Abstract
Background
Benralizumab induces rapid and near-complete depletion of eosinophils from blood and lung tissue. We investigated whether benralizumab could attenuate the allergen-induced late asthmatic response (LAR) in participants with allergic asthma.
Methods
Participants with allergic asthma who demonstrated increased sputum eosinophils and LAR at screening were randomised to benralizumab 30 mg or matched placebo given every 4 weeks for 8 weeks (3 doses). Allergen challenges were performed at weeks 9 and 12 when blood, sputum, bone marrow and bronchial tissue eosinophils and LAR were assessed.
Results
46 participants (mean age 30.9 years) were randomised to benralizumab (n=23) or placebo (n=23). Eosinophils were significantly reduced in the benralizumab group compared with placebo in blood at 4 weeks and sputum and bone marrow at 9 weeks after treatment initiation. At 7 h after an allergen challenge at week 9, sputum eosinophilia was significantly attenuated in the benralizumab group compared to placebo (least squares mean difference −5.81%, 95% CI −10.69– −0.94%; p=0.021); however, the LAR was not significantly different (least squares mean difference 2.54%, 95% CI 3.05–8.12%; p=0.363). Adverse events were reported for seven (30.4%) and 14 (60.9%) participants in the benralizumab and placebo groups, respectively.
Conclusion
Benralizumab administration over 8 weeks resulted in a significant attenuation of blood, bone marrow and sputum eosinophilia in participants with mild allergic asthma; however, there was no change in the LAR, suggesting that eosinophils alone are not a key component of allergen-induced bronchoconstriction.
Shareable abstract
The administration of benralizumab to deplete eosinophils in patients with allergic asthma had no effect on early- or late-phase bronchoconstriction to inhaled allergen, suggesting that eosinophils are not important drivers of these responses https://bit.ly/4crZjIW
Introduction
Asthma is a chronic inflammatory disease characterised by variable airflow limitation [1]. Allergic asthma is more common among younger-onset patients, is triggered by exposure to an allergen and is often accompanied by eosinophilic airway inflammation [1–4].
Benralizumab is an afucosylated, humanised, monoclonal antibody against interleukin-5 receptor α (IL-5Rα). Benralizumab induces a direct, rapid and near-complete depletion of eosinophils and basophils from blood and airway mucosa tissue through enhanced antibody-dependent, cell-mediated cytotoxicity [5, 6]. In patients with severe uncontrolled eosinophilic asthma, benralizumab is well tolerated, is effective at reducing asthma exacerbations, helps to control symptoms when added to maintenance therapy and can reduce the need for systemic corticosteroids [7–9]. Benralizumab can also significantly deplete eosinophil precursor cells in blood, airway mucosa and bone marrow and can significantly reduce basophil counts, likely because these cells express IL-5Rα [5, 10, 11].
Allergen inhalation in allergic asthmatic subjects induces an early asthmatic response (EAR) characterised by bronchoconstriction that peaks within 30 min and resolves in ∼1 h. Some individuals also develop a second, delayed bronchoconstrictor response, known as the late asthmatic response (LAR), which is associated with increased and prolonged airway hyperresponsiveness (AHR) to non-specific direct-acting stimuli (e.g. methacholine) and pronounced airway eosinophilia [12, 13].
An experimental whole lung allergen inhalation challenge (AIC) model has been useful for identifying mechanisms underlying inflammation in allergic asthma and for examining the effects of anti-inflammatory and immunomodulatory agents [14–16]. Studies based on AIC have suggested a possible role for eosinophils in the LAR, including suppression of allergen-induced sputum eosinophils associated with attenuation of the LAR following tezepelumab administration [17] and higher allergen-induced sputum eosinophilia associated with increased LAR after regular short-acting β-agonist (SABA) treatment [18]. However, an anti-IL-5 monoclonal antibody, mepolizumab, administered in participants with allergic asthma, reduced post-challenge eosinophil counts in blood and sputum but had no effect on the LAR [19]. Other allergen challenge studies have revealed increased basophil numbers in bronchial mucosa and altered protein expression patterns on basophils during the LAR [20–22] and increases in basophils and mast cells in sputum during the LAR [23].
Considering the potential involvement of eosinophils and basophils in the allergen-induced LAR, and the depletion of both cell types from circulation by benralizumab, the objective of this study was to determine whether benralizumab treatment could attenuate the allergen-induced LAR.
Methods
Study design and participants
This randomised, double-blind, parallel-group, placebo-controlled, phase 3 trial was conducted at six university hospitals in Canada between 11 October 2016 and 22 October 2019 (ClinicalTrials.gov: NCT02821416).
Participants were aged 18–65 years and had mild, stable, allergic asthma that was treated with only SABAs used no more than twice weekly as needed for control of symptoms. All participants provided written informed consent, and the study was performed in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki after approval by institutional review boards and/or ethics committees at each site.
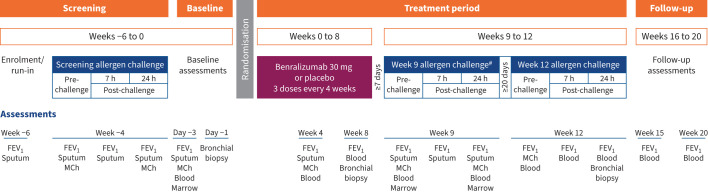
The study design is summarised in figure 1. Eligibility required a positive skin prick test to an aeroallergen, a forced expiratory volume in 1 s (FEV1) ≥70% predicted, a positive methacholine test based on a provocative concentration causing a 20% decrease in FEV1 (PC20) ≤16 mg·mL−1, allergen-induced EAR (maximum FEV1 decrease of ≥20% within 2 h), LAR (maximum FEV1 decrease of ≥15% at 3–7 h) and increased sputum eosinophils 7 h after AIC. Full eligibility criteria and additional details on study methods are provided in the supplementary material. Participants who demonstrated a return to asthma stability were randomised 1:1 to benralizumab 30 mg or placebo by subcutaneous injection at weeks 0, 4 and 8, and AIC was performed at weeks 9 and 12. Methacholine PC20 and sputum, blood, bone marrow and endobronchial measurements were conducted at baseline and before and after allergen challenges during the treatment period. A final visit was scheduled for 12 weeks after the last study treatment for follow-up.
FIGURE 1.
Study schematic. FEV1: forced expiratory volume in 1 s (pre-bronchodilator); MCh: methacholine (assessment for airway hyperresponsiveness). #: primary end-point assessment.
All participants, investigators and study staff were masked to treatment allocation, and eosinophil and basophil counts were redacted from interim reports to prevent investigators from identifying treatment assignments.
Assessments
Spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines [24]. The methacholine inhalation challenge was performed using the 2-min tidal breathing method at 24 h before and after AICs [25], and the PC20 was calculated. Aeroallergen for AIC was selected based on skin sensitivity, and together with methacholine PC20 was used to predict allergen PC20 [26]. During the screening period AIC was conducted by stepwise inhalation of allergen extracts over 2 min of tidal breathing from a Wright nebuliser followed by FEV1 measurement 10 min later. Inhaled allergen concentrations were doubled until a ≥20% fall in FEV1 was reached, and FEV1 was then measured at regular intervals for 7 h to calculate the area under the curve (AUC) of FEV1–time response from 0 to 2 h (EAR) and 3 to 7 h (LAR). During the treatment period, AIC was conducted using the highest three concentrations of allergen inhaled during screening. The challenge was stopped after one or two concentrations only if the participant had already reached a level of bronchoconstriction making it unsafe to administer the full dose. Sputum eosinophils were assessed 24 h before and at 7 and 24 h after AIC.
Sputum, blood, bone marrow and endobronchial biopsies were collected to evaluate eosinophil and basophil counts, and levels of IL-5, eotaxin-1, eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP). Safety was based on the frequency of treatment-emergent adverse events (TEAEs) according to the Medical Dictionary for Regulatory Activities (MedDRA; version 22.0).
End-points
Co-primary end-points were the allergen-induced change in percentage sputum eosinophils at 7 h after the allergen challenge and the maximum percentage decrease in FEV1 during the LAR at week 9. Secondary end-points included changes in methacholine PC20, EAR and eosinophil and basophil counts in sputum, blood, bone marrow and endobronchial tissue at 24 h after the allergen challenge.
Statistical analysis
Analyses were performed on the full/safety set (randomised participants who received at least one dose of study drug) and the primary efficacy set (those in the full analysis set who also received the same allergen dose at screening and week 9 AICs). Primary and secondary end-points were compared using a repeated-measures analysis with screening values selected as the covariate. The co-primary end-point of the maximum percentage decrease in FEV1 in LAR3–7 h was assessed using ANCOVA in which the treatment group was an explanatory variable, and the maximum percentage decrease in FEV1 during LAR3–7 h in the screening challenge was a covariate. For both primary end-points, the difference between treatment groups is presented as the least squares (LS) mean difference (95% confidence interval) for benralizumab versus placebo with a two-sided p-value.
For time-based secondary end-points, p-values were determined by using t-tests for the means of each group at each visit, while end-points based on repeated-measures estimates or ANCOVA were performed as described above. Methacholine PC20 was log-transformed prior to analysis. Statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). See the supplementary material for more information.
Results
Of 126 individuals initially screened, 46 met the eligibility criteria and were randomised to treatment with benralizumab 30 mg (n=23) or placebo (n=23) (supplementary figure E2). Demographics and baseline characteristics were generally similar between groups (table 1), and no notable differences in sputum eosinophil counts or lung function were observed in response to AIC during the screening period (supplementary figure E3).
TABLE 1.
Demographic and baseline characteristics
| Benralizumab (n=23) | Placebo (n=23) | |
|---|---|---|
| Age, years | 30.4±12.3 | 31.5±13.3 |
| Female | 14 (60.9) | 20 (87.0) |
| BMI, kg·m−2 | 25.5±4.5 | 27.5±6.3 |
| Race | ||
| White | 16 (69.6) | 19 (82.6) |
| Asian | 6 (26.1) | 3 (13.0) |
| Other | 1 (4.3) | 1 (4.3) |
| Ethnicity | ||
| Hispanic or Latino | 0 | 3 (13.0) |
| Not Hispanic or Latino | 23 (100) | 20 (87.0) |
| Allergy duration, years | 27.8±15.2 | 22.5±11.7 |
| Asthma duration, years | 19.0±14.4 | 18.7±12.2 |
| Lung function | ||
| FEV1, L | 3.24±0.80 | 2.96±0.54 |
| FVC, L | 4.35±1.33 | 3.77±0.69 |
| FEV1/FVC, % | 76.23±11.46 | 78.96±7.65 |
| FEV1, % pred | 88.12±11.36 | 89.61±9.05 |
| FVC, % pred | 97.69±14.01 | 95.78±10.89 |
| Sputum eosinophils | ||
| Cell count, g−1 | 6.76 (0.00–160.08) | 7.77 (0.00–314.60) |
| % | 1.80 (0.0–69.6) | 1.60 (0.0–48.4) |
| Sputum basophils | ||
| Cell count, µg−1 | 0.03 (0.00–1.24) | 0.08 (0.00–1.24) |
| Methacholine PC20, mg·mL−1 (geometric mean (CV, %)) | 1.76 (453.73) | 2.21 (244.87) |
Data are presented as mean±sd, n (%) or median (minimum–maximum range), unless otherwise stated. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PC20: provocative concentration causing a 20% decrease in FEV1; CV: coefficient of variation.
Four participants did not complete the study, including three in the benralizumab group (two withdrew consent and one was lost to follow-up) and one in the placebo group (TEAE of asthma exacerbation after completing study treatment). Missing data from some participants was due to protocol deviations and/or the inability to produce sufficient biological samples. Five participants had important protocol deviations (supplementary table E1), one of which required exclusion from the primary analysis set (allergen doses during the week 9 and 12 challenges exceeded the screening dose for a participant in the placebo group). Two participants in the benralizumab group received prednisone as rescue mediation after the completion of the allergen challenge day requiring 24 h post-allergen data to be removed from analysis, but with no impact on co-primary outcomes at 7 h post-challenge. Allergen doses were reduced for tolerability at week 9 (benralizumab n=3; placebo n=3) and week 12 (benralizumab n=1; placebo n=4) as permitted by the study protocol.
Allergen-induced eosinophils
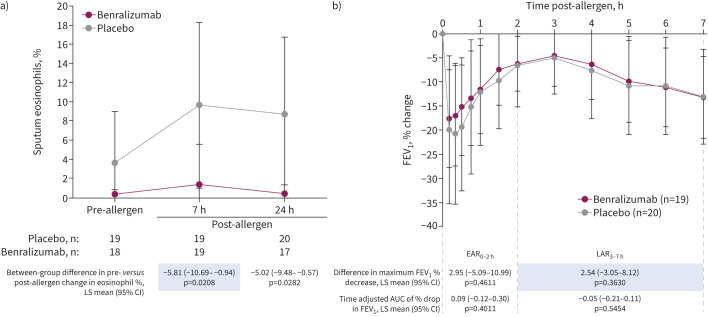
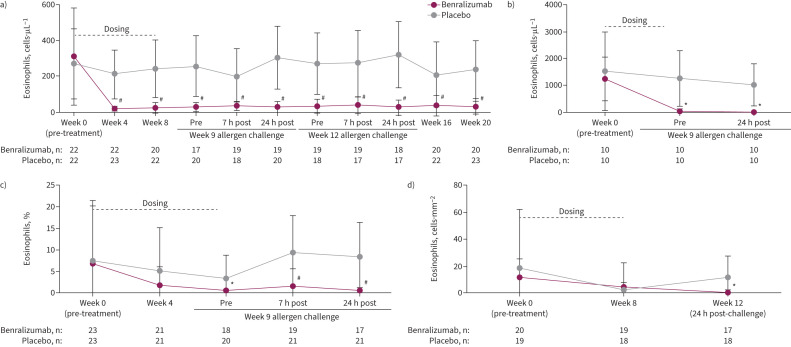
During the week 9 allergen challenge, mean±sd sputum eosinophils in the placebo group increased from a pre-challenge level of 3.6±5.4% to post-challenge levels of 9.7±8.6% (7 h) and 8.7±8.1% (24 h) and in the benralizumab group from 0.4±0.5% before challenge to 1.4±4.2% (7 h) and 0.4±0.9% (24 h) after (figure 2a). When these changes were compared between groups (primary end-point), benralizumab significantly inhibited an allergen-induced increase in the percentage eosinophil levels post-challenge at 7 h (p=0.021) and 24 h (p=0.028) versus placebo (figure 2a). Inhibition by benralizumab was also apparent when sputum eosinophil counts were expressed as cells·g−1 of mucus at 7 h (p=0.051) and 24 h (p=0.012) (supplementary table E2). Blood eosinophils remained significantly reduced compared with placebo through the week 12 allergen challenge (figure 3a and supplementary figure E4a).
FIGURE 2.
Effect of benralizumab on a) sputum eosinophils and b) change in forced expiratory volume in 1 s (FEV1) during the week 9 allergen challenge. Data are presented as mean±sd. Shaded values are co-primary end-points. Analyses are based on the primary efficacy analysis set that excluded one patient in the placebo group who received a higher dose of allergen at week 9 than in the screening challenge. Tabular results are based on repeated-measures analyses that estimated the mean change from before to after allergen exposure in the benralizumab group compared with the placebo group. The model for the sputum eosinophils percentage estimates was: allergen-induced change = treatment group +time post-allergen challenge + treatment group × time post-allergen challenge + allergen-induced change at screening. The model for the maximum percentage decrease in FEV1 estimates was: maximum percentage decrease in FEV1 in airway response = treatment group + maximum percentage decrease in airway response at screening. LS: least squares; AUC: area under the curve; EAR0–2 h: early asthmatic response (hours 0–2); LAR3–7 h: late asthmatic response (hours 3–7).
FIGURE 3.
Effect of benralizumab versus placebo on eosinophil levels measured before and after allergen challenge in a) blood, b) bone marrow, c) sputum and d) bronchial submucosa. Data are presented as mean±sd based on the full analysis set. Blood eosinophils were measured using the Advia 2120i system, bone marrow eosinophils from smears stained with DiffQuik and haemocytometer total leukocyte count, sputum eosinophils stained with DiffQuik on a cytospin and expressed as a percentage of leukocytes, and endobronchial eosinophils measured in haematoxylin/eosin-stained submucosa. *: p<0.05; #: p<0.0001 based on t-tests between means of treatment groups at each visit. Comparisons of between-group changes in eosinophils are presented in supplementary table E2.
Allergen-induced LAR
The maximum percentage decrease in FEV1 of the LAR during the week 9 challenge (co-primary end-point) was not significantly different between groups (LS mean difference 2.54%, 95% CI −3.05–8.12%; p=0.363) (figure 2b). Additionally, no significant effects were observed for benralizumab versus placebo on the EAR at week 9 (figure 2b) or on the LAR or EAR during the week 12 allergen challenge (supplementary figure E4b). The results of an a priori sensitivity analysis that was limited to participants who received the same allergen doses at screening and both allergen challenges with no important protocol deviations were consistent with the results of the primary end-point analyses (supplementary table E3).
Eosinophil levels in blood, bone marrow, sputum and bronchial tissue
Eosinophil levels were significantly reduced from baseline in all compartments following benralizumab administration (figure 3). The mean blood eosinophil count was significantly reduced in the benralizumab group versus placebo as early as the first assessment after treatment initiation (week 4), at which time the respective group mean±sd values were 18.6±13.9 and 210.4±133.2 cells·µL−1 (p<0.001) and remained stable for the entire observation period (figure 3a). In bone marrow, the mean±sd eosinophil count for the benralizumab group at week 9 was 0.03±0.09 compared with 1.25±1.03×109 cells·µL−1 for the placebo group (p=0.005) (figure 3b). In sputum, the percentage of eosinophils in the benralizumab group decreased from baseline by 5.59% at week 4 (p=0.194 versus placebo) and 7.98% at week 9 (p=0.005 versus placebo) to a median in the benralizumab group of 0% (figure 3c). In bronchial submucosa, there was no significant change in the eosinophil count·mm−2 at week 8 (figure 3d). This interpretation was limited by the high variability of eosinophil count and low eosinophil levels at baseline.
At 24 h after each allergen challenge, increases in blood eosinophils could be detected in the placebo group but not the benralizumab group (figure 3a); however, the change was not significant between groups at week 9 (p=0.085) or week 12 (p=0.079) (supplementary table E2 and supplementary figure E4). In bone marrow, the decrease in eosinophils observed in the benralizumab group before allergen exposure was maintained for 24 h after the challenge (figure 3b). In bronchial submucosa, the mean±sd eosinophil count·mm−2 was significantly higher in the placebo group than the benralizumab group 24 h after the week 12 challenge (12.35±15.25 versus 0.75±2.06 cells·mm−2; p=0.005) (figure 3d), and the change from week 0 (pre-treatment) to this time-point was significant between groups (LS mean difference for benralizumab versus placebo of −12.43 (95% CI −24.49– −0.37) cells·mm−2; p=0.044) (supplementary table E2).
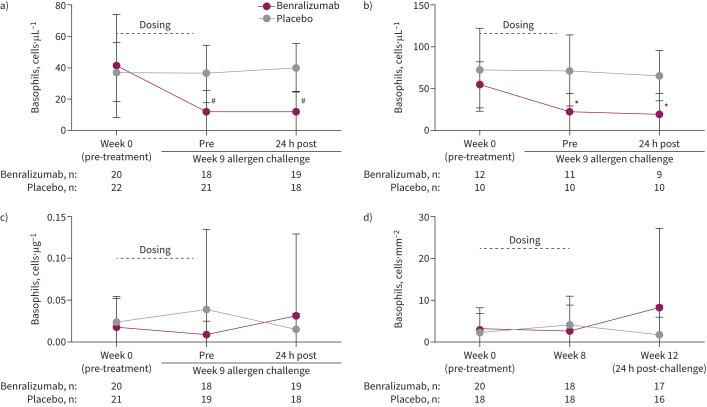
Basophil levels in blood, bone marrow, sputum and bronchial tissue
Basophil levels in blood (figure 4a) and bone marrow (figure 4b) were significantly lower in the benralizumab group versus placebo before the week 9 AIC and remained significantly lower for at least 24 h after challenge (both p<0.05). However, benralizumab had no significant effect on airway basophil counts at any time, including during and after allergen exposure, in sputum (figure 4c) or bronchial tissue (figure 4d).
FIGURE 4.
Effect of benralizumab versus placebo on basophil levels measured before and after allergen challenge in a) blood, b) bone marrow, c) sputum and d) bronchial submucosa. Data are presented as mean±sd based on the full analysis set. Blood, bone marrow and sputum basophils were measured by flow cytometry, taken as an average of CD45+FcεRI+CD123+, CD45+FcεRI+CRTH2+ or CD45+FcεRI+CCR3+; endobronchial basophils were measured using 2D7 antibody and immunofluorescence microscopy. *: p<0.05; #: p<0.0001 based on t-tests between means of treatment groups at each visit. In ANCOVA analyses performed to compare between-group changes in basophils from week 0 to 9 (24 h post-challenge), the least squares (LS) mean difference for benralizumab versus placebo was −26.9 (95% CI −37.2–16.5) cells·µL−1 (p<0.0001) in blood (n=17 versus 20), −39.1 (95% CI −64.5– −13.7) cells·µL−1 (p=0.005) in bone marrow (n=11 versus 10) and −0.29 (95% CI −0.77–0.19) cells·µg−1 (p=0.231) in sputum (n=18 versus 19). In the ANCOVA analysis for bronchial submucosa comparing changes from baseline to week 8, the LS mean difference for benralizumab (n=18) versus placebo (n=17) was −1.5 (95% CI −6.1–3.2) cells·mm−2 (p=0.522).
Biomarkers
Biomarker assessments revealed significant increases in eotaxin-1 in serum from baseline in the benralizumab group versus placebo (weeks 4 and 9) and bone marrow (week 9) and significant decreases in serum EDN and ECP at both time-points (p<0.05) (table 2). The level of IL-5 in serum by week 9 after dosing in the benralizumab group versus placebo was 2-fold higher, but this was not statistically different. When these biomarkers were examined for allergen-induced changes, a significant attenuation in serum and sputum EDN levels was observed in the benralizumab group versus placebo after allergen exposure during the week 9 challenge (p<0.05) (table 3).
TABLE 2.
Biomarker levels in blood, sputum and bone marrow over time (full analysis set)
| Benralizumab | Placebo | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 9 | Baseline | Week 4 | Week 9 | |
| Serum | ||||||
| IL-5 (ng·L−1) | 21.3±37.8 | 18.3±21.7 | 26.0±35.2 | 15.0±22.2 | 14.9±24.2 | 12.6±24.6 |
| Eotaxin-1 (ng·L−1) | 178.8±101.7 | 264.3±128.6* | 270.0±157.5* | 158.6±67.2 | 154.5±78.5 | 162.9±84.8 |
| EDN (µg·L−1) | 55.5±46.1 | 7.1±2.1* | 6.7±2.1* | 50.9±37.8 | 38.5±28.3 | 40.0±24.7 |
| ECP (µg·L−1) | 34.3±29.2 | 8.4±6.4* | 7.3±6.4* | 33.7±23.5 | 27.0±21.4 | 33.8±28.3 |
| Bone marrow | ||||||
| IL-5 (ng·L−1) | 9.6±15.5 | 15.0±22.2 | 17.7±34.4 | 16.2±30.9 | ||
| Eotaxin-1 (ng·L−1) | 268.4±183.6 | 626.9±345.1* | 346.3±224.4 | 249.2±110.63 | ||
| EDN (µg·L−1) | 255.5±148.0 | 85.0±42.9* | 238.1±139.0 | 250.7±156.8 | ||
| Sputum | ||||||
| IL-5 (ng·L−1) | 3.4±0.8 | 3.7±2.1 | 3.5±1.0 | 4.8±5.6 | 4.5±3.8 | 5.0±6.1 |
| Eotaxin-1 (ng·L−1) | 23.4±15.7 | 28.5±23.3 | 32.6±26.1 | 23.7±15.8 | 27.9±21.1 | 26.1±19.5 |
| EDN (µg·L−1) | 107.9±151.6 | 15.9±18.1 | 11.7±9.8 | 113.6±152.7 | 88.2±124.8 | 78.8±138.9 |
Data are presented as mean±sd. IL-5: interleukin 5; EDN: eosinophil-derived neurotoxin; ECP: eosinophil cationic protein. *: p<0.05 versus the corresponding time-point in the placebo group based on an unpaired t-test with Bonferroni correction.
TABLE 3.
Allergen-induced biomarker levels in blood, sputum and bone marrow during the week 9 allergen challenge (primary efficacy analysis set)
| Benralizumab (n=23) | Placebo (n=22) | |||||
|---|---|---|---|---|---|---|
| Pre-allergen challenge | Post-allergen challenge | Pre-allergen challenge | Post-allergen challenge | |||
| 7 h | 24 h | 7 h | 24 h | |||
| Serum | ||||||
| IL-5 (ng·L−1) | 26.0±35.2 | 26.3±29.8 | 43.0±57.2 | 13.0±25.2 | 15.6±26.1 | 15.5±24.2 |
| Eotaxin-1 (ng·L−1) | 270.0±157.5 | 268.0±156.7 | 272.1±143.7 | 162.0±86.8 | 151.5±86.8 | 157.0±76.8 |
| EDN (µg·L−1) | 6.7±2.1 | 7.5±3.5 | 6.4±1.3* | 40.7±25.1 | 43.2±31.9 | 52.7±32.1 |
| ECP (µg·L−1) | 7.3±6.4 | 8.6±5.9 | 7.6±5.1 | 34.7±28.7 | 29.3±21.6 | 41.7±39.9 |
| Bone marrow | ||||||
| IL-5 (ng·L−1) | 15.0±22.2 | 40.7±43.3 | 16.2±30.9 | 17.6±31.4 | ||
| Eotaxin-1 (ng·L−1) | 626.9±345.1 | 548.2±254.2 | 249.2±110.6 | 338.1±235.3 | ||
| EDN (µg·L−1) | 85.0±42.9 | 74.5±57.7 | 250.7±156.8 | 185.9±127.2 | ||
| Sputum | ||||||
| IL-5 (ng·L−1) | 3.5±1.0 | 20.7±45.8 | 23.5±52.7 | 5.1±6.3 | 20.5±37.5 | 10.3±12.5 |
| Eotaxin-1 (ng·L−1) | 32.6±26.1 | 45.0±42.1 | 65.3±98.0 | 26.6±19.9 | 33.8±37.0 | 29.7±15.3 |
| EDN (µg·L−1) | 11.7±9.8 | 15.7±13.7* | 19.3±20.5* | 82.1±141.7 | 205.5±210.3 | 258.6±226.0 |
Data are presented as mean±sd. IL-5: interleukin 5; EDN: eosinophil-derived neurotoxin; ECP: eosinophil cationic protein. *: p<0.05 versus the corresponding time-point in the placebo group based on the unpaired t-test with Bonferroni correction.
AHR to methacholine
No effect of benralizumab versus placebo on methacholine PC20 was detected at any time-point, as shown by no change from baseline to week 4 (LS mean difference 0.93 (95% CI 0.54–1.60) mg·mL−1; p=0.795) and week 9 before allergen challenge (LS mean difference 0.90 (95% CI 0.47–1.70) mg·mL−1; p=0.729) (supplementary table E4). During the week 9 allergen challenge the methacholine PC20 change from before to after allergen exposure fell by approximately 0.8 doubling doses in the placebo group and 0.4 doubling doses in the benralizumab group; however, no significant difference in PC20 was detected between groups at 24 h after challenge (LS mean difference 1.30 (95% CI 0.70–2.42) mg·mL−1; p=0.397).
Safety
Of the participants in the benralizumab and placebo groups, respectively, seven (30.4%) and 14 (60.9%) experienced TEAEs, including one (4.3%) and three (13.0%) who experienced events considered related to study treatment (table 4). Most TEAEs were of mild or moderate severity; severe events were reported for one participant (4.3%) in each group (migraine in the benralizumab group and musculoskeletal pain in the placebo group), neither of which was considered related to the study drug nor led to treatment discontinuation. No clinically meaningful laboratory, vital sign or electrocardiography findings occurred.
TABLE 4.
Treatment-emergent adverse events (TEAEs)
| Benralizumab (n=23) | Placebo (n=23) | |
|---|---|---|
| Any TEAE | 7 (30.4) | 14 (60.9) |
| Serious adverse event | 0 | 0 |
| TEAE related to study drug | 1 (4.3) | 3 (13.0) |
| Adverse event leading to drug discontinuation | 0 | 0 |
| Death | 0 | 0 |
| TEAEs in >1 patient | ||
| Headache | 1 (4.3) | 3 (13.0) |
| Nasopharyngitis | 1 (4.3) | 3 (13.0) |
| Asthma | 1 (4.3) | 1 (4.3) |
| Bronchitis | 0 | 2 (8.7) |
| Oropharyngeal pain | 0 | 2 (8.7) |
| Dizziness | 1 (4.3) | 1 (4.3) |
| Migraine | 1 (4.3) | 1 (4.3) |
| Myalgia | 1 (4.3) | 1 (4.3) |
Data are presented as n (%).
Discussion
In this study, despite effective and sustained depletion of baseline eosinophils and basophils in the blood and bone marrow, eosinophils in sputum and attenuation of allergen-induced airway eosinophilia, benralizumab did not attenuate either the allergen-induced EAR or LAR, baseline AHR to methacholine or increased AHR to methacholine after allergen challenge in participants with mild allergic asthma. These results are consistent with a previous study in which the anti-IL-5 biologic mepolizumab also depleted blood and sputum eosinophils before and after the allergen challenge but had no effect on the allergen-induced LAR [19]. Although that study was criticised for being underpowered to adequately evaluate the effect of IL-5 blockade on allergen-induced airway responses [27], the current study was sufficiently powered to find a significant effect on the allergen-induced LAR.
Studies examining the anti-thymic stromal lymphopoietin agent tezepelumab [17] and other anti-inflammatory asthma therapies, such as inhaled corticosteroids and leukotriene inhibitors [28–32], have reported a coincident decrease in blood and sputum eosinophils after allergen challenge with concomitant inhibition of the allergen-induced EAR and LAR; however, there is no direct evidence linking eosinophil activity to allergen-induced airway responses in allergic asthma. Furthermore, attenuation of the LAR by leukotriene inhibitors as well as corticosteroids and tezepelumab, especially paired with reduction of the EAR, suggests the allergen-induced pathophysiology involves the mast cell which does not appear to be a major target of anti-IL-5/IL-5Rα therapy. The current study was designed for allergen challenges to coincide with peak benralizumab concentrations in blood and lung tissue and with expected steady-state trough levels. As expected, we observed a robust reduction in eosinophils and in their activation marker EDN in blood, bone marrow and sputum following benralizumab administration and a significant attenuation of eosinophilia in sputum following allergen exposure. Near-complete depletion of eosinophils in bronchial tissue also occurred after allergen at week 12; however, assessment of bronchial tissue eosinophilia was limited to a single post-allergen challenge sample collected 24 h after the week 12 challenge, and the results were difficult to interpret because of low and variable eosinophil levels in these participants. Nonetheless, our results add to the evidence that eosinophils likely do not participate in allergen-induced airway responses in participants with mild asthma; this is in contrast to these cells playing a significant role through a mechanism to be determined in severe asthma exacerbation, as evidenced by a profound reduction in exacerbation rates following eosinophil depletion by benralizumab. Regardless of whether the low numbers of eosinophils recruited to airways in response to allergen challenge after benralizumab treatment are IL-5 independent or related to changes in eotaxin levels, or represent an important inflammatory phenotype [33–35], the complexity of eosinophil effector functions in the asthmatic airway requires further investigation.
Pharmacological interventions to reduce histamines and cysteinyl leukotrienes, which are produced by mast cells and basophils, have demonstrated partial attenuation of the EAR and complete attenuation of the LAR [32], and data suggest that mast cells and basophils may be important for the induction of the LAR in allergic asthma [36]. Yet, despite significant reductions of basophils in blood and bone marrow following benralizumab administration in our study, measurable basophil counts persisted during treatment, especially in the airways, and possibly driven in part by increased levels of eotaxin-1 and IL-5, both of which have been reported previously [37–40]. Basophils and mast cells remain as possible effector cells that drive allergen-induced airway responses through their release of mediators, including histamine, cysteinyl leukotrienes and prostaglandin D2. Assessment of these mediators in sputum after allergen challenge would provide a more thorough assessment of mechanisms of allergen-induced responses in benralizumab-treated patients.
Despite using a robust clinical model widely used to examine the mechanisms of allergic asthma and the effects of drugs, this study was limited by the available cellular assessments, particularly in bronchial biopsies, in participants with mild disease. These assessments yielded low cell counts at baseline for some of the cell types and the inability to confirm whether bronchial tissue eosinophils were fully depleted.
In conclusion, benralizumab 30 mg administered over 8 weeks resulted in the significant depletion of eosinophils in blood, bone marrow and sputum in participants with mild allergic asthma. Allergen-induced eosinophilia in sputum was also significantly attenuated with benralizumab treatment compared with placebo; however, there was no observable effect on the LAR. While eosinophils alone are not a key component of allergen-induced bronchoconstriction and likely do not play a significant role in the LAR in mild asthma, allergen-induced eosinophilia can be evaluated as a separate study end-point that is independent of the LAR.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00512-2024.Supplement (731.4KB, pdf)
Shareable PDF
Acknowledgements
Medical writing support was provided by Nate Connors and Dan Jackson (CiTRUS Health Group; www.citrushealthgroup.com), which was in accordance with Good Publication Practice (GPP 2022) guidelines. This support was funded by AstraZeneca (Cambridge, UK).
Footnotes
This clinical trial was prospectively registered at ClinicalTrials.gov with identifier number NCT02821416.
Ethics statement: The study protocol, informed consent forms and other relevant documents were reviewed by the independent ethics committee or institutional review board for each centre. The study was conducted in accordance with the International Conference on Harmonisation E6 Guidelines for Good Clinical Practice that have their origin in the Declaration of Helsinki, and all subjects provided written informed consent.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01316-2024
Conflict of interest: G.M. Gauvreau reports support for the present manuscript from AstraZeneca, grants from AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, and receipt of equipment, materials, drugs, medical writing, gifts or other services from AstraZeneca. R. Sehmi reports support for the present study from AstraZeneca. R. Leigh reports support for the present study from AstraZeneca, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca. D.W. Cockcroft reports support for the present manuscript from AstraZeneca, and grants from SHRF, Biohaven, AllerGen, University of Saskatchewan College of Medicine and CIHR. I. Mayers reports grants from AstraZeneca Canada and Boehringer Ingelheim, consultancy fees from Sanofi Canada and AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, and payment for expert testimony from Alberta Justice. L-P. Boulet reports grants from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron, royalties or licences from UptoDate and Taylor & Francis, consultancy fees from AstraZeneca, Novartis, GlaxoSmithKline, Merck and Sanofi-Regeneron, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Novartis, Merck and Sanofi, and leadership role as Past-Chair of the Global Initiative for Asthma (GINA) Board of Directors, Past President of the Global Asthma Organisation (Interasma), Past Member of the Canadian Thoracic Society Respiratory Guidelines Committee and Past Laval University Chair on Knowledge Transfer, Prevention and Education in Respiratory and Cardiovascular Health. T. Ho reports grants from Fisher & Paykel, consultancy fees from Valeo and AstraZeneca, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca and GlaxoSmithKline. I. Satia reports grants from Merck, GlaxoSmithKline and Bellus, consultancy fees from Merck, Genentech, Respiplus and GlaxoSmithKline/Bellus, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Merck, GlaxoSmithKline, AstraZeneca and Sanofi. P.D. Mitchell reports grants from Teva, consultancy fees from Pfizer and GlaxoSmithKline, and support for attending meetings from AstraZeneca. I.P. Magee reports payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline. C. Bergeron reports support for the present study from AstraZeneca, grants from AstraZeneca, Sanofi and Regeneron, consultancy fees from ValeoPharma, Sanofi, AstraZeneca and GlaxoSmithKline, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, ValeoPharma, Sanofi and Grifols. M. Bhutani reports grants from CIHR, GlaxoSmithKline, AstraZeneca and Sanofi, consultancy fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Sanofi, Covis and Valeo, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Sanofi and Covis. V. Werkström was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. T. Durżyński was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. K. Shoemaker was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. R.K. Katial was an employee of AstraZeneca at the time of the study and reports personal fees from AstraZeneca. M. Jison was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. C. McCrae was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. P.M. O'Byrne reports support for the present study from AstraZeneca, grants from AstraZeneca, Merck and Biohaven, consultancy fees from AstraZeneca, GlaxoSmithKline, Sage, Teva and Affibody, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Chiesi, GlaxoSmithKline and Covis. The remaining authors have no potential conflicts of interest to disclose.
Support statement: This study was funded by AstraZeneca (Cambridge, UK). The funders of the study participated in the study design and had access to the raw data. All authors, including those employed by the funders of the study, participated in the data collection, data analysis, data interpretation and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication. The funder provided support for study implementation and the statistical analysis plan. Data analyses and interpretation were completed by the funder in conjunction with the authors. All authors contributed to writing the report and made the decision to submit the report for publication. Medical writers, paid by the funder, assisted with the preparation of the report. All authors vouch for the integrity, completeness and accuracy of the data and analyses and confirm that they had full access to all the data in the study. Funding information for this article has been deposited with the Crossref Funder Registry.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of COPD. 2022. Available from: http://goldcopd.org/
- 2.Pakkasela J, Ilmarinen P, Honkamaki J, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med 2020; 20: 9. doi: 10.1186/s12890-019-1040-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoratti E, Havstad S, Wegienka G, et al. Differentiating asthma phenotypes in young adults through polyclonal cytokine profiles. Ann Allergy Asthma Immunol 2014; 113: 25–30. doi: 10.1016/j.anai.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract 2014; 2: 645–648. doi: 10.1016/j.jaip.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132: 1086–1096. doi: 10.1016/j.jaci.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham TH, Damera G, Newbold P, et al. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med 2016; 111: 21–29. doi: 10.1016/j.rmed.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 7.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376: 2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 8.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 10.Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol 2018; 141: 1529–1532. doi: 10.1016/j.jaci.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 11.Wright AKA, Weston C, Rana BMJ, et al. Human group 2 innate lymphoid cells do not express the IL-5 receptor. J Allergy Clin Immunol 2017; 140: 1430–1433. doi: 10.1016/j.jaci.2017.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weersink EJ, Aalbers R, Koëter GH, et al. Partial inhibition of the early and late asthmatic response by a single dose of salmeterol. Am J Respir Crit Care Med 1994; 150: 1262–1267. doi: 10.1164/ajrccm.150.5.7952550 [DOI] [PubMed] [Google Scholar]
- 13.Gauvreau GM, Watson RM, O'Byrne PM. Kinetics of allergen-induced airway eosinophilic cytokine production and airway inflammation. Am J Respir Crit Care Med 1999; 160: 640–647. doi: 10.1164/ajrccm.160.2.9809130 [DOI] [PubMed] [Google Scholar]
- 14.Gauvreau GM, El-Gammal AI, O'Byrne PM. Allergen-induced airway responses. Eur Respir J 2015; 46: 819–831. doi: 10.1183/13993003.00536-2015 [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Davis BE, Boulet LP, et al. The links between allergen skin test sensitivity, airway responsiveness and airway response to allergen. Allergy 2005; 60: 56–59. doi: 10.1111/j.1398-9995.2004.00612.x [DOI] [PubMed] [Google Scholar]
- 16.Gauvreau GM, FitzGerald JM, Boulet LP, et al. The effects of a CCR3 inhibitor, AXP1275, on allergen-induced airway responses in adults with mild-to-moderate atopic asthma. Clin Exp Allergy 2018; 48: 445–451. doi: 10.1111/cea.13114 [DOI] [PubMed] [Google Scholar]
- 17.Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370: 2102–2110. doi: 10.1056/NEJMoa1402895 [DOI] [PubMed] [Google Scholar]
- 18.Gauvreau GM, Jordana M, Watson RM, et al. Effect of regular inhaled albuterol on allergen-induced late responses and sputum eosinophils in asthmatic subjects. Am J Respir Crit Care Med 1997; 156: 1738–1745. doi: 10.1164/ajrccm.156.6.96-08042 [DOI] [PubMed] [Google Scholar]
- 19.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000; 356: 2144–2148. doi: 10.1016/S0140-6736(00)03496-6 [DOI] [PubMed] [Google Scholar]
- 20.Salter BM, Nusca G, Tworek D, et al. Expression of activation markers in circulating basophils and the relationship to allergen-induced bronchoconstriction in subjects with mild allergic asthma. J Allergy Clin Immunol 2016; 137: 936–938. doi: 10.1016/j.jaci.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 21.Pelikan Z. Expression of surface markers on the blood cells during the delayed asthmatic response to allergen challenge. Allergy Rhinol 2014; 5: 96–109. doi: 10.2500/ar.2014.5.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macfarlane AJ, Kon OM, Smith SJ, et al. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol 2000; 105: 99–107. doi: 10.1016/S0091-6749(00)90184-2 [DOI] [PubMed] [Google Scholar]
- 23.Gauvreau GM, Lee JM, Watson RM, et al. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med 2000; 161: 1473–1478. doi: 10.1164/ajrccm.161.5.9908090 [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Hargreave FE, Woolcock AJ. Measure of airway responsiveness to inhaled histamine or methacholine; method of continuous aerosol generation and tidal breathing inhalation. In: Hargreave FE, Woolcock AJ, eds. Airway Responsiveness: Measurement and Interpretation. Mississauga, Astra Pharmaceuticals Canada Ltd, 1985; pp. 22–28. [Google Scholar]
- 26.Cockcroft DW, Murdock KY, Kirby J, et al. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis 1987; 135: 264–267. doi: 10.1164/arrd.1987.135.1.264 [DOI] [PubMed] [Google Scholar]
- 27.O'Byrne PM, Inman MD, Parameswaran K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. J Allergy Clin Immunol 2001; 108: 503–508. doi: 10.1067/mai.2001.119149 [DOI] [PubMed] [Google Scholar]
- 28.Gauvreau GM, Doctor J, Watson RM, et al. Effects of inhaled budesonide on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med 1996; 154: 1267–1271. doi: 10.1164/ajrccm.154.5.8912734 [DOI] [PubMed] [Google Scholar]
- 29.Gauvreau GM, Boulet LP, FitzGerald JM, et al. A dual CysLT1/2 antagonist attenuates allergen-induced airway responses in subjects with mild allergic asthma. Allergy 2016; 71: 1721–1727. doi: 10.1111/all.12987 [DOI] [PubMed] [Google Scholar]
- 30.Inman MD, Watson RM, Rerecich T, et al. Dose-dependent effects of inhaled mometasone furoate on airway function and inflammation after allergen inhalation challenge. Am J Respir Crit Care Med 2001; 164: 569–574. doi: 10.1164/ajrccm.164.4.2007063 [DOI] [PubMed] [Google Scholar]
- 31.Gauvreau GM, Boulet LP, Postma DS, et al. Effect of low-dose ciclesonide on allergen-induced responses in subjects with mild allergic asthma. J Allergy Clin Immunol 2005; 116: 285–291. doi: 10.1016/j.jaci.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 32.Davis BE, Illamperuma C, Gauvreau GM, et al. Single-dose desloratadine and montelukast and allergen-induced late airway responses. Eur Respir J 2009; 33: 1302–1308. doi: 10.1183/09031936.00169008 [DOI] [PubMed] [Google Scholar]
- 33.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016; 126: 3279–3295. doi: 10.1172/JCI85664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly EA, Esnault S, Liu LY, et al. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am J Respir Crit Care Med 2017; 196: 1385–1395. doi: 10.1164/rccm.201611-2234OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Percopo CM, Brenner TA, Ma M, et al. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol 2017; 101: 321–328. doi: 10.1189/jlb.3A0416-166R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson BM, Oliveria JP, Tworek D, et al. Allergen inhalation increases circulating basophil activation in early and dual responding mild allergic asthmatics. Am J Respir Crit Care Med 2015; 191: A4146. [Google Scholar]
- 37.Lommatzsch M, Marchewski H, Schwefel G, et al. Benralizumab strongly reduces blood basophils in severe eosinophilic asthma. Clin Exp Allergy 2020; 50: 1267–1269. doi: 10.1111/cea.13720 [DOI] [PubMed] [Google Scholar]
- 38.Eck S, Castro M, Sinibaldi D, et al. Benralizumab effect on blood basophil counts in adults with uncontrolled asthma. Eur Respir J 2014; 44: Suppl. 58, P297. doi: 10.1183/09031936.00107914 [DOI] [Google Scholar]
- 39.Damera G, Brightling C, Bleecker E, et al. Pharmacodynamic effect of benralizumab on blood basophils and serum biomarkers in adults with COPD with sputum eosinophilia. Eur Respir J 2015; 46: Suppl. 59, PA2118. doi: 10.1183/13993003.congress-2015.PA2118 [DOI] [Google Scholar]
- 40.Sridhar S, Liu H, Pham TH, et al. Modulation of blood inflammatory markers by benralizumab in patients with eosinophilic airway diseases. Respir Res 2019; 20: 14. doi: 10.1186/s12931-018-0968-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00512-2024.Supplement (731.4KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00512-2024.Shareable (588.9KB, pdf)
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure