Abstract
Objective:
We aimed to investigate the relationship between sociodemographic and clinical characteristics, as well as the utilization of diabetes technologies, with diabetes management in individuals with type 1 diabetes (T1D).
Materials and Methods:
Our study included 134 cases diagnosed with T1D who were followed for at least 1 year with T1D.
Results:
Of the cases, 67.2% were using insulin pens as their insulin regimen, while 37.8% were using insulin pumps. The rate of continuous glucose monitoring (CGM) usage was 29.9%. The rate of CGM usage was 5% in families with low income levels. Glycosylated hemoglobin A1c (HbA1c) level of children with working mothers was found to be higher compared to those with non-working mothers (median 9.2% vs. 8%; P = .009). Cases with 3 or more siblings had higher HbA1c levels compared to those with 2 or fewer siblings (median 8.7% vs. 8.1%; P = .044).The median HbA1c was 8.7% in cases using insulin pens and checking fingerstick blood glucose (SMBG); 8.3% in cases using insulin pumps and SMBG; 7.6% in cases using insulin pens with CGM, and 7.5% in cases using insulin pumps with CGM (P = .003).
Conclusion:
The utilization of insulin pumps with CGM in T1D cases exhibited lower HbA1c levels. Similarly, even the usage of insulin pens with CGM demonstrated improved diabetes management. Maternal employment and having a higher number of siblings may negatively affect diabetes management due to increased caregiver burden. We believe that personalized healthcare delivery tailored to the individual needs of T1D patients based on family and clinical characteristics could have positive effects on diabetes management.
Keywords: Continuous glucose monitoring, diabetes management, insulin pump, sociodemographic factors, type 1 diabetes
What is already known on this topic?
The management of type 1 diabetes is a lifelong process that requires close collaboration between the child, their family, and their healthcare providers.
Sociodemographic factors and utilization of diabetes technologies are important for diabetes communication and management.
Using insulin pumps with continuous glucose monitoring in type 1 diabetes cases demonstrates a positive impact on diabetes management.
What this study adds on this topic?
Insulin pump usage without continuous glucose monitoring did not provide an advantage over using insulin pens.
Maternal employment and having a higher number of siblings may negatively affect diabetes management due to increased caregiver burden.
Introduction
Type 1 diabetes (T1D) is a chronic condition where pancreatic β cells are destroyed due to autoimmune attacks, leading to decreased insulin production.1-3 This condition results in the body’s inability to produce adequate insulin and control blood glucose levels. Typically starting in childhood or young adulthood, this condition often requires lifelong insulin therapy for treatment. For this purpose, insulin pens with multiple dose injections or insulin pumps, which continuously deliver insulin subcutaneously in small and portable devices, are commonly used. Additionally, for optimal diabetes management, a balanced diet plan, regular exercise, and monitoring of blood glucose levels are also important. In glucose monitoring, traditional intermittent fingerstick glucose measurements (self-monitoring blood glucose, SMBG) can be used, or with advancing technology, continuous glucose monitors (CGM) can be utilized. CGM devices employ sensors placed under the skin to continuously monitor blood glucose levels. These sensors measure glucose levels at regular intervals and transmit the data to a receiver device, allowing the user to see their glucose levels in real-time. CGM can also help identify fluctuations in glucose levels and assist in adjusting insulin therapy more precisely.1,4
The management of T1D is a lifelong process that requires close collaboration between the child, their family, and their healthcare providers. Sociodemographic factors, including age, sex, education level, income level, ethnic background, and geographical location, which influence family characteristics and individuals’ lifestyles, behaviors, and health habits, are important for diabetes communication and management.5,6 Various studies indicate that sociodemographic factors can have a significant impact on glucose control and diabetes management. Diabetes technologies play a crucial role in diabetes management and are continually evolving, with sociocultural factors believed to influence access to and the ability to utilize diabetes technologies.6,7
Researching the relationship between diabetes care and various factors will help us understand the significant role that sociodemographic and clinical characteristics may play in the management of individuals with T1D. Such insights can contribute to making diabetes care and management strategies more effective and personalized. For this purpose, we aimed to investigate the associations between sociodemographic and clinical characteristics, as well as the utilization of diabetes technologies, particularly CGM and insulin pump usage, with diabetes management in individuals with T1D.
Materials and Methods
Participant Selection and Clinical Investigations
Our study was designed as a single-center, cross-sectional study. Between January 2012 and January 2022, a total of 134 cases diagnosed with T1D, with a minimum of 1 year of follow-up at the Pediatric Endocrinology Clinic of our hospital, were included in our study. We established the diagnosis of T1D in patients based on the criteria outlined in guidelines of the International Society for Pediatric and Adolescent Diabetes (ISPAD).8 Data recorded from digital medical records included plasma glucose levels on admission, C-peptide levels, glycosylated hemoglobin A1c (HbA1c) levels, and T1D-associated autoantibody status (defined as the presence of at least one positive result for antibodies to glutamic acid decarboxylase, islet cell antibodies, or insulin antibodies). These cases were not in the honeymoon period and did not have any other chronic diseases aside from T1D. The parents of the participants were requested to complete a questionnaire assessing sociodemographic characteristics between January and March 2023, over a period of 3 months. This questionnaire encompassed details such as age, sex, age of parents, parental education level, occupation of parent, family income level, and number of siblings. Family income was determined based on parents’ self-reports. The total income considered included salaries of working parents, rent, and other additional income. According to the data collected by the Turkish Statistical Institute (TÜİK) in the year when sociodemographic characteristics were gathered, those below the poverty line were classified as “poor,” those able to meet basic family needs and allocate some budget for additional expenses were classified as “moderate,” and those able to make luxury expenditures without difficulty beyond basic needs were classified as “high.” The clinical characteristics included factors such as the duration of diabetes, insulin regimen (pen or pump), method of glucose monitoring (SMBG or CGM), whether the patient had experienced diabetic ketoacidosis (DKA) in the last 6 months, and the mean HbA1c levels over the past year. Furthermore, patients underwent anthropometric assessments, including measurements of body weight using an electronic scale. Height measurements were obtained using a wall-mounted stadiometer in both the upright standing position and during deep inspiration. Body mass index (BMI) was calculated by dividing weight by the square of height (kg/m2). Standard deviation scores (SDS) for height, weight, and BMI were determined using reference values specific to Turkish children.9 T1D cases were categorized based on their clinical characteristics and utilization of diabetes technologies, and subsequently compared with each other regarding their mean HbA1c levels over the previous year.
Statistical Analysis
We performed statistical analyses using the Statistical Package for the Social Sciences version 23.0 for Windows (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as median [Interquartile range (IQR)] or mean ± standard deviation, while categorical variables were presented as frequencies and percentages. Normality was assessed using the Shapiro–Wilk test, and distribution was checked for continuous variables. The Mann-Whitney U-test was employed to compare non-normally distributed parameters between 2 groups. Kruskal–Wallis tests were utilized as the parameters did not exhibit a normal distribution when comparing more than 2 groups. If the Kruskal–Wallis test was significant, Bonferroni-corrected Mann–Whitney U-test was used as a post-hoc test. Correlation analysis was performed with the Spearman’s rho test. For correlational coefficient r values, a relationship is considered very weak if r < 0.25, weak if r ranges from 0.26 to 0.49, medium if r ranges from 0.50 to 0.69, high if r ranges from 0.70 to 0.89, and very high if r ranges from 0.90 to 1.0. The Pearson chi-square test was used to compare proportions between independent groups. Statistical significance was determined at P < .05.
Ethics Committee Approval
Prior to commencing the study, approval was obtained from the local ethics committee of Akdeniz University (approval number: TBAEK-173, date: April 2, 2024). Informed consent was obtained from the parents of all participants before their inclusion in the study. The research strictly adhered to the principles outlined in the Declaration of Helsinki and followed ethical guidelines.
Results
Clinical and Sociodemographic Characteristics of Children with T1D
The age of the cases, age at diabetes diagnosis, anthropometric measurements, HbA1c levels, and other clinical features, along with parent and family characteristics, are provided in Table 1. The female-to-male ratio was 1.03. Among the cases, 67.2% utilized insulin pens as their insulin regimen, while 37.8% used insulin pumps. The utilization rate of CGM was 29.9%. The rate of cases using carbohydrates (CH) counting method was 59%. Among those who counted CH, 64.5% had fathers with a high school or university education, while 35.5% had fathers who were not literate or had only primary school education. Similarly, 63.2% of mothers had a high school or university education, and 36.8% of mothers were not literate or had only primary school education. In the group not using CH counting method, 29.1% of fathers and 43.6% of mothers were either not literate or had only primary school education. When comparing the educational levels of mothers and fathers between the groups using and not using CH counting method, no statistically significant difference was found (P = .420; P = .441, respectively).
Table 1.
Clinical and Sociodemographic Characteristics of Children with Type 1 Diabetes
| Variable | Results (n = 134) |
|---|---|
| Age (years) | 10 (5) |
| Sex F (n, %) M (n, %) |
68 (50.8) 66 (49.2 ) |
| Height SDS | 0.28 (1.2) |
| BMI SDS | 0.23 (1.4) |
| Age at T1D diagnosis (years) | 7.7 (6) |
| Diabetes duration (years) | 2.5 (4) |
| DKA+ (n, %) | 12 (9) |
| Insulin regimen Pen Pump |
90 (67.2 %) 44 (37.8 %) |
| CGM + (n, %) | 40 (29.9) |
| HbA1c (%) | 8.3 (2.6) |
| CH counting + (n, %) | 79 (59) |
| Mother’s age (years) | 38.9 ± 5.7 |
| Mother’s educational status Not literate Primary school graduation High school graduation University graduation |
3 (2.2%) 49 (36.6%) 48 (35.8%) 34 (25.4%) |
| Mother’s employment status Not employed Employed |
43 (32.1%) 91 (67.9%) |
| Father’s age (years) | 42.8 ± 6.1 |
| Father’s educational status Not literate Primary school High school University |
– 44 (32.8%) 53 (38.8%) 38 (28.4%) |
| Mother’s employment status Not employed Employed |
118 (88.1%) 16 (11.9%) |
| Family income Poor Moderate High |
19 (14.2%) 101 (75.4%) 14 (10.4%) |
| Number of siblings ≤ 2 ≥ 3 |
89 (66.4%) 45 (33.6%) |
Data are expressed as median (IQR), mean ± standard deviation or as number (percent).
BMI, body mass index; CGM, continuous glucose monitoring; DKA, diabetic ketoacidosis; F, female; HbA1c, glycosylated hemoglobin; M, male; SDS, standard deviation score; T1D, type 1 diabetes.
Examination of Diabetes Management Across Different Subgroups Through HbA1c
When evaluating HbA1c levels by age group; we observe that the median HbA1c level for children < 12 years old was median 8%, whereas for those ≥ 12 years old, the median HbA1c level was 8.7%. This difference was statistically significant, with a P-value of .005, indicating that adolescents had higher HbA1c levels compared to younger children.
When evaluating HbA1c levels among subgroups of cases, it was higher in females compared to males (median 8.6% vs. 8%; P = .048). No difference was observed based on maternal education level, but the average HbA1c level was higher in cases with working mothers compared to those with non-working mothers (median 9.2% vs. 8%; P = .009). The HbA1c level was also higher in those not using the CH counting method (median 8.9% vs. 7.9%; P = .001). No difference in HbA1c was found based on paternal education and employment status. Cases with 3 or more siblings had higher HbA1c levels compared to those with 2 or fewer siblings (median 8.7% vs. 8.1%; P = .044) (Table 2). All subgroup comparisons were also analyzed separately for groups < 12 and ≥ 12 years old, and the results are presented in Table 2. No correlation was found between HbA1c and age at diagnosis, diabetes duration, maternal age, or paternal age (Table 3).
Table 2.
HbA1c Levels Among Different Subgroups of Children with Type 1 Diabetes
| HbA1c of Total Group (n = 134) |
P | HbA1c of Children (< 12 years, n = 83) |
P | HbA1c of Adolescents (≥ 12 years, n = 51) |
P | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| F | 8.6 (2.9) | .048 | 8.3 (2.6) | .083 | 9.3 (3.8) | .358 |
| M | 8 (2.3) | 7.7 (2.4) | 8.4 (3.7) | |||
| Insulin regimen | ||||||
| Pump | 7.8 (2.2) | .047 | 7.5 (1.7) | .096 | 8.4 (3.1) | .081 |
| Pen | 8.5 (2.8) | 8.2 (2.2) | 9.7 (3.8) | |||
| CGM usage | ||||||
| CGM (+) | 7.5 (1.8) | < .001 | 7.5 (1.8) | .041 | 7.6 (1.8) | .013 |
| CGM (−) | 8.6 (2.7) | 8.3 (2.0) | 9.7 (3.6) | |||
| CH counting method | ||||||
| Using | 7.9 (2.3) | .001 | 7.6 (1.7) | < .001 | 8.6 (3.8) | .533 |
| Not using | 8.9 (2.4) | 9.1 (2.3) | 8.9 (3.4) | |||
| Mother’s educational status | ||||||
| Not literate or primary school graduation | 8.7 (2.6) | .380 | 8.4 (2.1) | .230 | 9.3 (3.5) | .887 |
| At least high school graduation | 8.0 (2.9) | 7.7 (1.4) | 8.7 (4.1) | |||
| Mother’s employment status | ||||||
| Employed | 9.2 (3.6) | .009 | 8.3 (1.9) | .253 | 11.4 (2.9) | .006 |
| Not employed | 8.0 (2.0) | 7.7 (1.9) | 8.4 (2.1) | |||
| Father’s educational status | ||||||
| Not literate or primary school graduation | 9.0 (2.9) | .078 | 9.1 (2.7) | .089 | 8.9 (3.6) | .583 |
| At least high school graduation | 8.1 (2.4) | 7.8 (1.4) | 8.4 (3.6) | |||
| Father’s employment status | ||||||
| Employed | 8.3 (2.7) | .748 | 7.9 (1.8) | .731 | 8.3 (2.2) | .213 |
| Not employed | 8.4 (3.0) | 9.1 (3.6) | 8.9 (3.7) | |||
| Family income | ||||||
| Poor | 8.7 (2.4) | .322 | 8.1 (3.4) | .832 | 8.8 (3.7) | .364 |
| Moderate-high | 8.2 (2.8) | 8.0 (1.8) | 8.3 (1.0) | |||
| Number of siblings | ||||||
| ≤ 2 | 8.1 (2.7) | .044 | 7.7 (2.5) | .040 | 8.6 (3.2) | .142 |
| ≥ 3 | 8.7 (2.7) | 8.6 (2.1) | 8.9 (4.4) | |||
Data are expressed as median (IQR).
BMI, body mass index; CGM, continuous glucose monitoring; F, female; HbA1c, glycosylated hemoglobin; M, male.
Table 3.
Evaluation of the Correlation Between HbA1c Levels and Various Clinical Characteristics
| HbA1c | |
|---|---|
| Age at T1D diagnosis | P = .998 (r < 0.001) |
| Diabetes duration | P = .121 (r = 0.135) |
| Mother’s age | P = .505 (r = 0.058) |
| Father’s age | P = .641 (r = 0.041) |
Spearman rho correlation.
HbA1c, glycosylated hemoglobin; T1D, type 1 diabetes.
Investigation of Diabetes Management Based on Diabetes Technologies
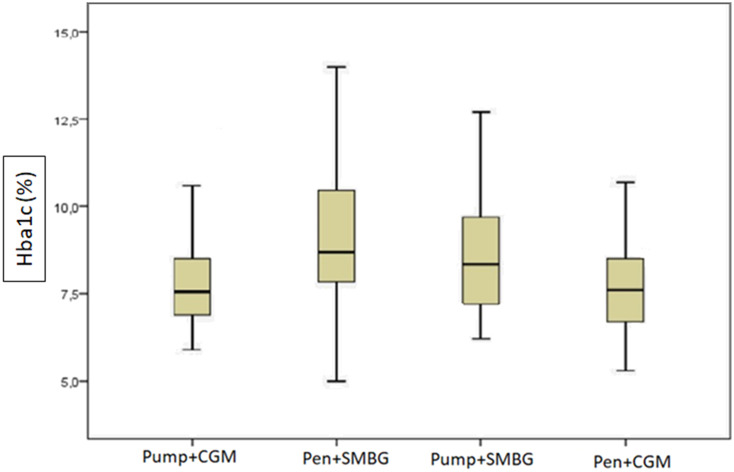
Among cases using insulin pens and performing SMBG (n = 71), the median HbA1c was 8.7%; for those using insulin pumps and performing SMBG (n = 22), the median HbA1c was 8.3%; for cases using insulin pens with CGM (n = 18), the median HbA1c was 7.6%; and for cases using insulin pumps with CGM (n = 22), the median HbA1c was 7.5% (P = .003) (Table 4, Figure 1).
Table 4.
Assessment of HbA1c Levels According to Insulin Regimen and Glucose Measurement Method
| Insulin pen + SMBG (n = 71) | Insulin pump + SMBG (n = 22) | Insulin pen + CGM (n = 19) | Insulin pump + CGM (n = 22) | P | |
|---|---|---|---|---|---|
| HbA1c (%) | 8.7 (2.7) | 8.3 (2.7) | 7.6 (1.9) | 7.5 (1.7) |
.003
a
.191b .014 c .005 d .048e .042 f .967g |
Data are expressed as median (IQR). The values marked by letters b, c, d, e, f, g were calculated using Mann–Whitney U-test with Bonferroni correction. Values in bold indicate statistical significance.
CGM, continuous glucose monitoring; HbA1c, glycosylated hemoglobin; SMBG, self-monitoring blood glucose (fingerstick capillary blood glucose).
aKruskal–Wallis test for all groups’ comparison.
bIinsulin pen+SMBG vs insulin pump+SMBG comparison.
cInsulin pen+SMBG vs insulin pen+CGM comparison.
dInsulin pen+SMBG vs insulin pump+CGM comparison.
eInsulin pump+SBMG vs insulin pen+CGM comparison.
fInsulin pump+SBMG vs insulin pump+CGM comparison.
gInsulin pen+CGM vs insulin pump+CGM comparison.
Figure 1.
Assessment of HbA1c levels according to insulin regimen and blood glucose measurement method.
Table 5 illustrates the rates of CGM and insulin pump usage according to family income status. For families with high income, the usage rates were higher, with 64.2% using CGM and 57.1% using an insulin pump.
Table 5.
Rates of CGM and Insulin Pump Usage According to Family Income Status
| Poor İncome (n = 19) | Moderate İncome (n = 101) | High İncome (n = 14) | P | |
|---|---|---|---|---|
| CGM | 1 (5) | 30 (29.7) | 9 (64.2) | .001 |
| Insulin pump | 3 (15.7) | 33 (32.6) | 8 (57.1) | .044 |
Data are expressed as n (%).
CGM, continuous glucose monitoring.
Discussion
There is a wealth of research indicating that sociodemographic factors can impact the management of T1D in children. Factors such as family income, level of education, age, sex, ethnic background, and geographic location are seen to play a decisive role in diabetes management.10,11 Certain researchers have explored the sex effect and found that girls tend to have higher HbA1c levels compared to boys like our study and female sex is indicated as a non-modifiable factor was associated with poor metabolic control.12-14 On the other hand, in our study, when we divided the cases into child and adolescent age groups, we observed no difference in HbA1c levels between boys and girls. Conversely, according to a review article, sex and age effects appear to be intertwined: no sex effect was noted before puberty or in young adults, yet pubescent girls exhibited higher HbA1c levels than boys during puberty, particularly between the ages of 13 and 21.15 The observed difference in those studies was attributed to a possible effect of the female pubertal hormones.16,17
In our study, when HbA1c levels were examined separately for children and adolescent age groups, the median HbA1c was lower in the children’s group. Young age has also been reported to be associated with optimal glycemic control in some studies.12,13,18 Despite initially achieving better glycemic control upon insulin therapy initiation, these children exhibited a greater decline in glycemic control during the first 5 years post-diagnosis compared to younger patients. Interestingly, this deterioration persisted despite older children maintaining stricter glycemic control throughout the study period.10,18 This situation has been linked to the notion that adherence to diabetes care is typically more rigorous among younger individuals who are under the management of their parents, as opposed to teenagers who frequently take on the responsibility of controlling their blood glucose levels independently. In this scenario, studies reveal varying outcomes regarding age and diabetes management depending on the level of caregivers’ involvement in diabetes management and the influence of other contributing factors. In our study, we observed lower HbA1c levels in cases where insulin dosing was adjusted based on CH counting by parents in the children age group. However, in the adolescent age group, HbA1c levels were statistically similar between those who counted CH and those who did not. This situation could be attributed to the indulgence in occasional meals or relaxation in adherence to strict rules during the adolescent period, affecting control management. On the other hand, it is difficult to make a definitive interpretation in our study since we did not compare the HbA1c levels before switching to the CH counting method with the HbA1c levels under the CH counting method. Contrary findings have been reported in the literature.19 Managing T1D in young children poses distinct challenges for families and caregivers. These little ones often have unpredictable eating habits and varying levels of physical activity on a day-to-day basis. At such a tender age, numerous children have not yet reached the developmental stage to understand, express, or even identify the symptoms of hypoglycemia and hyperglycemia to their caregivers.20,21 Therefore, cases in the younger age group are often dependent on caregivers while developing their daily skills and attempting to integrate them into diabetes management. This situation complicates diabetes management.
It is a recognized fact that families with lower income levels may encounter greater challenges in managing the costs associated with diabetes care and might face hurdles in accessing necessary treatments.22 Likewise, parents with lower levels of education may lack sufficient knowledge about diabetes management, leading to difficulties in implementing effective treatment plans.23 In our study, although cases with higher levels of parental education and better family income tended to exhibit lower median HbA1c levels, this difference did not reach statistical significance. This discrepancy could be attributed to the small sample size in our study. Furthermore, it was observed that children with T1D whose mothers were not employed and those with fewer siblings tended to have better HbA1c levels. This phenomenon was attributed to the decreased burden of responsibility on caregivers, as T1D requires demanding and continuous care and close monitoring. As caregiver responsibilities lessen, it appears that T1D management becomes more effective.
The unique nature of treating T1D significantly impacts the mental and social well-being of patients, as well as their lifestyle. This influence arises from changes in dietary patterns and physical activity, the necessity for ongoing treatment and monitoring, imposed limitations, the requirement for insulin injections, and the potential complications associated with diabetes.24 For the purpose of facilitating diabetes management, achieving optimal glucose levels, and enhancing daily comfort for patients, insulin pump and CGM systems have been developed. The use of diabetes technologies has shown greater improvements in glycemic control in both adult and pediatric patients compared to traditional methods.25-29 However, access to diabetes technologies varies depending on the healthcare policies of countries. While it is often suggested that disparities in diabetes care and the adoption of diabetes technologies stem from factors such as parental income and education rather than racism,7 findings from a study illustrate a notable contrast. The study revealed that 68% of White children with parents holding college or graduate degrees were using insulin pumps, whereas only 34% of non-Hispanic Black children with parents at similar education levels were utilizing insulin pump therapy. This discrepancy has been linked to disparities in health insurance coverage.30 Since all participants in our study were Turkish children of the same ethnic background, racial differences could not be evaluated. However, in Türkiye, the support provided by non-private health insurance for insulin pump costs amounts to 16 000 TL (~$470), which remains relatively low compared to the total cost of the pump. Additionally, monthly expenses for sets and reservoirs add to the economic burden. CGM, on the other hand, is not covered by non-private health insurance. Following the earthquake that affected 10 provinces in 2023, children with T1D residing in earthquake-affected areas were provided with CGM support for 1 year; however, this does not cover the period and region in which our study data were collected. Considering these circumstances, access to diabetes technologies is directly influenced by the economic status of families. In our study, when patients were grouped according to the use of insulin pumps and CGM, we observed the best HbA1c levels in cases where insulin pumps and CGM were used together. Due to economic reasons and different preferences of families, we observed that the use of pumps without CGM did not result in significant improvement in HbA1c compared to insulin pen and SMBG. This may be due to the difficulty in tracking blood glucose fluctuations, increases, and decreases trends without CGM, and the flexibility provided by pump use in terms of meal times and frequency compared to insulin pen use. On the other hand, we observed a significant decrease in HbA1c levels in cases where CGM support was used even with insulin pen. This suggests that in cases where the combined use of insulin pumps and CGM is not financially feasible, families may be encouraged to at least opt for CGM use. The ISPAD 2022 guideline recommends the use of CGM for all insulin-treated children under the age of 7.1 In our study, the usage rate of CGM in children from families with low income levels was notably low at 5%. The suggested blood glucose monitoring frequency of 4-6 times per day often proves insufficient in attaining desired glucose and HbA1c levels. A significant portion of time is spent outside the target glycemic range. Even with increased monitoring frequency, such as 7 or 10 checks per day, undetected hypoglycemic and hyperglycemic episodes remain prevalent in especially preschool children receiving insulin treatment.1,31,32 Caregivers’ qualitative feedback indicates that the use of CGM as part of remote monitoring can foster feelings of security, reduce anxiety, and enhance confidence in other caregivers.1,33
Our study has certain limitations. First, it is a cross-sectional study and does not cover long-term follow-ups of individuals, including parameters related to diabetes management before and after the initiation of diabetes technology devices and their longitudinal course as well as before and after using CH counting method. Secondly, when we divided the cases into 4 subgroups based on insulin pump and CGM usage, the number of cases decreased in small groups. Thirdly, HbA1c level alone does not provide information regarding the optimal status of diabetes management. Parameters such as time spent within the target glucose range and the frequency of hypoglycemic episodes, which we did not calculate for each case, are also important indicators of the effectiveness of diabetes management. Lastly, some data in our study, such as family income level and parents’ education status, are based on self-reports and do not include evidence-based methods, thus there is a possibility of error.
In our study, we found that cases using CGM had lower HbA1c levels compared to other groups. We observed that the usage rates of diabetes technologies varied according to family income levels. Factors such as a mother’s employment and having multiple siblings may negatively affect diabetes management due to increased caregiver workload. We believe that individualized healthcare delivery based on family, environmental, and clinical characteristics could have positive effects on diabetes management in individuals with T1D.
Funding Statement
This study received no funding.
Footnotes
Ethics Committee Approval: This study was approved by Ethics Committee of Akdeniz University (approval number: TBAEK-173; date: April 2, 2024).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Z.D., M.P.; Design – Z.D., M.P.; Supervision – Z.D., M.P.; Resources – Z.D., E.B.Ç., H.T., M.P.; Materials – Z.D., E.B.Ç., H.T., M.P.; Data Collection and/or Processing – Z.D., E.B.Ç., H.T., M.P.; Analysis and/or Interpretation – Z.D.; Literature Search – Z.D., M.P.; Writing – Z.D.; Critical Review – M.P.
Declaration of Interests: The authors have no conflicts of interest to declare.
References
- 1. de Bock M, Codner E, Craig ME, et al. ISPAD Clinical Practice Consensus Guidelines 2022: glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr Diabetes. 2022;23(8):1270 1276. ( 10.1111/pedi.13455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donbaloğlu Z, Tuhan H, Tural Kara T, et al. The examination of the relationship between COVID-19 and new-onset Type 1 diabetes mellitus in children. Turk Arch Pediatr. 2022;57(2):222 227. ( 10.5152/TurkArchPediatr.2022.21284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dündar İ, Akıncı A, Çamtosun E, Kayaş L, Çiftçi N, Özçetin E. Type 1 diabetes incidence trends in a cohort of Turkish children and youth. Turk Arch Pediatr. 2023;58(5):539 545. ( 10.5152/TurkArchPediatr.2023.23036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee S, Ooi L, Lai Y. Children and adolescents: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl1):180 199. [DOI] [PubMed] [Google Scholar]
- 5. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial–ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306 313. ( 10.1089/dia.2020.0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donbaloğlu Z, Barsal Çetiner E, İnan Yüksel A, et al. Sleep disturbances in children and adolescents with type 1 diabetes mellitus: prevalence, and relationship with diabetes management. Sleep Med. 2024;115:55 60. ( 10.1016/j.sleep.2024.01.031) [DOI] [PubMed] [Google Scholar]
- 7. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care. 2021;44(1):14 16. ( 10.2337/dci20-0048) [DOI] [PubMed] [Google Scholar]
- 8. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(suppl 27):155 177. ( 10.1111/pedi.12701) [DOI] [PubMed] [Google Scholar]
- 9. Neyzi O, Bundak R, Gökçay G, et al. Reference values for weight, height, head circumference, and body mass index in Turkish children. J Clin Res Pediatr Endocrinol. 2015;7(4):280 293. ( 10.4274/jcrpe.2183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogugua CF, Chikani UN, Okiche CY, Ibekwe UM. Sociodemographic determinants of glycaemic control among children with type 1 diabetes in South Eastern Nigeria. Pan Afr Med J. 2021;38:250. ( 10.11604/pamj.2021.38.250.19790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zuijdwijk CS, Cuerden M, Mahmud FH. Social determinants of health on glycemic control in pediatric type 1 diabetes. J Pediatr. 2013;162(4):730 735. ( 10.1016/j.jpeds.2012.12.010) [DOI] [PubMed] [Google Scholar]
- 12. Pironetti R, Saha MT, Luukkaala T, Keskinen P. Sociodemographic factors affecting glycaemic control in Finnish paediatric patients with type 1 diabetes. Endocrinol Diabetes Metab. 2023;6(6):e452. ( 10.1002/edm2.452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell MS, Schatz DA, Chen V, et al. A contrast between children and adolescents with excellent and poor control: the T1D exchange clinic registry experience. Pediatr Diabetes. 2014;15(2):110 117. ( 10.1111/pedi.12067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbauer J, Dost A, Karges B, et al. Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35(1):80 86. ( 10.2337/dc11-0993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gloaguen E, Bendelac N, Nicolino M, Julier C, Mathieu F. A systematic review of non-genetic predictors and genetic factors of glycated haemoglobin in type 1 diabetes one year after diagnosis. Diabetes Metab Res Rev. 2018;34(8):e3051. ( 10.1002/dmrr.3051) [DOI] [PubMed] [Google Scholar]
- 16. Elamin A, Omer MI, Zein K, Tuvemo T. Epidemiology of childhood type I diabetes in Sudan, 1987-1990. Diabetes Care. 1992;15(11):1556 1559. ( 10.2337/diacare.15.11.1556) [DOI] [PubMed] [Google Scholar]
- 17. Kyokunzire C, Matovu N, Mayega RW. Factors associated with adherence to diabetes care recommendations among children and adolescents with type 1 diabetes: a facility-based study in two urban diabetes clinics in Uganda. Diabetes Metab Syndr Obes. 2018;11:93 104. ( 10.2147/DMSO.S156858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clements MA, Lind M, Raman S, et al. Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res Care. 2014;2(1):e000039. ( 10.1136/bmjdrc-2014-000039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Commissariat PV, Boyle CT, Miller KM, et al. Insulin pump use in young children with type 1 diabetes: sociodemographic factors and parent-reported barriers. Diabetes Technol Ther. 2017;19(6):363 369. ( 10.1089/dia.2016.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinzimer SA, Swan KL, Sikes KA, Ahern JH. Emerging evidence for the use of insulin pump therapy in infants, toddlers, and preschool-aged children with type 1 diabetes. Pediatr Diabetes. 2006;7(suppl 4):15 19. ( 10.1111/j.1399-543X.2006.00172.x) [DOI] [PubMed] [Google Scholar]
- 21. Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034 2054. ( 10.2337/dc14-1140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karachaliou F, Simatos G, Simatou A. The challenges in the development of diabetes prevention and care models in low-income settings. Front Endocrinol (Lausanne). 2020;11:518. ( 10.3389/fendo.2020.00518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baharvand P, Hormozi M. Can parents’ educational level and occupation affect perceived parental support and metabolic control in adolescents with type 1 diabetes? J Educ Health Promot. 2019;8:11. ( 10.4103/jehp.jehp_215_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mousavi SI, Alizadeh Chaharborj T, Sheikh MR. Frequency of psychiatric disorder symptoms in diabetic patients of Yasuj city in 2014. J Neyshabur. Univ Med Sci. 2016;4:65 71. [Google Scholar]
- 25. Ahern JA, Boland EA, Doane R, et al. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatr Diabetes. 2002;3(1):10 15. ( 10.1034/j.1399-5448.2002.30103.x) [DOI] [PubMed] [Google Scholar]
- 26. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311 320. ( 10.1056/NEJMoa1002853) [DOI] [PubMed] [Google Scholar]
- 27. Blackman SM, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15(8):564 572. ( 10.1111/pedi.12121) [DOI] [PubMed] [Google Scholar]
- 28. Sherr JL, Hermann JM, Campbell F, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87 91. ( 10.1007/s00125-015-3790-6) [DOI] [PubMed] [Google Scholar]
- 29. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035 2037. ( 10.2337/dc12-1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424 434. ( 10.1542/peds.2014-1774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiMeglio LA, Kanapka LG, DeSalvo DJ, et al. Time spent outside of target glucose range for young children with type 1 diabetes: a continuous glucose monitor study. Diabet Med. 2020;37(8):1308 1315. ( 10.1111/dme.14276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sundberg F, Forsander G. Detection and treatment efficacy of hypoglycemic events in the everyday life of children younger than 7 yr. Pediatr Diabetes. 2014;15(1):34 40. ( 10.1111/pedi.12057) [DOI] [PubMed] [Google Scholar]
- 33. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493 498. ( 10.1089/dia.2019.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a