/16 patients) with abnormal left ventricular ejection fraction (short Axis, %) were male. In the 16 cases with abnormal left ventricular ejection fraction (short Axis, %), there were 6 cases (37.5%) in NYHA I class, 4 (25%) in NYHA II class and 3 (18.8%) each in NYHA III and IV class respectively. Amongst our analysed cohort, mean native T1 (ms) was 994.4 ± 29.2 ms, post-contrast T1 was 521.4 ± 37.5 ms, mean T2 basal was 49.3 ± 2.5 ms, mean T2 mid was 50.3 ± 3.1 ms, mean T2 apical was 52.5 ± 3.8 ms, while extracellular volume was 27.5 ± 4.4%.
Table 3.
Cardiac magnetic resonance imaging results.
| Reference range | Abnormal | ||
|---|---|---|---|
| Left ventricular ejection fraction (short axis, %) | 60.8 ± 5.7 | 57–77* | 16 (17.4) |
| Left ventricular ejection fraction (axial, %) | 60.8 ± 5.1 | – | – |
| Left ventricular end diastolic volume (ml) | 155.9 ± 35.2 | – | – |
| Right ventricular ejection fraction (short axis, %) | 59.6 ± 5.5 | Female, 51–71; male, 52–72* | 8 (9.5) |
| Right ventricular end diastolic volume (ml) | 161.1 ± 36.7 | – | – |
| Native T1 (ms) | 994.4 ± 29.2 | 990.8 ± 34.3** | – |
| Post-contrast T1 (ms) | 521.4 ± 37.5 | – | |
| Extracellular volume % | 27.5 ± 4.4 | – | – |
| T2 basal (ms) | 49.3 ± 2.5 | 47.3 ± 1.8*** | – |
| T2 mid (ms) | 50.3 ± 3.1 | 47.7 ± 2.2*** | – |
| T2 apical (ms) | 52.5 ± 3.8 | 49.8 ± 3.1*** | – |
| Left atrium size (cm2) | 20.7 ± 3.8 | Female, 13–27; male, 15–29* | 5 (5.2) |
| Right atrium size (cm2) | 20.3 ± 3.9 | 14–30* | 1 (1.0) |
| Inferior vena cava size (cm) | 1.7 ± 0.3 | – | – |
| Stress perfusion imaging (abnormal) | 0 (0) | – | – |
| Late gadolinium enhancement (abnormal) | 2 (2.2) | – | – |
| Pericardial effusion | – | – | |
| Minimal | 3 (3.1) | – | – |
| Small | 4 (4.2) | – | – |
| Moderate | 1 (1.0) | – | – |
Data expressed as number (%) or mean ± SD. Data for T2 basal and mid, left and right atrium size and pericardial effusion was available for 96, left ventricular ejection fraction (axial, %) and native T1 for 95, and Inferior vena cava size for 94, post-contrast T1 and late gadolinium enhancement for 93, left ventricular ejection fraction (short axis, %) and left/right ventricular end diastolic volume for 92, stress perfusion imaging for 89, extracellular volume for 87, and right ventricular ejection fraction (short axis, %) for 84 cases.
*Petersen et al.38. **Reference values derived from 18 age, sex and scanner matched controls. ***Reference values derived from 28 age, sex and scanner matched controls.
Results of our electrocardiography analysis are displayed as Supplementary Table 1 and Supplementary Fig. 5, and discussed in detail in our Supplementary appendix.
Clinical follow-up at 1 year
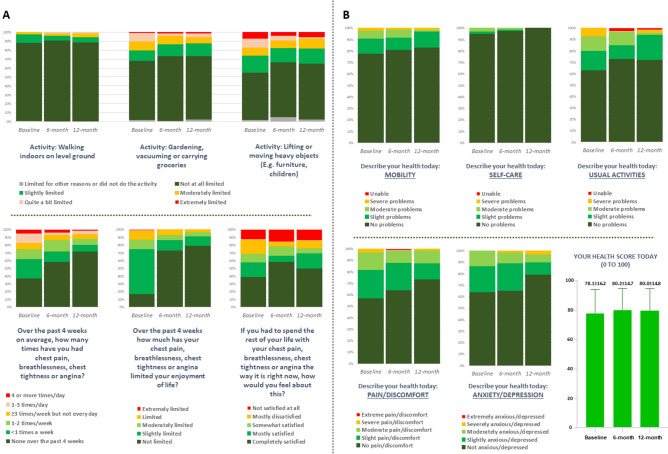
In total 99, 100 and 97 patients completed their 1, 6 and 12-month telephone follow-ups respectively. There were no significant events or overnight hospitalizations reported at 6-months follow-up time point, and one case of hospitalization for 7 nights for fever of unknown origin reported at 12 months follow-up. All 100 patients completed their SAQ7 and EQ-5D-5L questionnaires at baseline, while 75 and 72 patients, respectively, completed both their questionnaires at 6- and 12-month follow-up timepoints; responses are displayed as Fig. 2. Improvement is evident over time in terms of symptoms as well as quality of life of included subjects though the follow-up period.
Fig. 2.
(A) Responses for modified version of SAQ7 (Seattle Angina Questionnaire, short form) at baseline, 6 months and 12 months follow-up. Individual panels highlight responses to each question at baseline, 6-month follow up and 12 month follow up. Note that as these were otherwise healthy patients, without a diagnosis of ischemic heart disease, and not prescribed regular or as required nitroglycerin, the question with regard to nitroglycerin was omitted. (B) Responses for EQ-5D-5L (EuroQol 5-dimension 5 level) quality-of-life questionnaire at baseline, 6 months and 12 months follow-up. Individual panels highlight responses to each question at baseline, 6-month follow up and 12 month follow up. The final panel displays the mean overall “Health Score” at baseline, 6 months and 12 months.
Study highlights are visually presented as Fig. 3.
Fig. 3.
SETANTA study highlights.
Discussion
Summary
This study demonstrates that in an unselected patient cohort, recovering from COVID-19, cardiac abnormalities on CMR were not infrequent, although generally mild, with late gadolinium enhancement present in only 2.2% and abnormal LVEF in 17.4% of patients, despite 83% of patients reporting persistent symptoms. In terms of the aetiology of these findings, while causality cannot be inferred, they might conceivably be a consequence of COVID-associated myocarditis18. However, given the propensity for pro-coagulatory states in the setting of COVID-19, they may also be a consequence of COVID-19 related myocardial infarction, microvascular thrombosis, or endothelial inflammation related to COVID-1919,20. There is also the possibility of an association with COVID-19 induced Takotsubo cardiomyopathy, although this seems less likely21. The presence of a pericardial effusion likely signifies an association with generalized inflammation, not necessarily specific to myocarditis, but may often be seen in this setting. Markers of coagulation and inflammation remained elevated in a significant number of patients at a median of 189 days since their index diagnosis. Overall, these findings are in keeping with the fact that COVID-19 may be viewed as an endothelial disease22–24.
Strengths and limitations
This is the first prospective study in an Irish setting on patients with a COVID-19 infection who underwent evaluation for cardiac abnormalities. It has the advantage of integrating a quantification of symptom burden, biochemical and immunological markers with CMR data, allowing for the assessment the effects of COVID-19 in several domains. Furthermore, to our knowledge, it is the only study of its kind involving patients identified through primary practice, rather than in an in-patient setting. This is significant as there may be marked differences between those patients who required immediate hospitalisation for COVID-19 compared to those who did not, or ended up being admitted at a later date.
Nonetheless, our study has several limitations. Firstly, although primary care centre records were used to identify consecutive patients who have had SARS-CoV-2 infection, it was the patient’s decision to undergo enrolment (or not). While selection bias for inclusion is less than in studies enrolling patients referred to hospital for assessment, patients with persistent or troubling symptoms were more likely to be interested in taking part, and the frequency of patients with persistent symptoms thus may be overestimated. The magnitude of this overestimation is likely to be at least moderate25. Secondly, lack of baseline pre-COVID-19 imaging or laboratory tests for context constitutes a major limitation. We cannot provide information on the change of a parameter over time, or whether it was elevated prior to, or at the time of the index COVID-19 infection. Thus, in the context of this observational cohort study, causality cannot be inferred. Of note, this is, however, similar to the approach of Puntmann et al.9, whose findings we sought to replicate and given the fact that by design, our recruits were healthy volunteers from primary practice, it would not be expected that these patients would have undergone extensive cardiac testing in the past. Furthermore, our study had no direct control arm, and comparison of our patients directly against healthy controls would have been ideal. However, age, sex and MRI scanner specific data from the general population was used to generate the reference range for native T1 and T2 values, in order to mitigate the effects of this issue to some degree. Furthermore, due to the evolution of the COVID-19 virus and the interval development of several new variants, the current relevance of this data relating to infections from the time period of February–September 2021 is less clear. Thirdly, the study cohort was relatively homogenous in terms of ethnicity and age, where a majority of participants were Caucasian and relatively young.
Comparison with existing literature
Our work is similar to previously published articles in that it confirms a significant burden of “long Covid”, i.e. post-COVID-19 syndrome. These findings are similar to an Italian study that followed 143 patients recently discharged from the hospital after recovery from COVID-19. At a mean of 60.3 days after onset of the first COVID-19 symptom, 87.4% of patients reported persistence of at least 1 symptom, particularly fatigue and dyspnoea26. A longitudinal analysis of previously well patients with initial mild illness carried out by Puntmann et al. showed a somewhat lower, but still significant, incidence of persistent symptoms at 57%9. There is evidence to suggest that residual symptoms in post-COVID-19 syndrome may be related to persistent endothelial dysfunction27.
In terms of the evolution of CMR findings in COVID-19, initial imaging studies on selected patients (those with COVID-19 and elevated troponin levels during hospitalization)28 and unselected patients (those with a range of COVID-19 symptoms)9 demonstrated a high frequency (up to 78%) of cardiac abnormalities. Subsequent CMR studies have identified patients with myocarditis-like imaging patterns at levels higher than in the general population. However, these findings appear less common than originally thought, and in particular, it is important to point out that the incidence of abnormal CMR findings, especially with regard to native and post contrast T1, and T2 values, are dependent on the particular imaging protocols, sequence parameters and postprocessing approaches used by a given centre or by a given study protocol. Joy et al. published results from a nested case–control study that included 74 seropositive participants along with 75 age-, sex-, and ethnicity-matched control subjects selected from volunteering seronegative subjects29. Disease was mild in 99%, with 25% asymptomatic and only 2 hospitalized. Cardiovascular abnormalities on CMR were found to be no more common in seropositive versus seronegative individuals 6 months post mild COVID-19 infection, including 4% with LGE myocardial scarring29. Of note, only 2 patients in this cohort were found to have reduced ejection fraction with a minimum LVEF of 50%. A subsequent study found left ventricular dysfunction present in 11% (17/148) of patients recently recovered from COVID-1930. Furthermore, findings comparing a cohort of patients specifically with the delta variant of COVID-19 (n = 44) and healthy controls revealed no patients with abnormal LVEF and 9% of patients with myocardial late gadolinium enhancement31. Finally, Orbach et al. studied 70 consecutive patients with prior COVID-19 and positive high sensitivity troponin-T requiring hospitalization and found only 9% had late gadolinium enhancement suggestive of myocardial scarring, with LVEF only reduced in 4% of patients32. The findings of our study are in keeping with this latter data, despite the fact that none of our patients had a positive high sensitivity troponin-T value. However, as our serum biomarkers were not taken at the time of the index case, it may simply reflect the fact that troponin levels have normalized by the time our patients had blood samples drawn. Taken together, our data tends to concur with the contemporary literature, in that incidence of myocardial injury in COVID-19 is not as high as originally reported.
There is evidence that infection with COVID-19 leads to changes with regard to protein composition in the serum of infected individuals, which in turn alters the coagulation and inflammation response of that individual33. Fogarty et al. demonstrated that severe COVID-19 infection in hospitalized patients was associated with a significant coagulopathy that correlates with disease severity34. D-Dimer levels may remain elevated in up to 25% of patients up to 4 months post-SARS-CoV-2 infection35,36. Interestingly, the lower levels seen in our study corroborate this, in that we confirm D-dimer levels are elevated in fewer patients in a cohort who did not require hospitalisation. On the other hand, WVF-Ag was, however, found to be elevated in significant proportion of patients, in keeping with data of Philippe et al.25. The relative significance of these contrasting findings merits further study.
Pro-inflammatory markers are frequently significantly raised in patients with severe COVID-1937. Several pro-inflammatory markers were elevated in our study, even in the absence of severe COVID requiring hospitalization. This raises questions with regard to the specificity of these markers for severe infection, which requires further dedicated study.
Implications for research and/or practice
Despite a clear evidence of significant persistent symptom burden, no correlation with objective markers of myocardial injury on CMR was found for most patients in this study. Further investigation is necessary to allow us to glean a better insight as to why symptoms persist.
In contrast, absence of a high prevalence of direct myocardial involvement is reassuring. Future research efforts should therefore be concentrated on a pragmatic approach to dealing with symptoms associated with post-COVID-19 syndrome in general practice. Anti-inflammatory therapies in such patients may prove to be a possible therapeutic option.
Conclusions
In conclusion, this study demonstrates that in patients recovering from COVID-19, cardiac abnormalities on CMR were not infrequent, though generally mild, with late gadolinium enhancement present in 2.2% and abnormal LVEF 17.4% of patients. A prolonged inflammatory state and hypercoagulability was observed in a significant minority of patients and further evaluation of the long-term natural history and clinical importance of these findings in survivors of acute COVID-19 is warranted, especially those with post-acute COVID-19 syndrome.
Supplementary Information
Author contributions
Author contributions are as following: Conception and design of the work (RC, HR, RJB, MH, JSO’D, RAB); Acquisition, analysis, and interpretation of data for the work (RC, HR, RJB, AM, RL, JB, HW, NB, NB, MF, GS, EH, MH, JSO’D, RAB); Drafting the manuscript or reviewing it critically for important intellectual content (RC, SF, HR, LMcG, RJB, AM, AC, RL, JB, SMcK, GC, DO’C, HW, NB, NB, MF, JMcN, GS, EH, MH, JSO’D, RAB); Final approval of the submitted manuscript (RC, SF, HR, LMcG, RJB, AM, AC, RL, JB, SMcK, GC, DO’C, HW, NB, NB, MF, JMcN, GS, EH, MH, JSO’D, RAB); Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (RC, SF, HR, LMcG, RJB, AM, AC, RL, JB, SMcK, GC, DO’C, HW, NB, NB, MF, JMcN, GS, EH, MH, JSO’D, RAB).
Funding
This work was partly supported by a research grant to RC from Women As One (2021 Escalator award- Research).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
This study was funded by a Women As One Escalator Research Award 2021, awarded to the lead author, Dr. R. Colleran. R.A. Byrne reports research or educational funding to the institution of current employment from Abbott Vascular, Biosensors, Boston Scientific and Translumina; none of the funding contributes to his personal remuneration. L. McGovern is funded by the European Commission under Horizons 2020 framework (Grant agreement number 965246—CORE-MD). The other authors declare no potential conflicts of interest.
Ethical approval
This study was approved by Mater Misericordiae University Hospital Institutional Review Board (Approval Ref. No. 1/378/2199).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71535-8.
References
- 1.Akhmerov, A. & Marban, E. COVID-19 and the heart. Circ. Res.126(10), 1443–1455. 10.1161/CIRCRESAHA.120.317055 (2020). 10.1161/CIRCRESAHA.120.317055 [DOI] [PubMed] [Google Scholar]
- 2.Giustino, G. et al. Characterization of myocardial injury in patients with COVID-19. J. Am. Coll. Cardiol.76(18), 2043–2055. 10.1016/j.jacc.2020.08.069 (2020). 10.1016/j.jacc.2020.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried, J. A. et al. The variety of cardiovascular presentations of COVID-19. Circulation141(23), 1930–1936. 10.1161/CIRCULATIONAHA.120.047164 (2020). 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1-epidemiology, pathophysiology, and diagnosis. Eur. Heart J.43(11), 1033–1058. 10.1093/eurheartj/ehab696 (2022). 10.1093/eurheartj/ehab696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammirati, E. et al. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation145(15), 1123–1139. 10.1161/CIRCULATIONAHA.121.056817 (2022). 10.1161/CIRCULATIONAHA.121.056817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cenko, E. et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology and Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res.117(14), 2705–2729. 10.1093/cvr/cvab298 (2021). 10.1093/cvr/cvab298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med.28(3), 583–590. 10.1038/s41591-022-01689-3 (2022). 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raman, B., Bluemke, D. A., Luscher, T. F. & Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J.43(11), 1157–1172. 10.1093/eurheartj/ehac031 (2022). 10.1093/eurheartj/ehac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puntmann, V. O. et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol.5(11), 1265–1273. 10.1001/jamacardio.2020.3557 (2020). 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, L. et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc. Imaging13(11), 2330–2339. 10.1016/j.jcmg.2020.05.004 (2020). 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng, M. Y. et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. JACC Cardiovasc. Imaging13(11), 2476–2478. 10.1016/j.jcmg.2020.08.012 (2020). 10.1016/j.jcmg.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starekova, J. et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol.6(8), 945–950. 10.1001/jamacardio.2020.7444 (2021). 10.1001/jamacardio.2020.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, X. et al. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg. Microbes Infect.9(1), 1269–1274. 10.1080/22221751.2020.1773324 (2020). 10.1080/22221751.2020.1773324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel, M. M. et al. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA324(17), 1781–1782. 10.1001/jama.2020.18796 (2020). 10.1001/jama.2020.18796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llitjos, J. F. et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost.18(7), 1743–1746. 10.1111/jth.14869 (2020). 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goshua, G. et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol.7(8), e575–e582. 10.1016/s2352-3026(20)30216-7 (2020). 10.1016/s2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability. Circulation93(5), 1043–1065. 10.1161/01.Cir.93.5.1043 (1996). 10.1161/01.Cir.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 18.Rafiee, M. J. & Friedrich, M. G. MRI of cardiac involvement in COVID-19. Br. J. Radiol.97(1160), 1367–1377. 10.1093/bjr/tqae086 (2024). 10.1093/bjr/tqae086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bangalore, S. et al. ST-segment elevation in patients with Covid-19—A case series. N. Engl. J. Med.382(25), 2478–2480. 10.1056/NEJMc2009020 (2020). 10.1056/NEJMc2009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burkert, F. R. et al. Case report of a COVID-19-associated myocardial infarction with no obstructive coronary arteries: The mystery of the phantom embolus or local endothelitis. Eur. Heart J. Case Rep.5(2), ytaa521. 10.1093/ehjcr/ytaa521 (2021). 10.1093/ehjcr/ytaa521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moady, G. & Atar, S. Stress-induced cardiomyopathy-considerations for diagnosis and management during the COVID-19 pandemic. Medicina (Kaunas)10.3390/medicina58020192 (2022). 10.3390/medicina58020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sardu, C. et al. Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med.10.3390/jcm9051417 (2020). 10.3390/jcm9051417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinciguerra, M. et al. SARS-CoV-2 and atherosclerosis: Should COVID-19 be recognized as a new predisposing cardiovascular risk factor?. J. Cardiovasc. Dev. Dis.10.3390/jcdd8100130 (2021). 10.3390/jcdd8100130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinaglia, T. et al. Coronavirus disease-19: The multi-level, multi-faceted vasculopathy. Atherosclerosis322, 39–50. 10.1016/j.atherosclerosis.2021.02.009 (2021). 10.1016/j.atherosclerosis.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millard, L. A. C. et al. Exploring the impact of selection bias in observational studies of COVID-19: A simulation study. Int. J. Epidemiol.52(1), 44–57. 10.1093/ije/dyac221 (2023). 10.1093/ije/dyac221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carfì, A., Bernabei, R. & Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA324(6), 603–605. 10.1001/jama.2020.12603 (2020). 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuchler, T. et al. Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis26(4), 547–563. 10.1007/s10456-023-09885-6 (2023). 10.1007/s10456-023-09885-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight, D. S. et al. COVID-19: Myocardial injury in survivors. Circulation142(11), 1120–1122. 10.1161/circulationaha.120.049252 (2020). 10.1161/circulationaha.120.049252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joy, G. et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc. Imaging14(11), 2155–2166. 10.1016/j.jcmg.2021.04.011 (2021). 10.1016/j.jcmg.2021.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotecha, T. et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J.42(19), 1866–1878. 10.1093/eurheartj/ehab075 (2021). 10.1093/eurheartj/ehab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, L. et al. Cardiac involvement in patients recovering from Delta Variant of COVID-19: A prospective multi-parametric MRI study. ESC Heart Fail.9(4), 2576–2584. 10.1002/ehf2.13971 (2022). 10.1002/ehf2.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orbach, A. et al. Low prevalence of late myocardial injury on cardiac MRI following COVID-19 infection. J. Magn. Reson. Imaging58(6), 1777–1784. 10.1002/jmri.28668 (2023). 10.1002/jmri.28668 [DOI] [PubMed] [Google Scholar]
- 33.Beltran-Camacho, L. et al. The serum of COVID-19 asymptomatic patients up-regulates proteins related to endothelial dysfunction and viral response in circulating angiogenic cells ex-vivo. Mol. Med.28(1), 40. 10.1186/s10020-022-00465-w (2022). 10.1186/s10020-022-00465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogarty, H. et al. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol.189(6), 1044–1049. 10.1111/bjh.16749 (2020). 10.1111/bjh.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend, L. et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J. Thromb. Haemost.19(4), 1064–1070. 10.1111/jth.15267 (2021). 10.1111/jth.15267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann, A. et al. Impact of persistent D-dimer elevation following recovery from COVID-19. PLoS One16(10), e0258351. 10.1371/journal.pone.0258351 (2021). 10.1371/journal.pone.0258351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin, C. et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis.71(15), 762–768. 10.1093/cid/ciaa248 (2020). 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen, S. E., Khanji, M. Y., Plein, S., Lancellotti, P. & Bucciarelli-Ducci, C. European Association of Cardiovascular Imaging expert Consensus Document: A comprehensive review of cardiovascular MR normal values of cardiac chamber size and aortic root in adults and recommendation for grading severity. Eur. Heart J. Cardiovasc. Imaging20(12), 1321–1331 (2019). 10.1093/ehjci/jez232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.