Abstract
Human T-lymphotropic virus type 1 (HTLV-1), the etiologic agent of adult T-cell leukemia/lymphoma, is transmitted through breast milk and seminal fluid, which are rich in prostaglandins (PGs). We demonstrate that PGE2 upregulates the HTLV-1 long terminal repeat promoter through the protein kinase A pathway, induces replication of HTLV-1 in peripheral blood mononuclear cells (PBMC) derived from asymptomatic carriers, and enhances transmission of HTLV-1 to cord blood mononuclear cells (CBMC). Furthermore, HTLV-1 Tax transactivates a promoter for cyclooxygenase 2, a PG synthetase, and induces PGE2 expression in PBMC or CBMC. Thus, HTLV-1 interacts with and benefits from PGs, constituents of its own vehicle for transmission.
Human T-lymphotropic virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia/lymphoma and is transmitted horizontally or vertically through blood, seminal fluid, or breast milk (reviewed in references 13 and 37).
Prostaglandins (PGs) are synthesized and secreted by most human tissues and cell types; however, they are especially abundant in seminal fluid and breast milk (2, 7, 9, 10). PGs, particularly those of the E series, are widely regarded as pleiotropic immunomodulatory molecules, and regulation of their expression appears to be critical for a number of immune responses (reviewed in references 26 and 29). There are two isoforms of cyclooxygenase (COX) that catalyze the formation of PGs from arachidonic acid. While COX-1 is a housekeeping gene that is expressed constitutively, COX-2 is an immediate-early response gene that is highly inducible by mitogenic and inflammatory stimuli and is considered essential for the induction of the aforementioned immune responses (reviewed in reference 32).
In this study we demonstrate that (i) PGE2 upregulates the HTLV-1 long terminal repeat (LTR) promoter through the protein kinase A (PKA) pathway, induces viral replication in peripheral blood mononuclear cells (PBMC) derived from asymptomatic HTLV-1 carriers, and enhances transmission of HTLV-1 to cord blood mononuclear cells (CBMC) and that (ii) HTLV-1 Tax transactivates a COX-2 promoter and induces PGE2 production in PBMC. These data suggest that HTLV-1 interacts with and benefits from PGs that are abundant in seminal fluid and breast milk, vehicles for virus transmission.
MATERIALS AND METHODS
Reagents.
PGE2, H7, HA-1004, mitomycin C (MMC), 3′-azido-3′-deoxythymidine (AZT), phorbol 12-myristate 13-acetate (PMA), and ionomycin were purchased from Sigma (St. Louis, Mo.). MMC treatment of cells was performed as described previously (1).
Infectious HTLV-1 stock which was rendered devoid of cell culture supernatants by directly pelleting virions was obtained from Advanced Biotechnologies, Inc. (Columbia, Md.). Glutathione S-transferase (GST) and GST-Tax protein were propagated as described previously (23).
Plasmids and transient-expression assays.
pU3R-luc, a generous gift of K.-T. Jeang (National Institute of Allergy and Infectious Diseases [NIAID], Bethesda, Md.), carries the luciferase gene under the control of the HTLV-1 LTR. Plasmids pMT-2T (parent plasmid), pMT-Tax (encoding HTLV-1 Tax), pMT-p65 (encoding a NF-κB p65 subunit), and pMT-IκB (encoding I-κBα) were kindly provided by U. Siebenlist (NIAID) (23). The human COX-2 promoter-luciferase reporter plasmids phPES(−1432/+59), phPES(−327/+59), phPES(−220/+59), phPES(−124/+59), phPES(−52/+59), phPES-KBM (with a mutation at the NF-κB site), phPES-ILM (with a mutation at the NF-IL6 site), phPES-CRM (with a mutation at the cyclic AMP-responsive element [CRE]), phPES-KBM/ILM (with mutations at both the NF-κB and NF-IL6 sites), phPES-KBM/CRM (with mutations at both the NF-κB site and the CRE), phPES-ILM/CRM (with mutations at both the NF-IL6 site and the CRE), and phPES-KBM/ILM/CRM (with mutations at all three elements) were described previously (14, 15).
Transfection of the human Jurkat T leukemia cell line or PBMC and luciferase assays for transient-expression assays were performed as described previously (22). Briefly, 20 million Jurkat cells or 40 million PBMC were transfected by electroporation at 300 or 320 V, respectively, and 975 μF with a Gene Pulser II (Bio-Rad, Hercules, Calif.). After electroporation, cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 37°C for 40 h. Luciferase activity was determined by using a luciferase assay kit (Promega, Madison, Wis.) with a TD-20e luminometer (Turner).
Measurement of PGE2.
The concentration of PGE2 in the cell culture supernatants was determined in triplicate by enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn.). Where indicated, cells were infected with cell-free HTLV-1 stock (1 μg of protein per 3 × 106 cells) or were treated with GST or GST-Tax at a concentration of 100 ng/ml before cell-free supernatants were collected for PGE2 measurement.
HTLV-1 infection.
For HTLV-1 infection studies, PBMC were obtained from buffy coats of asymptomatic HTLV-1 carriers (Nagasaki Red-Cross Center, Nagasaki, Japan) as described previously (21). The cells were incubated in 24-well culture dishes at a concentration of 3 × 106 per ml in RPMI 1640 supplemented with 10% FBS in the presence or absence of PGE2 (100 nM).
For HTLV-1 transmission studies, cord blood samples were provided by the Pre- and Post-Natal Care Unit, Nagasaki University School of Medicine Hospital, with consent by donors, and CBMC were cocultured with MMC-treated PBMC obtained from HTLV-1 carriers in the presence or absence of PGE2 immediately after isolation. Cell-free culture supernatants were collected on day 7 for measurement of HTLV-1 p19 antigen by ELISA (Cellular Products Inc., Buffalo, N.Y.).
RESULTS AND DISCUSSION
PGE2 upregulates HTLV-1 LTR activity.
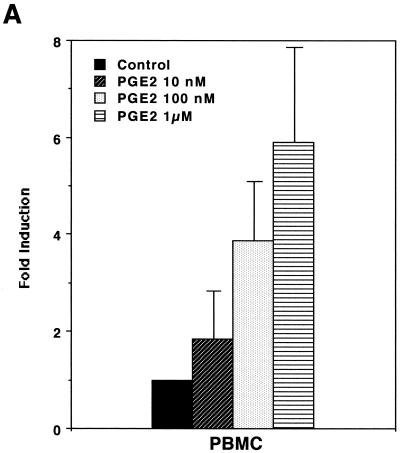
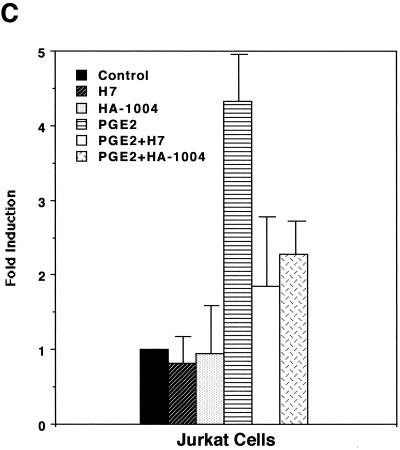
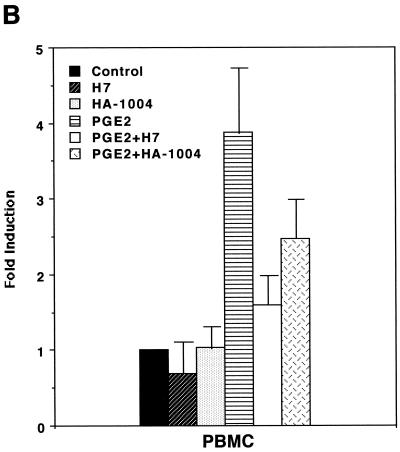
In order to assess whether PGE2 could influence expression of HTLV-1, we first performed transient-expression assays. HTLV-1 LTR activity was upregulated by stimulation with PGE2 in a dose-dependent manner (Fig. 1A). Since PGE2 levels in seminal fluid and breast milk whey were reported to be 1 to ∼70 μM (2, 7, 27) and ∼30 nM (10), respectively, and since an overproduction of PGE2 (up to 100 μM) is seen in a number of medical situations (e.g., allergy, hyper-immunoglobulin E syndrome, Hodgkin's lymphoma, trauma, sepsis, and transplantation) (29), the PGE2-mediated effect appears to be physiologically relevant. PGE2 induction of HTLV-1 LTR activity was markedly abolished by H7 (a selective serine/threonine kinase inhibitor that can inhibit PKA, PKC, and PKG) and HA-1004 (a PKA and PKG inhibitor) (Fig. 1B and C). Since PGE2 has been shown to elevate the level of intracellular cyclic AMP, which activates PKA (27), it is reasonable to assume that PKA activity is required for transcriptional activity of HTLV-1.
FIG. 1.
PGE2 upregulates HTLV-1 LTR activity. (A) PGE2 upregulates HTLV-1 LTR activity in a dose-dependent manner. A total of 40 million PBMC were transfected with 10 μg of pU3R-luc and were left unstimulated or were stimulated with the indicated amount of PGE2, and luciferase activity of the transfected cell lysates was assayed 2 days posttransfection. Fold induction is the luciferase activity relative to that obtained without PGE2 treatment. Results are means ± standard errors of the means from three independent experiments. (B and C) PKA activity is involved in PGE2 induction of expression from the HTLV-1 LTR. A total of 40 million PBMC (B) or 20 million Jurkat cells (C) were transfected with 10 μg of pU3R-luc, and luciferase activity of the transfected cell lysates was assayed 2 days posttransfection. Where indicated, cells were treated with PGE2 (100 nM) in the presence or absence of H7 (1 μM) or HA-1004 (1 μM) for 8 h before harvest. Fold induction is the luciferase activity relative to that obtained in the absence of the reagents. Results are means ± standard errors of the means from six independent experiments.
PGE2 induces HTLV-1 replication.
In order to determine whether PGE2 could actually induce replication of HTLV-1, we incubated PBMC obtained from asymptomatic HTLV-1 carriers in the presence or absence of PGE2. Stimulation with PGE2 enhanced HTLV-1 infection four- to fivefold as determined by HTLV-1 p19 antigen (Ag) levels (Table 1). Treatment with AZT (2 μM) resulted in a markedly decreased expression of p19 Ag (Table 1). Since AZT has no antiretroviral effect on PBMC already infected with HTLV-1 (18), profound suppression of p19 expression by AZT would indicate that induction of HTLV-1 expression in the presence of PGE2 required the expansion of HTLV-1 infection. Addition of PGE2 to the cell culture medium did not appear to influence cell viability as determined by trypan blue exclusion (data not shown). Thus, PGE2, a constituent of seminal fluid and breast milk, appears to induce replication of HTLV-1.
TABLE 1.
PGE2 induces HTLV-1 replicationa
| Carrier no. | p19 Ag level (pg/ml) with treatment
|
|||
|---|---|---|---|---|
| None | AZT | PGE2 | PGE2 and AZT | |
| 1 | 64 ± 5 | 45 ± 4 | 222 ± 23 | 108 ± 4 |
| 2 | 56 ± 1 | 50 ± 5 | 303 ± 45 | 98 ± 18 |
| 3 | 80 ± 8 | 89 ± 6 | 326 ± 31 | 142 ± 9 |
A total of 3 × 106 unfractionated PBMC derived from asymptomatic HTLV-1 carriers were propagated in RPMI 1640 supplemented with 10% FBS at 37°C for 7 days. The cells were either left untreated or treated with PGE2 (100 nM), AZT (2 μM), or both. Levels of p19 Ag in cell-free culture supernatants were determined by ELISA. Results are means ± standard errors of the means of duplicate wells.
We next investigated whether PGE2 could accelerate transmission of HTLV-1 to CBMC. Uninfected CBMC were cocultured with MMC-treated carriers' PBMC at a ratio of 10:1 in the presence or absence of PGE2. It has been shown that MMC-treated cells can no longer support new cycles of retroviral infection (1). As shown in Table 2, the p19 level in the coculture supernatants was increased by treatment with PGE2. PGE2 treatment increased p19 Ag levels in cultures of MMC-treated carriers' PBMC; however, they were much lower than those in coculture of these cells with CBMC. These results suggest that expansion to susceptible CBMC is required for the induction of HTLV-1 replication. It has been reported that enterally administered milk leukocytes can invade suckling neonates and become distributed in their lymphoid tissues (16, 36). Therefore, although the exact mechanisms and the target cells in the mucosa of milk-borne infection remain unknown, these results imply that PGE2 may accelerate viral transmission from maternal lymphocytes in breast milk to neonatal lymphocytes.
TABLE 2.
Expansion by PGE2 of HTLV-1 infection to CBMCa
| Culture or coculture | p19 Ag level (pg/ml) with treatment
|
|
|---|---|---|
| None | PGE2 | |
| CBMC alone | <25 | <25 |
| Carrier #4 PBMC alone | 33 ± 5 | 46 ± 6 |
| CBMC plus carrier's PBMC | 68 ± 3 | 186 ± 14 |
| CBMC alone | <25 | <25 |
| Carrier #5 PBMC alone | 42 ± 4 | 54 ± 9 |
| CBMC plus carrier's PBMC | 89 ± 10 | 287 ± 23 |
A total of 3 × 106 unfractionated CBMC derived from uninfected individuals were cocultured with 3 × 105 HTLV-1 carriers' PBMC or cultured individually. Carriers' PBMC had been pretreated with MMC (0.25 mg/ml) for 30 min to render the cells incapable of proliferating and supporting a new round of the viral replicative cycle. The cocultures were maintained at 37°C for 7 days in the presence or absence of PGE2 (100 nM). Levels of p19 Ag in culture supernatants were determined by ELISA. Similar results were obtained for two other donors (data not shown). Results are means ± standard errors of the means of duplicate wells.
HTLV-1 Tax transactivates the COX-2 promoter.
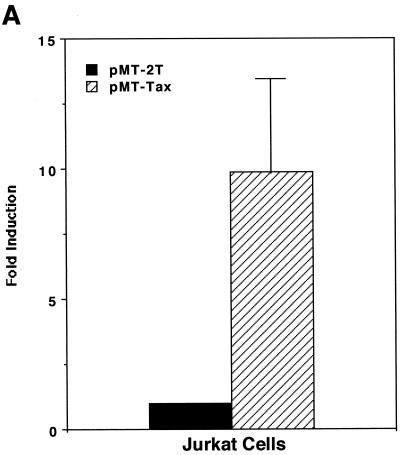
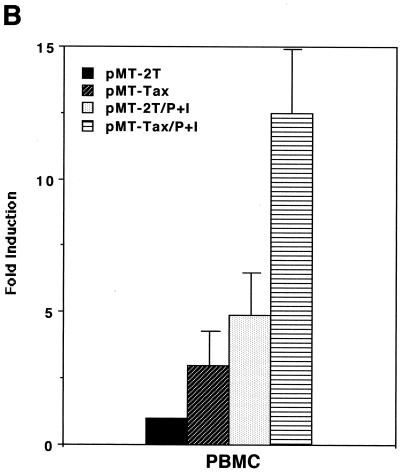
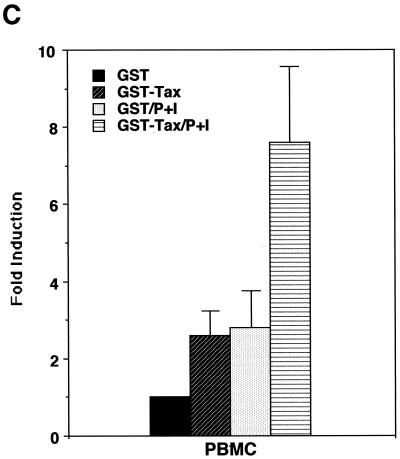
As shown above, induction of PGE2 production is likely to favor HTLV-1 infection and transmission; therefore, we next wanted to investigate whether HTLV-1 infection could influence expression of PGE2. The HTLV-1 Tax transactivator has been shown to upregulate expression of a number of viral and cellular genes, which usually contributes to viral replication or transformation (reviewed in references 6, 31, and 35). We therefore tested whether Tax could upregulate promoter activity of COX-2, a PG synthetase. As shown in Fig. 2A, coexpression of Tax alone upregulated the COX-2 promoter-luciferase reporter activity in the Jurkat human T-cell line. Tax alone was a relatively weak transactivator for the COX-2 promoter in PBMC (Fig. 2B); however, stimulation with PMA plus ionomycin in addition to Tax synergistically upregulated the COX-2 promoter activity in PBMC (Fig. 2B). Such synergy has been observed for other Tax-mediated effects (11, 23). We have also demonstrated that COX-2 promoter activity was upregulated in PBMC treated with purified Tax protein (GST-Tax) (Fig. 2C).
FIG. 2.
HTLV-1 Tax transactivates the COX-2 promoter. Jurkat cells (A) or PBMC (B) were transfected with 10 or 40 μg of phPES(−1432/+59), respectively, along with 10 μg of pMT-2T or pMT-Tax, respectively. Where indicated, PBMC were stimulated with PMA (1 μM) plus ionomycin (1 μM) for 8 h. Fold induction is the luciferase activity relative to that obtained with phPES(−1432/+59) plus pMT-2T in the absence of PMA plus ionomycin. Results are means ± standard errors of the means from six independent experiments. (C) PBMC were transfected with 40 μg of phPES(−1432/+59) and treated with GST or GST-Tax (100 ng/ml) for 2 days. Results are means ± standard errors of the means from three independent experiments.
In order to confirm that HTLV-1 infection or Tax protein induces PGE2 production, PBMC were infected with HTLV-1 or exposed to purified Tax protein, and PGE2 levels in the cell culture supernatants were determined by ELISA. HTLV-1-infected cells secrete soluble activity that exerts a number of biological activities on neighboring cells in a paracrine manner, and Tax protein released from infected cells is attributed, at least in part, to the soluble activity (4, 17, 19, 23). Experiment results listed in Tables 3 and 4 clearly demonstrate that HTLV-1 infection or Tax protein could induce PGE2 production.
TABLE 3.
HTLV-1 induces PGE2 productiona
| Donor | PGE2 level (pg/ml) in infection
|
||
|---|---|---|---|
| Mock | HTLV-1 | Inactivated HTLV-1 | |
| A | 42 ± 5 | 165 ± 18 | ND |
| B | <39 | 66 ± 20 | ND |
| C | 50 ± 9 | 182 ± 35 | ND |
| D | <39 | 80 ± 18 | <39 |
| E | 58 ± 8 | 210 ± 38 | 78 ± 15 |
A total of 3 × 106 PBMC obtained from uninfected volunteers were mock infected or infected with cell-free HTLV-1 stock (0.1 μg of protein). In experiments with PBMC derived from donors D and E, HTLV-1 stock was heat inactivated (56°C, 60 min). PGE2 levels in the culture supernatants were determined by ELISA on day 7 postinfection. Results are means ± standard errors of the means of triplicate wells. ND, not determined.
TABLE 4.
Tax protein induces PGE2 productiona
| Donor | PGE2 level (pg/ml) in the presence of:
|
|
|---|---|---|
| GST | GST-Tax | |
| D | 80 ± 19 | 252 ± 48 |
| E | 92 ± 20 | 304 ± 33 |
| F | 20 ± 7 | 121 ± 23 |
A total of 3 × 106 PBMC obtained from uninfected volunteers were incubated in the presence of either GST or GST-Tax (100 ng/ml) for 2 days. PGE2 levels in the culture supernatants were determined by ELISA. Results are means ± standard errors of the means of triplicate wells.
Both NF-κB and NF-IL6 sites are required in Tax activation of the COX-2 promoter.
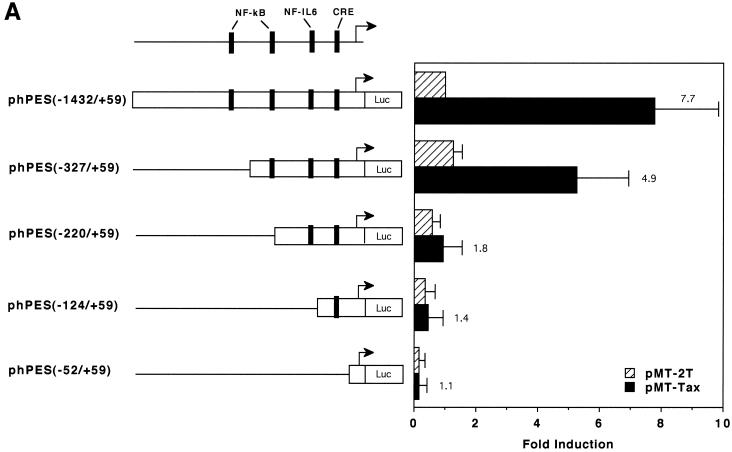
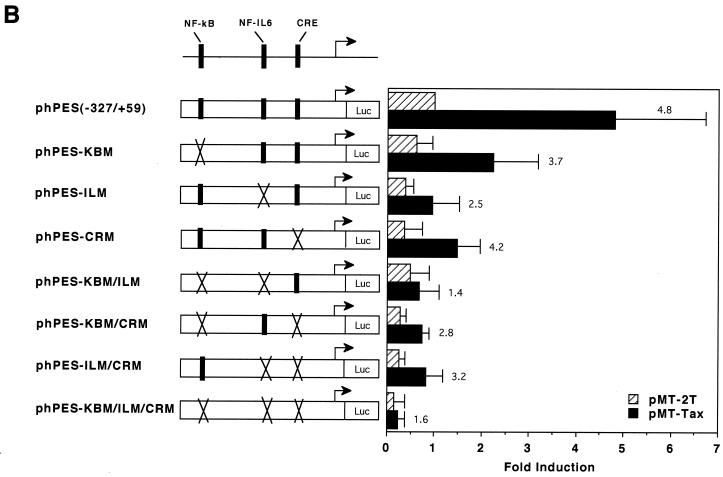
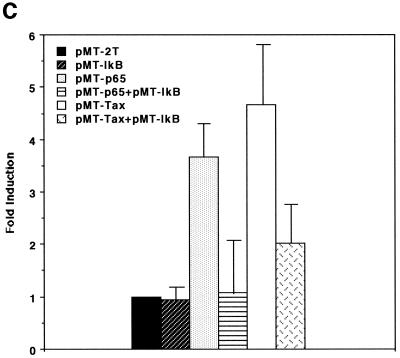
HTLV-1 Tax protein is not able to directly bind to DNA; instead, it interacts with certain cellular transcription factors to exert its transregulatory functions (reviewed in references 6, 31, and 35). The COX-2 promoter sequence contains several cis-acting elements (reviewed in reference 38), some of which have been shown to be involved in Tax-mediated transactivation in other promoter contexts. In order to determine the cis-acting elements involved in Tax-mediated transactivation of the COX-2 promoter, we tested a number of COX-2 promoter constructs in transient-expression assays. As shown in Fig. 3A, 5′ truncation of the promoter sequence down to position −327 modestly reduced responsiveness to Tax activation, while truncation down to −220 markedly reduced responsiveness. To further delineate the Tax-responsive region in the promoter sequence spanning −327 to +59 relative to the transcription start site, site-directed mutations were introduced individually or in combination. As shown in Fig. 3B, elements for NF-κB (which is located between −223 and −214) and for NF-IL6 (which is located between −132 and −124) are both involved in Tax-mediated transactivation of the COX-2 promoter: mutation at either of them alone modestly abrogated Tax induction of COX-2 promoter activity, while mutations at both elements markedly abrogated Tax induction of COX-2 promoter activity. In contrast, mutation at the CREB/ATF site (which is located between −59 and −53) had little effect on it. Involvement of NF-κB in Tax activation of the COX-2 promoter was also investigated by overexpressing I-κBα, a specific inhibitor for NF-κB. As expected, while cotransfection of the NF-κB p65 expression vector with the COX-2 promoter upregulated the promoter activity, I-κBα counteracted p65-mediated activation (Fig. 3C). Similarly, overexpression of I-κBα markedly abrogated Tax-mediated activation of the COX-2 promoter (Fig. 3C), suggesting that the NF-κB pathway is critical for its effect. Another NF-κB site is located between −1432 and −327 (H. Inoue and T. Tanabe, unpublished data) and may explain the partial responsiveness of the region to Tax (Fig. 3A).
FIG. 3.
Both the NF-κB and NF-IL6 sites are involved in Tax activation of the COX-2 promoter. (A) Jurkat cells were transfected with 10 μg of the luciferase reporter plasmid along with 10 μg of pMT-2T or pMT-Tax. Results are means ± standard errors of the means from four independent experiments. Fold induction by Tax of the respective luciferase reporter is shown to the right of the bars. (B) Jurkat cells were transfected with 10 μg of the luciferase reporter plasmid along with 10 μg of pMT-2T or pMT-Tax. Results are means ± standard errors of the means from six independent experiments. Fold induction by Tax of the respective luciferase reporter is shown to the right of the bars. (C) Jurkat cells were transfected with 10 μg of phPES(−1432/+59) and 10 μg of pMT-2T, pMT-p65, or pMT-Tax along with pMT-2T or pMT-IκB. Results are means ± standard errors of the means from four independent experiments.
In the present study we have demonstrated that HTLV-1, which is transmitted vertically or horizontally through breast milk or seminal fluid, respectively, and PGs, which are abundant in these body fluids, benefit from each other. These results confirm a recent study demonstrating that PKA activity is required for HTLV-1 LTR activity (34) and further extend the understanding of reciprocal interactions between HTLV-1 and PGs. PGE2 enhances HTLV-1 replication by upregulating the HTLV-1 LTR promoter, and HTLV-1 Tax transactivates a promoter for COX-2, a PG synthetase, leading to production of PGE2. Therefore, it is likely that such mutual aid helps accelerate viral transmission through seminal fluid or breast milk. This hypothesis will require experimental evidence.
PGs and other eicosanoids have been shown to play a key role in the regulation of both humoral and cell-mediated immunity, generally tipping the balance in favor of Th2- and Th3-type responses by modulating cytokine and immunoglobulin production as well as T-cell proliferation and activation (reviewed in references 24 and 26). It therefore is not surprising if PGs influence the pathogenesis of infection with a number of pathogens. In fact, recent studies suggested that PGE2 can modify infection with human immunodeficiency virus type 1, another human retrovirus that is also transmitted through breast milk or seminal fluid (5, 33). Thus, constituents such as PGs in these body fluids may variably influence viral transmission. We are currently investigating PGE2 levels in asymptomatic HTLV-1 carriers as well as effects of other constituents in these body fluids on HTLV-1 infection. Preliminary results have indicated that several other constituents also benefit HTLV-1 infection (M. Moriuchi and H. Moriuchi, unpublished data).
Although HTLV-1 has oncogenic potential, the mode of oncogenesis by HTLV-1 is poorly understood. It has been shown that HTLV-1-encoded Tax protein is necessary and sufficient for cell immortalization (reviewed in references 20 and 35). Several studies showed that Tax-transgenic mice develop several types of malignancy, including leukemia, mesenchymal tumors, and neurofibromas (3, 8, 12, 25). Tax is able to interact with a number of cellular factors involved in transcription and/or cell cycling and is considered to induce cell transformation through such interactions (reviewed in references 20 and 35). Since PGs have also been associated with carcinogenesis (reviewed in reference 30), Tax activation of COX-2 through cellular transcription factors (i.e., NF-κB/Rel family proteins) may contribute to HTLV-1-induced oncogenicity. In fact, a recent study has demonstrated that a number of HTLV-1-transformed cell lines do, but most HTLV-1-uninfected cell lines do not, express COX-2 mRNA (N. Mori [Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan], personal communication), implying a critical role in HTLV-1-induced oncogenicity.
In summary, our studies have identified PGs as another target for HTLV-1 Tax, and reciprocal interactions between HTLV-1 and PGs may have implications for its transmission and oncogenesis.
ACKNOWLEDGMENTS
We thank K.-T. Jeang and U. Siebenlist for reagents, Nagasaki Red-Cross Center and Pre- and Post-Natal Care Unit (Nagasaki University Hospital) for providing blood samples, and K. Iijima and M. Yokoyama for assistance with the figures. We also thank T. Tanabe for critical reading of the manuscript.
REFERENCES
- 1.Busch M P, Lee T H, Heitman J. Allogeneic leukocytes but not therapeutic blood elements induce reactivation and dissemination of latent human immunodeficiency virus type 1 infection: implications for transfusion support of infected individuals. Blood. 1992;80:2128–2135. [PubMed] [Google Scholar]
- 2.Bydgeman M, Gillespie A. Prostaglandins in seminal fluids. In: Lee J B, editor. Prostaglandins in human reproduction. New York, N.Y: Elsevier; 1982. p. 215. [Google Scholar]
- 3.Coscoy L, Gonzalez-Dunia D, Tangy F, Syan S, Brahic M, Ozden S. Molecular mechanism of tumorigenesis in mice transgenic for the human T-cell leukemia virus Tax gene. Virology. 1998;248:332–341. doi: 10.1006/viro.1998.9298. [DOI] [PubMed] [Google Scholar]
- 4.Dhib-Jalbut S, Hoffman P M, Yamabe T, Sun D, Xia J, Eisenberg H, Berger G, Ruscetti F W. Extracellular human T-cell lymphotropic virus type 1 Tax protein induces cytokine production in adult human microglial cells. Ann Neurol. 1994;36:787–790. doi: 10.1002/ana.410360516. [DOI] [PubMed] [Google Scholar]
- 5.Dumais N, Barbeau B, Olivier M, Tremblay M J. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-κB-dependent and -independent signaling pathways. J Biol Chem. 1998;273:27306–27314. doi: 10.1074/jbc.273.42.27306. [DOI] [PubMed] [Google Scholar]
- 6.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphoma virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 7.Gerozissis K, Jouannet P, Soufir J C, Dray F. Origin of prostaglandins in human semen. J Reprod Fertil. 1982;65:401–404. doi: 10.1530/jrf.0.0650401. [DOI] [PubMed] [Google Scholar]
- 8.Grossman W J, Kimata J T, Wong F-H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammerström S. Leukotrienes. Annu Rev Biochem. 1983;52:355–377. doi: 10.1146/annurev.bi.52.070183.002035. [DOI] [PubMed] [Google Scholar]
- 10.Hawkes J S, Bryan D L, James M J, Gibson R A. Cytokines (IL-1β, IL-6, TNF-α, TGF-β1, and TGF-β2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res. 1999;46:194–199. doi: 10.1203/00006450-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Himes S R, Katsikeros R, Shannon M F. Costimulation of cytokine gene expression in T cells by the human T leukemia/lymphotropic virus type I trans activator Tax. J Virol. 1996;70:4001–4008. doi: 10.1128/jvi.70.6.4001-4008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinrichs S H, Nerenberg M, Reynolds R K, Khoury G, Jay G. A transgenic mouse model for human neurofibromatosis. Science. 1987;237:1340–1343. doi: 10.1126/science.2888191. [DOI] [PubMed] [Google Scholar]
- 13.Höllsberg P, Hafler D A. Pathogenesis of disease induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H, Tanabe T. Transcriptional role of the nuclear factor kappa B site in the induction by lipopolysaccharide and suppression by dexamethasone of cyclooxygenase-2 in U937 cells. Biochem Biophys Res Commun. 1998;244:143–148. doi: 10.1006/bbrc.1998.8222. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 16.Jain L, Vidyasagar D, Xanthou M, Ghai V, Shimada S, Blend M. In vivo distribution of human milk leukocytes after ingestion by newborn baboons. Arch Dis Child. 1989;64:930–933. doi: 10.1136/adc.64.7_spec_no.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindholm P F, Marriott S J, Gitlin S D, Bohan C A, Brady J N. Induction of nuclear NF-κB DNA binding activity after exposure of lymphoid cells to soluble Tax1 protein. New Biol. 1990;2:1034–1043. [PubMed] [Google Scholar]
- 18.Macchi B, Faraoni I, Zhang J, Grelli S, Favalli C, Mastino A, Bonmassar E. AZT inhibits the transmission of human T cell leukemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J Gen Virol. 1997;78:1007–1016. doi: 10.1099/0022-1317-78-5-1007. [DOI] [PubMed] [Google Scholar]
- 19.Marriott S J, Lindholm P F, Reid R L, Brady J N. Soluble HTLV-I Tax1 protein stimulates proliferation of human peripheral blood lymphocytes. New Biol. 1991;3:678–686. [PubMed] [Google Scholar]
- 20.Mesnard J-M, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 21.Moriuchi H, Moriuchi M, Combadiere C, Murphy P M, Fauci A S. CD8+ T-cell-derived factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriuchi H, Moriuchi M, Fauci A S. NF-κB potently upregulates the promoter activity for RANTES, a chemokine that inhibits HIV-1 infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- 23.Moriuchi H, Moriuchi M, Fauci A S. Factors secreted by HTLV-I-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J Exp Med. 1998;187:1689–1697. doi: 10.1084/jem.187.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morteau O. COX-2: promoting tolerance. Nat Med. 1999;5:867–868. doi: 10.1038/11301. [DOI] [PubMed] [Google Scholar]
- 25.Nerenberg M, Hincrichs S H, Reynold R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type I induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 26.Phipps R P, Stein S H, Roper R L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 27.Quayle A J, Kelly R W, Hargreave T B, James K. Immunosuppression by seminal prostaglandins. Clin Exp Immunol. 1989;75:387–391. [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen H, Goodman D B, Tenenhouse A. The role of cyclic AMP and calcium in cell activation. Crit Rev Biochem. 1972;1:95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- 29.Roper R L, Phipps R P. Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res. 1994;22:101–110. [PubMed] [Google Scholar]
- 30.Shiff S J, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal anti-inflammatory drugs (NSAIDs) J Exp Med. 1999;190:445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith M R, Greene W. Molecular biology of type I human T-cell leukemia/lymphoma virus (HTLV-I) and adult T-cell leukemia. J Clin Investig. 1991;87:761–766. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith W L, DeWitt D L. Prostaglandin endopeptidase H synthetase-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 33.Thivierge M, Le Gouill C, Tremblay M J, Stañková J, Rola-Pleszczynski M. Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood. 1998;92:40–45. [PubMed] [Google Scholar]
- 34.Turgeman H, Aboud M. Evidence that protein kinase A activity is required for the basal and tax-stimulated transcriptional activity of human T-cell leukemia virus type-I long terminal repeats. FEBS Lett. 1998;428:183–187. doi: 10.1016/s0014-5793(98)00513-4. [DOI] [PubMed] [Google Scholar]
- 35.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Weiler I J, Hickler W, Sprenger R. Demonstration that milk cells invade the suckling neonatal mouse. Am J Reprod Immunol. 1983;4:95–98. doi: 10.1111/j.1600-0897.1983.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi K. Human T-lymphotropic virus type I in Japan. Lancet. 1994;343:213–216. doi: 10.1016/s0140-6736(94)90994-6. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto S, Yamamoto K, Kurobe H, Yamashita R, Yamaguchi H. Transcriptional regulation of fatty acid cyclooxygenases-1 and -2. Int J Tissue React. 1998;XX:17–22. [PubMed] [Google Scholar]