Abstract
Higher cardiorespiratory fitness has been associated with improved cognitive control in preadolescent children, with various studies highlighting related brain health benefits. This cross-sectional study aimed to provide novel insights into the fitness-cognition relationship by investigating task-related changes in effective connectivity within two brain networks involved in cognitive control: the cingulo-opercular and fronto-parietal networks. Twenty-four higher-fit and twenty-four lower-fit preadolescent children completed a modified flanker task that modulated inhibitory control demand while their EEG and task performance were concurrently recorded. Effective connectivity for correct trials in the theta band was estimated using directed transfer function. The results indicate that children with higher fitness levels demonstrated greater connectivity in specific directions within the cingulo-opercular network (average effect size, d = 0.72). Brain-behavior correlations demonstrated a positive association between the majority of these connections and general task accuracy, which was also higher in higher fit children (average correlation coefficient, ρ = 0.34). The findings further support a positive relationship between fitness and cognitive performance in children. EEG findings offer novel insights into the potential brain mechanisms underlying the fitness-cognition relationship. The study suggests that increased task-related connectivity within the cingulo-opercular network may mediate the cognitive benefits associated with higher fitness levels in preadolescent children.
Keywords: Physical fitness, Preadolescent children, Cognitive control, Connectivity, EEG, Directed transfer function
Subject terms: Cognitive control, Human behaviour
Introduction
In modern societies, the growing prevalence of physical inactivity among children is a significant public health concern1. Inadequate physical activity levels not only increase the risk of chronic diseases2 but also pose a risk for poorer cognitive functioning3. On the other hand, accumulating evidence highlights the positive associations between higher physical activity levels and cognitive performance. However, the underlying brain mechanisms are still not fully understood4–6. This study aimed to shed more light on the possible mechanisms linking cardiorespiratory fitness (related to physical activity levels), cognitive performance, and brain outcomes in children. To achieve this aim, we employed a novel approach to unravel task-related communication of brain networks that might subserve cognitive control in lower-fit and higher-fit children.
Cognitive control refers to an individual's capacity to adapt to environmental demands and maintain behavioral goals over extended periods7,8. An important aspect of cognitive control is inhibition, which involves filtering out task-irrelevant information and overriding prepotent incorrect responses in favor of a correct one9,10. Inhibition is commonly investigated through interference tasks such as flanker tasks11. During this task, participants react to a centrally located target while gating out the potentially conflicting surrounding flanking stimuli. The task requires modulation of top-down attention to targets and inhibition of flankers. The flankers can either match the target (congruent condition) or differ from it (incongruent condition). Compared with the congruent condition, the incongruent condition results in longer reaction times and less accurate responses. Successful task performance is indicated by a greater ability to manage interference associated with the flanking stimuli and improved general behavioral performance (shorter reaction times, higher accuracy)12.
Successful inhibition plays a crucial role in academic settings in developing populations. It enables children to inhibit impulsive behavior and stay on task13,14. Children who score well on inhibition tasks (as well as in other cognitive control tasks) tend to achieve greater academic success13,14. Therefore, understanding factors that might enhance children's cognitive control is important to support their development.
Research supports the idea that cardiorespiratory fitness, which depends on physical activity levels, positively correlates with improved inhibition and focused attention in children15. Higher-fit children exhibit better task performance and lower interference costs (the difference between congruent and incongruent trials) than their lower-fit counterparts15,16. These behavioral benefits often coincide with favorable differences in brain function (detailed below), which may underlie the fitness-cognition relationship.
Despite numerous studies on the relationship between fitness and cognition in children, the mechanisms underlying this connection are, to date, not fully understood. To elucidate the neural mechanisms involved, previous investigations primarily relied on event-related brain potential studies5,17,18. Collectively, these findings suggest that higher-fit children exhibit enhanced attentional allocation capabilities (as evidenced by larger P3 amplitude and shorter P3 latency5,17, and smaller N2 amplitude5,18 (indicating reduced interference at the neural level).
Functional magnetic resonance imaging (fMRI) studies provided further insight into the fitness-inhibition relationship by providing knowledge of differential patterns of brain activation in lower-fit and higher-fit children. Voss et al.6 revealed that higher fitness levels in children were linked to more efficient activation in brain networks responsible for inhibition, task-set maintenance, and top-down regulation, processes that play a crucial role in cognitive control. More specifically, children with higher levels of fitness exhibited increased activity in control-related brain structures, which positively correlated with task performance (both in congruent and incongruent conditions). Other studies have employed a functional connectivity approach to study the coordinated activity of brain networks that might underlie the fitness-cognition relationship. The functional connectivity approach identifies patterns of synchronized activity between different brain regions during rest (without an explicit cognitive task being performed, i.e., resting-state) or during a task (task-induced). Most studies thus far have assessed resting-state functional connectivity and suggested that greater fitness levels in children might be associated with enhanced connectivity within brain networks responsible for cognitive control, including inhibition19–22 (for a contrasting finding of reduced connectivity, see23).
Enhanced connectivity within cognitive networks is generally considered advantageous as it may indicate more efficient information processing, leading to better executive functioning. However, while an increase in resting-state functional connectivity in the brain may indicate positive changes related to cognition, it does not always translate into improved task performance19. To gain a more targeted understanding of the neural networks involved in specific cognitive tasks, researchers have employed task-induced functional connectivity. This approach allows for manipulating and comparing different task conditions, facilitating more systematic investigations into functional connectivity. However, there appears to be limited research on the relationship between children's fitness levels and task-induced functional connectivity, with only one electroencephalography (EEG) study conducted by Kamijo et al.4. This study investigated EEG functional connectivity patterns in children with different fitness levels who performed two conditions of a visual search task: identifying targets among distractors that either shared or did not share a basic feature with the target. Higher-fit children exhibited higher response accuracy relative to lower-fit children across two task conditions. Moreover, higher-fit children showed increased frontoparietal functional connectivity during a task condition that required heightened top-down control modulation (searching for targets among distractors that shared some similar features), whereas lower-fit children faced challenges in upregulating top-down control mechanisms. These findings suggest that higher-fit children perform better in a visual tasks and might be characterized by increased task-related functional connectivity in more demanding tasks conditions. Still, additional research is necessary to understand the associations between children's fitness and task-induced connectivity during cognitive control tasks.
To gain further insights into the potential neural mechanisms underlying the association between fitness and cognitive control in children, we conducted an investigation focusing on task-induced connectivity within two specific regional systems that have been widely recognized for their significance in cognitive control24–26. One of these systems is the cingulo-opercular network (CON), which plays a vital role in domain-independent task performance and encompasses anterior regions of the cingulate cortex, insula, and prefrontal cortex24,27. This network exhibits sustained activity throughout the entire duration of a goal-directed task and likely regulates goal-directed behavior by maintaining stable task sets. The other system is the frontoparietal network (FPN), consisting of the dorsolateral prefrontal cortex and intraparietal sulcus, which adapts control on a trial-by-trial basis, enabling flexible modulation and control in response to ongoing performance24,28. Both networks are strongly intraconnected and quite separate from each other. Although both networks serve cognitive control, they carry out dissociable control functions and affect cognitive processing either on a trial-by-trial basis (FPN) or in a more stable fashion CON24. Overall, given the crucial roles of the CON and FPN in sustained attention, task-set maintenance, and flexible cognitive control, these networks provide an interesting platform to gain a nuanced understanding of how fitness may be related to cognitive control in children.
This study employed a novel approach in the field of health neuroscience that assessed effective connectivity within the FPN and CON networks. Unlike functional connectivity, which examines the temporal correlation of activation across brain regions, effective connectivity focuses on understanding the causal interactions among brain regions29. In other words, while functional connectivity examines whether two brain regions activate simultaneously, effective connectivity goes further by evaluating the temporal direction and strength of influence one region exerts on another. Moreover, utilizing effective connectivity analysis on EEG signals offers the advantage of directly measuring neuronal activity, contrasting with fMRI connectivity, which primarily relies on BOLD-mediated signals. Amongst different effective connectivity methods, we utilized a well-established method called the directed transfer function (DTF). DTF is based on Granger causality principles and multivariate autoregressive modeling (MVAR)30–32 and allowed us to determine the direction and strength of the information flow within CON and FPN cortical regions in lower-fit and higher-fit children. By adopting this approach, we aimed to provide a more nuanced understanding of the relationship between children's fitness levels and brain connectivity during an inhibition task. We focused on interactions between structures within CON and FPN networks in the theta band. Power changes in theta activity have been consistently linked to cognitive control, interference detection, and top-down processes28,33–35.
Overall, previous connectivity findings have consistently indicated higher resting-state and task-induced connectivity in higher-fit children4,19–21. In line with these findings, we expect to observe greater general (across conditions) task-induced connectivity within the cingulo-opercular (CON) and frontoparietal (FPN) networks in higher-fit children, as these networks play a crucial role in executing cognitive control tasks36. This effect would reflect improvement in general task performance across congruent and incongruent conditions. Previous studies utilizing inhibition tasks have also shown lower interference costs in higher fit children, as illustrated, for instance, by reduced congruent versus incongruent N2 component5,18. Consequently, we expect that higher-fit children will exhibit reduced interference-related connectivity within both the CON and FPN networks. This deactivation would indicate reduced interference costs and a decreased need for cognitive control adjustments.
Methods
The analyses presented herein were conducted on a subset of previously published data5. Our decision to utilize this pre-existing dataset stemmed from the pioneering nature of our analyses. To verify the relationships between effective connectivity patterns and children's fitness, we opted for a well-documented cross-sectional dataset featuring extreme fitness groups and pronounced behavioral differences across lower- and higher-fit children. The study by Pontifex et al.5 investigated the relationship between cardiorespiratory fitness and cognitive control in preadolescent children categorized into higher-fit and lower-fit groups. It revealed behavioral and event-related brain potential benefits for higher-fit children. While Pontifex et al.5. employed both compatible (respond to the direction of the target stimulus) and incompatible (respond opposite the direction of the target stimulus) stimulus–response versions of the flanker task, we focused exclusively on the conventional, compatible subset of the dataset (which contains both congruent and incongruent trials) to explore the connection between fitness and effective connectivity using a more established task. This study aimed to follow the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines37. For more methodological details, please refer to Pontifex et al5.
Cardiorespiratory fitness assessment
Cardiorespiratory fitness data are described in the participants section. Maximal oxygen consumption (VO2max) was evaluated using a computerized indirect calorimetry system (ParvoMedics True Max 2400, Sandy, UT). Oxygen uptake (VO2) and respiratory exchange ratio (RER) were averaged every 20 s. A modified Balke protocol38was used, involving a motor-driven treadmill set. Participants walked/ran at a constant speed with incline increases of 2.5% every 2 min until the participant reached volitional exhaustion. Heart rate (HR) was monitored (Polar WearLink + 31; Polar Electro, Lake Success, NY), and ratings of perceived exertion (RPE) were recorded every 2 min utilizing the children’s OMNI scale39. Relative peak oxygen consumption was expressed in ml/kg/min, based on maximal effort. Criterion for achieving maximal effort included achieving one or more of the following: (1) a peak heart rate of ≥ 185 bpm and a heart rate plateau40; (2) an RER of ≥ 1.041;and/or (3) a rating on the children’s OMNI scale of perceived exertion of ≥ 839.
Participants
Forty-eight preadolescent children from the east-central Illinois region were recruited for the study. All participants provided written assent, and their legal guardians provided written informed consent. The study's experimental procedures complied with the directives of the Helsinki Declaration and were approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign. Prior to testing, legal guardians completed a health history and demographics questionnaire, reporting that their child was free of neurological diseases, or physical disabilities; and indicated normal or corrected-to-normal vision. Participants were bifurcated into higher-fit (n = 24, 13 girls) and lower-fit (n = 24, 10 girls) groups based on their VO2max, with the higher-fit group having VO2max values above the 70th percentile and the lower-fit group having values below the 30th percentile according to normative data provided by Shvartz and Reibold42. The mean age of the participants was 10.1 years (SD = 0.6) for the lower-fit group and 10.0 years (SD = 0.6) for the higher-fit group, with no significant difference between the groups (p = 0.46). Participants, in collaboration with their legal guardians, completed the Tanner Staging System43, indicating their pubertal status on a 5-point scale. Both groups had a mean Tanner stage of 1.7(SD = 0.5), indicating the same pubertal development (i.e., prepubescent) (p = 0.98). Intellectual ability, measured using the K-BIT composite IQ score44, averaged 113.2 (SD = 14.9) for the lower-fit group and 115.3 (SD = 8.6) for the higher-fit group, with no significant difference between the groups (p = 0.41). ADHD symptoms were evaluated using the ADHD Rating Scale IV45, with the lower-fit group having a mean score of 6.3 (SD = 4.7) and the higher-fit group having a mean score of 6.9 (SD = 4.5), showing no significant difference in attentional disorder between the groups (p = 0.52). Socioeconomic status (SES) was assessed using a composite score based on parental education, occupation, and participation in free or reduced-price lunch programs at school46. The lower-fit group had a mean SES score of 2.8 (SD = 0.6), while the higher-fit group had a mean SES score of 2.6 (SD = 0.7), with no significant difference between the groups (p = 0.71). Fitness levels, measured by VO2max, showed a significant difference between the groups (p < 0.01). The lower-fit group had a mean VO2max of 35.7 ml/kg/min (SD = 5.3) and a percentile rank of 8.8 (SD = 5.3), whereas the higher-fit group had a mean VO2max of 52.6 ml/kg/min (SD = 4.2) and a percentile rank of 83.3 (SD = 4.1). Where applicable (VO2max , IQ, ADHD) raw scores were converted into age-based standard scores using normed data provided by the publishing company42,44,45. To assess the adequacy of our sample size, a post hoc power analysis was conducted using G*Power47. This analysis, based on the observed mean effect of connectivity estimates (d = 0.72) and a significance level (α) of 0.05, indicated an achieved power of 0.80, suggesting that our study had a sufficient number of participants to reliably detect the observed effect.
Task
Participants engaged in a modified version of the Eriksen flanker task11. They were instructed to respond as quickly and accurately as possible to the direction of a centrally presented arrow, which was flanked by either congruous (e.g., < < < < < or > > > > >) or incongruous (e.g., < < > < < or > > < > >) arrows. The incongruent condition, compared to the congruent condition, requires greater interference control to inhibit the responses elicited by the flanking arrows and execute the correct response based on the central target arrow48.
The task comprised two blocks of 100 trials each, with equal probabilities for congruent and incongruent conditions. The stimuli consisted of white arrows, each 3 cm tall, arranged in a 16.5 cm wide array, presented on a black background. The visual angle was 1.32° vertically and 7.26° horizontally. Each array was shown for 200 ms with a fixed interstimulus interval of 1700 ms. Reaction times (RT) for correct responses and accuracy were recorded separately for congruent and incongruent conditions.
Procedure
During the initial laboratory visit, participants completed informed consent, tests and questionnaires, and a cardiorespiratory fitness assessment. Before the fitness assessment, they were equipped with a Polar heart rate monitor (Polar WearLink + 31; Polar Electro). Their height was measured using a stadiometer, and their weight was recorded with a Tanita WB-300 Plus digital scale. Participants with VO2max falling above the 70th or below the 30th percentile, based on normative data42, were invited for the second day of testing. On the second visit to the laboratory, participants underwent EEG testing in a sound-attenuated room after being fitted with a 64-channel Quik-Cap (Compumedics Neuroscan, 2003). Task instructions were provided, allowing participants to ask questions, followed by 40 practice trials before the formal testing commenced.
Data analysis
All statistical analyses were performed and visualized using the R 4.0.3 (R Core Team, 2021) and JASP version 0.16.3 (JASP Team 2022) software. In both behavioral and connectivity data analyses, t-tests were employed to explore potential differences between lower-fit and higher-fit children across variables of interest: accuracy, reaction time (RT), and effective connectivity measures. Therefore, for all analyses, we reduced the congruency factor by calculating additional measures (as detailed below). This decision reflects a specific research context. The results presented by Pontifex et al.5 did not reveal a significant interaction between group and congruency in behavioral outcomes. Consequently, reducing the congruency factor provided a more straightforward framework for data interpretation and brain-behavior correlations. Moreover, pooling conditions together offers practical advantages for calculating connectivity estimates. This approach allows for average correlation matrices, which describe a basic structure of the relations in the dataset, later translated into the transmission pattern expressing properties characteristic for all joined conditions. This approach not only aligns connectivity and behavioral analyses but also enhances the statistical properties of the estimated model parameters, thereby bolstering the robustness of DTF estimates. Normality screening was conducted for all relevant variables using the Kolmogorov–Smirnov test. The analysis revealed no significant deviations from normality for any variables (all p > 0.05).
Behavioral analyses
In behavioral analyses, we focused on accuracy and RT data. As a manipulation check, the interference effect in RT and accuracy (i.e., the difference across the incongruent and the congruent condition) was tested using paired sample t-tests. Then, general (across-conditions) task performance indices and interference costs were calculated. General task performance was calculated as mean RT and mean accuracy across task conditions (congruent + incongruent / 2; general accuracy, general RT). Interference costs were calculated as the difference across task conditions (congruent—incongruent for accuracy, incongruent – congruent for RT). To examine whether there were differences in general task performance and interference costs between lower-fit and higher-fit children, we conducted independent t-tests.
EEG recording
EEG activity was recorded from 64 electrode sites arranged in an extended montage based on the International 10–10 System using a Neuroscan Quik-Cap (Compumedics, Charlotte, NC). Refer to Pontifex et al.5 for additional details.
EEG preprocessing
EEG data were preprocessed using the Atlantis toolbox (http://atlantis.psychologia.uj.edu.pl). The preprocessing was based on the approach proposed by Mantini et al.49 and further extended by Spadone et al.50. The signal was filtered in a 2–46 Hz range with windowed sinc linear phase FIR filters (HP order: 2460; LP order 550) and then segmented using a -0.2 to 1-s window relative to stimuli onset. Noisy channels were detected using an IQR-based extreme outliers rejection algorithm (the threshold for channel variance set to Q1/Q3 ± 5 IQR, based on a visual inspection of the data distribution33), calculated from EOG-corrected signals after the RLS method. Surviving original channels (without EOG correction) were re-referenced to the average value across all channels. Trial-based artifact rejection consisted of extreme outlier removal based on variance (threshold set to Q1/Q3 ± 3 IQR), maximum trial voltage difference (< 250 µV), and muscle artifact identification (based on elevated spectral power in a 35–46 Hz frequency). The mean number of excluded trials showed no significant difference between the lower-fit (M = 6.02%, SD = 3.14) and higher-fit (M = 5.42%, SD = 2.20) groups (t(46) = 0.771, p = 0.44). Remaining trials were decomposed with fastICA with deflation and pow3 nonlinearity, and resulting components were classified using a previously trained model (with topography, spectral power, pre/post-stimulus variance, and correlation with EOG signals) into brain and non-brain independent components (ICs). The brain ICs were localized using their weight matrices with the minimum norm estimation (MNE) method51 based on MNI standard templates (5 mm regular grid) and the 3-layer Boundary Element Method ('bemcp') volume conductor model52.
Regions of interest (ROIs)
Locations of the regions of interest (ROIs) were selected based on our hypotheses and previous literature24,50,53–55. For the frontoparietal network (FPN) the following ROI were chosen: L/R dorsolateral PFC (DLPFC; -43 18 29 / 43 18 29); L/R intraparietal sulci (IPS; -32 -48 44 / 32 -52 50). For the cingulo-opercular network (CON), the following ROIs were chosen: L/R anterior prefrontal cortex (aPFC; -28 51 15 / 27 50 23); dorsal anterior cingulate (dACC; -1 -10 46); L/R anterior insula / frontal operculum (aI; -35 14 5 / 36 16 4). The ROIs were reconstructed as a sum of IC signals in the respective (closest) source dipole obtained as a product of a particular IC time course and respective weight components separately for all spatial directions. The scalar values of ROI signals were calculated from three spatial components using the PCA by taking the first component .
Effective connectivity analyses
To control for spurious correlation between estimated source time courses, leakage correction was applied using a symmetric multivariate orthogonalization procedure56. Connectivity between the ROIs was estimated for theta (4-8 Hz) frequency band using a non-normalized Directed Transfer Function (DTF31), a method based on Granger causality assumptions. Determination of model order (order = 7) was guided by the Yule-Walker method. Theta band was chosen as power changes in theta activity have been consistently linked to cognitive control, interference detection, and top-down processes28,33–35. We estimated DTF for both general task- and interference-related connectivity. For general (across conditions) task-related connectivity, DTF estimates were calculated for all correct trials, irrespective of the congruency. For interference-related connectivity, DTF estimates were calculated as the difference across the incongruent and congruent correct trials. In both cases, multivariate DTF estimates were calculated for all possible connections between selected ROIs within FPN and CON. To investigate potential differences in general task-related and interference-related connectivity between lower-fit and higher-fit children, we used unpaired t-test contrasts. Given the multiple comparisons in connectivity data, we applied false discovery rate (FDR) corrections57. Extreme outliers were removed using the criterion Q1/Q3 ± 3 IQR33. The number of remaining participants by group and connections is reported in Tables 2 and 3, along with the DTF results.
Table 2.
Effective connectivity results.
| General task-related connectivity | Interference-related connectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Direction | t | Df | p | d | n (HF / LF) | t | Df | p* | d | n (HF / LF)1 |
| dACC → LaI | − 2.03 | 41 | 0.024* | − 0.620 | 21/22 | − 0.420 | 41 | 0.662 | − 0.129 | 20/23 |
| dACC → RaI | 0.18 | 39 | 0.569 | 0.055 | 21/20 | − 1.320 | 40 | 0.903 | − 0.408 | 21/19 |
| dACC → RaPFC | 0.24 | 42 | 0.593 | 0.071 | 21/23 | − 1.285 | 40 | 0.897 | − 0.396 | 21/21 |
| dACC → LaPFC | 0.06 | 41 | 0.524 | 0.018 | 20/23 | 0.480 | 45 | 0.317 | 0.140 | 23/24 |
| LaI → dACC | 0.80 | 42 | 0.787 | 0.243 | 21/23 | 0.254 | 42 | 0.400 | 0.077 | 21/23 |
| LaI → RaI | − 0.49 | 40 | 0.314 | − 0.151 | 22/20 | − 0.278 | 40 | 0.609 | − 0.086 | 21/21 |
| LaI → RaPFC | − 1.50 | 41 | 0.071 | − 0.458 | 21/22 | 1.261 | 39 | 0.107 | 0.395 | 19/22 |
| LaI → LaPFC | − 0.74 | 39 | 0.471 | − 0.023 | 21/20 | 0.043 | 37 | 0.483 | 0.014 | 21/18 |
| RaI → dACC | 0.37 | 38 | 0.644 | 0.118 | 19/21 | − 0.548 | 38 | 0.706 | − 0.173 | 20/20 |
| RaI → LaI | − 2.39 | 44 | 0.011* | − 0.695 | 23/23 | 1.849 | 44 | 0.036 | 0.545 | 23/23 |
| RaI → RaPFC | − 3.77 | 41 | < .001* | − 1.150 | 22/21 | − 0.060 | 40 | 0.524 | − 0.019 | 21/21 |
| RaI → LaPFC | − 1.14 | 42 | 0.131 | − 0.344 | 21/23 | 0.308 | 41 | 0.380 | 0.094 | 21/22 |
| RaPFC → dACC | − 3.39 | 43 | < .001* | − 1.009 | 23/22 | 0.058 | 39 | 0.477 | 0.018 | 20/21 |
| RaPFC → LaI | − 2.00 | 42 | 0.026 | − 0.605 | 21/23 | 1.912 | 42 | 0.031 | 0.577 | 22/22 |
| RaPFC → RaI | − 0.65 | 40 | 0.260 | − 0.200 | 21/22 | − 0.597 | 35 | 0.723 | − 0.196 | 19/18 |
| RaPFC → LaPFC | − 2.31 | 43 | 0.013* | − 0.686 | 22/23 | 0.978 | 43 | 0.167 | 0.292 | 23/22 |
| LaPFC → dACC | − 0.63 | 41 | 0.265 | − 0.193 | 21/22 | 0.630 | 42 | 0.266 | 0.190 | 22/22 |
| LaPFC → LaI | − 1.33 | 39 | 0.096 | − 0.416 | 20/21 | 0.488 | 39 | 0.314 | 0.152 | 21/20 |
| LaPFC → RaI | − 0.79 | 40 | 0.217 | − 0.244 | 22/20 | 0.176 | 39 | 0.431 | 0.055 | 19/22 |
| LaPFC → RaPFC | − 0.013 | 40 | 0.495 | − 0.004 | 19/19 | − 0.351 | 41 | 0.636 | − 0.107 | 20/23 |
Independent t-test between Lower-Fit (LF) and Higher-fit (HF) children for all connections within the cingulo-opercular network. dACC = Dorsal anterior cingulate cortex; LaI = Left anterior insula; RaI = Right anterior insula; RaPFC = Right anterior prefrontal cortex; LaPFC = Left anterior prefrontal cortex; the bolded text indicates connectivity assessments that are statistically significant; *significant after FDR correction; 1after extreme outlier removal.
Table 3.
Effective connectivity results.
| General task-related connectivity | Interference-related connectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Direction | t | Df | p | d | n (HF / LF) | t | Df | p* | d | n (HF / LF)1 |
| lDLPFC → lIPS | − 0.956 | 42 | 0.172 | − 0.289 | 21/23 | 1.022 | 40 | 0.156 | 0.316 | 20/22 |
| lDLPFC → rDLPFC | 0.042 | 39 | 0.516 | 0.013 | 21/20 | 1.534 | 39 | 0.066 | 0.481 | 22/19 |
| lDLPFC → rIPS | 0.565 | 41 | 0.712 | 0.173 | 20/20 | − 0.141 | 36 | 0.556 | − 0.046 | 19/19 |
| lIPS → lDLPFC | 0.220 | 39 | 0.586 | 0.069 | 20/21 | − 0.688 | 38 | 0.752 | − 0.220 | 21/19 |
| lIPS → rDLPFC | 0.330 | 40 | 0.628 | 0.102 | 20/22 | 0.945 | 41 | 0.175 | 0.289 | 23/20 |
| lIPS → rIPS | − 0.039 | 40 | 0.484 | − 0.012 | 22/20 | 0.330 | 38 | 0.372 | 0.105 | 21/19 |
| rDLPFC → lDLPFC | 0.060 | 43 | 0.524 | 0.018 | 23/22 | − 0.824 | 35 | 0.792 | − 0.271 | 18/19 |
| rDLPFC → lIPS | 0.090 | 41 | 0.536 | 0.027 | 21/22 | 0.191 | 41 | 0.425 | 0.058 | 21/22 |
| rDLPFC → rIPS | − 0.435 | 38 | 0.333 | − 0.138 | 20/20 | 0.087 | 36 | 0.466 | 0.028 | 19/19 |
| rIPS → lDLPFC | − 1.091 | 40 | 0.141 | − 0.337 | 20/22 | − 0.760 | 38 | 0.774 | − 0.240 | 20/20 |
| rIPS → lIPS | − 1.346 | 40 | 0.093 | − 0.417 | 22/20 | − 1.568 | 40 | 0.938 | − 0.484 | 21/21 |
| rIPS → rDLPFC | 0.880 | 40 | 0.808 | 0.272 | 21/21 | − 0.560 | 40 | 0.711 | − 0.174 | 22/20 |
Independent t-test between Lower-Fit and Higher-fit children for all connections within the fronto-parietal network. lDLPFC = Left dorso-lateral prefrontal cortex; rDLPFC = Right dorsolateral prefrontal cortex; lIPS = Left intraparietal sulci; rIPS = Right intraparietal sulci; 1after extreme outlier removal.
Brain-behavior correlation analyses
Additional brain-behavioral correlation analyses were conducted when significant differences in DTF estimates between lower-fit and higher-fit children were observed. By performing these correlations, we aimed to determine whether the observed changes in DTF estimates could be linked to behavioral performance. To perform these correlations, we utilized two-sided correlations while controlling for potential confounding variables, including the sex and age of participants. Given the relatively low sample size within each fitness group, these relationships were assessed across the whole sample to increase the statistical power and reliability of the results. By doing so, we aimed to identify general trends and associations between brain connectivity and behavioral outcomes that may not be detectable within smaller subgroups. As such, the results of these correlation analyses provide insight into the potential mechanisms underlying the observed cognitive benefits associated with higher fitness levels.
Results
Behavioral performance
Table 1 presents the mean response accuracy and RT categorized by fitness groups.
Table 1.
Behavioral results (M ± SD) for lower- and higher-fit children.
| Lower-fit | Higher-fit | |
|---|---|---|
| Response accuracy (%) | ||
| General performance* | 78.91 ± 8.89 | 84.71 ± 7.14 |
| Interference effect | 12.01 ± 11.37 | 10.00 ± 7.63 |
| Congruent* | 84.92 ± 9.01 | 89. 71 ± 8.18 |
| Incongruent* | 72.91 ± 11.90 | 79.71 ± 8.02 |
| Response time (ms) | ||
| General performance | 541 ± 95 | 538 ± 106 |
| Interference effect | 55 ± 56 | 76 ± 53 |
| Congruent | 515 ± 97 | 503 ± 96 |
| Incongruent | 570 ± 100 | 580 ± 123 |
*significant difference between lower- and higher-fit children as assessed by independent t-test.
The observed results correspond to the pattern reported by Pontifex et al.5, encompassing both compatible and incompatible versions of the flanker task. Overall, the analyses performed on a compatible subset of the Pontifex et al.5 data confirmed the presence of the interference effects and greater general task accuracy of higher-fit children. Specifically, participants exhibited higher accuracy (87.31 ± 8.84%) and shorter reaction times (509 ± 95 ms) in the congruent condition compared to the incongruent condition (76.31 ± 10.61%; 575 ± 111 ms) [t(47) = 7.91, p < 0.01, d = 1.14; t(47) = 8.27, p < 0.01, d = 1.19 for accuracy and reaction time, respectively]. Regarding general task accuracy, higher-fit children displayed significantly higher performance than lower-fit children (78.91 ± 8.89% vs. 84.71 ± 7.14%; t(46) = − 2.49, p = 0.008, d = − 0.72). However, there were no significant differences between lower-fit and higher-fit children regarding general task reaction time, interference effect for accuracy, or reaction time.
Effective connectivity
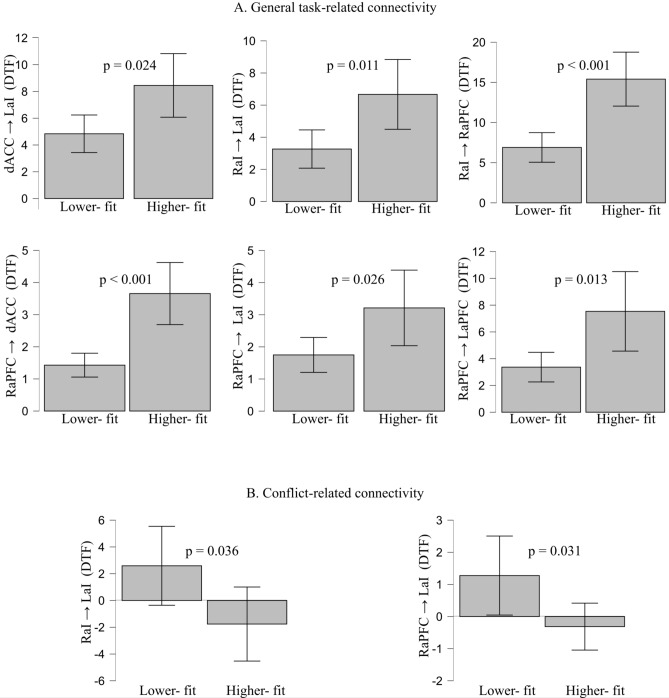
Tables 2 and 3 present the lower-fit and higher-fit group comparisons for DTF estimates among ROIs within the CON and FPN, respectively. Figure 1 depicts the significant changes in DTF estimates observed across lower-fit and higher-fit children and highlights brain-behavior correlations. Figure 2 shows bar plots illustrating significant differences in DTF estimates between lower-fit and higher-fit children. Within the cingulo-opercular network, higher-fit children exhibited greater general task-related connectivity compared to lower-fit children in five specific directions: from dACC to lAI [t(41) = − 2.03, p = 0.02, d = − 0.62], from rAI to lAI [t(44) = -2.39, p = 0.011, d = − 0.70], from rAI to raPFC [t(41) = − 3.77, p < 0.001, d = − 1.15], from raPFC to dACC [t(43) = -3.39, p < 0.001, d = − 1.01], and from RaPFC to LaPFC [t(43) = − 2.31, p = 0.013, d = − 0.69. The connection from raPFC to lAI [t(42) = -2.00, p = 0.026, d = − 0.65] did not survive the FDR correction. Additionally, higher-fit children demonstrated decreased interference-related connectivity in two directions: from rAI to lAI [t(44) = − 1.85, p = 0.036, d = 0.55] and from raPFC to lAI [t(42) = 1.92, p = 0.025, d = 0.58]. Yet, these connections were not significant after the FDR correction. In contrast, no significant differences between lower-fit and higher-fit children in DTF estimates were observed between any ROIs within the fronto-parietal network.
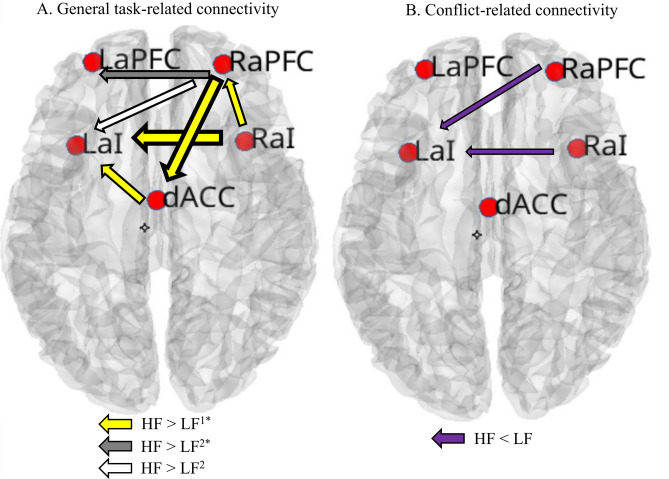
Fig. 1.
Significant Differences in Effective Connectivity between lower-fit (LF) and higher-fit (HF) children within the Cingulo-Opercular Network. Panel (A) displays the differences in general task-related connectivity. The arrows represent connections that are stronger in HF children than in LF children. The thickness of the arrows is proportional to the magnitude of the estimated effect size for each group difference (note that this representation is for illustrative purposes only, such that thicker arrows reflect larger effect sizes)1. The yellow arrows indicate connections positively correlated with task performance, meaning that higher estimates of effective connectivity were associated with greater general accuracy2. Gray arrows (FDR corrected) and white arrows (FDR uncorrected) indicate connections that were not correlated with task performance. Panel( B) shows the differences in interference-related connectivity (incongruent minus congruent trials) between LF and HF children. The purple arrows indicate stronger connections in LF children than in HF children. Brain-behavior correlations were not significant here. No significant differences in effective connectivity within the fronto-parietal network were observed between LF and HF children. * Results significant after FDR corrections. dACC = Dorsal anterior cingulate cortex; LaI = Left anterior insula; RaI = Right anterior insula; RaPFC = Right anterior prefrontal cortex; LaPFC = Left anterior prefrontal cortex; lDLPFC = Left dorso-lateral prefrontal cortex; rDLPFC = Right dorsolateral prefrontal cortex; lIPS = Left intraparietal sulci; rIPS = Right intraparietal sulci.
Fig. 2.
Bar plots illustrating significant differences in Effective Connectivity between lower-fit and higher-fit children within the Cingulo-Opercular Network. Panel( A) depicts differences in general task-related connectivity. Panel( B) shows differences in interference-related connectivity (incongruent minus congruent trials).
Brain-behavioral correlations
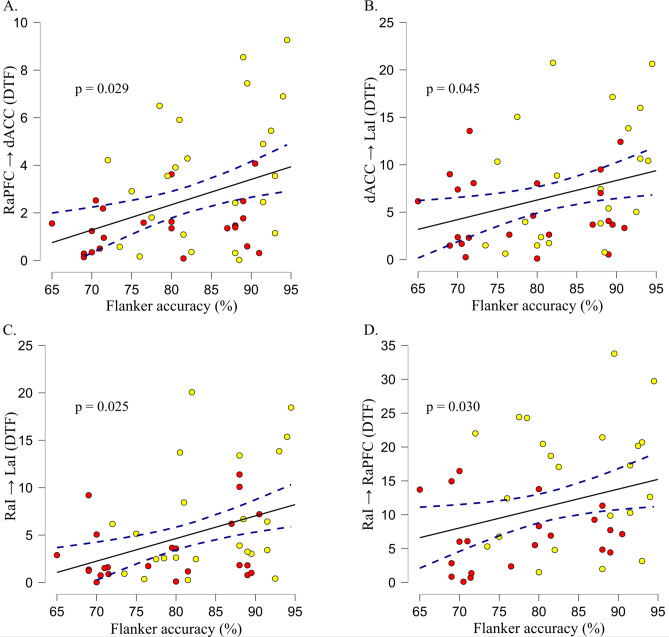
Brain-behavioral correlations were performed between general task performance scores (accuracy, RT) and estimates of general task-related connectivity for five directions that differed across lower-fit and higher-fit children (FDR-corrected): (1) dACC to lAI; (2) rAI to lAI; (3) rAI to raPFC; (4) raPFC to dACC, and (5) raPFC to lAI. The first four of these connections were positively related to general task accuracy: (1) dACC to lAI [rho = 0.31, p = 0.045 ]; (2) rAI to lAI [rho = 0.34, p = 0.025]; (3) rAI to raPFC [rho = 0.34, p = 0.030]; 4) raPFC to dACC [rho = 0.33, p = 0.029], and 5) raPFC to LaPFC [rho = 0.01, p = 0.931]. Figure 3 shows scatter plots illustrating significant correlations. Moreover, interference-related connectivity from rAI to lAI and from raPFC to lAI (FDR-not-corrected) was examined for correlations with interference scores. However, none of these effects reached significance (all p-values > 0.05).
Figure3.
Brain-behavioral correlations. Scatterplots with regression lines are presented for the significant relationships between connectivity estimates and general flanker accuracy. Each plot delineates connections that exhibited differences across lower- and higher-fit children. Low-fit individuals are represented by red circles, while high-fit individuals are represented by yellow circles. Specifically, Panel( A) depicts the connectivity from the right anterior prefrontal cortex to the dorsal anterior cingulate cortex (RaPFC → dACC); Panel ( B)displays the connectivity from the dorsal anterior cingulate cortex to the left anterior insula (dACC → LaI). Panel ( C)showcases the connectivity from the right anterior insula to the left anterior insula (RaI → LaI). Panel ( D)demonstrates the connectivity from the right anterior insula to the right anterior prefrontal cortex (RaI → RaPFC).
Discussion
This study investigated the relationship between cardiorespiratory fitness, cognitive performance in an inhibition task, and neural effective connectivity in preadolescent children. We confirmed that children with higher cardiorespiratory fitness levels demonstrated significantly better general response accuracy than their less fit counterparts. Novel to this investigation, the effective connectivity patterns indicated that the higher accuracy of higher-fit children was linked to enhanced task-induced connectivity within the cingulo-opercular network but not the frontal-parietal network. Brain-behavior correlation analyses revealed that most of the connections that were stronger in higher-fit children positively correlated with general task accuracy.
Behavioral findings
In line with previous investigations and the analysis performed by Pontifex et al.5 on a complete dataset, higher-fit children exhibited higher accuracy compared to lower-fit children17,18. These performance differences were not attributed to a tradeoff between response speed and accuracy, as there were no group differences in reaction times. These findings are consistent with other studies examining inhibitory tasks in preadolescent children, wherein effects have been predominantly observed in response accuracy rather than response speed16. The current findings strengthen the argument that response accuracy is a more meaningful outcome measure for children, given their tendency to prioritize response speed over accuracy10. However, unlike some prior studies, our investigation found no significant relationships between fitness and interference scores, which would have indicated a selective association between fitness and cognitive control6,22. Instead, our findings align with studies reporting the association between fitness and general (across conditions) performance5,58–61. It is important to acknowledge that the absence of group effects for interference scores could be attributed to the specific samples tested, as selective associations were reported in a reanalysis of a large aggregate dataset of over 700 children16. Results reported by Raine et al.16 also suggested that the relationships between fitness and interference scores may be more subtle than the relationship with general accuracy. Thus, the effect may not consistently emerge in all tested samples.
Connectivity findings
The novel aspect of our investigation was the evaluation of task-related effective connectivity estimates within two essential networks implicated in cognitive control: the CON, responsible for implementing general task sets across trials, and the FPN, which adapts control on a trial-by-trial basis24–27. Our findings revealed distinct connectivity patterns within the CON, but not the FPN, between lower-fit and higher-fit children.
Consistent with our hypothesis, higher-fit children exhibited greater across-condition connectivity within the CON. Heightened connectivity was observed in five (six when FDR uncorrected) specific connections between structures involved in this network (see Fig. 1 Panel A). Each of these structures plays a vital role in cognitive control. The dorsal anterior cingulate cortex (dACC) is involved in the preparation and maintenance of control signals during the task62; the anterior insula region (aI, sometimes labeled ventrolateral prefrontal cortex) is identified as a network hub responsible for representing general, across-trials task rules63,64; and finally, the anterior prefrontal cortex (aPFC) is associated with representations of more complex task strategies65,66. Thus, increased connectivity within the CON suggests that higher-fit children had more active domain-independent "task mode"8,24. That is, higher-fit children may outperform lower-fit children on inhibition tasks because of effective connectivity differences within the CON, which underlie domain-general rules that govern, amongst other, cognitive control. Brain-behavioral correlations further strengthen this reasoning. Most of the connections that were strengthened in higher-fit children (four out of five) displayed a positive correlation with general task accuracy. This not only supports the functional significance of the CON as a domain-general network but also implies that differences in CON connectivity between higher-fit and lower-fit children bear relevance to behavioral differences.
In addition to the heightened general connectivity, our hypothesis was partially supported by the observation of lower interference-related connectivity within the CON in higher-fit children, indicating fewer across-condition adjustments within this network (less interference costs). That is, previous reports have demonstrated lower behavioral interference in higher-fit compared to lower-fit children using flanker tasks6,22, and the interference-related findings in the CON reported herein appear to support, or at least are consonant with, these prior findings. However, this effect was limited to only two directions, did not pass the FDR corrections, and had no significant relationship with behavioral performance, thus providing partial support (see Fig. 1, Panel B). Regardless, this effect might be interpreted as greater neural efficiency of higher-fit children since they demonstrated reduced connectivity strength in circuits not directly associated with task performance. Instead, higher-fit children exhibited stable activity in CON network that subserved better task performance. It is also possible that higher interference-related connections in lower-fit children represent higher neural interference costs that were not detected at the behavioral level. Thus, it might be possible that effective connectivity measures may be more sensitive than behavioral measures in detecting interference costs. Clearly, future work will need to unpack these connectivity and behavioral findings to better understand their relationship.
The findings related to connectivity within the CON align with behavioral observations, suggesting that higher-fit children displayed general rather than selective inhibitory performance enhancement. The activation of the CON remains elevated throughout the entire task performance period in higher-fit children, with no excess task-irrelevant activity. In summary, these findings suggest that heightened connectivity within the cingulo-opercular network mediates the cognitive advantages linked to elevated fitness levels.
Contrary to our expectations, the estimates of effective connectivity within the FPN showed no significant differences between lower-fit and higher-fit children. This finding contradicts previous studies that reported increased functional connectivity within different networks as a function of fitness in children during resting-state fMRI measurements19–21,67. Notably, however, network patterns during rest can significantly differ from those assessed during tasks and may not necessarily translate to task performance outcomes68. For enhanced comparability with fMRI results, future EEG studies should include measurements of effective connectivity during the resting state. Still, the lack of observed effects within the FPN is also inconsistent with findings from a task-related EEG functional connectivity study4. Our study focused on effective connectivity, which, unlike functional connectivity, examines direct influences between two brain structures. While functional connectivity captures connections between structures coactivating simultaneously, effective connectivity assesses whether one structure directly influences another. Thus, in our approach, a connection would only be present if there is a demonstrable influence between the two structures rather than solely their simultaneous activation. Finally, the Kamijo et al.4 study used a visual search task rather than a flanker task, and greater task-related functional connectivity in higher-fit children was only observed under more demanding task conditions. In contrast, lower-fit children showed no difference in connectivity assessment across the conditions. This selective effect reported by Kamijo et al. aligns with the postulated function of the FPN, which is thought to exert top-down control of attention on a trial-by-trial basis24. On the other hand, our study observed only behavioral effects across task conditions, which might explain the lack of effects in the FPN. Higher-fit and lower-fit children did not differ significantly in interference scores, suggesting that their trial-to-trial adjustment of cognitive control, realized by the FPN, could be similar. To better understand differences between lower-fit and higher-fit children in FPN activation, future studies should investigate whether differences in effective connectivity arise when using tasks that better modulate trial-by-trial inhibitory demand, leading to larger interference scores.
Overall, the synthesis of behavioral and neural findings in this study strongly supports the notion that higher-fit children outperform their lower-fit counterparts, both behaviorally and in terms of brain-related advantages. This study suggests that the benefits of being fit extend beyond specific cognitive functions to general improved functioning, but future research should continue to pursue the general vs. selective nature of this relationship. Still, increased connectivity across trials in the CON suggests that higher-fit children can better maintain a domain-general task set than their less-fit peers. This ability enables individuals to perform a wide range of tasks across different domains or contexts, and therefore, it could be particularly crucial in academic settings, suggesting that these children may excel in various school tasks. Specifically, higher-fit children may possess superior abilities to sustain a task set while executing different goals. Their attentional may bias cognitive processes even before the task begins, enabling them to sustain it throughout the task. This ability may enable them to concentrate more effectively on a given task, stay on course, and, in case of distraction, quickly return to the task at hand.
Strengths and limitations
A key strength of this study is the novel application of effective connectivity measures, which allows for a deeper understanding of the underlying brain mechanisms associated with fitness-related benefits. By estimating effective connectivity within the CON and FPN, we could examine the interactions between the involved brain regions and gain valuable insights into the neural basis of the fitness-cognitive control relationship. The findings revealed dependencies within the CON, which not only correlated with task performance but also provided evidence of the functional relevance of this network in supporting cognitive control. Furthermore, the DTF method proved valuable in elucidating the brain basis of cognitive processes and understanding the connectivity dynamics within the networks under investigation. It is important to acknowledge the limitations of this investigation. The cross-sectional design of the study does not allow for causal conclusions. Therefore, future research employing randomized control trials would be valuable in establishing causality and further understanding the effects of cardiorespiratory fitness on cognitive control and underlying patterns of effective connectivity. Such intervention studies could provide valuable insights into the role of enhancing cardiorespiratory fitness as a strategy to improve cognitive control and overall brain health, not only in healthy children but also in those with various neurological or developmental conditions69. Nonetheless, these findings are specific to neurotypical children aged 8–10 years who were able to complete maximal exercise testing. Therefore, future research should explore whether these results are applicable to neurodivergent children, children unable to complete treadmill tests, and older age groups such as adolescents and teenagers. Another limitation is the spatial resolution of EEG measurements. While the methods we used provided reasonable spatial accuracy, we relied on MNI coordinates from previous studies when selecting regions of interest (ROIs). As MNI is a template of averaged brains, this approach may introduce potential errors regarding the exact localization of structures in individual brains. Future studies should consider utilizing individual MRI scans of children to determine the precise location of ROI individually for each individual. Finally, in this study, our focus was on two brain networks that play a crucial role in cognitive control. However, these networks are not the sole players in cognitive control. Other neural networks are likely involved, and future research should also explore their contributions and mutual interactions. Employing different research approaches, such as full brain connectivity analysis, would be valuable in gaining a more comprehensive understanding of the complex neural mechanisms underlying cognitive control.
Conclusions
In conclusion, the findings presented in this study replicate and extend previous research that highlights positive associations between children's fitness, cognitive performance, and brain health. By employing the effective connectivity method, the study provides a novel and deeper understanding of how cognitive processes operate at the neural level in preadolescent children of varied fitness. The study also underscores the significance of effective connectivity as a powerful tool for investigating the intricate neural underpinnings of cognitive functioning.
Acknowledgements
During the work on the manuscript, TSL was supported by the National Science Centre, Poland (2021/43/D/HS6/02959) and a grant funded by the Strategic Program Excellence Initiative at the Jagiellonian University.
Author contributions
TSL: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing; LBR: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing; MBP: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing; MW: Data curation, Methodology, Software, Supervision, Validation, Writing – review & editing; AFK: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing; CHH: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Data availability
All current study data are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee, B. Y. et al. Modeling the economic and health impact of increasing children’s physical activity in the United States. Health Aff (Millwood)36, 902–908 (2017). 10.1377/hlthaff.2016.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haskell, W. L. et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation116, 1081–1093 (2007). 10.1161/CIRCULATIONAHA.107.185649 [DOI] [PubMed] [Google Scholar]
- 3.Hillman, C. H., Erickson, K. I. & Kramer, A. F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci.9, 58–65 (2008). 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- 4.Kamijo, K., Takeda, Y., Takai, Y. & Haramura, M. The relationship between childhood aerobic fitness and brain functional connectivity. Neurosci. Lett.632, 119–123 (2016). 10.1016/j.neulet.2016.08.051 [DOI] [PubMed] [Google Scholar]
- 5.Pontifex, M. B. et al. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J. Cognitive Neurosci.23, 1332–1345 (2011). 10.1162/jocn.2010.21528 [DOI] [PubMed] [Google Scholar]
- 6.Voss, M. W. et al. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience199, 166–176 (2011). 10.1016/j.neuroscience.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. Conflict monitoring and cognitive control. Psychol. Rev.108, 624–652 (2001). 10.1037/0033-295X.108.3.624 [DOI] [PubMed] [Google Scholar]
- 8.Braver, T. S. & Barch, D. M. Extracting core components of cognitive control. Trends Cogn Sci.10, 529–532 (2006). 10.1016/j.tics.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 9.Barkley, R. A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull121, 65–94 (1997). 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- 10.Davidson, M. C., Amso, D., Anderson, L. C. & Diamond, A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia44, 2037–2078 (2006). 10.1016/j.neuropsychologia.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksen, B. A. & Eriksen, C. W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys.16, 143–149 (1974). 10.3758/BF03203267 [DOI] [Google Scholar]
- 12.Falkenstein, M., Hoormann, J. & Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. (Amst)101, 267–291 (1999). 10.1016/S0001-6918(99)00008-6 [DOI] [PubMed] [Google Scholar]
- 13.Bull, R., Johnston, R. S. & Roy, J. A. Exploring the roles of the visual-spatial sketch pad and central executive in children’s arithmetical skills: Views from cognition and developmental neuropsychology. Dev. Neuropsychol.15, 421–442 (1999). 10.1080/87565649909540759 [DOI] [Google Scholar]
- 14.St Clair-Thompson, H. L. & Gathercole, S. E. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quart. J. Exp. Psychol.59, 745–759 (2006). 10.1080/17470210500162854 [DOI] [PubMed] [Google Scholar]
- 15.Van Waelvelde, H., Vanden Wyngaert, K., Mariën, T., Baeyens, D. & Calders, P. The relation between children’s aerobic fitness and executive functions: a systematic review. Infant Child Dev.29, e2163 (2020). 10.1002/icd.2163 [DOI] [Google Scholar]
- 16.Raine, L. B. et al. A large-scale reanalysis of childhood fitness and inhibitory control. J. Cogn. Enhanc.2, 170–192 (2018). 10.1007/s41465-018-0070-7 [DOI] [Google Scholar]
- 17.Hillman, C. H., Castelli, D. M. & Buck, S. M. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med. Sci. Sports Exerc.37, 1967–1974 (2005). 10.1249/01.mss.0000176680.79702.ce [DOI] [PubMed] [Google Scholar]
- 18.Hillman, C. H. et al. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience159, 1044–1054 (2009). 10.1016/j.neuroscience.2009.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaddock-Heyman, L. et al. Brain network modularity predicts improvements in cognitive and scholastic performance in children involved in a physical activity intervention. Front. Human Neurosci.3(14), 346 (2020). 10.3389/fnhum.2020.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteban-Cornejo, I. et al. Physical fitness, hippocampal functional connectivity and academic performance in children with overweight/obesity: the activebrains project. Brain, Behav. Immun.91, 284–295 (2021). 10.1016/j.bbi.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 21.Logan, N. E. et al. The differential effects of adiposity and fitness on functional connectivity in preadolescent children. Med. Sci. Sports Exerc.54, 1702–1713 (2022). 10.1249/MSS.0000000000002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, R. D. et al. Aerobic fitness and intra-individual variability of neurocognition in preadolescent children. Brain Cognition82, 43–57 (2013). 10.1016/j.bandc.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishihara, T., Drollette, E. S., Ludyga, S., Hillman, C. H. & Kamijo, K. The effects of acute aerobic exercise on executive function: a systematic review and meta-analysis of individual participant data. Neurosci. Biobehav. Rev.128, 258–269 (2021). 10.1016/j.neubiorev.2021.06.026 [DOI] [PubMed] [Google Scholar]
- 24.Dosenbach, N. U. F. et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci.104, 11073–11078 (2007). 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dosenbach, N. U. F. et al. Prediction of Individual Brain Maturity Using fMRI. Science329, 1358–1361 (2010). 10.1126/science.1194144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marek, S., Hwang, K., Foran, W., Hallquist, M. N. & Luna, B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol.13, e1002328 (2015). 10.1371/journal.pbio.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sestieri, C., Corbetta, M., Spadone, S., Romani, G. L. & Shulman, G. L. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J. Cogn. Neurosci.26, 551–568 (2014). 10.1162/jocn_a_00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper, P. S. et al. Theta frontoparietal connectivity associated with proactive and reactive cognitive control processes. NeuroImage108, 354–363 (2015). 10.1016/j.neuroimage.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 29.Friston, K. J. Functional and effective connectivity: a review. Brain Connect1, 13–36 (2011). 10.1089/brain.2011.0008 [DOI] [PubMed] [Google Scholar]
- 30.Blinowska, K. J., Kuś, R. & Kamiński, M. Granger causality and information flow in multivariate processes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys.70, 050902 (2004). 10.1103/PhysRevE.70.050902 [DOI] [PubMed] [Google Scholar]
- 31.Kaminski, M. J. & Blinowska, K. J. A new method of the description of the information flow in the brain structures. Biol. Cybern.65, 203–210 (1991). 10.1007/BF00198091 [DOI] [PubMed] [Google Scholar]
- 32.Ligeza, T. S., Wyczesany, M., Tymorek, A. D. & Kamiński, M. Interactions between the prefrontal cortex and attentional systems during volitional affective regulation: an effective connectivity reappraisal study. Brain Topogr.29, 253–261 (2016). 10.1007/s10548-015-0454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamczyk, A. K. & Wyczesany, M. Theta-band connectivity within cognitive control brain networks suggests common neural mechanisms for cognitive and implicit emotional control. J. Cognitive Neurosci.35, 1656–1669 (2023). 10.1162/jocn_a_02034 [DOI] [PubMed] [Google Scholar]
- 34.Hanslmayr, S. et al. The electrophysiological dynamics of interference during the Stroop task. J. Cogn. Neurosci.20, 215–225 (2008). 10.1162/jocn.2008.20020 [DOI] [PubMed] [Google Scholar]
- 35.Oehrn, C. R. et al. Human hippocampal dynamics during response conflict. Curr. Biol.25, 2307–2313 (2015). 10.1016/j.cub.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 36.Cai, W. et al. Causal interactions within a frontal-cingulate-parietal network during cognitive control: convergent evidence from a multisite-multitask investigation. Cereb. Cortex26, 2140–2153 (2016). 10.1093/cercor/bhv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med.147, 573–577 (2007). 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 38.American College of Sports Medicine, Riebe, D., Ehrman, J. K., Liguori, G. & Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription. (2018). [DOI] [PubMed]
- 39.Utter, A. C., Robertson, R. J., Nieman, D. C. & Kang, J. Children’s OMNI scale of perceived exertion: walking/running evaluation. Med. Sci. Sports Exercise34, 139 (2002). 10.1097/00005768-200201000-00021 [DOI] [PubMed] [Google Scholar]
- 40.Freedson, P. S. & Goodman, T. L. Measurement of oxygen consumption. In Pediatric laboratory exercise testing: Clinical guidelines (ed. Rowland, T. W.) 91–113 (Human Kinetics, 1993). [Google Scholar]
- 41.Bar-Or, O. Pediatric Sports Medicine for the Practitioner. (Springer, New York, NY, 1983). 10.1007/978-1-4612-5593-2.
- 42.Shvartz, E. & Reibold, R. C. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat. Space Environ. Med.61, 3–11 (1990). [PubMed] [Google Scholar]
- 43.Tanner, J. M. Growth at Adolescence; with a General Consideration of the Effects of Hereditary and Environmental Factors upon Growth and Maturation from Birth to Maturity (Blackwell Scientific Publications, 1962). [Google Scholar]
- 44.Kaufman, A. S., Kaufman, N. L., & American Guidance Service. K-BIT : Kaufman Brief Intelligence Test. (1990).
- 45.DuPaul, G. J., Power, T. J., Anastopoulos, A. D. & Reid, R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation. viii, 79 (The Guilford Press, New York, NY, US, 1998).
- 46.Birnbaum, A. S. et al. Survey development for assessing correlates of young adolescents’ eating. Am. J. Health Behav.26, 284–295 (2002). 10.5993/AJHB.26.4.5 [DOI] [PubMed] [Google Scholar]
- 47.Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods39, 175–191 (2007). 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 48.Spencer, K. M. & Coles, M. G. The lateralized readiness potential: relationship between human data and response activation in a connectionist model. Psychophysiology36, 364–370 (1999). 10.1017/S0048577299970749 [DOI] [PubMed] [Google Scholar]
- 49.Mantini, D. et al. A signal-processing pipeline for magnetoencephalography resting-state networks. Brain Connect.1, 49–59 (2011). 10.1089/brain.2011.0001 [DOI] [PubMed] [Google Scholar]
- 50.Spadone, S., Wyczesany, M., Della Penna, S., Corbetta, M. & Capotosto, P. Directed flow of beta band communication during reorienting of attention within the dorsal attention network. Brain Connect11, 717–724 (2021). 10.1089/brain.2020.0885 [DOI] [PubMed] [Google Scholar]
- 51.Hämäläinen, M. S. & Ilmoniemi, R. J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput.32, 35–42 (1994). 10.1007/BF02512476 [DOI] [PubMed] [Google Scholar]
- 52.Fuchs, M., Kastner, J., Wagner, M., Hawes, S. & Ebersole, J. S. A standardized boundary element method volume conductor model. Clin. Neurophysiol.113, 702–712 (2002). 10.1016/S1388-2457(02)00030-5 [DOI] [PubMed] [Google Scholar]
- 53.Lacadie, C. M., Fulbright, R. K., Rajeevan, N., Constable, R. T. & Papademetris, X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage42, 717–725 (2008). 10.1016/j.neuroimage.2008.04.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDonald, A. W., Cohen, J. D., Stenger, V. A. & Carter, C. S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science288, 1835–1838 (2000). 10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- 55.Papademetris, X. et al. BioImage suite: an integrated medical image analysis suite: an update. Insight J.2006, 209 (2006). [PMC free article] [PubMed] [Google Scholar]
- 56.Colclough, G. L., Brookes, M. J., Smith, S. M. & Woolrich, M. W. A symmetric multivariate leakage correction for MEG connectomes. Neuroimage117, 439–448 (2015). 10.1016/j.neuroimage.2015.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc.57, 289–300 (1995). 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 58.Chaddock, L. et al. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev. Neurosci.32, 249–256 (2010). 10.1159/000316648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kao, S.-C. et al. Aerobic Fitness Is Associated With Cognitive Control Strategy in Preadolescent Children. J Mot Behav49, 150–162 (2017). 10.1080/00222895.2016.1161594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scudder, M. R. et al. Aerobic capacity and cognitive control in elementary school-age children. Med. Sci. Sports Exerc.46, 1025–1035 (2014). 10.1249/MSS.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, C.-T. et al. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology25, 333–341 (2011). 10.1037/a0022167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crottaz-Herbette, S. & Menon, V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci.18, 766–780 (2006). 10.1162/jocn.2006.18.5.766 [DOI] [PubMed] [Google Scholar]
- 63.Badre, D. & Wagner, A. D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia45, 2883–2901 (2007). 10.1016/j.neuropsychologia.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 64.Bunge, S. A., Ochsner, K. N., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. E. Prefrontal regions involved in keeping information in and out of mind. Brain124, 2074–2086 (2001). 10.1093/brain/124.10.2074 [DOI] [PubMed] [Google Scholar]
- 65.Bunge, S. A. et al. Neural circuitry underlying rule use in humans and nonhuman primates. J. Neurosci.25, 10347–10350 (2005). 10.1523/JNEUROSCI.2937-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crone, E. A., Wendelken, C., Donohue, S. E. & Bunge, S. A. Neural evidence for dissociable components of task-switching. Cereb. Cortex16, 475–486 (2006). 10.1093/cercor/bhi127 [DOI] [PubMed] [Google Scholar]
- 67.Moore, D., Jung, M., Hillman, C. H., Kang, M. & Loprinzi, P. D. Interrelationships between exercise, functional connectivity, and cognition among healthy adults: a systematic review. Psychophysiology59, e14014 (2022). 10.1111/psyp.14014 [DOI] [PubMed] [Google Scholar]
- 68.Arbabshirani, M. R., Havlicek, M., Kiehl, K. A., Pearlson, G. D. & Calhoun, V. D. Functional network connectivity during rest and task conditions: a comparative study. Human Brain Mapping34, 2959–2971 (2013). 10.1002/hbm.22118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koirala, G. R., Lee, D., Eom, S., Kim, N.-Y. & Kim, H. D. Altered brain functional connectivity induced by physical exercise may improve neuropsychological functions in patients with benign epilepsy. Epilepsy Behav.76, 126–132 (2017). 10.1016/j.yebeh.2017.06.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All current study data are available from the corresponding author on request.