Abstract
Pomacea canaliculata is one of the most successful invader in worldwide, adversely affecting native ecosystem through direct predation or indirect competition, while the mechanism of indirect effects on native species remain poorly understood. To clarify the effects of P. canaliculata on the native near-niche species, Bellamya purificata, a widespread freshwater gastropod in China, was selected as the research subject. The changes of mortality, histology, antioxidant system as well as the intestinal flora diversity of B. purificata were explored in present study. The results showed that the median lethal dose of P. canaliculata culture solution for B. purificata was 23.76 ind/L and a concentration-dependent damage of both the gonad and hepatopancreas were observed, the gonadal villi were dissolved and the hepatopancreas cells were broken at 20 ind/L. Furthermore, different concentrations of P. canaliculata culture solution leading to the antioxidant damage on the enzyme or non-enzyme systems of B. purificata at various degrees. Additionally, a decrease in the diversity of the intestinal flora was observed, accompanied by an increase in the abundance of pathogenic bacteria such as Pseudomonas and Aeromonas after the exposure of the culture solution of P. canaliculata. Last, after being recovered in freshwater for 24 h, the antioxidant damage of B. purificata and the disturbance of intestinal flora diversity were still not recovered especially in the high concentration group. The indirect competitive mechanism of P. canaliculata culture solution on B. purificata were explored from the aspects of tissue, biochemical level and intestinal flora, which enriched the research of P. canaliculata invasion on native snails in China, and provided new insights for the study of the invasion strategy of P. canaliculata.
Keywords: Pomacea canaliculata, Invasion mechanism, Histological damage, Antioxidant system, Intestinal microbiota
Subject terms: Biochemistry, Cell biology, Ecology, Microbiology, Zoology
Introduction
Biological invasion is a serious ecological and environmental problem under the background of economic globalization, which is one of the five major global environmental problems of the twenty-first century1. Invasive species has caused significant economic losses in the invaded area2,3 and threaten the public health safety4, as well as the huge damage to biodiversity and ecosystem function5. Pomacea canaliculata, is one of the most malignant invasive species worldwide6, which was introduced into China in 1981, causing great ecological impact and economic losses. P. canaliculata has a higher tolerance to environmental conditions than other native snail species, resulting in its expansive ecological niche7. A study has shown that P. canaliculata can accumulate uric acid in allantoin as a non-enzymatic antioxidant to survive its aestivation8. Moreover, P. canaliculata has multiple escape strategies and can quickly respond to predator pheromones or similar injured chemical signals9. After being attacked by predators, P. canaliculata can quickly repair the broken shells by hemolymph10. These life-history strategies give them an absolute advantage in the ecosystem, facilitating the gradual proliferation of invasive species populations and exerting substantial pressure on other species, further leading to reduced agricultural crop production and loss of local biodiversity11,12. P. canaliculata has the advantage of displacing native snail species in competition for food and space13. It has been reported that the food consumption and fecundity of P canaliculata invading the everglades in United States are higher than those of the native snail Pomacea paludosa14. The detrimental impact of P. canaliculata on aquatic plants in invasive areas has been extensively documented14,15. During the periods of plant scarcity, P. canaliculata also prey on native snails16,17, exerting both indirect competitive pressure and direct predatory threat on native snails. In addition, invasive species exert significant impacts on local ecosystem processes through their diverse life history strategies, resulting in alterations to the structure or functional groups of microbial communities in native species18–20, which can ultimately lead to ecological exhaustion such as community structure simplicity and recession21. Consequently, the P. canaliculata population have more survival advantages than native snails in freshwater ecological environment22.
Invasive species exert both directly or indirectly influences on microorganisms and other organisms within the ecosystem through the release of allelochemicals. A classic intrusion model in ecological studies is the global invasive crayfish, Procambarus clarkia. The presence of predatory invasive crayfish affects the development of local frog tadpoles, necessitating increased energy expenditure for adaptation and resulting in an inevitable decline in their populations23. The excretions and secretions of invading pufferfish Siganus rivulatus affected the microbial functional groups of the invaded area, which changed the nutrient content and the phytoplankton structure in aquatic environment, leading to the unpredictable profound influence on the invasion of water24. P. canaliculata have the same invasive strategy, including deterioration of water quality and eutrophication25,26. By studying the composition of the excreta of P. canaliculata, it can be found that the soluble excreta of P. canaliculata contains a large amount of ammonia, which is the main cause of eutrophication of water quality27. These environmental changes have led to the death of native species, providing P. canaliculata populations with expanded living space and food resources to exploit vacant niches within the ecosystem. However, there are few studies on the response mechanism of native species under indirect stress of P. canaliculata.
Bellamya purificata is a common freshwater gastropod molluscs in China28, which prefers to inhabit in silt and devour the surrounding organic debris and algae29. Besides, it can effectively improve water quality30, and often used as an environmental indicator species31,32. The ecological niche similarity between B. purificata and P. canaliculata leads to constant invasion of the former’s habitat, resulting in population suppression of B. purificata by P. canaliculata33. Thus, B. purificata represents an ideal model species for investigating the effects of P. canaliculata invasion on native species.
Although the direct effects of P. canaliculata on native species have been extensively studied, the indirect invasion strategy and the targets as well as the potential mechanism of P. canaliculata on native species have not been addressed adequately. To clarify the impact of the existence and population density of P. canaliculata in freshwater environment on the survival and reproduction of native snails, the near-niche species B. purificata were exposed to the culture solution of P. canaliculata at different concentrations. The median lethal dose (LC50) was determined, and the indirect mechanism of P. canaliculata was analyzed by detecting the histology, antioxidant system and intestinal microbial community of native snails. This research will provide valuable insights for life history strategy research of invasive species P. canaliculata and the potential risk assessment in native ecosystems.
Materials and methods
Animals culture and sampling
Bellamya purificata were captured from Weishan Lake in Shandong Province, China, where they exist as a wild population and had no contact with P. canaliculata in their natural habitat. Pomacea canaliculata were acquired from Zhaoqing, Guangdong Province, China. All the snails transported to the laboratory within 24 h with icebags and then temporarily reared in a 5 L plastic aquarium with 3 L aerated freshwater. B. purificata and P. canaliculata were cultured separately, and the temperature maintained at 23 ± 1 °C and 27 ± 1 °C, respectively. The light–dark cycle was set at a ratio of 12 h: 12 h. Prior to the experiment, both species were fed with the homogenate benthic feed (Tetra, German) every 48 h, and the culture solutions were renewed after 24 h of feeding.
Culture solution collection
The preparation of P. canaliculata culture solution was referred to the research of Zhou et al.34. The shell height of P. canaliculata was measured using a vernier caliper. Snails with a shell height of 25.0 ± 1 mm will be used to collect culture fluid and there were no mortalities during the collection process. A total of 80 snails were randomly selected and placed into cylindrical plastic containers with 1 L of sterile water. After fasted for 24 h, the snails were picked out and the culture solution were collected as the stocking solution for subsequent experiment. As described in the study of A. Vega (2012), to prevent the impact of feces and other insoluble particles on subsequent experiments, the original stock solution was filtered through filter paper to remove these particles before dilution35. Then, the stock solution of P. canaliculata (80 ind/L) were diluted with sterile water to simulate exposure solution concentrations of 1, 5, 10, 20, and 40 ind/L, respectively. These concentration settings included the actual outbreak density (10 ind/L) of P. canaliculata in freshwater ecosystem, as well as the simulated extreme outbreaks of invasive species36. The group with no P. canaliculata adding with 500 mL sterile water was set as control group. Water quality analyzer (Proplus, YSI, USA) was used to detect the dissolved oxygen (8.678 ± 0.18 mg/L) and pH (7.73 ± 0.12) of P. canaliculata culture solution at different concentrations, and no significant difference between the groups (P > 0.05).
Experiment design
The experiment was conducted in an 800 mL cylindrical plastic aquarium (lower diameter 9.6 cm, upper diameter 15 cm, height 6 cm), adding with 500 mL culture solution of P. canaliculata in different concentrations. A total of 126 adult B. purificata with equal size (3.5 ± 0.5 g) were selected for the experiment. These animals were divided into 7 concentration groups (0, 1, 5, 10, 20, 40, 80 ind/L). Each group had 6 replicated systems, with 3 individuals placed in each system. Before the experiment commenced, the snails were fasted for 24 h. The death of B. purificata was judged when soft tissue ectropion, visceral mass dissolution or no response to shell shedding. The survival status of snails was checked every 6 h, and the dead individuals were removed from the system in time. The median lethal concentration of B. purificata was calculated according to Four-parameter Logistic model by GraphPad Prism 8.0.2 after experiment.
At the end of experiment, the snails were washed by sterile water, and carefully broke the shell. Subsequently, the hepatopancreas, gut and gonads of B. purificata were dissected on ice. As the main immune organ and antioxidant organ of B. purificata37, 9 hepatopancreas samples from each group were frozen in − 80 °C immediately for subsequent antioxidant biochemical tests. In order to detect the intestinal flora of B. purificata, three guts sample from each treatment group were rinsed with 0.9% sterile saline for three times, then frozen in − 80 °C for 16S rRNA sequence. Furthermore, three hepatopancreas and gonads for each group were fixed for 24 h using 4% paraformaldehyde solution and preserved in 75% ethanol solution for histological observation.
To study the repair effects in antioxidant system and intestinal flora community structure, the rest of survival B. purificata were recovered in 500 mL sterile water for 24 h, dissection and sample collection were performed as above.
Histological analysis
Three gonads and hepatopancreas samples were fixed by 4% paraformaldehyde, and then dehydrated with gradient alcohol. The tissues were hyalinized by immersion in xylene, followed by embedding in paraffin wax. Then the embedded specimens were sliced into sections with a thickness of 4 µm using a rotary microtome and three sections were made for each tissue. Furthermore, the sections were stained with hematoxylin and eosin (H&E) dye to enhance visualization. The slices were observed under a microscope at a magnification of 20 x, and images were captured by Nikon software and three different microscope fields were selected for photographing.
Antioxidant biochemical assays
We detected the antioxidant substances of superoxide dismutase (SOD) and catalase (CAT). SOD disproportionates superoxide anion radicals in cells into hydrogen peroxide and oxygen, and CAT catalyzes hydrogen peroxide into oxygen and water38. At the same time, the non-enzymatic antioxidant glutathione (GSH) was added. GSH binds to free radicals in the body through sulfhydryl groups, which can directly reduce free radicals into acidic substances, thereby accelerating the excretion of free radicals and resisting the damage of free radicals to important organs38. Finally, we will detect malondialdehyde (MDA), which is the product of peroxidation of membrane lipids under the action of free radicals38.
The hepatopancreas of B. purificata from treatment group and recovery group were weighed and homogenized with sterilized saline water on ice. Each sample was homogenized from the hepatopancreas of three B. purificata snails, and three samples were detected in each treatment group. After centrifugation at 2500 rpm for 10 min, the supernatant was taken to measure malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, China), according to the manufacturer' s instructions. The concentrations of total protein were detected using BCA protein kit as standard. All the assays were performed in duplicate.
Intestinal microbial detection
Intestinal microbiota analysis was detected with gut samples from treatment groups and recovery groups, including B. purificata treated by 0, 5, 20 ind/L P. canaliculata culture solution (T0, T5, T20), and B. purificata recovered in sterile water after treated by 0, 5, 20 ind/L P. canaliculata culture solution (R0, R5, R20). All gut samples were washed by sterile saline and put into the sterile Eppendorf tube immediately, the frozen samples are transported to Novogene (Beijing, China) with dry ice for sequencing analysis. The CTAB/SDS method was used to extract the total genome DNA in samples. DNA concentration and purity were monitored on 1% agarose gels. The bacterial 16S rRNA gene V4 region was amplified with the primers (515F: CCTAYGGGRBGCASCAG, 806R: GGACTACNNGGGTATCTAAT) and sequenced on an Illumina NovaSeq platform.
Statistical analysis of antioxidant biochemical assays
All the data analyses were performed by SPSS Statistics 27 software, and expressed as means ± standard error. One-way analysis of variance (ANOVA) was used to compare the experimental data in the treatment group or recovery group, used the Bonferroni correction method for correction and post hoc comparisons determined using the Least Significant Difference (LSD) test. In order to compare the changes of B. purificata treated with the same concentration of P. canaliculata culture solution in treatment group and recovery group, a paried Student’s T test was used for analysis. The probabilities < 0.05 were identified as a significant difference. The median lethal concentration of B. purificata was calculated according to Four-parameter Logistic model.
High-throughput sequencing data analysis
In order to analyze bacterial data, sequence assembly, data filtering and chimera removal steps were performed to obtain effective sequences. The original 16S rRNA sequence was processed using QIIME (version 1.9.1). Sequences with 97% or higher similarity were assigned to the same operational taxonomic units (OTUs) using UPARSE software (v.7.0.1001). The SILVA database (v. 138.1) was used to identify each representative sequence, and remove rare reads. The α diversity was determined based on the abundance of OTUs. QIIME software was used to calculate α-diversity indices such as Shannon index, Chao 1 index, simpson's index and community richness was evaluated. If the data were normally distributed, the significance between two groups was determined using two-tailed Student's t test, and analysis of variance (ANOVA) followed by the Tukey test was used for multiple test groups. One-way ANOVA with Kruskal–Wallis test was performed when the data did not conform to the normal distribution. Pairwise comparison was performed using the Wilcoxon Rank Sum Test, and P < 0.05 was defined as statistically significant.
Results
Effect of P. canaliculata culture solution on the survival of B. purificata
After 72 h of exposure to B. purificata, the mortality rate was 11.11% in the control group, 22.22% in 1 ind/L and 5 ind/L groups, 38.89% in 10 ind/L group, 44.44% in 20 ind/L group, and 88.89% in 40 ind/L and 80 ind/L groups.
The concentration of P. canaliculata culture solution was logarithmically transformed and construct a nonlinear model with the death rate of B. purificata by Four-parameter Logistic model method. The fitting curve was showed in Fig. 1, and the median lethal concentration (LC50) was 23.76 ind/L. After 72 h-exposure, the mortality of snail was increased with the culture solution concentrations of P. canaliculata, reaching a peak mortality at 80 ind/L (88.89%), only about 10% of the experimental individuals could survive under the high concentration of culture solution exposure.
Fig. 1.
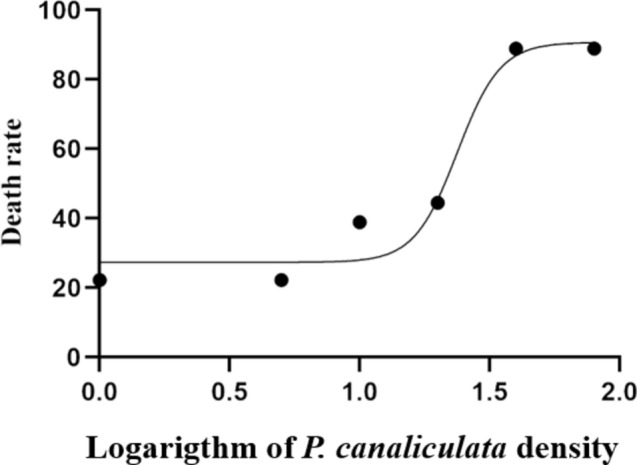
The effects of P. canaliculata culture solution on the death rate of B. purificata after exposed for 72 h.
Effect of P. canaliculata culture solution on histology in gonads and hepatopancreas
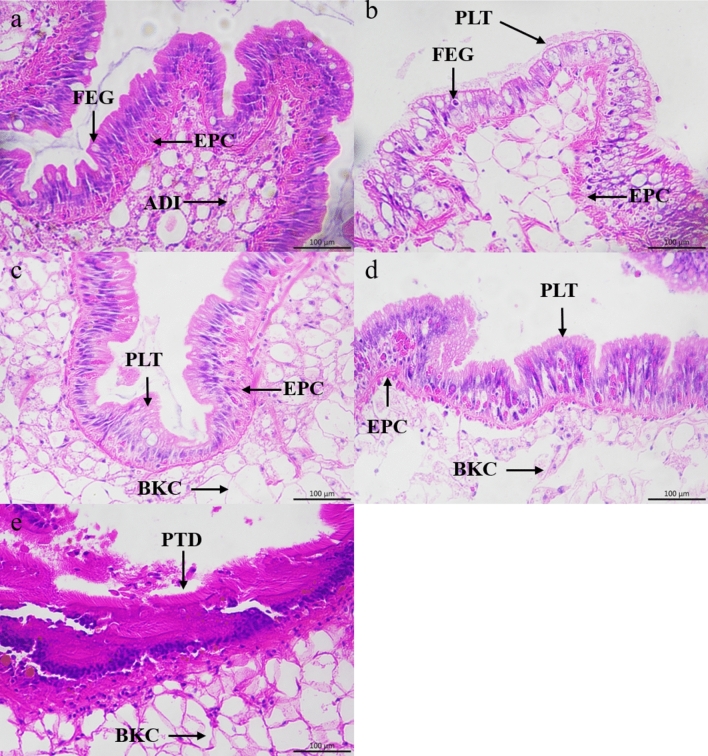
As shown in Fig. 2, the gonad of B. purificata was mainly composed of adipocytes, epithelial cell and placental tissues. The adipocytes were enveloped by epithelial cells, and the placental tissues covered them. The fertilized eggs were attached to the placental tissues and developed into larvae before being discharged into the tissue cavity (Fig. 2). In the control group, the placental tissues were arranged tightly and the boundary between villus and adipocytes was clear (Fig. 2a). After exposed in the culture solution of P. canaliculata, the adipocytes gradually evacuated under the treatment of P. canaliculata culture solution at 1 ind/L (Fig. 2b) and 5 ind/L (Fig. 2c), resulting in edema formation. Under the treatment of 5 ind/L and 10 ind/L group, the adipocytes in the hepatopancreas have been broken (Fig. 2c,d). Treatment with the highest concentration of 20 ind /L led to dissolution of the placental tissues (Fig. 2e), indicating that gonadal rupture of B. purificata and impaired attachment of fertilized egg to the villi tissue, resulting in reproductive disorders.
Fig. 2.
Gonadal sections of B. purificata under different concentrations of P. canaliculata culture solution. (a) Control group, (b–e) 1 ind/L, 5 ind/L, 10 ind/L and 20 ind/L treatment group, respectively. PLT Placental tissues, EPC Epithelial cell, ADI Adipocytes, FEG Fertilized eggs, BKC Broken cell, PTD Placental tissues dissolution.
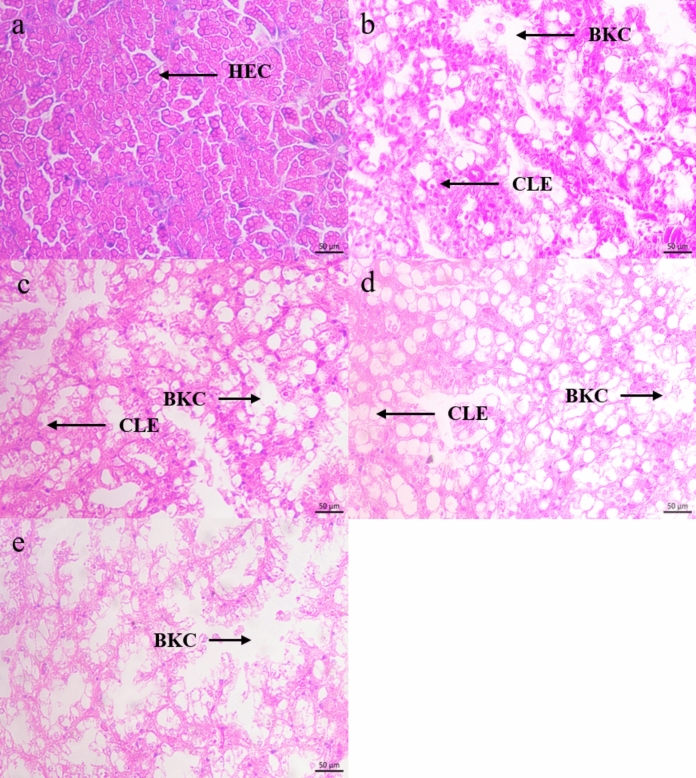
With the increase concentration of P. canaliculata culture solution, the hepatopancreas cells of B. purificata gradually appeared edema until rupture (Fig. 3). In the control group, the intracellular and extracellular boundaries of the hepatopancreas cells were clear and the cell size was equal and arranged neatly (Fig. 3a). Under the treatment of 1 ind/L, 5 ind/L and 10 ind/L P. canaliculata culture solution, the hepatopancreas cells showed edema, and the cell burst led to the appearance of vacuoles. When the concentration reached 20 ind/L, the tissue was necrotic in a large area, and it was difficult to find the complete cell morphology under the microscope.
Fig. 3.
Hepatopancreas sections of B. purificata under different concentrations of P. canaliculata culture solution. (a) Control group, (b–e) 1 ind/L, 5 ind/L, 10 ind/L and 20 ind/L treatment group, respectively. HEC Hepatopancreas cells, BKC Broken cell, CLE Cellular edema.
Effects of the P. canaliculata culture solution on the antioxidant system of B. purificata
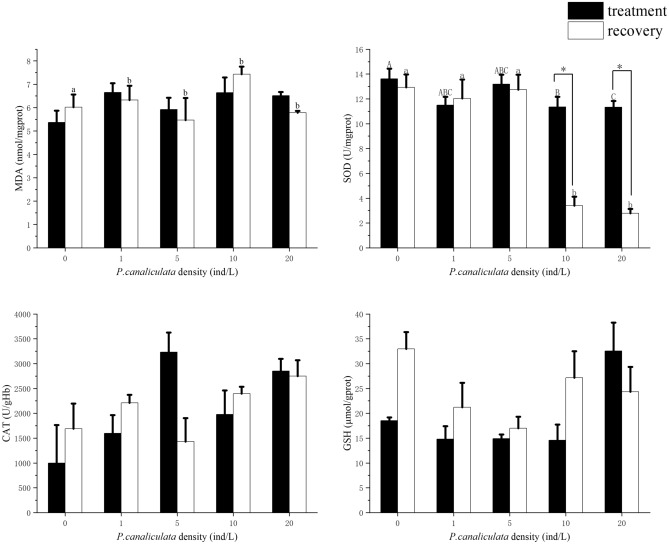
MDA content of B. purificata increased after treatment with P. canaliculata culture solution without significance (P > 0.05). After B. purificata was recovered in sterile water for 24 h, the amount of MDA varied among all concentration of culture fluid (P < 0. 05, F = 1.132), and the highest amount appeared in the recovery group of 10 ind/L (7.44 nmol/mg prot) (Fig. 4a).
Fig. 4.
Antioxidant enzyme activity and lipid peroxidation of B. purificata in treatment and recovery group. (a) MDA. (b) SOD. (c) CAT. (d) GSH. Different concentrations of treatment group and control group were analyzed by ANOVA. Different capital letters represent significant differences between the treatment groups (P < 0.05), and different lowercase letters represent significant differences between the recovery groups (P < 0.05). * Means the statistical differences P < 0.05 between the treatment group and recovery group, statistics by double-tailed paired samples Student ‘s t test.
SOD activity of B. purificata was inhibited in high concentration group at 10 ind/L and 20 ind/L, and there was a significant difference between the control group to 10 ind/L group and 20 ind/L group (P < 0.05, F = 2.401). After recovered in sterile water for 24 h, the activity of SOD in control group was significantly higher than the 10 ind/L group and 20 ind/L (P < 0.05, F = 14.338) group and the activity of recovery group was significant lowered than the treatment group (P < 0.05). The activity of SOD in B. purificata hepatopancreas was inhibited after exposure to 10 and 20 ind/L culture solution of P. canaliculata, and this inhibition persisted after the disappearance of the stimulation source (Fig. 4b).
After 72 h exposure to P. canaliculata culture solution, the highest CAT activity was observed in the treatment group at 5 ind/L (3232.30 U/g Hb) (Fig. 4c). Under the treatment of P. canaliculata culture solution, CAT responded more active than SOD. The activity of GSH did not change significantly during acute exposure to P. canaliculata culture solution (Fig. 4d), the highest value of GSH appeared at 20 ind/L (32.57 μmol/g prot). After 24 h of recovery in sterile water, except for 20 ind/L, the GSH activities of the other groups were higher than that of the treatment group.
Intestinal microbial diversity analysis
A total of 3419 operational taxonomic units (OTUs) were observed in T0, T5 T20 groups and a total of 2957 OTUs were observed in R0, R5, R20 groups. The OTUs of recovery group was lower than the treatment group, and in the R20 group it was significantly lower than the T0 group (P < 0.05). The indexes of Simpson, Shannon and Chao 1 were used to evaluate the abundance and diversity of the microbiota (Table 1). The diversity and abundance of intestinal flora of B. purificata decreased after exposure to the culture solution of P. canaliculata. The Simpson index of T5 group and R20 group was significantly lowered than that of T0 group. The Chao 1 index was decreased with the increasing concentration of P. canaliculata, the lowest value of the Shannon and Chao 1 index was appeared in the R20 group, which was significantly different from the T0 group (P < 0.05).
Table 1.
Intestinal microbial diversity of B. purificata under different treatments. Different letters represent the significant difference (P < 0.05) between the groups.
| Group | OTUs | Simpson | Shannon | Chao1 |
|---|---|---|---|---|
| T0 | 934.333 ± 329.333a | 0.955 ± 0.068a | 7.368 ± 1.619a | 936.625 ± 331.625a |
| T5 | 471.667 ± 232.333ab | 0.613 ± 0.366b | 3.278 ± 2.371bc | 474.707 ± 294.097a |
| T20 | 354.333 ± 91.333ab | 0.867 ± 0.059ab | 4.584 ± 1.085abc | 355.235 ± 90.985ab |
| R0 | 899.000 ± 430.000a | 0.915 ± 0.131ab | 6.908 ± 1.888ab | 905.679 ± 430.54a |
| R5 | 720.000 ± 478.000ab | 0.908 ± 0.122ab | 6.194 ± 2.455abc | 727.821 ± 485.678ab |
| R20 | 253.667 ± 260.333b | 0.569 ± 0.346b | 2.739 ± 2.496c | 236.657 ± 278.281b |
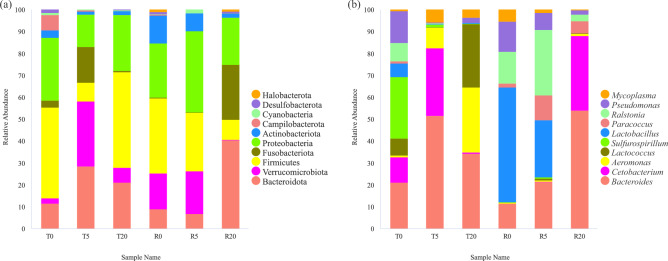
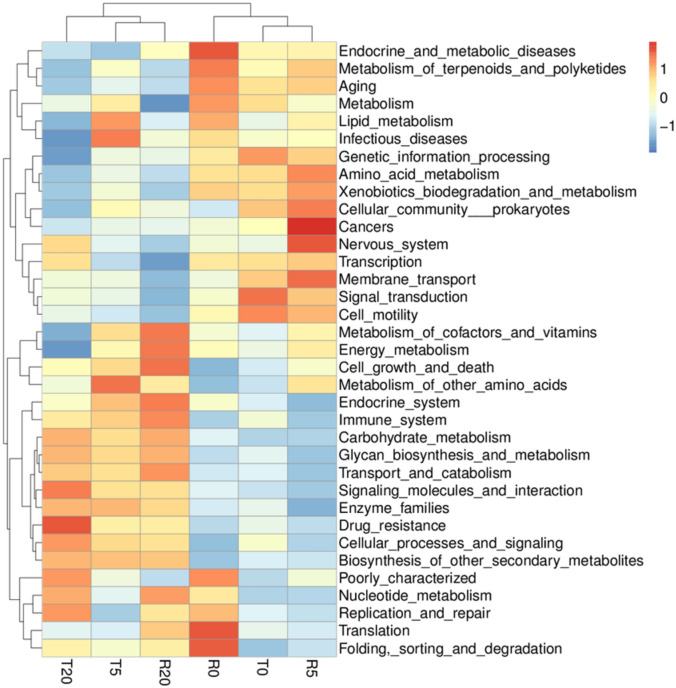
While, P. canaliculata culture solution exposure and the recovery treatment have a great impact on the composition of the intestinal microbiota of B. purificata (Fig. 5). Firmicutes, Proteobacteria, Bacteroidota and Campilobacterota are the most abundant bacteria in T0 in the phylum level. The abundance of Bacteroidota, Verrucomicrobiota and Fusobacteriota are increased in T5 group. Firmicutes and Bacteroidota are increased in T20, but the abundance of Fusobacteriota is decreased. Verrucomicrobiota had the lowest content in T0, increased abundance in T5, and maintained high abundance in R5.
Fig. 5.
The composition of intestinal symbiotic bacteria of B. purificata under different treatments. (a) Phylum, (b) genus, N = 3.
Bacteroides maintained a low relative abundance in T0 group, R0 group and R5 group, but the relative abundance in T5 group, T20 group and R20 group was higher. The highest relative of Sulfurospirillum abundance was in T0 group. The relative abundance of Cetobacterium increased in T5 group and decreased in T20 group, it was completely reversed after recovery. And the relative abundance of Lactobacillus was the highest in R0 group and decreased in R5 group. Lactococcus had the highest relative abundance in T20 group.
As shown in Fig. 6, the treatment of P. canaliculata culture solution inhibited the metabolic function of B. purificata with a concentration-dependent effect, and the effect recovered poorly after the inhibition was relieved. In the control group, the immune system was not activated, and the immune function was improved with the increase of P. canaliculata concentration. After the invasion was relieved, the recovery effect of the medium concentration group (R5) was better, while the inhibition effect of high concentration group (R20) was persistent. The culture solution of P. canaliculata increase the drug-resistant intestinal microorganisms of B. purificata but does not have a concentration dependent effect, and a faster recovery speed was observed following alleviation of invasion. Exposure to the culture solution of P. canaliculata increased the abundance of bacteria related to cell growth and death in the intestine of B. purificata. After being recovered in sterile water for 24 h, the abundance of those bacteria decreased in the low concentration group and increased in the high concentration group.
Fig. 6.
Predictive analysis of the function of the intestinal microbiota Tax4fun in B. purificata under different treatments.
Discussion
P. canaliculata is widely distributed in freshwater ecosystems in China, accounting for 40–50% of the snail population. In paddy fields invaded by P. canaliculata, the average density of snails can reach 8.8 ind/m239. The outbreak density of P. canaliculata directly determines the destructive impact on the ecological environment40. In this study, the survival rate of B. purificata was significantly inhibited by the exposure of P. canaliculata culture solution for 72 h. By fitting the curve, exposure to P. canaliculata culture solution with a concentration of 23.76 ind/L for 72 h resulted in a 50% mortality rate for B. purificata. In the experiment, the mortality of B. purificata reached 88.89% under the treatment of 80 ind/L culture solution. According to the fitting curve, we speculated that the mortality reached 100% when the concentration reached 90.7 ind/L according to the fitting curve (Fig. 1). Although the outbreak density of P. canaliculata in the actual aquatic environment does not reach the LD50, the long-term exposure of P. canaliculata can still pose a risk to native near-niche species and other aquatic organisms41. Furthermore, the P. canaliculata culture solution caused obvious pathological changes in the gonad and hepatopancreas of B. purificata, resulting in vacuolization, villus cell degeneration, and extensive tissue necrosis in the gonads (Fig. 2). Gonadal damage in B. purificata led to the decrease of reproductive capacity, affecting the reproduction of native snail populations, and further augmenting the population competitive advantage of P. canaliculata in the ecological environment. Gonad is an important target of toxicity, the destruction of its tissue structure resulting in impaired individual reproductive ability, which often used to control the population of mollusks42. Hepatopancreas serves as the primary detoxification organ in mollusks and play a crucial role in defending against oxidative damage37. Studies have demonstrated that microcystins can trigger a significant production of reactive oxygen species (ROS), resulting in the disorganization and damage of hepatopancreas cells in Sinotaiahistrica43,44. These findings are consistent with the outcomes of our study, suggesting that the culture solution of P. canaliculata may induce the accumulation of ROS in native snail cells, which subsequently causes hepatopancreas damage.
At present, it remains unclear the mechanism of P. canaliculata on native species, triggering oxidative stress and antioxidative damage may be the most common pathway. MDA is a byproduct of cell membranes lipid peroxidation and frequently used as a marker of cell membrane damage caused by oxidative stress45, which is a sensitive indicator of lipid oxidation. In this study, the contents of MDA in treatment groups were higher than that in the control group without statistical significance. The treatment of P. canaliculata culture solution induced the lipid peroxidation in B. purificata, resulting in the accumulation of intracellular ROS and lipid peroxidation of cell membrane, which may further lead to hepatopancreas cell swelling and damage to the cell membrane of B. purificata46. In this study, compared with the control group, the high concentration culture solution of P. canaliculata had significantly inhibit SOD activity. The activity of antioxidant enzymes was inhibited under the treatment of P. canaliculata culture solution at high concentrations Furthermore, GSH reached the highest value in the high concentration group of 20 ind/L. GSH serves as the primary substance responsible for eliminating reactive oxygen species in the non-enzymatic system, playing an important role in scavenging free radicals and reactive oxygen species47. The findings suggest that B. purificata activates the non-enzymatic system as a response to oxidative damage when exposed to a high concentration of P. canaliculata culture solution. At the same time, except 20 ind/L group, GSH concentration of the recovery groups appeared higher than that in treatment groups under the same P. canaliculata density. The high level of GSH usually meansstrong antioxidant capacity in cells38. However, when B. purificata is treated with a high concentration of P. canaliculata culture solution, the antioxidant capacity of GSH was destroyed and hard to be restored after recovery. These results indicate that the oxidative damage caused by P. canaliculata culture solution to B. purificata is persistent, and the antioxidant enzyme system is inhibited under high concentration exposure, and the function of non-enzyme system is also damaged.
Intestinal microorganisms play a crucial role in maintaining the intestinal health of the host, which helps in host metabolism, maintaining normal function of the digestive and immune system, and protecting against the toxicity of pathogenic bacteria48. When the external environment undergoes adverse changes, the structure and abundance of the intestinal microbial community can be changed. These changes may disrupt the host's intestinal community, making the host more susceptible to disease, intensifying inflammatory responses, and even jeopardizing the survival of the host49,50. Intestinal flora is highly correlated with the occurrence and development of inflammatory reaction, which can disrupt the balance of intestinal flora, potentially causing further damage51. Moreover, the microbiota also serves as the primary barrier of intestinal immunity, significantly impacting the state of the host body52–54. The intestinal flora of mollusks is highly correlated with the surrounding environment. Exposure to P. canaliculata culture solution reduces the biodiversity of the intestinal microbiota of B. purificata and causes the imbalance of intestinal microbial community structure55. Additionally, exposure to P. canaliculata culture solution elevates the relative abundance of Aeromonas in the intestine of B. purificata, making it as one of the dominant microbial groups. Aeromonas is a common opportunistic pathogen that can cause in humans and other aquatic organisms, leading to multisystem inflammation, hematological diseases, and even death56. The exposure to P. canaliculata culture solution increased the relative abundance of Aeromonas in the intestine of B. purificata, making it one of the most abundant microbial groups in B. purificata. It suggests a potential correlation between the colonization of opportunistic pathogens and the composition of P. canaliculata secretions. The T5 group and T20 group had a relatively high abundance of Bacteroides, which was highly correlated with the host’s intestinal immunity and homeostasis and immune system development57. Exposure to P. canaliculata stimulated the proliferation of Bacteroides, causing stress in B. purificata, and initiating the immune defense function of intestinal flora. The abundance of intestinal microorganisms in snails is strongly linked to nutrients metabolism. The higher relative abundance of Lactococcus in the intestinal tract of T20 group. Lactococcus is a kind of common intestinal probiotics, which can convert xylose in plants into lactic acid and help the host to resist oxidative damage58. It suggests that the invasion of P. canaliculata may alter the nutrient reception and metabolic pathways of the native species55. According to the predictions regarding relative abundance and microbial function, exposure to P. canaliculata appears to enhance the resistance of intestinal microbiota of B. purificata, which may lead to the enrichment of resistance genes in B. purificata and even the dissemination of drug resistance.
Conclusion
In present study, the culture solution of P. canaliculata exerts toxical effect on B. purificata, with a half lethal dose of 23.76 ind/L, which provides a theoretical basis for the harmful outbreak density of P. canaliculata in freshwater environment. Oxidative damage is the main response mechanism of P. canaliculata culture solution on B. purificata, which may lead to tissue damage. And gonadal damage further reduces the reproductive capacity of B. purificata and lead to a decrease in population. Additionally, it also disturbs the diversity of intestinal microbiota and elevates the abundance of pathogenic bacteria which accelerated the death of B. purificata. The existence of P. canaliculata in aquatic environment indirectly affects the survival of B. purificata population by inducing oxidative damage and destroying the stability of intestinal flora. This study enriches the research on the invasion strategy of P. canaliculata and highlights a new idea for the management of P. canaliculata invasion.
Acknowledgements
We sincerely acknowledge Miss Na Li and Mr. Pengfei Ma for their their invaluable cooperation in preparing this research before they graduated from Dalian Ocean University.
Author contributions
All the authors have contributed to the conception and design of the study. Dr. Q.Z. and J.L. proposed the initial idea of this study. C.S. and M.L. conducted the experimental design and completed the main experiment of this study. The first draft of this manuscript was written by C.S. S.C., B.W. and T.Z. made figures in the manuscript. S.C., R.H. and W.Z. were responsible for the statistical analysis of the data. Y.W., W.Z. and Z.Q. searched and checked the references and proofread all the words in the manuscript. Sponsorship support for this study was provided by Professor Y.L., Dr. Q.Z. and Dr. J.L. Before the manuscript was uploaded, all authors have already read the manuscript and expressed their opinions. All authors read and approved the final manuscript.
Funding
This study was funded by Modern Agro-industry Technology Research System (CARS-49), Innovation Support Program for High-level Talents of Dalian City (2019RD12), Basic scientific research project of Education Department of Liaoning province (LJKMZ20221107), Scientific Research Joint Fund of Liaoning Province (2023-MSLH-007), the Overseas Training Program for Innovation Team of Liaoning Province (201818), Innovation and Entrepreneurship Project for High-Level Talents of Dalian (2020RQ109), the Project of the Educational Department of Liaoning Province (No. LJ2020012), Laboratory Animal Domestication of Lampetra reissneri (JYTMS20231060).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
All authors declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Responses of survival, antioxidant system and intestinal microbiota of native snail Bellamya purificata to the invasive snail Pomacea canaliculata”.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qian Zhang, Email: zhangqian726@sina.com.
Jun Li, Email: lj_bio@lnnu.edu.cn.
References
- 1.MEA. Millenium Ecosystem Assessment: Ecosystems and Human Well-Being—Synthesis (2005).
- 2.Pimentel, D., Zuniga, R. & Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ.52, 273–288. 10.1016/j.ecolecon.2004.10.002 (2005). 10.1016/j.ecolecon.2004.10.002 [DOI] [Google Scholar]
- 3.Xu, H. et al. The distribution and economic losses of alien species invasion to China. Biol. Invasions8, 1495–1500. 10.1007/s10530-005-5841-2 (2006). 10.1007/s10530-005-5841-2 [DOI] [Google Scholar]
- 4.McMichael, A. J. & Beaglehole, R. The changing global context of public health. Lancet356, 495–499. 10.1016/S0140-6736(00)02564-2 (2000). 10.1016/S0140-6736(00)02564-2 [DOI] [PubMed] [Google Scholar]
- 5.Simberloff, D. et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol.28, 58–66. 10.1016/j.tree.2012.07.013 (2013). 10.1016/j.tree.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 6.Garnas, J. R., Augerrozenberg, M. A. & Roques, A. The 100th of the world’s worst invasive alien species (2016).
- 7.Martín, P., Seuffert, M. E., Tamburi, N. E., Burela, S. & Saveanu, L. Behaviour and ecology of Pomacea canaliculata from Southern Pampas (Argentina). In Biology and Management of Invasive Apple Snails (eds Joshi, R. C. et al.) 241–256 (PhilRice, 2017). [Google Scholar]
- 8.Giraud-Billoud, M., Abud, M. A., Cueto, J. A., Vega, I. A. & Castro-Vazquez, A. Author’s personal copy Uric acid deposits and estivation in the invasive apple-snail, Pomacea canaliculata. Comp. Biochem. Physiol. Part A Physiol.158, 506–512 (2011). 10.1016/j.cbpa.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 9.Eriko, U. & Yoichi, Y. Antipredator behaviour in response to single or combined predator cues in the apple snail Pomacea canaliculata. J. Molluscan Stud.81, 51–57 (2015). 10.1093/mollus/eyu057 [DOI] [Google Scholar]
- 10.Liu, Q. et al. Regeneration of excised shell by the invasive apple snail Pomacea canaliculata. Mar. Freshw. Behav. Physiol.50, 1–13 (2017). 10.1080/10236244.2016.1261455 [DOI] [Google Scholar]
- 11.Cowie, R. H. Apple Snails (Ampullariidae) as Agricultural Pests: Their Biology, Impacts and Management (CABI Publishing, 2002). [Google Scholar]
- 12.Matsukura, K., Izumi, Y., Yoshida, K. & Wada, T. Cold tolerance of invasive freshwater snails, Pomacea canaliculata, P. maculata, and their hybrids helps explain their different distributions. Freshw. Biol.61, 80–87. 10.1111/fwb.12681 (2016). 10.1111/fwb.12681 [DOI] [Google Scholar]
- 13.Fang, L., Wong, P. K., Lin, L. I., Lan, C. & Qiu, J. W. Impact of invasive apple snails in Hong Kong on wetland macrophytes, nutrients, phytoplankton and filamentous algae. Freshw. Biol.55, 1191–1204. 10.1111/j.1365-2427.2009.02343.x (2010). 10.1111/j.1365-2427.2009.02343.x [DOI] [Google Scholar]
- 14.Morrison, W. E. & Hay, M. E. Feeding and growth of native, invasive and non-invasive alien apple snails (Ampullariidae) in the United States: Invasives eat more and grow more. Biol. Invasions13, 945–955. 10.1007/s10530-010-9881-x (2011). 10.1007/s10530-010-9881-x [DOI] [Google Scholar]
- 15.Naylor, R. L. Invasions in agriculture: Assessing the cost of the golden apple snail in Asia. 443–448 (1996).
- 16.Cowie, R. H. Patterns of introduction of non-indigenous non-marine snails and slugs in the Hawaiian Islands. Biodivers. Conserv.7, 349–368 (1998). 10.1023/A:1008881712635 [DOI] [Google Scholar]
- 17.Kwong, K. L. & Chan, R. The potential of the invasive snail Pomacea canaliculata as a predator of various life-stages of five species of freshwater snails. Malacologia51, 343–356 (2009). 10.4002/040.051.0208 [DOI] [Google Scholar]
- 18.Wright, J. T., Gribben, P. E., Byers, J. E. & Monro, K. Invasive ecosystem engineer selects for different phenotypes of an associated native species. Ecology93, 1262–1268. 10.1890/11-1740.1 (2012). 10.1890/11-1740.1 [DOI] [PubMed] [Google Scholar]
- 19.Zhang, et al. Dietary flexibility aids Asian earthworm invasion in North American forests RID B-4422-2008. Ecology91(7), 2070–2079 (2010). 10.1890/09-0979.1 [DOI] [PubMed] [Google Scholar]
- 20.Cui, B. S., Qiang, H. & An, Y. Spartina alterniflora invasions and effects on crab communities in a western Pacific estuary. Ecol. Eng.37, 1920–1924 (2011). 10.1016/j.ecoleng.2011.06.021 [DOI] [Google Scholar]
- 21.Hulvey, K. B. & Zavaleta, E. S. Abundance declines of a native forb have nonlinear impacts on grassland invasion resistance. Ecology93, 378–388. 10.1890/11-0091.1 (2012). 10.1890/11-0091.1 [DOI] [PubMed] [Google Scholar]
- 22.Chaichana, R. & Sumpan, T. The potential ecological impact of the exotic snail Pomacea canaliculata on the Thai native snail Pila scutata. Scienceasia40, 11–15. 10.2306/scienceasia1513-1874.2014.40.011 (2014). 10.2306/scienceasia1513-1874.2014.40.011 [DOI] [Google Scholar]
- 23.Melotto, A., Manenti, R. & Ficetola, G. F. Rapid adaptation to invasive predators overwhelms natural gradients of intraspecific variation. Nat. Commun.11, 3608. 10.1038/s41467-020-17406-y (2020). 10.1038/s41467-020-17406-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escalas, A. et al. An invasive herbivorous fish (Siganus rivulatus) influences both benthic and planktonic microbes through defecation and nutrient excretion. Sci. Total Environ.838, 156–207. 10.1016/j.scitotenv.2022.156207 (2022). 10.1016/j.scitotenv.2022.156207 [DOI] [PubMed] [Google Scholar]
- 25.Wang, J., Lu, X., Zhang, J., Wei, G. & Xiong, Y. Regulating soil bacterial diversity, community structure and enzyme activity using residues from golden apple snails. Sci. Rep.10, 16302 (2020). 10.1038/s41598-020-73184-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlsson, N. O. L. & Brönmark, C. Size-dependent effects of an invasive herbivorous snail (Pomacea canaliculata) on macrophytes and periphyton in Asian wetlands. Freshw. Biol.51, 695–704 (2010). 10.1111/j.1365-2427.2006.01523.x [DOI] [Google Scholar]
- 27.Vega, I. A., Giraud-Billoud, M., Koch, E., Gamarra, C. & Castro-Vazquez, A. Uric acid accumulation within intracellular crystalloid corpuscles of the midgut gland in Pomacea canaliculata (Caenogastropoda, Ampullariidae). Veliger48, 276–283 (2007). [Google Scholar]
- 28.Gu, Q. H. et al. The perplexing population genetic structure of Bellamya purificata (Gastropoda: Viviparidae): Low genetic differentiation despite low dispersal ability. J. Molluscan Stud.81, 466–475 (2015). 10.1093/mollus/eyv017 [DOI] [Google Scholar]
- 29.Reavell, P. E. A study of the diets of some British freshwater gastropods. J. Conchol.30, 253–271 (1980). [Google Scholar]
- 30.Brnmark, C. & Vermaat, J. E. Complex Fish–Snail–Epiphyton Interactions and Their Effects on Submerged Freshwater Macrophytes (Springer, 1998). [Google Scholar]
- 31.Hou, Y. et al.Effect of Bellamya purificata on Organic Matter Degradation in Surface Sediment as Revealed by Amino Acids (Inter-Research Science Center, 2021). [Google Scholar]
- 32.Liu, F. & Lu, J. Ecological engineering approaches to restoring the aquatic biological community of an urban pond ecosystem and its effects on water quality—A case study of the urban Xixi National Wetland Park in China. Knowl. Manag. Aquat. Ecosyst.422, 24 (2021). 10.1051/kmae/2021024 [DOI] [Google Scholar]
- 33.Liu, M.-Y., Zhang, Y.-J., Chen, N.-F., Ma, H.-N. & Zou, W. Ecological squeezing effect of the invasive species Pomacea canaliculata on the indigenous species Bellamya purificata. Chin. J. Zool.56, 673. 10.13859/j.cjz.202105004 (2021). 10.13859/j.cjz.202105004 [DOI] [Google Scholar]
- 34.Zhou, Q. et al. Predator kairomone triggers sexual reproduction of Daphnia population via increasing population density. Freshw. Biol.1644–1655, 2022. 10.1111/fwb.13969 (2022). 10.1111/fwb.13969 [DOI] [Google Scholar]
- 35.Vega, I. A., Arribére, M. A., Almonacid, A. V., Guevara, S. R. & Castro-Vazquez, A. Apple snails and their endosymbionts bioconcentrate heavy metals and uranium from contaminated drinking water. Environ. Sci. Pollut. Res.19, 3307–3316 (2012). 10.1007/s11356-012-0848-6 [DOI] [PubMed] [Google Scholar]
- 36.Hu, A. et al. Investigation of Pomacea canaliculata and rodent densities and their infection status with Angiostrongylus cantonensis in Nan’ao Island of Guangdong Province. Chin. J. Parasitol. Parasit. Dis.35, 239–245 (2017). [Google Scholar]
- 37.Pinho, G. L. L. et al. Toxic effects of microcystins in the hepatopancreas of the estuarine crab Chasmagnathus granulatus (Decapoda, Grapsidae)—ScienceDirect. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol.135, 459–468. 10.1016/S1532-0456(03)00147-9 (2003). 10.1016/S1532-0456(03)00147-9 [DOI] [PubMed] [Google Scholar]
- 38.Bailly, C. et al. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plantarum97, 104–110 (1996). 10.1111/j.1399-3054.1996.tb00485.x [DOI] [Google Scholar]
- 39.Hu, Q. A. et al. Investigation of Pomacea canaliculata and rodent densities and their infection status with Angiostrongylus cantonensis in Nan’ao Island of Guangdong Province. Chin. J. Parasitol. Parasit. Dis.35, 239–245 (2017). [Google Scholar]
- 40.Marie, K., Dagoc, F., Ayn, M., Penaredondo, E. & Mag-Aso, A. V. Density and fecundity of Pomacea canaliculata (Lamarck, 1822) in selected areas of Mindanao, Philippines: Implications on pest management strategies. Adv. Environ. Biol.9, 154–159 (2015). [Google Scholar]
- 41.Maldonado, M. A. & Martin, P. R. Dealing with a hyper-successful neighbor: Effects of the invasive apple snail Pomacea canaliculata on exotic and native snails in South America. Curr. Zool.65, 11 (2019). 10.1093/cz/zoy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adewunmi, C. & Sofowora, E. Preliminary screening of some plant extracts for molluscicidal activity. Planta Medica39, 57–65. 10.1055/s-2008-1074903 (1980). 10.1055/s-2008-1074903 [DOI] [Google Scholar]
- 43.Xie, L. et al. Inhibitory effect of naringin on microcystin-LR uptake in the freshwater snail Sinotaia histrica—ScienceDirect. Environ. Toxicol. Pharmacol.38, 430–437. 10.1016/j.etap.2014.07.006 (2014). 10.1016/j.etap.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 44.Zaidi, H., Amrani, A., Sedrati, F., Maaref, H. & Nasri, H. Histological and chemical damage induced by microcystin-LR and microcystin-RR on land snail Helix aspersa tissues after acute exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol.1, 2. 10.1016/j.cbpc.2021.109031 (2021). 10.1016/j.cbpc.2021.109031 [DOI] [PubMed] [Google Scholar]
- 45.Mendes, R., Cardoso, C. & Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem.112, 1038–1045. 10.1016/j.foodchem.2008.06.052 (2009). 10.1016/j.foodchem.2008.06.052 [DOI] [Google Scholar]
- 46.Firoze Khan, M. & Wang, Z. D. Environmental agents, oxidative stress and autoimmunity. Curr. Opin. Toxicol.7, 22–27 (2018). 10.1016/j.cotox.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, W. & Chen, Y. Response of juvenile crucian carp (Carassius auratus) to long-term ammonia exposure: Feeding, growth, and antioxidant defenses. J. Freshw. Ecol.26, 563–570 (2011). [Google Scholar]
- 48.Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature10.1038/nature11552 (2012). 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 49.Audebert, C. et al. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci. Rep.6, 25255 (2016). 10.1038/srep25255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science336, 489–493 (2012). 10.1126/science.1219328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wlodarska, M. et al. An integrative view of microbiome–host interactions in inflammatory bowel diseases—ScienceDirect. Cell Host Microbe17, 577–591. 10.1016/j.chom.2015.04.008 (2015). 10.1016/j.chom.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong, M. et al. Growth, body composition, intestinal enzyme activities and microflora of juvenile Jian carp (Cyprinus carpio var Jian) fed graded levels of dietary valine. Aquac. Nutr.10.1111/j.1365-2095.2011.00926.x (2013). 10.1111/j.1365-2095.2011.00926.x [DOI] [Google Scholar]
- 53.Nie, L., Zhou, Q. J., Qiao, Y. & Chen, J. Interplay between the gut microbiota and immune responses of ayu (Plecoglossus altivelis) during Vibrio anguillarum infection. Fish Shellfish Immunol.68, 479–487. 10.1016/j.fsi.2017.07.054 (2017). 10.1016/j.fsi.2017.07.054 [DOI] [PubMed] [Google Scholar]
- 54.Wen, H. et al. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol.40, 275–287. 10.1016/j.fsi.2014.07.004 (2014). 10.1016/j.fsi.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 55.De Schryver, P. & Vadstein, O. Ecological theory as a foundation to control pathogenic invasion in aquaculture. ISME J.8, 2360–2368. 10.1038/ismej.2014.84 (2022). 10.1038/ismej.2014.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michael, J. J. & Abbott, S. L. Evolving concepts regarding the genus Aeromonas: An expanding Panorama of species, disease presentations, and unanswered questions. Clin. Infect. Dis.27, 332–344 (1998). 10.1086/514652 [DOI] [PubMed] [Google Scholar]
- 57.Lanning, D., Sethupathi, P., Rhee, K. J., Zhai, S. K. & Knight, K. L. Intestinal microflora and diversification of the rabbit antibody repertoire. J. Immunol.165, 2012–2019. 10.4049/jimmunol.165.4.2012 (2000). 10.4049/jimmunol.165.4.2012 [DOI] [PubMed] [Google Scholar]
- 58.Mermod, M., Mourlane, F., Waltersperger, S., Oberholzer, A. E. & Solioz, M. Structure and function of CinD (YtjD) of Lactococcus lactis, a copper-induced nitroreductase involved in defense against oxidative stress. J. Bacteriol.192, 4172 (2010). 10.1128/JB.00372-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.