Abstract
Ovarian serous carcinoma is a gynecological malignancy associated with a high mortality rate, which is commonly diagnosed in the first instance at a late stage and has a propensity to develop resistance to platinum-based chemotherapy. Identifying reliable biomarkers for platinum sensitivity is critical for improving patient outcomes. The present retrospective study included 64 patients with high-grade serous ovarian carcinoma (Federation of Gynecology and Obstetrics stages III or IV). Patients were classified as platinum-sensitive (no relapse within 6 months of the last platinum administration) or platinum-resistant (relapse within 6 months). Immunohistochemical analysis was performed to evaluate Fyn expression in tumor tissues, and Fyn knockdown experiments were performed using the OVSAHO ovarian cancer cell line to assess carboplatin sensitivity. Fyn expression was significantly higher in platinum-resistant patients compared with in platinum-sensitive patients (P<0.01). A weighted Fyn expression score was developed and a cutoff score of 6 was determined to predict platinum sensitivity with a specificity of 65.5% and a sensitivity of 62.9%. Patients with low Fyn expression (score ≤6) exhibited higher platinum sensitivity and longer overall survival (P<0.05). Multivariate analysis identified Fyn expression and postoperative residual tumor size as independent predictors of platinum sensitivity (P=0.033 and P=0.023, respectively). In vitro, Fyn knockdown significantly increased carboplatin sensitivity in ovarian cancer cells (P<0.05). Fyn, a member of the Src family of kinases, serves a crucial role in various cellular functions and has been implicated in chemotherapy resistance. The results demonstrated a notable association between Fyn expression and platinum sensitivity in ovarian serous carcinoma. The findings suggested that Fyn may serve as a predictive biomarker for response to platinum-based chemotherapy, offering the potential for more personalized treatment strategies. To the best of our knowledge, the present study is the first to establish an association between Fyn expression and platinum sensitivity in advanced ovarian serous carcinoma. Prospective studies with larger, multi-center cohorts and comprehensive biomarker analyses are recommended to validate and extend these results, ultimately improving therapeutic strategies and patient prognosis.
Keywords: ovarian carcinoma, chemotherapy, carboplatin, paclitaxel, Fyn, apoptosis, platinum sensitivity
Introduction
Ovarian serous carcinoma is a significant cause of cancer-related deaths among females worldwide, primarily due to late-stage diagnosis and high mortality rates (1). It is the most lethal gynecological malignancy with the poorest prognosis compared to other gynecological cancers (2). Most patients with ovarian serous carcinoma are diagnosed when the cancer is in an advanced stage (III or IV) due to the absence of specific symptoms and reliable early-stage biomarkers (3). The typical treatment protocol includes maximal debulking surgery followed by chemotherapy with a combination of platinum and taxane agents (3). The National Comprehensive Cancer Network Guidelines recommend the use of paclitaxel or carboplatin as the first-line treatment for epithelial ovarian carcinoma (4). Even though there is a strong initial effectiveness of platinum-based chemotherapy, many patients eventually develop resistance to these first-line treatments (1,3,5). The effectiveness of resuming platinum-based chemotherapy upon recurrence is determined by the interval without platinum, which is the duration between the last dose of platinum-based chemotherapy and the recurrence of cancer. A recurrence within 6 months classifies the cancer as ‘platinum-resistant’, whereas a recurrence after 6 months classifies it as ‘platinum-sensitive’. (1,5–7). This classification is a critical prognostic factor for overall and progression-free survival (8,9). Patients who develop platinum resistance have a significantly poorer prognosis, with a median survival of <16 months (10). While germline BRCA1/2 mutations and homologous recombination mutations are predictive of better overall survival and platinum sensitivity (11–13), these biomarkers have not yet provided definitive guidance for treatment. There is an urgent need for noninvasive pretreatment methods to identify patients unlikely to benefit from platinum-based therapies, enabling the selection of alternative treatments.
The Src family of kinases (SFKs) play a vital role in controlling numerous cellular functions, such as migration, proliferation, invasion, survival, angiogenesis, differentiation, and motility across various cancer types. This is achieved via phosphorylating tyrosine residues on target proteins that are part of multiple signaling pathways (14,15). One member of the SFK family of proteins, Fyn, is a non-receptor tyrosine kinase with a molecular weight of 59 kDa and is encoded by a gene on chromosome 6q21 (14,15). In cancer, Fyn contributes to cancer development and progression by inhibiting apoptosis and promoting proliferation, invasion, migration, and metastasis. Overexpression of Fyn enhances the anti-apoptotic activity of Akt through phosphorylation of focal adhesion kinase (FAK) and activation of the PI3K/AKT pathway (14–16). Thus, Fyn is considered a significant molecule in conferring resistance to anti-cancer agents that induce apoptosis, which can be relevant in platinum-based chemotherapy drugs for ovarian carcinoma.
In the present study, the relationship between Fyn expression and platinum sensitivity in patients with advanced-stage high-grade serous carcinoma was assessed. A novel biomarker that could be used to predict platinum sensitivity was identified, and may have potential for improving the prognosis of patients with ovarian serous carcinoma.
Materials and methods
Patients
This retrospective analysis encompassed 64 cases of ovarian high-grade serous carcinoma at FIGO stages III or IV, all of which were histologically confirmed as high-grade serous carcinoma. The patients underwent primary debulking surgery followed by chemotherapy (175 mg/m2 of paclitaxel combined with AUC6 of carboplatin administered every 3 weeks) between January 1, 2005, and December 31, 2014, at Osaka City University Hospital. The patients were divided into two groups: Platinum-sensitive group, did not relapse within 6 months after the last platinum administration, and platinum-resistant group, relapsed within 6 months. The characteristics of the patients, including age, serum CA125 tumor marker levels, FIGO stage, and the size of the residual tumor post-surgery were compared between the two groups. This study received approval from the Institutional Review Board of Osaka Metropolitan University Hospital (approval no. 2022-108; Osaka, Japan). All patients provided written informed consent for participation.
Immunohistochemistry
Fyn expression was evaluated using immunohistochemical analysis on paraffin-embedded tissue sections containing ovarian cancer tissues obtained via surgery. Four-micrometer-thick sections were first deparaffinized and rehydrated, followed by immersion for 10 min in 3% hydrogen peroxide at room temperature to inhibit the activity of endogenous peroxidase. Antigen retrieval was conducted by placing the sections in 10 mM citrate buffer (pH 9.0; cat. no. S2367; Agilent Technologies, Inc.) and heating them in an autoclave at 121°C for 20 min. The sections were incubated overnight at 4°C with a rabbit polyclonal anti-Fyn antibody (cat. no. ab184276; Abcam; 1:250). To visualize antibody binding, sections were treated with Dako REAL EnVision Detection System Peroxidase/DAB+, Rabbit/Mouse (cat. no. K5007; Agilent Technologies, Inc.) for 3 min at room temperature. Then, we incubated them with a streptavidin-peroxidase complex, using 3,3′-diaminobenzidine as the chromogen.
As the final step, the sections were counterstained at room temperature with hematoxylin for 1 min. The scoring of Fyn expression was determined by the weighted score method described by Sinicrope et al (17). Briefly, the proportion of positive cells was categorized as: 0, <5%; 1, 5–25%; 2, 5–50%; 3, 50–75%; and 4, >75%. The staining intensity was evaluated as follows: 0, no staining; 1, weak staining; 2, moderate staining; or 3, strong staining. The final Fyn expression score, ranging from 0–12, was obtained by multiplying the score for percentage positivity by the score for staining intensity.
Cell culture
The OVSAHO human ovarian serous carcinoma cell line (cat. no. JCRB1046; National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan) was grown in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 units/ml penicillin, and 100 units/ml streptomycin. The cells were maintained at 37°C in a humidified incubator supplied with 5% CO2. The culture medium was replaced every two days to maintain optimal cell growth and viability.
Cell viability assay and siRNA transfection
OVSAHO cells were plated into 96-well plates at a concentration of 1×104 cells/well. The cells were allocated into two groups, a control group transfected with control siRNA (cat. no. SIC001_10NMOL; MilliporeSigma) and a siFyn group transfected with Fyn-specific siRNA (cat. no. sc-29321; Santa Cruz Biotechnology, Inc.) The sequence of the siFyn construct was: Sense, 5′-CAUCGAGCGCAUGAAUUAU-3′ and antisense 5′-AUAAUUCAUGCGCUCGAUG-3′. The manufacturer did not disclose the sequence of the control siRNA. After the cells had adhered, the cells in the siFyn group were cultured in fresh medium with Fyn siRNA transfection complexes, while the control group was cultured in fresh medium with control siRNA. Both groups were incubated at 37°C for 24 h. Following transfection, the cells were cultured in medium containing varying concentrations of carboplatin (25, 50, 100, or 200 µM) and incubated for an additional 24 h at 37°C. Cell viability was assessed using a Cell Counting Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc.). A 10 µl aliquot of the CCK-8 solution was added to each well, and the cells were incubated at 37°C for 1 h. Absorbance was measured at 450 nm using a microplate reader (Corona Electric Co., Ltd.).
Reverse transcription-quantitative PCR (RT-qPCR)
RT-qPCR was conducted to verify the knockdown of Fyn at the mRNA level. Total RNA was extracted from the cells using the RNeasy Mini kit (Qiagen GmbH). Subsequently, cDNA was synthesized from the extracted RNA using the High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, Inc.). TaqMan chemistry was employed in accordance with the manufacturer's instructions, utilizing TaqMan primers and probes for Fyn (cat. no. Hs00941613_m1) and hypoxanthine phosphoribosyl transferase 1 (HPRT1; cat. no. Hs02800695_m1; Thermo Fisher Scientific, Inc.) as the internal control. RT-qPCR was performed with TaqMan Fast Universal PCR Master Mix (Thermo Fisher Scientific, Inc.). The RT-qPCR was performed using the following thermocycling cycling conditions: Initial denaturation, 95°C for 20 sec; followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec. All steps were performed following the manufacturer's protocols. Gene expression changes were quantified relative to the control by employing the 2−ΔΔCq method (18).
Statistical analysis
Data are presented as the median (range). Associations between categorical variables in the two groups were assessed using a Fisher's exact test, while comparisons of median values and mean values between groups were performed using a Mann-Whitney U test or an unpaired Student's t-test, respectively. A Receiver Operating Characteristic (ROC) curve was plotted to determine the optimal Fyn score cutoff for predicting platinum sensitivity. Survival analysis between the groups was performed using the Kaplan-Meier method with log-rank tests. To identify independent factors for platinum sensitivity, a multivariate logistic regression analysis was used. RT-qPCR experiments were replicated five times, and cell viability assays were performed with 10 replicates. P<0.05 was considered to indicate a statistically significant differences. All statistical analyses were performed using GraphPad Prism Version 9 (GraphPad Software, Inc.).
Results
Patient characteristics
Table I presents the patients' characteristics. The platinum-sensitive group consisted of 35 patients, while the platinum-resistant group included 29 patients. The median age for the two groups was 63 and 62 years, respectively, with no significant difference between them (P=0.656). Similarly, the distribution of the FIGO stage showed no significant statistical difference between the groups (P=0.368). The median CA125 value was 929 U/ml for the platinum-sensitive group and 1,422 U/ml for the platinum-resistant group, with no significant difference (P=0.246). Regarding the size of postoperative residual tumors, the distributions were as follows: No residual tumor in 9 vs. 3 cases, residual tumor ≤1 cm in 13 vs. 3 cases, and residual tumor >1 cm in 13 vs. 23 cases between the platinum-sensitive group and the platinum-resistant group, respectively. This distribution showed a significant statistical difference (P<0.01).
Table I.
Patient characteristics.
| Characteristic | Platinum-sensitive group | Platinum-resistant group | P-value |
|---|---|---|---|
| No. of patients | 35 | 29 | |
| Median age, years (range) | 63 (36–81) | 62 (39–76) | 0.656a |
| FIGO stage, n | 0.368b | ||
| IIIA | 2 | 0 | |
| IIIB | 3 | 1 | |
| IIIC | 27 | 21 | |
| IVA | 2 | 4 | |
| IVB | 1 | 3 | |
| Median CA125, U/ml (range) | 929 (25–30,075) | 1,422 (209–9,478) | 0.246a |
| Postoperative residual tumor, n | <0.01b | ||
| None | 9 | 3 | |
| ≤1 cm | 13 | 3 | |
| >1 cm | 13 | 23 |
Mann-Whitney U test;
Fisher's exact test. FIGO, Federation of Gynecology and Obstetrics.
Weighted score of Fyn expression and cutoff value to predict platinum sensitivity
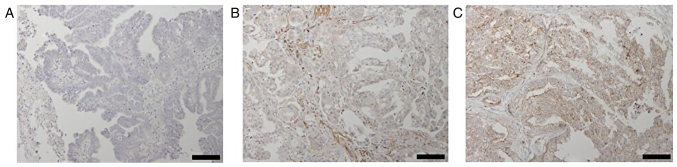
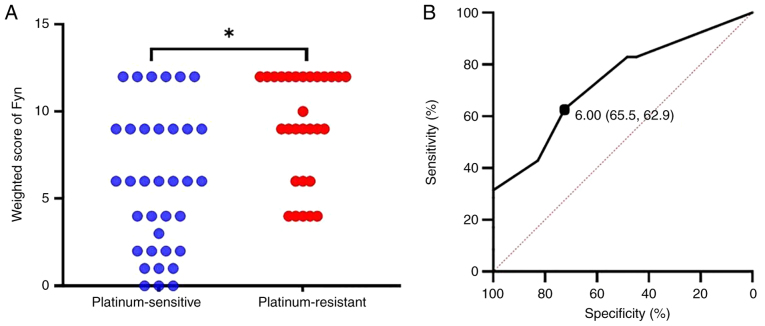
The weighted score of Fyn expression and its cutoff value to predict platinum sensitivity were next evaluated. Fyn expression was primarily observed in the cytoplasm and at the cell membrane (Fig. 1). The median weighted score was 6 for the platinum sensitive group and 9 for the platinum resistant group, with the latter being significantly higher (P<0.01, Fig. 2A). To determine the cutoff value of the Fyn weighted score for predicting platinum sensitivity, a ROC curve was constructed. The analysis revealed a cutoff value of 6, which predicted platinum sensitivity with a specificity of 65.5%, a sensitivity of 62.9%, an AUC of 0.733, and a 95% confidence interval of 0.614-0.852 (Fig. 2B).
Figure 1.
Immunohistochemical staining of Fyn in surgically obtained ovarian serous carcinoma specimens counterstained with hematoxylin. Representative images corresponding to different Fyn expression levels as assessed by weighted scores: (A) 0, no staining; (B) 6, moderate staining; and (C) 12, strong staining. Scale bar, 100 µm.
Figure 2.
Comparison of Fyn weighted scores between platinum-sensitive and platinum-resistant groups, and ROC curve analysis for predicting platinum sensitivity. (A) Comparison of Fyn weighted scores showed significantly lower scores in the platinum-sensitive group compared to the platinum-resistant group. (B) ROC curve analysis using Fyn weighted scores revealed a cutoff value of 6 for predicting platinum sensitivity, with a sensitivity of 62.9% and specificity of 65.5%. The AUC is 0.733, with a 95% confidence interval of 0.614–0.852. *P<0.01. ROC, Receiver Operating Characteristic; AUC, area under the curve.
Association between platinum sensitivity and overall survival based on Fyn expression
Using a cutoff value of 6 for the weighted score of Fyn expression, the patients were divided into two groups, a low expression group with a weighted score of Fyn ≤6 and a high-expression group with a weighted score of Fyn ≥8. The characteristics of the patients in both groups are described in Table II. The low-expression group consisted of 30 patients, while the high-expression group consisted of 34 patients. The median ages of the patients were 60.5 and 63.0 years, respectively, with no significant difference (P=0.228). Similarly, there was no significant difference in the distribution of FIGO stages between the groups (P=0.142). The median CA125 values were 1,227.5 U/ml for the low expression group and 1,415.5 U/ml for the high-expression group, with no statistically significant difference (P=0.360). Additionally, the distribution of size of postoperative residual tumors showed no significant difference between the groups (P=0.052). Platinum sensitivity was compared between the two groups. In the low expression group, 73.3% of the patients were platinum-sensitive, whereas in the high-expression group, only 38.3% were platinum-sensitive. This indicated that the platinum sensitivity rate was significantly greater in the low-expression group than in the high-expression group (P<0.01, Table III). Additionally, overall survival (OS) was compared between the two groups. The OS was significantly longer in the low-expression group compared with the high-expression group (P=0.023, Fig. 3).
Table II.
Characteristics of the patients based on Fyn expression.
| Characteristic | Low expression group | High expression group | P-value |
|---|---|---|---|
| No. of patients | 30 | 34 | |
| Median age, years (range) | 60.5 (36–79) | 63.0 (39–81) | 0.228a |
| FIGO stage, n | 0.142b | ||
| IIIA | 2 | 0 | |
| IIIB | 2 | 2 | |
| IIIC | 23 | 25 | |
| IVA | 3 | 3 | |
| IVB | 0 | 4 | |
| Median CA125, U/ml (range) | 1,227.5 (25–30,075) | 1,415.5 (113–12,300) | 0.360a |
| Postoperative residual tumor, n | 0.052b | ||
| None | 8 | 4 | |
| ≤1 cm | 10 | 6 | |
| >1 cm | 12 | 24 |
Mann-Whitney U test;
Fisher's exact test. FIGO, Federation of Gynecology and Obstetrics.
Table III.
Association between Fyn expression and platinum sensitivity.
| Fyn expression, n (%) | Platinum-sensitive | Platinum-resistant | P-value |
|---|---|---|---|
| Low expression, score ≤6 | 22 (73.3%) | 8 (26.7%) | <0.01a |
| High expression, score ≥8 | 13 (38.3%) | 21 (61.7%) |
Fisher's exact test.
Figure 3.
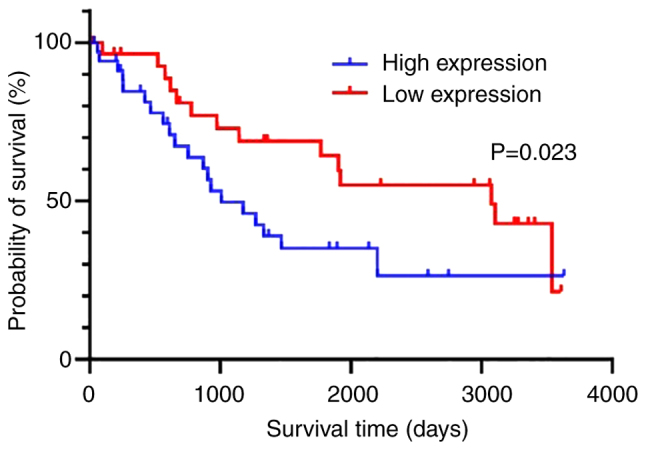
Kaplan-Meier survival analysis demonstrating overall survival. Patients with low Fyn expression experienced significantly extended overall survival than those with high Fyn expression.
Multivariate analysis of factors independently related to platinum sensitivity
Multivariate analysis was performed to identify factors independently related to platinum sensitivity, using factors identified as significant in the univariate analysis (the size of postoperative residual tumors and the Fyn weighted score). Table IV demonstrates that both Fyn expression and the size of postoperative residual tumors were independently associated with platinum sensitivity. The odds ratio for Fyn expression was 0.295 (95% CI: 0.096-0.906) with a P-value of 0.033, while the odds ratio for the size of postoperative residual tumors was 0.400 (95% CI: 0.181-0.882) with a P-value of 0.023.
Table IV.
Multivariate analysis for detecting independent factors of platinum sensitivity.
| 95% confidence interval | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Odds ratio | Lower | Upper | P-value |
| Fyn expression, low/high | 0.295 | 0.096 | 0.906 | 0.033a |
| Postoperative residual tumors, 0 cm/<1 cm/≥1 cm | 0.400 | 0.181 | 0.882 | 0.023a |
Logistic regression analysis.
Effect of Fyn knockdown on the sensitivity of ovarian cancer cells to carboplatin
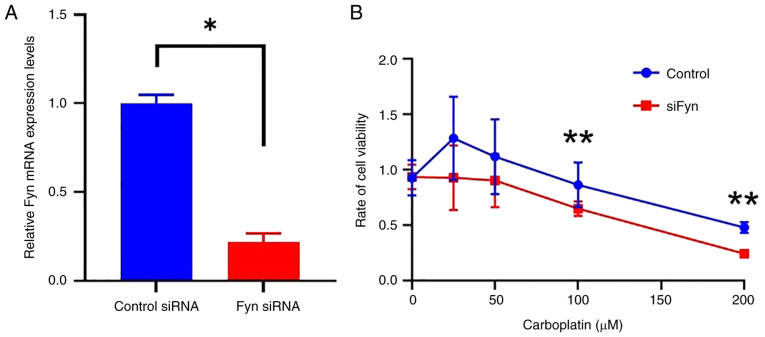
Ovarian cancer cells were allocated into two groups, a control group transfected with control siRNA and a siFyn group transfected with Fyn-specific siRNA. The successful knockdown of Fyn expression using siRNA was confirmed by RT-qPCR. This examination demonstrated a significant decrease in Fyn mRNA levels in cells treated with Fyn-specific siRNA compared to those treated with control siRNA (P<0.01, Fig 4A). Subsequently, the sensitivity of cells to carboplatin between the control group and the siFyn group was compared by assessing cell viability after administering various doses of carboplatin. The results showed that at doses of ≥100 µM, cell viability was significantly lower in the siFyn group compared to the control group (P<0.05, Fig 4B). This indicates that reducing Fyn expression increases ovarian cancer cells' sensitivity to carboplatin.
Figure 4.
Effects of Fyn knockdown on carboplatin sensitivity in ovarian cancer cells. (A) Reverse transcription-quantitative PCR analysis showing a significant decrease in Fyn mRNA expression in cells treated with Fyn-specific siRNA compared to those treated with control siRNA, confirming successful knockdown. (B) Cell viability assays showed decreased viability following Fyn knockdown and treatment with varying concentrations of carboplatin (≥100 µM) compared to control cells. Data are expressed as the mean ± standard deviation. *P<0.01, **P<0.05 vs. siFyn or as indicated. si, small interfering.
Discussion
Ovarian cancer remains a formidable disease with a high mortality rate and poor prognosis, even with the introduction of advanced therapeutic options such as PARP inhibitors and VEGF inhibitors (19,20). Despite initial responsiveness to chemotherapy, a large subset of patients eventually experience recurrence and develop resistance to platinum-based treatments, which are the cornerstone of ovarian cancer therapy (3,5). Platinum resistance is a significant barrier in the effective management of advanced ovarian carcinoma, leading to treatment failure and disease progression (7,21). Several theories have been proposed to explain platinum resistance, including decreased cellular import and increased export of the drug through transporters, enhanced DNA damage repair, intracellular drug inactivation by detoxifying enzymes, and the inactivation of cell death signaling pathways (7,22–24). These multiple mechanisms may concurrently contribute to platinum resistance (25–28).
Tyrosine kinases are classified into two primary categories: Receptor tyrosine kinases and non-receptor tyrosine kinases. Non-receptor tyrosine kinases comprise families such as Src, Abl, Janus kinase, and FAK, whereas receptor tyrosine kinases including the vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR), and mesenchymal-epithelial transition factor, respond to signals from soluble ligands (29). When these tyrosine kinases are dysregulated, they can cause cancer by disrupting cellular function, growth, and morphology, which are key characteristics of malignancy (29). The SFKs, including Fyn, c-Src, Yes, Lck, Lyn, Fgr, Blk, and Hck, are among these non-receptor kinases, with Fyn, c-Src, and Yes broadly expressed across various tissues, unlike the others, which instead have more limited expression patterns (29). Fyn, which is a non-receptor tyrosine kinase, is involved in various biological processes by phosphorylating tyrosine residues of the key molecules involved in different signal pathways, such as signal transduction through T cell receptors, signal transduction in neurons (impacting brain function), and adhesion-mediated signaling in physiological conditions (14,29). Moreover, Fyn is crucial in the onset and advancement of multiple cancer types, regulating cell growth, apoptosis, motility, migration, and morphogenic transformation (14,29). Multiple malignancies such as glioma, melanoma, breast cancer, prostate cancer, head and neck squamous cell carcinoma, chronic myeloid leukemia, cholangiocarcinoma, thyroid cancer, gastric cancer, and esophageal squamous cell carcinoma exhibit Fyn involvement (30–35). In cancer, Fyn is involved in receptor tyrosine kinase pathways, including those involving VEGFR, EGFR, fibroblast growth factor, and platelet-derived growth factor receptor. It conveys signals via Ras-independent pathways (including PIK3/Akt, FAK, STAT3, VAV1, β-catenin, paxillin, and/or SHC) and Ras-dependent pathways (via Ras/MEK/ERK) (12,28). These pathways enable Fyn to mediate anti-apoptotic effects of Akt/PKB mediated by growth factor (16,36). Numerous studies have correlated Fyn expression with response to anti-cancer drugs because of its role in regulating apoptosis. For example, Fyn knockdown enhances apoptosis induced by doxorubicin and boosts the sensitivity of cells resistant to doxorubicin to this drug by inactivating MAPK signaling (14,29). Furthermore, Fyn has been identified as one of the hub genes of the interaction network in cisplatin-resistant ovarian cancer cells (37). In pancreatic ductal adenocarcinoma, increased Fyn expression decreases chemosensitivity to gemcitabine via regulation of miR-125a-3p (38). Moreover, the effectiveness of PP2, an SFK inhibitor, is significantly influenced by Fyn expression levels, as PP2 induces apoptosis (39). Thus, Fyn is recognized as a critical molecule in conferring resistance to anti-cancer agents, primarily through its role in apoptosis induction (14,29).
Fyn expression was found to be associated with platinum sensitivity and OS in patients with ovarian serous carcinoma in the current study. Immunohistochemical evaluation using a weighted scoring system revealed that Fyn expression was significantly lower in the platinum-sensitive group compared to the platinum-resistant group. Additionally, low Fyn expression (weighted score ≤6) was significantly correlated with both increased platinum sensitivity and a longer OS. Furthermore, multivariate analysis demonstrated that Fyn expression was an independent factor associated with platinum sensitivity, demonstrating the highest odds ratio. Additionally, in vitro experiments confirmed that Fyn knockdown using siRNA enhanced the effectiveness of carboplatin against ovarian cancer cells, further supporting the potential of Fyn as a therapeutic target to improve treatment outcomes for patients with ovarian serous carcinoma.
However, there are several limitations to the current study. Firstly, its retrospective nature inherently limits the ability to establish causation. Additionally, the sample size is relatively small, with only 64 cases, which can limit the generalizability of the findings and reduce the statistical power for the detection of significant differences or associations. The study was conducted at a single institution, meaning the results may not be representative of broader patient populations or other ethnicities. Furthermore, the immunohistochemical evaluation and the weighted scoring method for Fyn expression are subject to variability and potential observer bias. The study also focused solely on Fyn expression, without exploring other potential biomarkers and molecular mechanisms influencing platinum sensitivity and resistance. Lastly, while the study shows an association between Fyn expression and platinum sensitivity, it does not thoroughly investigate the underlying biological mechanisms or pathways through which Fyn influences resistance to chemotherapy. These limitations suggest that further research is required to confirm the findings and expand on the underlying mechanisms. Prospective studies with larger, multi-center cohorts and comprehensive biomarker analyses including those biomarkers, such as UCP2, PRMT1, and TBX2, which our research team has reported as predictors of platinum sensitivity (25–27) are necessary to validate and extend these results. While immunohistochemistry may influence the reliability of the results to some extent, it remains a straightforward and practical method in clinical practice, making it a valuable technique for assessing sensitivity to platinum-based chemotherapy. While the current study lacked mechanistic insights, establishing a correlation between Fyn expression and platinum sensitivity provides a foundational step toward exploring the underlying mechanisms of platinum sensitivity in ovarian cancer patients.
As far as we are aware, this study is the first to establish a link between Fyn expression and platinum sensitivity in patients with advanced ovarian serous carcinoma. Understanding the mechanisms underpinning platinum sensitivity in advanced ovarian serous carcinoma is crucial for developing therapeutic strategies that improve prognosis. In conclusion, the results of this study highlight the potential of using Fyn expression for predicting sensitivity to platinum-based chemotherapy for ovarian serous carcinoma.
Acknowledgements
The authors would like to thank Dr Yukimi Kira (Research Support Platform, Osaka Metropolitan University Graduate School of Medicine, Osaka, Japan) for their technical assistance and expertise.
Funding Statement
This study received financial support from the Osaka Medical Research Foundation for Intractable Diseases (grant no. 29-1-46).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
EU, TF and TSu conceptualized and designed the study. EU, TSe, TN, YA, TW, RT, MY and TY performed the experiments and collected the data. EU, TF and TSu analyzed and interpreted the data. EU and TF drafted the manuscript. EU and TF confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Osaka Metropolitan University Hospital (approval no. 2022-108). Written informed consent was obtained from all participants.
Patient consent for publication
All participants provided written informed consent for the publication of this article.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Falzone L, Scandurra G, Lombardo V, Gattuso G, Lavoro A, Distefano AB, Scibilia G, Scollo P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review) Int J Oncol. 2021;59:53. doi: 10.3892/ijo.2021.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 3.Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, Marth C, Thigpen T, Trimble E, participants of 4th Ovarian Cancer Consensus Conference (OCCC); Gynecologic Cancer Intergroup 2010 gynecologic cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: Report from the fourth ovarian cancer consensus conference. Int J Gynecol Cancer. 2011;21:750–755. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 4.Daly MB, Pal T, Maxwell KN, Churpek J, Kohlmann W, AlHilli Z, Arun B, Buys SS, Cheng H, Domchek SM, et al. NCCN Guidelines® insights: Genetic/Familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2024. J Natl Compr Canc Netw. 2023;21:1000–1010. doi: 10.6004/jnccn.2023.0051. [DOI] [PubMed] [Google Scholar]
- 5.Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S, Pujade-Lauraine E, Sehouli J, et al. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21:771–775. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL., Jr Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 7.St Laurent J, Liu JF. Treatment approaches for platinum-resistant ovarian cancer. J Clin Oncol. 2024;42:127–133. doi: 10.1200/JCO.23.01771. [DOI] [PubMed] [Google Scholar]
- 8.Kyrgiou M, Salanti G, Pavlidis N, Paraskevaidis E, Ioannidis JP. Survival benefits with diverse chemotherapy regimens for ovarian cancer: Meta-analysis of multiple treatments. J Natl Cancer Inst. 2006;98:1655–1663. doi: 10.1093/jnci/djj443. [DOI] [PubMed] [Google Scholar]
- 9.Havasi A, Cainap SS, Havasi AT, Cainap C. Ovarian cancer-insights into platinum resistance and overcoming it. Medicina (Kaunas) 2023;59:544. doi: 10.3390/medicina59030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMullen M, Madariaga A, Lheureux S. New approaches for targeting platinum-resistant ovarian cancer. Semin Cancer Biol. 2021;77:167–181. doi: 10.1016/j.semcancer.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Khan MA, Vikramdeo KS, Sudan SK, Singh S, Wilhite A, Dasgupta S, Rocconi RP, Singh AP. Platinum-resistant ovarian cancer: From drug resistance mechanisms to liquid biopsy-based biomarkers for disease management. Semin Cancer Biol. 2021;77:99–109. doi: 10.1016/j.semcancer.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo PE, Taoum C, Fabbro M, Quesada S, Rouanet P, Ray-Coquard I. Impact of molecular testing on the surgical management of advanced epithelial ovarian cancer. Crit Rev Oncol Hematol. 2024;202:104469. doi: 10.1016/j.critrevonc.2024.104469. [DOI] [PubMed] [Google Scholar]
- 14.Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn: A novel molecular target in cancer. Cancer. 2010;116:1629–1637. doi: 10.1002/cncr.24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Liu C, Tang Y. Role of Fyn in hematological malignancies. J Cancer Res Clin Oncol. 2023;149:6759–6767. doi: 10.1007/s00432-023-04608-2. [DOI] [PubMed] [Google Scholar]
- 16.Nanno S, Fukuda T, Noda T, Uchikura E, Awazu Y, Imai K, Yamauchi M, Yasui T, Sumi T. Fyn expression is associated with the response of patients with locally advanced uterine cervical squamous cell carcinoma to neoadjuvant chemotherapy. Mol Clin Oncol. 2022;17:147. doi: 10.3892/mco.2022.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.O'Malley DM, Krivak TC, Kabil N, Munley J, Moore KN. PARP inhibitors in ovarian cancer: A review. Target Oncol. 2023;18:471–503. doi: 10.1007/s11523-023-00970-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda T, Noda T, Uchikura E, Awazu Y, Tasaka R, Imai K, Yamauchi M, Ichimura T, Yasui T, Sumi T. Real-world efficacy and safety of bevacizumab for advanced or recurrent müllerian cancer: A single-institutional experience. Anticancer Res. 2023;43:3097–3105. doi: 10.21873/anticanres.16481. [DOI] [PubMed] [Google Scholar]
- 21.Friedlander ML, Stockler MR, Butow P, King MT, McAlpine J, Tinker A, Ledermann JA. Clinical trials of palliative chemotherapy in platinum-resistant or -refractory ovarian cancer: Time to think differently? J Clin Oncol. 2013;31:2362. doi: 10.1200/JCO.2012.47.7927. [DOI] [PubMed] [Google Scholar]
- 22.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 24.Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res. 2016;106:27–36. doi: 10.1016/j.phrs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Matsubara H, Fukuda T, Awazu Y, Nanno S, Shimomura M, Inoue Y, Yamauchi M, Yasui T, Sumi T. PRMT1 expression predicts sensitivity to platinum-based chemotherapy in patients with ovarian serous carcinoma. Oncol Lett. 2021;21:162. doi: 10.3892/ol.2020.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawanishi M, Fukuda T, Shimomura M, Inoue Y, Wada T, Tasaka R, Yasui T, Sumi T. Expression of UCP2 is associated with sensitivity to platinum-based chemotherapy for ovarian serous carcinoma. Oncol Lett. 2018;15:9923–9928. doi: 10.3892/ol.2018.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasaka R, Fukuda T, Shimomura M, Inoue Y, Wada T, Kawanishi M, Yasui T, Sumi T. TBX2 expression is associated with platinum-sensitivity of ovarian serous carcinoma. Oncol Lett. 2018;15:3085–3090. doi: 10.3892/ol.2017.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda T, Kawanishi M, Awazu Y, Nanno S, Shimomura M, Inoue Y, Matsubara H, Yamauchi M, Kasai M, Hashiguchi Y, et al. Neutrophil-to-lymphocyte ratio is associated with sensitivity to platinum-based chemotherapy and prognosis in patients with advanced serous ovarian carcinoma. Mol Clin Oncol. 2021;15:217. doi: 10.3892/mco.2021.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng S, Fu Y. FYN: Emerging biological roles and potential therapeutic targets in cancer. J Transl Med. 2023;21:84. doi: 10.1186/s12967-023-03930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Zhou J, Nie Y, Guo G, Wang A, Zhu X. A new finding in the key prognosis-related proto-oncogene FYN in hepatocellular carcinoma based on the WGCNA hub-gene screening trategy. BMC Cancer. 2022;22:380. doi: 10.1186/s12885-022-09388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu SC, Han DD, Li XL, Ma J, Wu Q, Dong HM, Bai C, He Q. Fyn knockdown inhibits migration and invasion in cholangiocarcinoma through the activated AMPK/mTOR signaling pathway. Oncol Lett. 2018;15:2085–2090. doi: 10.3892/ol.2017.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Zhou Z, Wei Z, Wu J, OuYang J, Huang W, He Y, Zhang C. FYN promotes gastric cancer metastasis by activating STAT3-mediated epithelial-mesenchymal transition. Transl Oncol. 2020;13:100841. doi: 10.1016/j.tranon.2020.100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Huang Z, Guo Y, Xiao T, Tang L, Zhao S, Wu L, Su J, Zeng W, Huang H, et al. The phosphorylation of CD147 by Fyn plays a critical role for melanoma cells growth and metastasis. Oncogene. 2020;39:4183–4197. doi: 10.1038/s41388-020-1287-3. [DOI] [PubMed] [Google Scholar]
- 34.Dong W, Sun SJ, Qin JJ, Liu GM. Fyn stimulates the progression of pancreatic cancer via Fyn-GluN2b-AKT axis. Eur Rev Med Pharmacol Sci. 2020;24:109–121. doi: 10.26355/eurrev_202001_19900. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Zhao D, Zhang L, Xiao Y, Wu Q, Wang Y, Chen J, Zhan Q. Src heterodimerically activates Lyn or Fyn to serve as targets for the diagnosis and treatment of esophageal squamous cell carcinoma. Sci China Life Sci. 2023;66:1245–1263. doi: 10.1007/s11427-022-2216-x. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Ditzel HJ. Fyn is an important molecule in cancer pathogenesis and drug resistance. Pharmacol Res. 2015;100:250–254. doi: 10.1016/j.phrs.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Sakhare SS, Rao GG, Mandape SN, Pratap S. Transcriptome profile of OVCAR3 cisplatin-resistant ovarian cancer cell line. BMC Bioinformatics. 2014;15:21. doi: 10.1186/1471-2105-15-S10-P21. [DOI] [Google Scholar]
- 38.Liu G, Ji L, Ke M, Ou Z, Tang N, Li Y. miR-125a-3p is responsible for chemosensitivity in PDAC by inhibiting epithelial-mesenchymal transition via Fyn. Biomed Pharmacother. 2018;106:523–531. doi: 10.1016/j.biopha.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 39.Noronha G, Barrett K, Boccia A, Brodhag T, Cao J, Chow CP, Dneprovskaia E, Doukas J, Fine R, Gong X, et al. Discovery of [7-(2,6-dichlorophenyl)-5-methylbenzo [1,2,4]triazin-3-yl]-[4-(2-pyrrolidin-1-ylethoxy)phenyl]amine-a potent, orally active Src kinase inhibitor with anti-tumor activity in preclinical assays. Bioorg Med Chem Lett. 2007;17:602–608. doi: 10.1016/j.bmcl.2006.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.