Extract
Long COVID (also known as “post-acute sequelae of COVID-19”) is a multi-system disorder that follows an acute bout of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. Although its exact prevalence is unknown, it is estimated to affect approximately 10% of SARS-CoV-2-infected individuals, though in reality the proportion is likely much higher owing to under-reporting of cases [1]. The prevalence is elevated in patients who have had acute SARS-CoV-2 pneumonia requiring hospitalisation, and lower in those who have been previously vaccinated or were infected with the Omicron variant [1]. In approximately 6% of the cases of long COVID, pulmonary symptoms such as dyspnoea, cough and wheeziness are prominent, leading to considerable disability and morbidity [2, 3]. While it is attractive to view long COVID as one disease, it is likely a very complex, heterogeneous disorder, with multiple different phenotypes, each driven by a unique set of molecules and pathways [1]. Even within an organ system (e.g. the lungs), there is likely to be significant heterogeneity in the phenotypes of disease. Here, we hypothesise that patients with long COVID with a predominance of pulmonary symptoms (which we will refer to in this viewpoint editorial as “pulmonary long COVID”, or PLC) have airway pathology that can be detected using conventional as well as emerging technologies, and careful phenotyping of this condition will provide important insights on its mechanism(s) and reveal novel biomarkers and therapeutic solutions for millions around the world with PLC.
Shareable abstract
A majority of patients with pulmonary long COVID have small airway disease, characterised by inflammation, which can be diagnosed with traditional and emerging technologies https://bit.ly/48WceB3
Introduction
Long COVID (also known as “post-acute sequelae of COVID-19”) is a multi-system disorder that follows an acute bout of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. Although its exact prevalence is unknown, it is estimated to affect approximately 10% of SARS-CoV-2-infected individuals, though in reality the proportion is likely much higher owing to under-reporting of cases [1]. The prevalence is elevated in patients who have had acute SARS-CoV-2 pneumonia requiring hospitalisation, and lower in those who have been previously vaccinated or were infected with the Omicron variant [1]. In approximately 6% of the cases of long COVID, pulmonary symptoms such as dyspnoea, cough and wheeziness are prominent, leading to considerable disability and morbidity [2, 3]. While it is attractive to view long COVID as one disease, it is likely a very complex, heterogeneous disorder, with multiple different phenotypes, each driven by a unique set of molecules and pathways [1]. Even within an organ system (e.g. the lungs), there is likely to be significant heterogeneity in the phenotypes of disease. Here, we hypothesise that patients with long COVID with a predominance of pulmonary symptoms (which we will refer to in this viewpoint editorial as “pulmonary long COVID”, or PLC) have airway pathology that can be detected using conventional as well as emerging technologies, and careful phenotyping of this condition will provide important insights on its mechanism(s) and reveal novel biomarkers and therapeutic solutions for millions around the world with PLC.
Diagnosis of pulmonary long COVID
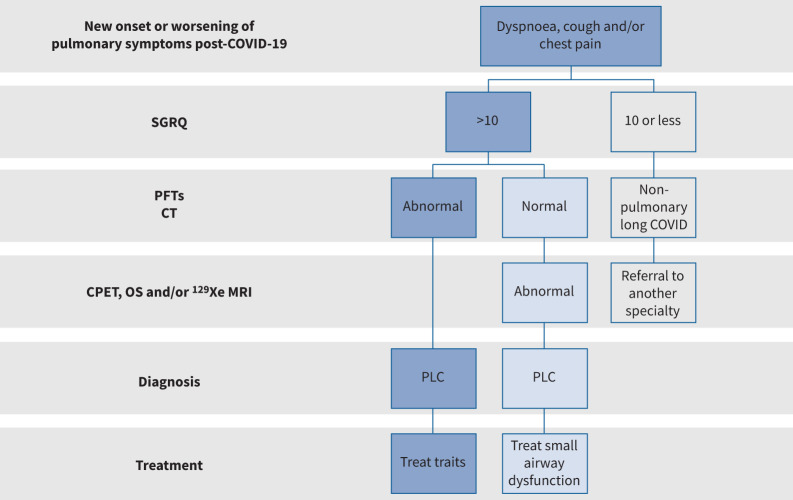
For discussion purposes, we will define PLC as patients who have a new onset or worsening of pulmonary symptoms that persists beyond 12 weeks post-COVID-19. Although technically the St George's Respiratory Questionnaire (SGRQ) is an instrument to measure health status rather than symptom burden, operationally in our clinics, we use a SGRQ total score of >10 to denote “significant” pulmonary symptoms (figure 1) [4]. It should be noted that other well-validated questionnaires, such as MRC [5] or the ATS Symptoms Questionnaire [6], can also be used for this purpose. In figure 1, we have summarised our general diagnostic approach to patients with PLC.
FIGURE 1.
A proposed approach to patients with long COVID and significant pulmonary symptoms. We suggest using a validated instrument such as the St George's Respiratory Questionnaire (SGRQ) to evaluate the symptom burden of patients and their health status. If SGRQ total scores are >10, we recommend pulmonary function testing (PFT) and computed tomography (CT) imaging of the lungs. If they are normal or if the symptoms are disproportionately greater than the CT and PFT abnormalities, we suggest measurement of small airway function using cardiopulmonary exercise testing (CPET), oscillometry (OS) and/or hyperpolarised xenon (129Xe) magnetic resonance imaging (MRI), guided by availability of these tests and local expertise. Significant abnormalities in PFT, CT and/or tests of small airway function would support the diagnosis of pulmonary long COVID (PLC) in symptomatic individuals. Although there is no good evidence for treatment, we recommend a “treatable trait” approach to PLC patients. For example, for patients with significant airflow limitation, we suggest using bronchodilators, and for those with perturbed gas exchange, consider the use of inhaled corticosteroids with or without a bronchodilator. Close follow-up should be instituted as there is good evidence that pulmonary symptoms spontaneously improve over time in some patients.
In our experience, approximately 10% of patients have abnormal pulmonary function tests (PFTs) and/or computed tomography (CT) data, though this proportion decreases over follow-up time [7]. The most common PFT abnormality is a reduced diffusing capacity of the lungs for carbon monoxide (DLCO), followed by a “restrictive” pattern of airflow limitation, characterised by a concomitant reduction in forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC). The most common abnormalities on CT scan are ground glass opacities and linear reticulations, which resolve (in many cases) over time with only a small percentage (∼5–10%) of patients having significant fibrotic changes by year 2 or 3 post-infection [7]. Addition of an expiratory CT may also unveil air trapping that may have been missed on PFTs or inspiratory CT alone [8]. In some cases, CT angiography may be necessary to rule out venous thromboembolic (VTE) disease. However, the overall prevalence of VTE disease is less than 1% of patients with long COVID [9], though it may be higher in patients who have been previously hospitalised for their initial infection [10]. Because CT angiography cannot detect microthrombi in small vessels, many more may have small vessel VTE disease, which may go unnoticed and undiagnosed. For this and other reasons, in most cases, even in patients who demonstrate PFT or CT abnormalities, the pulmonary symptoms are disproportionately greater compared with the severity of the physiological or imaging changes. In a vast majority of patients, both the PFT and CT scans are within normal limits [11].
What should be done when PFTs and CT scans are normal?
For conventional medical CT instruments, the lower limit of resolution is 1–2 mm [12] and most imaging centres routinely perform only inspiratory scans. Thus, subtle abnormalities in the small airways (defined as airways <2 mm), alveoli or pulmonary microcirculation can be missed on CT imaging. Similarly, standard spirometry is not sensitive enough to detect changes in small airways, unless >50% of the airways are lost or become remodelled by disease [13]. A more sensitive test is a cardiopulmonary exercise test (CPET). Ingul et al. [14] showed that 23% of patients who were previously hospitalised with COVID-19 pneumonia demonstrated exercise intolerance (defined by a peak oxygen consumption (V′O2) <80% predicted) at 12 months of follow-up, though they had normal PFTs. Importantly, they showed that patients with persistent dyspnoea at 12 months had 9% lower peak V′O2 compared with individuals without persistent dyspnoea. As many physiological factors contribute to reduced exercise tolerance, the exact aetiology for reduced peak V′O2 in PLC patients remains largely a mystery.
Another diagnostic test for small airways disease is oscillometry, which measures airway resistance and reactance [15]. However, the studies using oscillometry in long COVID patients have been relatively small and the findings have been inconsistent [16, 17]. A more sensitive method of detecting small airways dysfunction is nitrogen multiple-breath washout test (MWT), which measures ventilation inhomogeneity. One study showed that 50% of long COVID patients with normal spirometry demonstrated ventilation inhomogeneity at the level of the small airways, which, in turn, significantly correlated with dyspnoea [18].
More striking data have been generated using hyperpolarised xenon magnetic resonance imaging (129Xe MRI). 129Xe MRI has shown subtle and usually peripheral ventilation abnormalities [19], highlighting the role of small airways dysfunction in long COVID. In addition to identifying ventilation defects, 129Xe MRI can also sensitively detect pulmonary gas exchange abnormalities, as inhaled 129Xe gas dissolves into the interstitial–alveolar membrane and then into pulmonary capillary red blood cells (RBC) [20]. In long COVID patients, many studies have now shown low 129Xe RBC-to-membrane ratio in the absence of any other CT or PFT abnormalities [21–23], likely reflecting microvascular abnormalities, such as pulmonary capillary inflammation, vasoconstrictive remodelling or micro-thrombosis, that inhibit transfer of gas. We have shown that approximately 30% of long COVID patients (all of whom had normal PFTs and inspiratory CT scans) demonstrated reduced 129Xe transfer to RBCs [11]. In another 30% of patients, there is an increase in 129Xe membrane uptake only, possibly reflecting interstitial inflammation or oedema that is not evident on CT. In about 10% of the patients, there is a mix of increase in 129Xe membrane uptake with concomitant reduced RBC transfer. This last group also demonstrate a restrictive pattern of abnormalities on PFTs, and mild interstitial changes on CT (e.g. reticulation, fibrosis), which may indicate post-COVID interstitial lung disease (ILD) [11].
What is the underlying pathophysiological mechanism(s) in the small airways?
In our experience, less than 10% of patients who are referred for PLC assessment at 1–2 years post-COVID-19 infection have abnormal PFTs or CT images that are diagnostic of airways disease or ILD [4, 11]. We thus postulate that patients with PLC who have a significant symptom burden have persistent inflammation in the small airways, which may not be detectable on PFTs and are beyond the resolution of medical CT images. To investigate this possibility, we performed bronchoscopy on patients with PLC (as well as healthy controls) with no prior history of airways disease or a significant smoking history and obtained bronchial brush samples from sixth to eighth generation airways and subjected these cells to single cell RNA sequencing (scRNAseq) [24]. All of the PLC patients were at least 1 year post-acute infection with a median time from COVID-19 of 2 years. The scRNAseq data showed increased number of neutrophils in the airway mucosa, along with increased expression of mucin genes, and an upregulation of interleukin (IL)-33 and T-receptor signalling in the secretory mucosal cells. Transcriptomic pathway analysis of the PLC airway mucosa revealed a pro-inflammatory pattern with enrichment in the neutrophil-associated activation signatures, including neutrophil degranulation [4]. The cell counts in the bronchoalveolar lavage fluid, however, were not significantly different between the PLC and control groups.
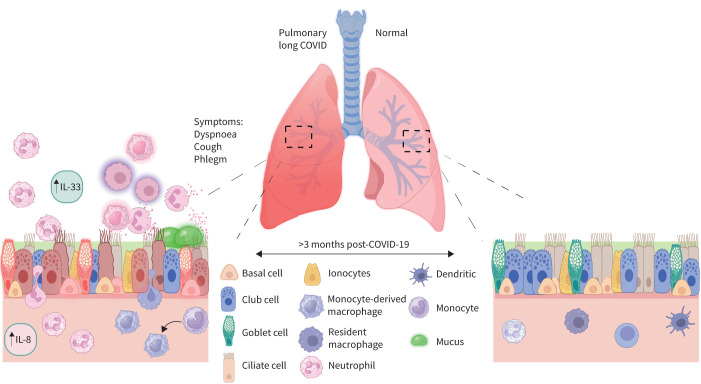
Why patients with PLC have evidence for ongoing small airway inflammation 1–3 years post-acute infection is a mystery. Son et al. [25] have suggested the generation of autoimmune antibodies in some individuals following their acute bout of SARS-CoV-2 infection. Whether these auto-antibodies deposit in the small airways and fuel the inflammatory process is not known. Another possibility is that the acute COVID-19 pneumonia may alter the airway microbiome [26], leading to a pro-inflammatory micro-milieu [18]. We also speculate that there may be “re-programming” of the airway progenitor cells (e.g. basal cells) during acute infection in some patients, leading to increased production and release of epithelial-derived cytokines (i.e. alarmins), including IL-33, IL-25 and/or thymic stromal lymphopoietin, that propagates the inflammatory process (figure 2). These and other possibilities should be tested in future studies. In figure 2, we provide a summary of the proposed inflammatory changes in the small airways of patients with PLC.
FIGURE 2.
A proposed small airway landscape in the pulmonary long COVID mucosa (left) compared to a healthy mucosa (right). Secretory cell expression of interleukin (IL)-8 and other cytokines leads to infiltration of neutrophils into the airway lumen from the vasculature. Upregulation of IL-33 by club cells further promotes a pro-inflammatory cascade consisting of activation and recruitment of neutrophils, infiltration of monocytes and their differentiation into tissue macrophages, among other changes. Enrichment in the neutrophil-associated activation signature results in the increase of mucin gene expression in the secretory cells, and consequently excess mucus production by these cells.
Where do we go from here?
We believe that patients with a significant PLC burden (e.g. SGRQ total score >10) should undergo full PFTs, including DLCO, and co-registered inspiratory/expiratory CT scans. In those who have normal findings or whose symptoms are disproportionately greater than would be expected based on their PFTs or CT scan, we recommend CPET, oscillometry, MWT and/or 129Xe MRI to detect small airways disease and impairments in gas exchange (figure 1). However, it should be noted these tests are often expensive and may not be available in certain settings. Moreover, their performance (e.g. sensitivity and specificity) in long COVID has not been established. As there are no robust cost-effectiveness analyses or data on their performance in the real-world for any of these diagnostic tests in PLC, these should be implemented based on local availability and resources. For management, we suggest a “treatable trait” approach to PLC. Significant airflow limitation can be treated with bronchodilators with or without inhaled corticosteroids. If the abnormality is predominantly in the small airways, we suggest using inhalers that are capable of generating particles or aerosols of sufficient size to penetrate the small airways. Patients with significant ILD changes should be referred to a multidisciplinary ILD clinic for assessment, similar to other ILD patients. With greater understanding of the pathophysiology of PLC, more targeted (and better) treatments can be developed. Until then, a “treatable trait” approach with close follow-up (as patient symptoms may spontaneously improve over time) is suggested.
Shareable PDF
Footnotes
Conflict of interest: F.V. Gerayeli has no potential conflicts of interest to disclose. R.L. Eddy reports grants or contracts from Michael Smith Health Research BC, and Canadian Respiratory Research Network, consulting fees from VIDA Diagnostics Inc, and an honorarium for a presentation from Thorasys Thoracic Medical Systems, outside the submitted work. D.D. Sin reports honoraria from GSK, AZ, and BI, outside the submitted work; and is chair of a data and safety monitoring board for a COPD clinical trial for NHLBI.
Support statement: This work was supported by the Canadian Institutes of Health Research (CIHR); D.D. Sin is a Tier 1 Canada Research Chair in COPD and de Lazzari Family Chair Holder. R.L. Eddy was supported by a Michael Smith Health Research BC trainee award and a Canadian Respiratory Research Network postdoctoral fellowship.
References
- 1.Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023; 21: 133–146. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowe B, Xie Y, Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat Med 2023; 29: 2347–2357. doi: 10.1038/s41591-023-02521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 2022; 28: 1706–1714. doi: 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerayeli FV, Park HY, Milne S, et al. Single cell sequencing reveals cellular landscape alterations in the airway mucosa of patients with pulmonary long COVID. Eur Respir J 2024; in press [ 10.1183/13993003.01947-2023]. [DOI] [Google Scholar]
- 5.Medical Research Council's Committee on the Aetiology of Chronic Bronchitis . Standardized questionaries on respiratory symptoms. Br Med J 1960; 2: 1665.13688719 [Google Scholar]
- 6.Comstock GW, Tockman MS, Helsing KJ, et al. Standardized respiratory questionnaires: comparison of the old with the new. Am Rev Respir Dis 1979; 119: 45–53. [DOI] [PubMed] [Google Scholar]
- 7.Guinto E, Gerayeli FV, Eddy RL, et al. Post-COVID-19 dyspnoea and pulmonary imaging: a systematic review and meta-analysis. Eur Respir Rev 2023; 32: 220253. doi: 10.1183/16000617.0253-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho JL, Villacreses R, Nagpal P, et al. Quantitative chest CT assessment of small airways disease in post-acute SARS-CoV-2 infection. Radiology 2022; 304: 185–192. doi: 10.1148/radiol.212170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah B, Ahmad MN, Khalid M, et al. Long COVID and wavering incidence of pulmonary embolism: a systematic review. J Community Hosp Intern Med Perspect 2023; 13: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology 2021; 298: E70–E80. doi: 10.1148/radiol.2020203557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddy RL, Mummy D, Zhang S, et al. Cluster analysis to identify long COVID phenotypes using 129Xe magnetic resonance imaging: a multicentre evaluation. Eur Respir J 2024; 63: 2302301. doi: 10.1183/13993003.02301-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washko GR, Parraga G. COPD biomarkers and phenotypes: opportunities for better outcomes with precision imaging. Eur Respir J 2018; 52: 1801570. doi: 10.1183/13993003.01570-2018 [DOI] [PubMed] [Google Scholar]
- 13.Koo HK, Vasilescu DM, Booth S, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med 2018; 6: 591–602. doi: 10.1016/S2213-2600(18)30196-6 [DOI] [PubMed] [Google Scholar]
- 14.Ingul CB, Edvardsen A, Follestad T, et al. Changes in cardiopulmonary exercise capacity and limitations 3–12 months after COVID-19. Eur Respir J 2023; 61: 2200745. doi: 10.1183/13993003.00745-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020; 55: 1900753. doi: 10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 16.Tamminen P, Kerimov D, Viskari H, et al. Lung function during and after acute respiratory infection in COVID-19 positive and negative outpatients. Eur Respir J 2022; 59: 2102837. doi: 10.1183/13993003.02837-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaramuzzo G, Ronzoni L, Campo G, et al. Long-term dyspnea, regional ventilation distribution and peripheral lung function in COVID-19 survivors: a 1 year follow up study. BMC Pulm Med 2022; 22: 408. doi: 10.1186/s12890-022-02214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merenstein C, Bushman FD, Collman RG. Alterations in the respiratory tract microbiome in COVID-19: current observations and potential significance. Microbiome 2022; 10: 165. doi: 10.1186/s40168-022-01342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooner HK, McIntosh MJ, Matheson AM, et al. Postacute COVID-19 syndrome: 129Xe MRI ventilation defects and respiratory outcomes 1 year later. Radiology 2023; 307: e222557. doi: 10.1148/radiol.222557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Bier EA, Swaminathan A, et al. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J 2019; 54: 1900831. doi: 10.1183/13993003.00831-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grist JT, Collier GJ, Walters H, et al. Lung abnormalities detected with hyperpolarized 129Xe MRI in patients with long COVID. Radiology 2022; 305: 709–717. doi: 10.1148/radiol.220069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matheson AM, McIntosh MJ, Kooner HK, et al. Longitudinal follow-up of postacute COVID-19 syndrome: DLCO, quality-of-life and MRI pulmonary gas-exchange abnormalities. Thorax 2023; 78: 418–421. doi: 10.1136/thorax-2022-219378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders LC, Collier GJ, Chan HF, et al. Longitudinal lung function assessment of patients hospitalized with COVID-19 using 1H and 129Xe lung MRI. Chest 2023; 164: 700–716. doi: 10.1016/j.chest.2023.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerayeli FV, Milne S, Yang CX, et al. Single-cell RNA sequencing of bronchoscopy specimens: development of a rapid minimal handling protocol. Biotechniques 2023; 75: 157–167. doi: 10.2144/btn-2023-0017 [DOI] [PubMed] [Google Scholar]
- 25.Son K, Jamil R, Chowdhury A, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J 2023; 61: 2200970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie L, Chen L, Li X, et al. Analysis of lung microbiome in COVID-19 patients during time of hospitalization. Pathogens 2023; 12: 944. doi: 10.3390/pathogens12070944 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02302-2023.Shareable (278.7KB, pdf)