Abstract
Decisions to disperse from a habitat stand out among organismal behaviours as pivotal drivers of ecosystem dynamics across scales. Encounters with other species are an important component of adaptive decision-making in dispersal, resulting in widespread behaviours like tracking resources or avoiding consumers in space. Despite this, metacommunity models often treat dispersal as a function of intraspecific density alone. We show, focusing initially on three-species network motifs, that interspecific dispersal rules generally drive a transition in metacommunities from homogeneous steady states to self-organized heterogeneous spatial patterns. However, when ecologically realistic constraints reflecting adaptive behaviours are imposed—prey tracking and predator avoidance—a pronounced homogenizing effect emerges where spatial pattern formation is suppressed. We demonstrate this effect for each motif by computing master stability functions that separate the contributions of local and spatial interactions to pattern formation. We extend this result to species-rich food webs using a random matrix approach, where we find that eventually, webs become large enough to override the homogenizing effect of adaptive dispersal behaviours, leading once again to predominately pattern-forming dynamics. Our results emphasize the critical role of interspecific dispersal rules in shaping spatial patterns across landscapes, highlighting the need to incorporate adaptive behavioural constraints in efforts to link local species interactions and metacommunity structure.
This article is part of the theme issue ‘Diversity-dependence of dispersal: interspecific interactions determine spatial dynamics’.
Keywords: dispersal, metacommunity, network, pattern formation, cross-diffusion
1. Introduction
Organismal behaviour plays a pivotal role in shaping ecosystems across scales. Dispersal in particular exerts a profound influence, affecting everything from the availability of local resources [1–3] and the distribution of predators [4] to the structure of ecological networks [5,6], and the dynamics of metacommunities [7–10]. However, understanding the effects of dispersal on metacommunity dynamics has been made difficult by the variation and complexity of behaviours that ultimately influence whether an organism leaves a given habitat [8,9,11]. Often, these dispersal decisions are influenced by encounters with other species, including with resources, competitors or consumers [12–15]. Despite the observable dependencies of dispersal on local diversity and species interactions, many metacommunity models still treat dispersal as a function of intraspecific density alone [7,9,16,17].
The consideration of dispersal rates that respond to species interactions is needed as previous studies suggest that they may have substantial impacts on metacommunity dynamics [10,14,16–20]. These dispersal responses are often represented as ‘cross-diffusion’ terms, where the movement of one species is influenced by the spatial gradient of another species’ density or abundance [21–23]. A recurring finding in recent spatial models incorporating cross-diffusion is the heightened sensitivity of spatially homogeneous steady states to spontaneous pattern formation driven by dispersal, i.e. Turing instabilities [24–28]. To ensure that these models capture ecologically relevant phenomena, it is important to incorporate cross-diffusion terms that reflect the signs of interspecific interactions, such that prey would avoid predators or consumers would actively track prey [24]. However, when such constraints on cross-diffusivity have been implemented in ecological models, they have mostly been applied to two-species systems [10,29], limiting the range of potential dynamical behaviours [28]. These simplified models fall short in capturing the dynamics of larger species interaction networks, leaving critical gaps in our understanding of the link between interaction-driven dispersal and the self-organized community structures that emerge at realistic scales [5,19,30–32].
Here, we assess the susceptibility of model metacommunities to spatial pattern formation, both with and without ecologically relevant adaptive constraints applied to the sign of cross-diffusion terms (i.e. predator avoidance and prey tracking). Our results consistently indicate that increasing the prevalence of unconstrained cross-diffusion facilitates spatial pattern formation. However, the introduction of ecological constraints that regulate cross-diffusion diminishes or even eliminates the tendency for pattern formation. We first examine ecologically relevant three-species interaction motifs (figure 1a–d ) via a rigorous computational scan of both non-spatial (trophic) and spatial (dispersal) model parameters. We then extend our analysis to systems comprising more than three species using a more efficient random matrix approach, showing that findings in our three-species motifs qualitatively hold when extended to larger metacommunities.
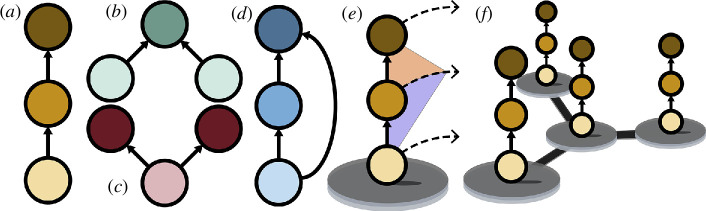
Figure 1.
Food web motifs and metacommunity connectivity. Solid arrows denote feeding relationships. Light, medium and dark shading represents primary producers, intermediate consumers and top consumers, respectively. Motifs are labelled as (a) food chain, (b) apparent competition, (c) resource competition and (d) intraguild predation. N > 3 food webs are not shown but may include one or more of these motifs. (e) Species may disperse from patches (dashed lines) in response to other species’ densities (shaded areas). For webs (a)–(c), a maximum of such interspecific dispersal responses are possible. When constrained, dispersal responses are opposite in sign from feeding relationships such that dispersal is higher for a species with locally high densities of its predators and low densities of its prey. (f) Dispersal connects habitats in spatial networks to create metacommunities.
2. Modelling and analysis framework
We employ a metacommunity modelling framework in order to study the effects of cross-diffusion on spatial food web dynamics. We assume these dynamics are governed by a set of deterministic reaction–diffusion equations on a spatial network describing species interactions and dispersal in separable terms. Nodes in the spatial network represent habitat patches where species interactions determine both local food web dynamics and decisions to emigrate to neighbouring patches. The dynamics of species on a given patch are then captured by:
| (2.1) |
where the generally non-linear function of species ’s biomass density and define the rates of trophic interactions and dispersal, respectively, and are assumed to be identical across all habitat patches. Connections between patches are represented by the Laplacian matrix , with A the network’s adjacency matrix. As exact solutions to equation (2.1) rarely exist, either direct simulation or analysis of the linearized system can be employed to understand the long-term dynamics.
We consider the dynamics of equation (2.1) following small perturbations from a spatially homogeneous, non-trivial steady state . These perturbations can be decomposed over eigenmodes of , each with a corresponding Laplacian eigenvalue analogous to the wavenumber in continuous space [10,30,33,34]. The allowed spatial signatures of all eigenmodes, as encoded in the respective eigenvectors, are predetermined by the structure of , unlike in continuous space where perturbations may be decomposed over arbitrarily high wavenumbers. To evaluate the potential dynamical behaviour across arbitrary patch networks, we therefore refrain from specifying a particular form of and instead consider as a real-valued, positive parameter of arbitrarily high magnitude. The exponential growth rate of the th mode for each species is then given by eigenvalues of
| (2.2) |
where the local Jacobian
| (2.3) |
encodes the linearized non-spatial component of the model (i.e. trophic interactions), while the connectivity matrix
| (2.4) |
encodes the linearized spatial response of species to species near [10,34].
The long-term behaviour of equation (2.1) can be qualitatively understood via the distribution of all possible eigenmodes’ maximum growth rates
| (2.5) |
where the right-hand side denotes the leading eigenvalue of the matrix , variably referred to as the dispersion relation [22,35] or the master stability function [10,34]. We delineate possible metacommunity outcomes into three qualitatively different dynamical behaviours based on the form of . The first, which we label ‘stable’ dynamics (st), occurs when perturbations decay on an isolated patch (i.e. ) and the homogeneous state is similarly maintained on any spatial patch network [10], such that for all . In contrast, ‘unstable’ dynamics (us) are characterized by an initially positive master stability function (i.e. ) such that stable coexistence of all species beginning from is impossible in isolation or on any spatial network. Finally, ‘pattern-forming’ dynamics (pf) occur when but crosses at some critical value. In these systems, certain spatial networks will cause the system to self-organize to a heterogeneous state where species exhibit variation in densities—either static or oscillatory— across patches in the spatial network.
To assess the tendency of ecological systems to exhibit any of the particular dynamical behaviours outlined above, it is important to account for the effects of parameter variation in equation (2.1). We quantify this tendency as the robustness of a given behaviour to variation in the parameter space of equation (2.1) [35,36]. Separating the effects of trophic and dispersal interactions, we define the local robustness , spatial robustness and total robustness as fractions of the appropriate parameter spaces yielding a particular behaviour (see electronic supplementary material). This definition is conceptually akin to that of feasibility domains discussed elsewhere in ecological literature [37]. Local robustness thus quantifies the fraction of possible interaction models, encoded in , which result in a particular dynamical outcome under a set of behavioural assumptions on dispersal, encoded in . Similarly, spatial robustness quantifies the fraction of considered dispersal behaviours with a common outcome under a set of assumptions on trophic interactions. Finally, the total robustness quantifies the frequency of a given outcome as both local and spatial parameters vary.
To construct the connectivity matrix , we first assume diagonal entries are strictly positive to accommodate mass-action diffusive effects. Off-diagonal elements may appear for each pairwise food web interaction resulting in a total of interspecific dispersal responses. To impose ecologically reasonable (i.e. adaptive) constraints we then restrict the signs of these off-diagonals as
| (2.6) |
such that the effects of prey tracking and predator avoidance are accounted for [24,29]. Rather than assume a specific form for , we focus on the linearized spatial responses in equation (2.4). While this prevents us from performing a direct simulation of the system, it allows for a comprehensive numerical sampling scheme to approximate the robustness of dynamical behaviours, for which analytical predictions become intractable for species [28].
3. Species motifs
We start by considering the effects of interspecific dispersal rules on conventional models of three-species ecological interaction motifs (figure 1). Local population dynamics on patch for each interaction motif are defined by a generalized system of equations:
| (3.1) |
where and are the intrinsic population growth rate and carry capacity, respectively, of producers, while , , and are the attack rate, half-saturation constant, conversion efficiency and mortality coefficient, respectively, of consumers. The relevant gain and loss terms due to predation interactions appear according to the interaction motif. This corresponds to ecological models with logistic producer growth, and consumers that exhibit type-II functional responses and density-dependent mortality.
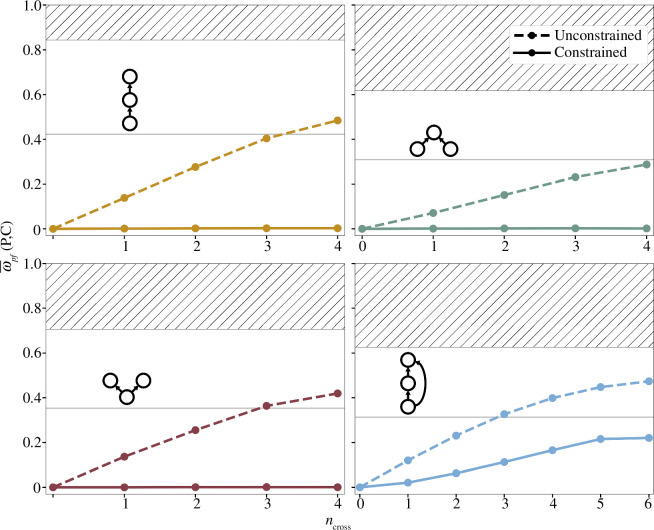
In accordance with previous studies on two-species systems, for all considered three-species systems we find that the inclusion of interspecific spatial responses has the potential to induce pattern formation which is not possible with intraspecific dispersal alone (i.e. ). This potential increases monotonically with the prevalence of cross-diffusion (i.e. with ) such that pattern-forming dynamics eventually become the most robust to variation in both local and spatial interaction parameters for 3 out of 4 food web motifs (figure 2). That is, a random parameterization of equation (3.1) is highly likely to yield a steady state susceptible to pattern formation if spatial interactions between species are common and no restrictions exist on the relative weight or sign of these interactions. The increase in with such unconstrained spatial responses is qualitatively similar across all interaction motifs, despite consisting of markedly different trophic structures and propensities for locally unstable dynamics.
Figure 2.
Robustness of pattern formation in food web motifs. Total robustness of pattern-forming dynamics shown is averaged over permutations of interspecific dispersal responses for a given local interaction motif. The robustness of unstable dynamics depends exclusively on local interactions and is indicated by the hatched area, providing an upper bound on . The robustness of stable dynamics (not shown) is simply . Thin horizontal line indicates the threshold above which pattern formation becomes the most robust dynamical behaviour.
However, under the behavioural constraints on dispersal in equation (2.6), the robustness of pattern-forming dynamics is significantly diminished. Regardless of the local interaction motif or the value of , stable dynamics become more robust to parameter variation than pattern-forming dynamics. For systems with only two directly interacting species, the constrained value of falls to nearly zero. This indicates that a locally stable steady state of food webs in figure 1a–c will be maintained regardless of the underlying spatial network or the particular model parameters, so long as the behaviours of prey tracking and predator avoidance are strictly adhered to. For the case of three direct interactions in food web (figure 1 d) , pattern-forming dynamics remain likely for a randomly parameterized metacommunity, but significantly less so relative to the case of unconstrained cross-diffusion. Thus, figure 2 reflects an increased tendency for the system in equation (3.1) to maintain spatial homogeneity rather than transition to a heterogeneous state when spatial interactions follow reasonable expectations for adaptive behaviours.
While the cumulative effect of adding interspecific dispersal terms on metacommunity behaviour is equivalently given by the first moment, i.e. the mean, of either robustness metric, higher moments can shed light on differential impacts for either spatial or local robustness. Thus, we compute the second moment, the variance, and the third moment, the skewness, about the shared mean for the distributions of local and spatial robustness at each value of . To draw a direct comparison of these quantities between each robustness metric, we consider the distributions over parameter samples of equation (3.1) which yield food webs that are both feasible and stable in isolation, i.e. we exclude webs which yield unstable dynamics.
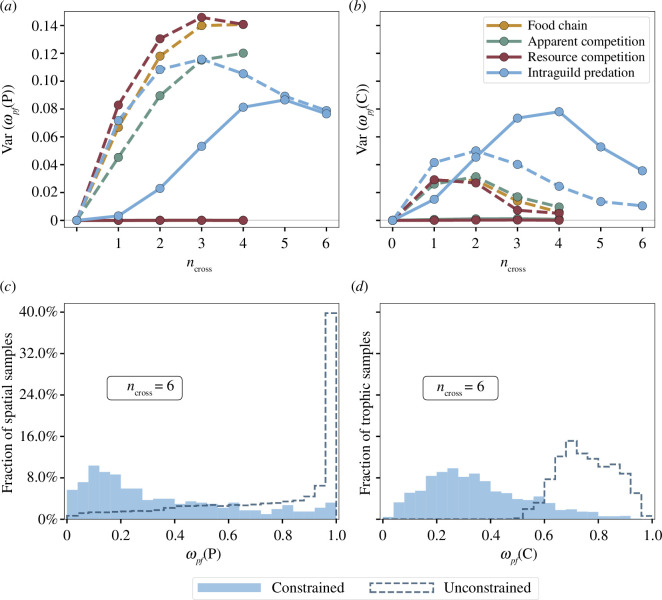
Beginning with the second moment, the variance provides a heuristic measure of model sensitivity to variation in species’ spatial responses when computed for or sensitivity to variation in the local parameters of equation (3.1) when computed for . As seen in figure 3a , the robustness of pattern-forming dynamics is highly dependent on the species’ spatial responses, encoded in the near-equilibrium dispersal rates of equation (2.4). For all local interaction motifs, this sensitivity is maximized at an intermediate value of approximately where the difference in total robustness between stable and pattern-forming dynamics is minimized (figure 2). For higher values of , susceptibility to pattern formation becomes increasingly common for initially stable steady states of equation (3.1), and thus decreases as the dynamical behaviour becomes less dependent on the particular set of dispersal parameters. In comparison, the sensitivity to local parameters of equation (3.1) shown in figure 3b is relatively low, and the maxima of occur prior to that of the distribution. This indicates that dispersal is the more decisive factor in determining dynamical behaviour relative to local interaction conditions for systems where species respond to one another spatially without any constraints on such responses.
Figure 3.
Distributions of spatial and local robustness. Local (a,c) and spatial (b,d) robustness distributions considered exclude locally unstable model parameterizations, such that may reach as opposed to figure 2. Without constraints on interspecific dispersal (dotted line), the sensitivity of pattern-forming behaviour to dispersal rates (a) is significantly higher than the sensitivity to local interaction parameters (b). Dispersal constraints (solid line) render these sensitivities comparable while diminishing or even prohibiting pattern formation. Example distributions (c) and (d) for the intraguild predation motif show that, at high , stabilization of homogeneous equilibria via dispersal constraints is reflected most prominently in the local robustness distribution.
The imposition of the constraints in equation (2.6) significantly alters these sensitivities. For motifs with only two direct interactions, the variance of either distribution is approximately zero as stable dynamics become virtually guaranteed. In contrast, the intraguild predation motif (figure 1d ) maintains a high sensitivity to near-equilibrium dispersal rates while increases such that the local and spatial sensitivities become comparable. Thus, when spatial responses are possible between all species comprising our metacommunity, the dynamical outcome of equation (2.1) is highly sensitive to the choice of parameters and susceptibility to pattern formation remains common for an initially stable food web under cross-diffusive constraints, even while stable dynamics become generally preferred.
Turning to the third central moment, the skewness indicates the tendency for randomly selected dispersal rates to yield values of local robustness higher or lower than the total robustness (i.e. the mean) when computed for , and similarly indicates this tendency for randomly selected local interaction parameters when computed for . We find that for low values of , the robustness of pattern-forming dynamics tends to be lower than the average shown in figure 2 in the absence of cross-diffusive constraints from equation (2.6). Imposition of the constraints exacerbates this effect, in particular with respect to the choice of local interaction parameters, and at higher the skew towards relatively low is comparable for both robustness distributions. In the absence of these constraints, however, the local robustness distribution skews towards relatively high , while the spatial robustness becomes symmetric about the mean.
Overall, when dispersal responses between species are few, ecologically motivated dispersal constraints result in a more significant shift towards preference of stable dynamics in the distribution compared with the distribution. However, when interspecific dispersal responses are prevalent, this stabilization of homogeneous equilibria is instead reflected most prominently in the local robustness distribution. This highlights the strong potential of interspecific dispersal responses, and their particular dependence on food web interactions, to determine the dynamical behaviour of spatially explicit metacommunities.
4. Large random metacommunities
To understand the impacts of interspecific dispersal rules on the dynamics of large metacommunities, we employed a method to generate random Jacobian matrices based on species interactions defined by the niche model [38]. To construct the network Jacobians, we first generate a food web topology by drawing niche values randomly from a uniform distribution for each of species in the metacommunity. These values depict each species’ position along a one-dimensional trophic niche axis. Niche ranges are then determined for each species by randomly sampling values from a beta distribution with parameters that depended on the desired connectance [38]. For species pair , if the niche value of falls within the range of , then is designated the consumer and as a resource. The corresponding Jacobian entries are modified accordingly: receives a positive entry drawn from a folded normal distribution , while receives a value drawn from . Finally, the diagonal entries are set to , reflecting density-dependent effects and self-regulation. Resulting webs were examined to ensure that paths exist between all species in the food web (i.e. there are no disconnected sub-webs), otherwise the web was discarded and regenerated. To populate the connectivity matrix , we define an auxiliary parameter which specifies the probability that the effect of species on will lead to a uniformly distributed dispersal kernel, such that , with the sign of optionally constrained by equation (2.6).
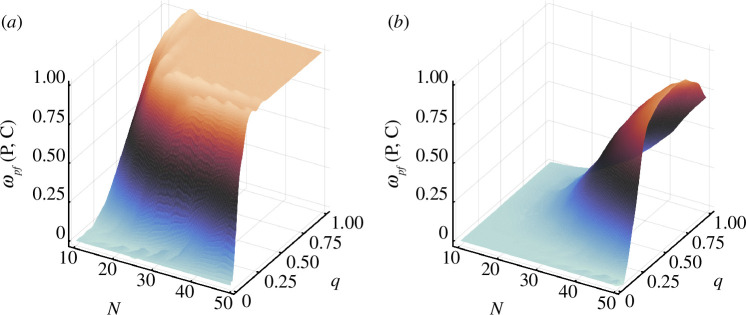
The effects of cross-diffusion observed in our three-species model with fully specified trophic interactions qualitatively hold when extended to larger metacommunities with interaction strengths in equation (2.3) randomly assigned. Firstly, the potential for pattern formation increases dramatically with the prevalence of interspecific dispersal responses . In the absence of constraints on these responses, locally stable matrices are guaranteed to be susceptible to pattern formation at sufficiently high for most food web sizes . A stronger tendency towards pattern formation is observed relative to our three species results, reflected in a higher maximum robustness and in a lower number of interspecific responses needed to reach the said maximum. Secondly, imposing the ecological constraints of equation (2.6) significantly diminishes , even eliminating the possibility of pattern formation for metacommunities with relatively few species. While the robustness of pattern-forming dynamics still scales with for large , pattern-forming dynamics never become guaranteed as it does for unconstrained cross-diffusion. Thus, the increased propensity for a homogeneous state under strict behavioural constraints on interspecific dispersal is a generic feature of our results, regardless of the number of species.
5. Discussion
In this paper, we show that dispersal driven by interspecific interactions has profound effects on spatial-pattern formation tendencies in metacommunities. Ecological studies commonly simplify dispersal as a linear function of intraspecific density with similar rates for all species, assumptions that tend to stabilize spatially uniform equilibrium states [22,23]. Generally, we find that assuming dispersal rates respond locally to both intra- and interspecific densities significantly increases susceptibility to pattern formation. However, when interspecific dispersal responses are constrained to follow empirically observed adaptive behaviours [12–15], i.e. prey tracking and predator avoidance, pattern formation is dramatically suppressed. This effect is observed regardless of food web size, although for sufficiently large number of species pattern-forming dynamics take precedent over homogeneous dynamics with or without adaptive dispersal constraints applied (figure 4).
Figure 4.
Pattern formation in large metacommunities. As in figure 3, locally unstable model parameterizations are excluded, such that may reach . Vertical axis and shading indicate total robustness of pattern-forming behaviour in randomly generated metacommunities (methods), with the probability of a food web interaction having a corresponding interspecific dispersal response either without (a) or with (b) the ecologically motivated constraints in equation (2.6).
The three-species interaction motifs investigated here showed minimal pattern formation when interspecific dispersal was constrained. The intraguild predation module was a notable exception as it exhibited significant pattern formation in both the unconstrained and constrained scenarios (figure 2). This motif is distinctive among the three-species motifs we examined because it involves interaction links spanning multiple trophic levels, potentially introducing additional dispersal feedbacks that can destabilize the system. Interestingly, we observed limited pattern formation in larger metacommunities with fewer than approximately 20 species (figure 4b ). While all motifs could potentially co-occur as building blocks of larger metacommunities, it appears that the homogeneous dynamics observed in certain motifs appear to dominate less speciose metacommunities. The presence of pattern-forming omnivory in such cases might be stabilized by other motifs or factors [9] or only become a frequent module in larger metacommunities. Studies that construct large food webs around specific interaction motifs could elucidate which ones are most important for pattern formation and represent an important future research direction [39,40].
The spatially heterogeneous metacommunity dynamics observed in our models may take different forms. Heterogeneous spatial patterns in metacommunities may include static differences in species densities—Turing-like patterns—and localized oscillations that may be synchronous or asynchronous across patches [20,22,30,41]. Certain forms of spatially asynchronous variability are expected to promote species persistence [31,32,42–44]. Internally generated patterns have been observed in nature [45], although empirical links between pattern formation and ecosystem resilience are few [46,47]. This is particularly the case of large-scale metacommunity dynamics [44], where variation may only manifest across longer temporal and spatial scales.
Regardless of the exact form, pattern formation mechanisms generate variation among patches even without underlying variation in the abiotic environment. However, environmental variation within metacommunities is commonplace and underpins notable metacommunity paradigms [48]. Models of large random metacommunities show that spatial environmental variation can promote community persistence, inverting the classic relationship between community complexity and instability [9,49,50]. Environmental variation may also drive dispersal, generating another path for altering cross-diffusion terms. By increasing the number and/or strength of community linkages, cross-diffusion terms would likely increase complexity and therefore reduce pattern formation when spatial environmental variation is present. Furthermore, high dispersal among certain species can provide sufficient dispersal to increase stability when overall dispersal is low [9,19]; this effect could be promoted by adaptive dispersal behaviour [51,52]. Whether constrained or unconstrained cross-diffusion leads to greater metacommunity complexity or influences pattern formation in spatially heterogeneous habitats is an open question.
Our models assume that dispersal decisions are based on local resource or predator densities. Emigrants dispersing from a patch then ‘resettle’ in neighbouring patches without an assessment of the new conditions there. In contrast, fitness-dependent models implement dispersal as a function of differences between both the ‘donor’ and ‘recipient’ habitats [16,17,51]. These alternative representations of dispersal lead to different outcomes regarding species coexistence and distributions among habitats [8,53]. Which model of dispersal best approximates the behaviour of real organisms remains an open question, and likely depends on the focal system in question (reviewed in [8]). However, it is probably unlikely that individuals can rapidly assess differences between community conditions at the scale over which most metacommunities operate (but see [54,55]).
Still, our assumption that dispersal is triggered directly by local resource or predator densities may not be general to all ecological systems. While we restrict interactions to only exist between consumers and resources, competing species are also known to drive each other’s emigration decisions [56]. Species responding to the presence of competitors may be especially common under contest competition (e.g. competition for space [57]). Given that direct dispersal responses to competitors would add cross-diffusion terms similar to ‘unconstrained’ ones in our model, we expect the inclusion of such responses to further increase the propensity of pattern-forming dynamics.
Furthermore, species may use both intra- and interspecific social cues when dispersing. Congregation behaviours may be reflected in directional biases in movement among patches that have been well studied in the context of animal grouping [21,58,59] and are known to facilitate spontaneous organization into heterogeneous patterns [10,60–62]. Organisms may also emigrate from habitat patches in groups [63] and the cues used to synchronize group movements may include those from other species [3,15]. While grouping behaviour and synchronized dispersal among group members are likely to also lead to pattern formation, the heterogeneous patterns that result may differ strongly from those in the systems we study here [33,34].
Teasing apart the contributions of internal and external drivers of observed spatial patterns continues to be a major empirical challenge. Dispersal shaped by interactions with other species is one of the many mechanisms that can generate self-organized variation in community compositions. Given the central role of dispersal behaviour in how organisms respond to their environment, future changes to environmental conditions may simultaneously alter external and internal drivers of spatial heterogeneity. Modelling studies such as ours can play an important role in understanding under what conditions internal pattern formation is possible, guiding empirical research.
Acknowledgements
We acknowledge this research was primarily conducted on unceded land of the Cahuilla, Tongva, Luiseño and Serrano peoples native to Southern California.
Contributor Information
Patrick Lawton, Email: patrick.lawton@email.ucr.edu.
Ashkaan K. Fahimipour, Email: afahimipour@fau.edu.
Kurt E. Anderson, Email: kurt.anderson@ucr.edu.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Code to reproduce all data and analyses is publicly available [64].
Supplementary material is avilable online [65].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
P.L.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; A.K.F.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, visualization, writing—original draft, writing—review and editing; K.E.A.: conceptualization, investigation, project administration, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
A.K.F. was supported by National Science Foundation grant EF-2222478 and K.E.A. was supported by National Science Foundation grant DEB-2225098.
References
- 1. Stier AC, Hein AM, Parravicini V, Kulbicki M. 2014. Larval dispersal drives trophic structure across Pacific coral reefs. Nat. Commun. 5 , 5575. ( 10.1038/ncomms6575) [DOI] [PubMed] [Google Scholar]
- 2. Fahimipour AK, Anderson KE. 2015. Colonisation rate and adaptive foraging control the emergence of trophic cascades. Ecol. Lett. 18 , 826–833. ( 10.1111/ele.12464) [DOI] [PubMed] [Google Scholar]
- 3. Gil MA, Hein AM. 2017. Social interactions among grazing reef fish drive material flux in a coral reef ecosystem. Proc. Natl Acad. Sci. USA 114 , 4703–4708. ( 10.1073/pnas.1615652114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hein AM, Gillooly JF. 2011. Predators, prey, and transient states in the assembly of spatially structured communities. Ecology 92 , 549–555. ( 10.1890/10-1922.1) [DOI] [PubMed] [Google Scholar]
- 5. Pillai P, Gonzalez A, Loreau M. 2011. Metacommunity theory explains the emergence of food web complexity. Proc. Natl Acad. Sci. USA 108 , 19293–19298. ( 10.1073/pnas.1106235108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fahimipour AK, Hein AM. 2014. The dynamics of assembling food webs. Ecol. Lett. 17 , 606–613. ( 10.1111/ele.12264) [DOI] [PubMed] [Google Scholar]
- 7. Amarasekare P. 2008. Spatial dynamics of foodwebs. Annu. Rev. Ecol. Evol. Syst. 39 , 479–500. ( 10.1146/annurev.ecolsys.39.110707.173434) [DOI] [Google Scholar]
- 8. Gross T, Allhoff KT, Blasius B, Brose U, Drossel B, Fahimipour AK, Guill C, Yeakel JD, Zeng F. 2020. Modern models of trophic meta-communities. Phil. Trans. R. Soc. B 375 , 20190455. ( 10.1098/rstb.2019.0455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson KE, Fahimipour AK. 2021. Body size dependent dispersal influences stability in heterogeneous metacommunities. Sci. Rep. 11 , 17410. ( 10.1038/s41598-021-96629-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fahimipour AK, Zeng F, Homer M, Traulsen A, Levin SA, Gross T. 2022. Sharp thresholds limit the benefit of defector avoidance in cooperation on networks. Proc. Natl Acad. Sci. USA 119 , e2120120119. ( 10.1073/pnas.2120120119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hein AM, Altshuler DL, Cade DE, Liao JC, Martin BT, Taylor GK. 2020. An algorithmic approach to natural behavior. Curr. Biol. 30 , R663–R675. ( 10.1016/j.cub.2020.04.018) [DOI] [PubMed] [Google Scholar]
- 12. Kreuzinger-Janik B, Gansfort B, Ptatscheck C. 2022. Population density, bottom-up and top-down control as an interactive triplet to trigger dispersal. Sci. Rep. 12 , 5578. ( 10.1038/s41598-022-09631-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Legrand D, Trochet A, Moulherat S, Calvez O, Stevens VM, Ducatez S, Clobert J, Baguette M. 2015. Ranking the ecological causes of dispersal in a butterfly. Ecography 38 , 822–831. ( 10.1111/ecog.01283) [DOI] [Google Scholar]
- 14. Fronhofer EA, et al. 2018. Bottom-up and top-down control of dispersal across major organismal groups. Nat. Ecol. Evol. 2 , 1859–1863. ( 10.1038/s41559-018-0686-0) [DOI] [PubMed] [Google Scholar]
- 15. Fahimipour AK, Gil MA, Celis MR, Hein GF, Martin BT, Hein AM. 2023. Wild animals suppress the spread of socially transmitted misinformation. Proc. Natl Acad. Sci. USA 120 , e2215428120. ( 10.1073/pnas.2215428120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abrams PA. 2007. Habitat choice in predator–prey systems: spatial instability due to interacting adaptive movements. Am. Nat. 169 , 581–594. ( 10.1086/512688) [DOI] [PubMed] [Google Scholar]
- 17. Amarasekare P. 2010. Effect of non-random dispersal strategies on spatial coexistence mechanisms. J. Anim. Ecol. 79 , 282–293. ( 10.1111/j.1365-2656.2009.01607.x) [DOI] [PubMed] [Google Scholar]
- 18. Quévreux P, Pigeault R, Loreau M. 2021. Predator avoidance and foraging for food shape synchrony and response to perturbations in trophic metacommunities. J. Theor. Biol. 528 , 110836. ( 10.1016/j.jtbi.2021.110836) [DOI] [PubMed] [Google Scholar]
- 19. Brechtel A, Gross T, Drossel B. 2019. Far-ranging generalist top predators enhance the stability of meta-foodwebs. Sci. Rep. 9 , 12268. ( 10.1038/s41598-019-48731-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pedersen EJ, Marleau JN, Granados M, Moeller HV, Guichard F. 2016. Nonhierarchical dispersal promotes stability and resilience in a tritrophic metacommunity. Am. Nat. 187 , E116–28. ( 10.1086/685773) [DOI] [PubMed] [Google Scholar]
- 21. Turchin P. 1998. Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 22. Murray JD. 2003. Mathematical biology: II: Spatial models and biomedical applications. New York, NY: Springer. ( 10.1007/b98869) [DOI] [Google Scholar]
- 23. Cantrell RS, Cosner C. 2004. Spatial ecology via reaction-diffusion equations. Hoboken, NJ: John Wiley & Sons. ( 10.1002/0470871296) [DOI] [Google Scholar]
- 24. Vanag VK, Epstein IR. 2009. Cross-diffusion and pattern formation in reaction-diffusion systems. Phys. Chem. Chem. Phys. 11 , 897–912. ( 10.1039/b813825g) [DOI] [PubMed] [Google Scholar]
- 25. Zemskov EP, Kassner K, Hauser MJB, Horsthemke W. 2013. Turing space in reaction-diffusion systems with density-dependent cross diffusion. Phys. Rev. E 87 . ( 10.1103/PhysRevE.87.032906) [DOI] [Google Scholar]
- 26. Fanelli D, Cianci C, Di Patti F. 2013. Turing instabilities in reaction-diffusion systems with cross diffusion. Eur. Phys. J. B 86 . ( 10.1140/epjb/e2013-30649-7) [DOI] [Google Scholar]
- 27. Madzvamuse A, Barreira R, Gerisch A. 2017. Cross-diffusion in reaction-diffusion models: analysis, Numerics, and applications. In Progress in industrial mathematics at ECMI (eds Quintela P, Barral P, Gómez D, Pena FJ, Rodríguez J, Salgado P, Vázquez-Méndez ME), pp. 385–392. Cham, Switzerland: Springer. ( 10.1007/978-3-319-63082-3) [DOI] [Google Scholar]
- 28. Krause AL, Gaffney EA, Maini PK, Klika V. 2021. Modern perspectives on near-equilibrium analysis of Turing systems. Phil. Trans. R. Soc. A 379 , 20200268. ( 10.1098/rsta.2020.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsyganov MA, Brindley J, Holden AV, Biktashev VN. 2004. Soliton-like phenomena in one-dimensional cross-diffusion systems: a predator–prey pursuit and evasion example. Phys. D Nonlinear Phenom. 197 , 18–33. ( 10.1016/j.physd.2004.06.004) [DOI] [Google Scholar]
- 30. Hata S, Nakao H, Mikhailov AS. 2014. Dispersal-induced destabilization of metapopulations and oscillatory Turing patterns in ecological networks. Sci. Rep. 4 , 3585. ( 10.1038/srep03585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayes SM, Anderson KE. 2023. Persistence in spatial multi-species food webs: the conflicting influences of isolated food web feasibility and spatial asynchrony. Commun. Nonlinear Sci. Numer. Simul. 119 , 107089. ( 10.1016/j.cnsns.2023.107089) [DOI] [Google Scholar]
- 32. Gouhier TC, Guichard F, Gonzalez A. 2010. Synchrony and stability of food webs in metacommunities. Am. Nat. 175 , E16–34. ( 10.1086/649579) [DOI] [PubMed] [Google Scholar]
- 33. Nakao H, Mikhailov AS. 2010. Turing patterns in network-organized activator–inhibitor systems. Nat. Phys. 6 , 544–550. ( 10.1038/nphys1651) [DOI] [Google Scholar]
- 34. Brechtel A, Gramlich P, Ritterskamp D, Drossel B, Gross T. 2018. Master stability functions reveal diffusion-driven pattern formation in networks. Phys. Rev. E 97 , 032307. ( 10.1103/PhysRevE.97.032307) [DOI] [PubMed] [Google Scholar]
- 35. Scholes NS, Schnoerr D, Isalan M, Stumpf MPH. 2019. A comprehensive network atlas reveals that Turing patterns are common but not robust. Cell Syst. 9 , 243–257.( 10.1016/j.cels.2019.07.007) [DOI] [PubMed] [Google Scholar]
- 36. Li Z, Liu S, Yang Q. 2017. Incoherent inputs enhance the robustness of biological oscillators. Cell Syst. 5 , 72–81.( 10.1016/j.cels.2017.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grilli J, Adorisio M, Suweis S, Barabás G, Banavar JR, Allesina S, Maritan A. 2017. Feasibility and coexistence of large ecological communities. Nat. Commun. 8 , 14389. ( 10.1038/ncomms14389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams RJ, Martinez ND. 2000. Simple rules yield complex food webs. Nature 404 , 180–183. ( 10.1038/35004572) [DOI] [PubMed] [Google Scholar]
- 39. Gellner G, McCann K. 2012. Reconciling the omnivory-stability debate. Am. Nat. 179 , 22–37. ( 10.1086/663191) [DOI] [PubMed] [Google Scholar]
- 40. Gellner G, McCann KS. 2016. Consistent role of weak and strong interactions in high- and low-diversity trophic food webs. Nat. Commun. 7 , 11180. ( 10.1038/ncomms11180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hata S, Nakao H, Mikhailov AS. 2014. Sufficient conditions for wave instability in three-component reaction-diffusion systems. Prog. Theor. Exp. Phys. 2014 , 13A01–0. ( 10.1093/ptep/ptt102) [DOI] [Google Scholar]
- 42. Abbott KC. 2011. A dispersal-induced paradox: synchrony and stability in stochastic metapopulations. Ecol. Lett. 14 , 1158–1169. ( 10.1111/j.1461-0248.2011.01670.x) [DOI] [PubMed] [Google Scholar]
- 43. Anderson KE, Hayes SM. 2018. The effects of dispersal and river spatial structure on asynchrony in consumer–resource metacommunities. Freshw. Biol. 63 , 100–113. ( 10.1111/fwb.12998) [DOI] [Google Scholar]
- 44. Guill C, Hülsemann J, Klauschies T. 2021. Self-organised pattern formation increases local diversity in metacommunities. Ecol. Lett. 24 , 2624–2634. ( 10.1111/ele.13880) [DOI] [PubMed] [Google Scholar]
- 45. Rietkerk M, van de Koppel J. 2008. Regular pattern formation in real ecosystems. Trends Ecol. Evol. 23 , 169–175. ( 10.1016/j.tree.2007.10.013) [DOI] [PubMed] [Google Scholar]
- 46. Zhang K, Yan J, He Q, Xu C, van de Koppel J, Wang B, Cui B, Liu QX. 2023. Self-organized mud cracking amplifies the resilience of an iconic ‘Red Beach’ salt marsh. Sci. Adv. 9 , eabq3520. ( 10.1126/sciadv.abq3520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maron JL, Harrison S. 1997. Spatial pattern formation in an insect host–parasitoid system. Science 278 , 1619–1621. ( 10.1126/science.278.5343.1619) [DOI] [PubMed] [Google Scholar]
- 48. Leibold MA, et al. 2004. The metacommunity concept: a framework for multi‐scale community ecology. Ecol. Lett. 7 , 601–613. ( 10.1111/j.1461-0248.2004.00608.x) [DOI] [Google Scholar]
- 49. Gravel D, Massol F, Leibold MA. 2016. Stability and complexity in model meta-ecosystems. Nat. Commun. 7 , 12457. ( 10.1038/ncomms12457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mougi A, Kondoh M. 2016. Food-web complexity, meta-community complexity and community stability. Sci. Rep. 6 , 24478. ( 10.1038/srep24478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Amarasekare P. 2007. Spatial dynamics of communities with intraguild predation: the role of dispersal strategies. Am. Nat. 170 , 819–831. ( 10.1086/522837) [DOI] [PubMed] [Google Scholar]
- 52. Mougi A. 2019. Adaptive migration promotes food web persistence. Sci. Rep. 9 , 12632. ( 10.1038/s41598-019-49143-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gramlich P, Plitzko SJ, Rudolf L, Drossel B, Gross T. 2016. The influence of dispersal on a predator–prey system with two habitats. J. Theor. Biol. 398 , 150–161. ( 10.1016/j.jtbi.2016.03.015) [DOI] [PubMed] [Google Scholar]
- 54. Fronhofer EA, Nitsche N, Altermatt F. 2017. Information use shapes the dynamics of range expansions into environmental gradients. Global Ecol. Biogeogr. 26 , 400–411. ( 10.1111/geb.12547) [DOI] [Google Scholar]
- 55. Ponchon A, Travis JMJ. 2022. Informed dispersal based on prospecting impacts the rate and shape of range expansions. Ecography 2022 , e06190. ( 10.1111/ecog.06190) [DOI] [Google Scholar]
- 56. Fronhofer EA, Klecka J, Melián CJ, Altermatt F. 2018. Condition‐dependent movement and dispersal in experimental metacommunities. Ecol. Lett. 18 , 954–963. ( 10.1111/ele.12475) [DOI] [PubMed] [Google Scholar]
- 57. Calcagno V, Mouquet N, Jarne P, David P. 2006. Coexistence in a metacommunity: the competition–colonization trade-off is not dead. Ecol. Lett. 9 , 897–907. ( 10.1111/j.1461-0248.2006.00930.x) [DOI] [PubMed] [Google Scholar]
- 58. Okubo A, Levin SA. 2001. Diffusion and ecological problems: modern perspectives, 2nd edn. New York, NY: Springer. ( 10.1007/978-1-4757-4978-6) [DOI] [Google Scholar]
- 59. Turchin P, Thoeny WT. 1993. Quantifying dispersal of southern pine beetles with mark–recapture experiments and a diffusion model. Ecol. Appl. 3 , 187–198. ( 10.2307/1941801) [DOI] [PubMed] [Google Scholar]
- 60. Anderson KE, Hilker FM, Nisbet RM. 2012. Directional biases and resource-dependence in dispersal generate spatial patterning in a consumer–producer model. Ecol. Lett. 15 , 209–217. ( 10.1111/j.1461-0248.2011.01727.x) [DOI] [PubMed] [Google Scholar]
- 61. Maimaiti Y, Yang W. 2023. Stability and pattern formation in a general class of reaction-diffusion-advection system. Acta Appl. Math. 185 . ( 10.1007/s10440-023-00576-4) [DOI] [Google Scholar]
- 62. Lewis MA. 1994. Spatial coupling of plant and herbivore dynamics: the contribution of herbivore dispersal to transient and persistent ‘waves of damage. Theor. Popul. Biol. 45 , 277–312. ( 10.1006/tpbi.1994.1014) [DOI] [Google Scholar]
- 63. Cote J, et al. 2017. Behavioural synchronization of large-scale animal movements—disperse alone, but migrate together? Biol. Rev. Camb. Philos. Soc. 92 , 1275–1296. ( 10.1111/brv.12279) [DOI] [PubMed] [Google Scholar]
- 64. Lawton P, Fahimipour AK, Anderson KE. 2023. Data from: Interspecific dispersal influences pattern formation in model metacommunities. Github. https://github.com/patricklawton/MetacomCrossDiffusion [DOI] [PMC free article] [PubMed]
- 65. Lawton P, Fahimipour A, Anderson K. 2024. Supplementary material from "Interspecific dispersal constraints suppress pattern formation in metacommunities. Figshare. ( 10.6084/m9.figshare.c.7293084) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Code to reproduce all data and analyses is publicly available [64].
Supplementary material is avilable online [65].