Abstract
Background:
Mild cognitive impairment (MCI), a prodromal phase of Alzheimer’s disease (AD), is heterogeneous with different rates and risks of progression to AD. There are significant gender disparities in the susceptibility, prognosis, and outcomes in patients with MCI, with female being disproportionately negatively impacted.
Objective:
The aim of this study was to identify sex-specific heterogeneity of MCI using multi-modality data and examine the differences in the respective MCI subtypes with different prognostic outcomes or different risks for MCI to AD conversion.
Methods:
A total of 325 MCI subjects (146 women, 179 men) and 30 relevant features were considered. Mixed-data clustering was applied to women and men separately to discover gender-specific MCI subtypes. Gender differences were compared in the respective subtypes of MCI by examining their MCI to AD disease prognosis, descriptive statistics, and conversion rates.
Results:
We identified three MCI subtypes: poor-, good-, and best-prognosis for women and for men, separately. The subtype-wise comparison (for example, poor-prognosis subtype in women versus poor-prognosis subtype in men) showed significantly different means for brain volumetric, cognitive test-related, also for the proportion of comorbidities. Also, there were substantial gender differences in the proportions of participants who reverted to normal function, remained stable, or converted to AD.
Conclusion:
Analyzing sex-specific heterogeneity of MCI offers the opportunity to advance the understanding of the pathophysiology of both MCI and AD, allows stratification of risk in clinical trials of interventions, and suggests gender-based early intervention with targeted treatment for patients at risk of developing AD.
Keywords: Alzheimer’s disease, comorbidity, gender differences, heterogeneity, mild cognitive impairment, subtypes
INTRODUCTION
Mild cognitive impairment (MCI) represents as an intermediate stage between normal cognitive aging and Alzheimer’s disease (AD) in which individuals demonstrate a slight, measurable decline in cognitive abilities but do not suffer significant impact on their daily functioning [1]. People with MCI may be three to five times more likely to develop AD relative to those without MCI [2]. Focusing on MCI allows to examine early disease symptoms, which may be most responsive to new treatments.
MCI is etiologically heterogeneous with different risks and rates of progression to dementia [2]. Some MCI patients will stay stable for ten years, or even return to normal cognitive status by timely interventions [3], whereas others will progress to AD rapidly or succumb after as little as three years [4]. Medical, environmental, and lifestyle risk factors as well as genetic variation contribute to such heterogeneity [5–7]. At least one out of three AD dementia cases can be linked to medical factors such as cardiovascular conditions, obesity, diabetes, and lifestyle factors such as physical activity, diet, social engagement, and educational attainment [8–10]. Understanding and characterizing such heterogeneity of MCI with respect to the trajectory of clinical outcomes including rates of cognitive decline, progression to AD or reversion to normal is essential as it would enable clinicians and researchers to identify individuals most in need of early intervention and maximally delay the progression of the condition. In addition, it would allow stratification of participants in clinical trials of new drugs aimed at slowing the progression of MCI to AD.
Many of the above-mentioned AD risk factors show gender effects, and, after advanced age, female sex is the major risk factor for AD [11, 12]. Research on gender-associated differences at MCI stage has focused on gender-differences in risk profiles [13], progression to AD [14, 15], and longitudinal rates of cognitive performance [16, 17]. However, the differences have not been characterized with respect to the heterogeneity of MCI. As gender differences exist in the disease risk factors, manifestation, and progression to AD, the study on gender-specific heterogeneity of MCI can be clinically very useful and for initiating new therapies for gender-based AD prevention trials. The heterogeneity of MCI can be identified via subtyping approaches [18], and it is useful for implementing precision medicine approaches for the understanding, prevention, treatment, and clinical trials for AD.
The focus of this study is to identify, quantify, and compare the differences in the subtypes of MCI between women and men that have distinct patterns of progression of MCI to AD. We separately classified female and male MCI subjects into clinically relevant subtypes based on features including AD-related comorbidities, lifestyle risk factors, demographics, brain imaging features, genetics, cognitive scores, and blood biomarkers with data that are derived from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (https://adni.loni.usc.edu). The ADNI data set has been widely used in many research studies on subtype analysis [18, 19]; otherwise it has not been used for examining gender-specific heterogeneity of MCI.
METHODS
Data used in the preparation of this article were obtained from the ADNI database. The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information, see https://www.adni-info.org.
Criteria for ADNI eligibility and diagnostic classifications are described at https://www.adni-info.org/Scientists/ADNIGrant/ProtocolSummary.aspx. ADNI criteria for MCI were: 1) Mini-Mental State Examination (MMSE) scores between 24 and 30; 2) subjective memory complaints; objective memory loss defined as scoring below an education-adjusted cut-off score on the Wechsler Memory Scale Logical Memory test (score = 8 for those with = 16 years of education; score = 4 for those with 8–15 years of education; score = 2 for those with 0–7 years of education); 3) a Clinical Dementia Rating score of 0.5; and 4) absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia. In this study, all ADNI-1 and ADNI-2/ADNI-GO MCI subjects with at least one post-baseline visit data were included. On average, for each subject, 6 post-baseline visit data were available. For each subject, a total of 30 features were included for gender-specific MCI subtype analysis, including AD-related comorbidities, lifestyles, and other AD-relevant data modalities at baseline have been considered and described below.
A total of 8 AD-related comorbidities [9, 10] were considered and the status of these medical conditions at baseline visit was determined using rating scales, physical measurements, treatment/medication use, or self-reported medical history based on the data availability in ADNI. Subjects self-reported medical history was screened based on the presence of related key words to determine the status of each comorbid condition (for example, the keywords used for Hypertension are ‘hypertension’, ‘high blood pressure’, and ‘HTN’). The details of how the status of each comorbidity was determined are provided below.
Hypertension – Based on the presence in the self-reported medical history of related keywords (“hypertension”, “high blood pressure”, or “HTN”), or treatment with antihypertensive medications.
Diabetes – Based on the presence in the self-reported medical history of related key words (“diabetes”, “type2 diabetes”), or the use of glucose-lowering medications.
High cholesterol – Based on the presence in the self-reported medical history of related key words (“Hypercholesterolemia”, “high cholesterol”, “elevated cholesterol”, or “Hyperlipidemia”), or treatment with lipid-lowering medications.
Depression – Geriatric Depression Scale (GDS-15) of 10, or treatment with antidepressants.
Obesity – Based on body mass index (BMI)>30.
Cardiovascular disease – Based on the presence in the self-reported medical history of related key words (“stroke”, “coronary artery disease”, “congestive heart failure”, “cerebrovascular disease”, “carotid artery stenosis”, “peripheral vascular disease”, or “Atrial fibrillation”).
Hearing Loss – Based on the presence in the self-reported medical history of related key words (“hearing loss”, “deaf”, or “presbycusis”).
Traumatic brain injury (TBI) – Based on the presence in the self-reported medical history of related key words (“concussion” or “head injury”).
In addition to AD-related comorbidities, an additional two lifestyle factors, smoking and alcohol use, were also considered. Both smoking and alcohol use were determined by subjective self-report from the medical record. Comorbidities and lifestyle factors were defined based on current/former/never status. Finally, a total of 7 data modalities were considered for gender-specific MCI subtype analysis.
Demographic data (Age), Education in years, Family history [5].
APOE gene status: Apolipoprotein E (APOE) ε2/ε4 carrier status was included as a genetic marker of AD [7].
The MRI volumetric features: Total ventricular volume, hippocampal volume (left plus right), middle temporal gyral volume (left plus right), and total entorhinal and fusiform volume [20, 21]. These MRI volumes were normalized by total intracranial volume (ICV).
Cognitive tests: The 11-item score and 13-item score from the Alzheimer’s Disease Assessment Scale (ADAS); total score from the Clinical Dementia Rating scale Sum of Boxes (CDRSB); total score from the Functional Assessment Questionnaire (FAQ); total score from the MMSE; and immediate score, learning score, forgetting score, and percentage forgetting score from the Rey’s Auditory Verbal Learning Test (RAVLT) [22, 23].
plasma neurofilament light (Nfl) and tau phosphorylated at threonine-181 (P-tau181) [24, 25].
Lifestyle factors: status of smoking, alcohol use [10].
Comorbidities: status of hypertension, diabetes, high cholesterol, depression, obesity, cardiovascular disease, hearing loss, and TBI [6].
A total of 325 MCI (146 females, 179 males) subjects from the ADNI study were included in the current analysis. Mixed-data clustering [26–28] was applied to female and male subjects separately to discover the MCI clusters within each gender. The clustering is performed based on all the above mentioned 30 baseline features (covariates) and the clinical relevance of each cluster was determined by examining the MCI to AD disease prognosis of subjects in the cluster based on the Kaplan-Meier survival analysis [29]. A 6-year follow-up window was considered, and the subjects who were lost to follow-up before the conversion was detected were censored. Log-rank test [30] was used to test for statistical differences between survivals of different clusters, where survival indicates that patients maintain a diagnosis of MCI rather than progress to AD. Furthermore, statistical differences between features of the subtypes were computed using the Fishers exact test for categorical variables and independent t-test for continuous variables. p values lower than 0.05 were considered significant.
RESULTS
Our study includes a total of 325 MCI subjects comprised of 146 women and 179 men. Table 1 summarizes the characteristics of this MCI cohort. Among MCI subjects in the study population, women were younger and had less education than men (p < 0.05). There was no statistically significant difference in APOE ε4 carrier status between women and men. Women performed significantly better with almost all the cognitive measures and had significantly larger ICV normalized volumes of hippocampus and middle temporal gyrus than men (p < 0.05). Compared with men, women had lower prevalence of cardiovascular (60.27% women, 72.62% men) and hearing problems (6.16% women, 26.81% men). These gender differences were based on characteristics of MCI patients before subtyping.
Table 1.
Gender-wise characteristics of MCI subjects
| Feature | Female | Male | Significance |
|---|---|---|---|
| Age (y, mean ± SD) | 69.99 ± 7.78 | 71.96 ± 6.79 | * |
| Education (years, mean ± SD) | 15.79 ± 2.65 | 16.49 ± 2.66 | * |
| Family History (%) | 96 (65.75) | 12 (64.80) | NS |
| APOE ε4 (% of >=1 allele) | 67 (45.89) | 87 (48.6) | NS |
|
| |||
| MRI volumetric features (volumes in mm3, mean ± SD) | |||
| Ventricles | 0.021 ± 0.012 | 0.026 ± 0.012 | ** |
| Hippocampus | 0.004 ± 8e-04 | 0.004 ± 8e-04 | ** |
| Middle temporal gyrus | 0.014 ± 0.001 | 0.013 ± 0.001 | * |
| Entorhinal | 0.002 ± 5e-04 | 0.002 ± 4e-04 | NS |
| Fusiform | 0.012 ± 0.001 | 0.012 ± 0.001 | NS |
|
| |||
| Cognitive tests (scores, mean ± SD) | |||
| ADAS11 | 8.51 ± 4.18 | 9.55 ± 4.21 | * |
| ADAS13 | 13.59 ± 6.57 | 15.34 ± 6.19 | * |
| CDRSB | 1.40 ± 0.89 | 1.54 ± 0.88 | ** |
| FAQ | 1.94 ± 3.17 | 3.38 ± 4.10 | ** |
| MMSE | 28.29 ± 1.74 | 27.97 ± 1.60 | NS |
| RAVLT immediate | 41.09 ± 11.54 | 33.84 ± 9.06 | ** |
| RAVLT learning | 5.19 ± 2.52 | 4.31 ± 2.46 | ** |
| RAVLT forgetting | 4.51 ± 2.81 | 4.73 ± 2.29 | NS |
| RAVLT % forgetting | 49.90 ± 33.26 | 59.05 ± 29.40 | * |
|
| |||
| Plasma biomarkers (pg/mL, mean ± SD) | |||
| Nfl | 38.59 ± 20.45 | 36.00 ± 16.10 | NS |
| P-tau181 | 16.85 ± 10.41 | 18.94 ± 13.50 | NS |
|
| |||
| Lifestyle factors (%) | |||
| Alcoholic | 5 (3.42) | 8 (4.46) | NS |
| Smoking | 55 (37.67) | 66 (36.87) | NS |
|
| |||
| Comorbidities, n (%) | |||
| Hypertension | 60 (41.09) | 89 (49.72) | NS |
| Diabetes | 12 (8.21) | 24 (13.40) | NS |
| Cholesterol | 66 (45.20) | 100 (55.86) | NS |
| Depression | 42 (28.76) | 51 (28.49) | NS |
| Obesity | 39 (26.71) | 40 (22.34) | NS |
| Cardiovascular | 88 (60.27) | 130 (72.62) | * |
| Hearing Problems | 9 (6.16) | 48 (26.81) | ** |
| TBI | 3 (2.05) | 12 (6.70) | NS |
MCI, mild cognitive impairment; APOE, Apolipoprotein; ADAS, Alzheimer’s Disease Assessment Scale, CDRSB, Clinical Dementia Rating scale Sum of Boxes; FAQ, Functional Assessment Questionnaire; MMSE, Mini-Mental State Examination; RAVLT, Rey’s Auditory Verbal Learning Test; Nfl, neurofilament light; P-tau181, tau phosphorylated at threonine-181; TBI, traumatic brain injury; NS, not significant. MRI volumes presented as a fraction of intracranial volume.
p < 0.05,
p < 0.01, NS p > 0.05.
Three subtypes: poor-, good-, and best-prognosis (PP, GP, BP) were identified for women and men separately. Figure 1a and 1b provided the Kaplan-Meier plots for female and male stratifications, respectively. Each gender-based stratification produced statistically significant survivals for all three subtypes with p < 0.001. These results demonstrated that there is significant heterogeneity within women and within men with MCI and this heterogeneity can be categorified into three subtypes with distinct clinical prognosis. The descriptive statistics of the features for female and male PP, GP, and BP subtypes were provided in Tables 2–4, respectively.
Fig. 1.
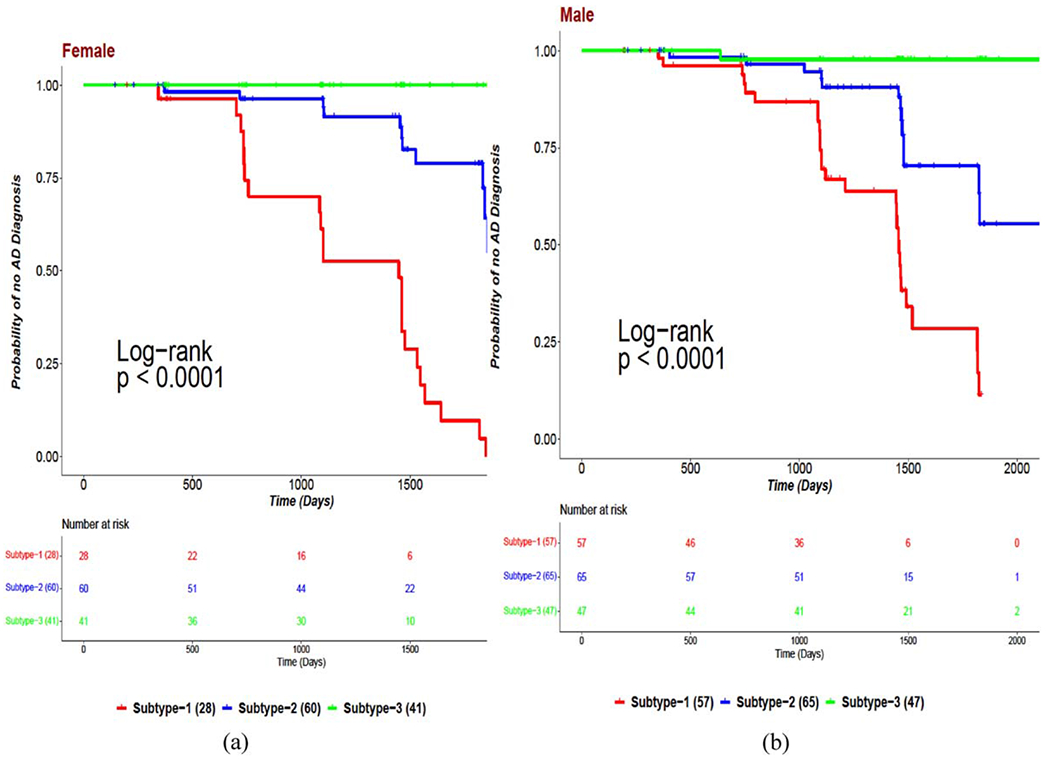
Kaplan-Meier plots for (a) female MCI subtypes and (b) male MCI subtypes.
Table 2.
Distribution of features in female-PP and male-PP subtypes
| Feature | Female-PP N = 28 | Male-PP N = 57 | Significance |
|---|---|---|---|
| Age (y, mean ± SD) | 71.65 ± 7.92 | 74.36 ± 6.55 | NS |
| Education (y, mean ± SD) | 15.05 ± 2.92 | 16.38 ± 2.94 | * |
| Family History, n (%) | 18 (64.28) | 34 (59.64) | NS |
| APOE ε4 (% of >=1 allele) | 20 (71.43) | 28 (49.12) | * |
|
| |||
| The MRI volumetric features (volumes in mm3, mean ± SD) | |||
| Ventricles | 0.026 ± 0.014 | 0.031 ± 0.013 | NS |
| Hippocampus | 0.004 ± 6e-04 | 0.004 ± 7e-04 | NS |
| Middle temporal gyrus | 0.012 ± 0.001 | 0.012 ± 0.001 | NS |
| Entorhinal | 0.002 ± 5e-04 | 0.002 ± 5e-04 | * |
| Fusiform | 0.011 ± 0.001 | 0.011 ± 0.001 | NS |
|
| |||
| Cognitive tests (scores, mean ± SD) | |||
| ADAS11 | 15.42 ± 3.59 | 12.61 ± 4.60 | * |
| ADAS13 | 23.10 ± 4.96 | 20.07 ± 6.21 | * |
| CDRSB | 2.30 ± 1.07 | 2.25 ± 0.92 | NS |
| FAQ | 5.85 ± 4.77 | 8.03 ± 4.01 | * |
| MMSE | 26.85 ± 1.97 | 27.29 ± 1.68 | NS |
| RAVLT immediate | 27.25 ± 4.50 | 28.45 ± 6.98 | NS |
| RAVLT learning | 2.5 ± 1.64 | 2.94 ± 2.02 | NS |
| RAVLT forgetting | 5.21 ± 1.64 | 5.12 ± 2.24 | NS |
| RAVLT % forgetting | 81.51 ± 21.63 | 76.02 ± 28.50 | NS |
|
| |||
| Plasma biomarkers (pg/mL, mean ± SD) | |||
| Nfl | 53.78 ± 29.40 | 40.28 ± 17.25 | * |
| P-tau181 | 22.79 ± 10.71 | 22.30 ± 18.08 | NS |
|
| |||
| Lifestyle factors, n (%) | |||
| Alcoholic | 3 (10.71) | 5 (8.77) | NS |
| Smoking | 7 (25) | 20 (35.08) | NS |
|
| |||
| Comorbidities, n (%) | |||
| Hypertension | 13 (46.42) | 33 (57.89) | NS |
| Diabetes | 0 (0) | 9 (15.78) | * |
| Cholesterol | 14 (50) | 30 (52.63) | NS |
| Depression | 5 (17.85) | 20 (35.08) | NS |
| Obesity | 5 (17.85) | 17 (29.82) | NS |
| Cardiovascular | 14 (50) | 44 (77.19) | * |
| Hearing Problems | 3 (10.71) | 17 (29.82) | * |
| TBI | 1 (3.57) | 4 (7.01) | NS |
PP, poor prognosis; APOE, Apolipoprotein; ADAS, Alzheimer’s Disease Assessment Scale, CDRSB, Clinical Dementia Rating scale Sum of Boxes; FAQ, Functional Assessment Questionnaire; MMSE, Mini-Mental State Examination; RAVLT, Rey’s Auditory Verbal Learning Test; Nfl, neurofilament light; P-tau181, tau phosphorylated at threonine-181; TBI, traumatic brain injury; NS, not significant. MRI volumes presented as a fraction of intracranial volume.
p < 0.05,
p < 0.01, NS p > 0.05.
Table 4.
Distribution of features in female-BP and male-BP subtypes
| Feature | Female-BP N = 55 | male-BP N = 53 | Significance |
|---|---|---|---|
| Age (y, mean ± SD) | 65.52 ± 5.27 | 68.63 ± 5.37 | * |
| Education (y, mean ± SD) | 16.55 ± 2.42 | 16.56 ± 2.32 | NS |
| Family History, n (%) | 43 (78.18) | 37 (69.81) | NS |
| APOE ε4 (% of >=1 allele) | 20 (36.37) | 20 (37.73) | NS |
|
| |||
| The MRI volumetric features (volumes in mm3, mean ± SD) | |||
| Ventricles | 0.015 ± 0.006 | 0.018 ± 0.009 | * |
| Hippocampus | 0.005 ± 6e-04 | 0.005 ± 6e-04 | NS |
| Middle temporal gyrus | 0.014 ± 0.001 | 0.014 ± 0.001 | NS |
| Entorhinal | 0.002 ± 3e-04 | 0.002 ± 3e-04 | NS |
| Fusiform | 0.012 ± 0.001 | 0.013 ± 0.001 | * |
|
| |||
| Cognitive tests (scores, mean ± SD) | |||
| ADAS11 | 5.08 ± 2.27 | 6.80 ± 2.56 | * |
| ADAS13 | 8.36 ± 2.90 | 9.96 ± 3.39 | * |
| CDRSB | 1.15 ± 0.68 | 1.24 ± 0.65 | NS |
| FAQ | 0.85 ± 1.28 | 1.30 ± 1.79 | NS |
| MMSE | 29.01 ± 1.17 | 25.91 ± 1.11 | NS |
| RAVLT immediate | 52.49 ± 7.12 | 40.30 ± 9.10 | * |
| RAVLT learning | 6.87 ± 1.77 | 5.71 ± 2.33 | * |
| RAVLT forgetting | 2.74 ± 2.37 | 3.69 ± 1.74 | * |
| RAVLT % forgetting | 21.11 ± 18.47 | 37.33 ± 19.34 | * |
|
| |||
| Plasma biomarkers (pg/mL, mean ± SD) | |||
| Nfl | 27.15 ± 9.21 | 25.72 ± 8.52 | NS |
| P-tau181 | 10.12 ± 5.23 | 13.10 ± 6.11 | * |
|
| |||
| Lifestyle factors, n (%) | |||
| Alcoholic | 2 (3.63) | 1 (1.88) | NS |
| Smoking | 23 (41.81) | 17 (32.07) | NS |
|
| |||
| Comorbidities, n (%) | |||
| Hypertension | 14 (25.45) | 16 (30.18) | NS |
| Diabetes | 3 (5.45) | 4 (7.54) | NS |
| Cholesterol | 21 (38.18) | 26 (49.05) | NS |
| Depression | 18 (32.72) | 17 (32.07) | NS |
| Obesity | 15 (27.27) | 13 (24.52) | NS |
| Cardiovascular | 26 (47.27) | 35 (66.03) | * |
| Hearing Problems | 1 (1.81) | 5 (9.43) | NS |
| TBI | 1 (1.81) | 3 (5.66) | NS |
BP, best prognosis; APOE, Apolipoprotein; ADAS, Alzheimer’s Disease Assessment Scale, CDRSB, Clinical Dementia Rating scale Sum of Boxes; FAQ, Functional Assessment Questionnaire; MMSE, Mini-Mental State Examination; RAVLT, Rey’s Auditory Verbal Learning Test; Nfl, neurofilament light; P-tau181, tau phosphorylated at threonine-181; TBI, traumatic brain injury; NS, not significant. MRI volumes presented as a fraction of intracranial volume.
p < 0.05,
p < 0.01, N. S p > 0.05.
The gender differences which exhibited before subtyping also appeared after subtyping, but were restricted to specific subtypes, like gender differences on age and cognitive tests were significant only for BP subtype, while education and brain volumetric features differed significantly for GP subtype. Plasma features did not show any gender differences before subtyping, but differences on plasma Nfl and P-tau181 were found significant for PP and BP subtypes, respectively. Before subtyping, only cardiovascular and hearing problems differed between women and men, but after subtyping, along with cardiovascular and hearing problems (in PP subtype), diabetes (PP subtype), and obesity (GP subtype) showed significant gender differences.
Table 5 provided the percentages of subjects who reverted to normal (“reverters”), who stayed at MCI stage (“stablers”), and who progressed to AD (“converters”) in each of the male and female MCI subtypes. For BP subtype, no MCI converter to AD was observed for both women and men. For PP subtype, no reverter from MCI to normal was observed for both women and men. The converters proportions among APOE ε4 carriers in both genders were close (24 % in female, 25% in male), when computed in the whole MCI population before subtyping. But the number of the converters among APOE ε4 carriers in the female-PP subtype was almost twice that among APOE ε4 carriers in the male-PP subtype (78.57% female, 49.12% male). These results show that the risk of AD onset or conversion from MCI was higher among women APOE ε4 carriers than among men APOE ε4 carriers is apparent after the stratifications, but not in the whole population without subtyping. Across subtypes, converters/reverters were mostly women compared to men. On the contrary, a higher proportion of men than women remained stable across each subtype. There were significant differences between stablers in male-PP and female-PP subtypes (21.4% versus 50.9%, p < 0.05); between converters in male-PP and female-PP subtypes (78.6% versus 49.1%, p < 0.05). For both male and female, first AD-conversion time (which is minimum over AD-conversion times of all MCI converters) among the subjects in PP group was less than 1 year and for the other group, it was over 1 year. These results demonstrate that gender-specific subtyping provided clinically relevant male and female stratifications of MCI subjects and gender-differences across subtypes with different levels of AD prognosis.
Table 5.
Distribution of Reverters to Normal, MCI stable, and Converters to AD for each of the subtypes in both female, male stratifications of MCI
| female-PP | male-PP | female-GP | male-GP | female-BP | male-BP | |
|---|---|---|---|---|---|---|
| Reverters (%) | 0 | 0 | 4.76 | 5.79 | 25.45 | 11.42 |
| Stablers (%) | 21.43 | 50.88 | 76.19 | 75.36 | 74.55 | 88.58 |
| Converters (%) | 78.57 | 49.12 | 19.05 | 18.84 | 0 | 0 |
| Mean conversion time to AD (Days) | 1199.04 | 1209.54 | 1410.58 | 1296.92 | – | – |
| First conversion time to AD (Days) | 343 | 352 | 371 | 404 | – | – |
| Converters among APOE ε4+ (%) | 79.17 | 48 | 19.64 | 21.31 | – | – |
PP, poor prognosis; GP, good prognosis; BP, best prognosis.
DISCUSSION
Our study investigated gender-specific heterogeneities in patients with MCI using multi-modal data based on the ADNI database. We utilized 30 AD relevant features including 8 AD-related comorbidities along with 22 others from demographics, APOE genotype, brain volumetric data, cognitive measurements, plasma biomarkers, and behavioral factors to understand and compare gender-specific variabilities in progression from MCI to AD. Using the mixed multi-modal data, we stratified female MCI subjects into three subtypes (female-PP, female-GP, female-BP) with poor-, good-, and best- AD prognosis; and male MCI subjects into three subtypes (male-PP, male-GP, male-BP) with poor-, good-, and best- AD prognosis. Gender differences were explored across female-PP versus male-PP, female-GP versus male-GP, and female-BP versus male-BP subtypes. Before subtyping, women were younger than men and had less education than men. After subtyping, age difference was significant only for BP subtype, and education difference was found for PP and GP subtypes. Females performed significantly better than men with almost all cognitive tests in the whole MCI population, i.e., before subtyping. These differences were found for BP (which is consistent with the study in [31]) and not for PP and GP subtypes. This may be explained by differences in overall age; subjects in PP and GP subtypes were older compared to BP; and younger subjects in general have a better overall health condition and more active lifestyle. Sex differences were found on 3 out of 5 ICV-normalized MRI volumetric features for GP subtype, with slightly larger normalized volumes for women than for men. In other subtypes, the differences were not significant, but had slightly larger normalized hippocampus volumes for women than for men, which is in concordance with another study in [32]. Before subtyping, only cardiovascular and hearing problems differed between women and men, but after subtyping, along with cardiovascular and hearing problems (in PP subtype), diabetes (PP subtype) and obesity (GP subtype) showed significant gender differences.
From the analysis of the MCI groups identified in both stratifications, we observe that no MCI subject in the BP subtype progressed to AD during the observation period, and conversely, none of MCI subjects in the PP subtype reverted to normal. The converters with positive APOE ε4 in the female PP subtype were almost double when compared with the converters with positive APOE ε4 in the male PP subtype (78.57% female, 49.12% male). On the other hand, the converters proportions among APOE ε4 carriers in both genders were very close (24 % in female, 25% in male) in the whole MCI population without subtyping. These results demonstrate that there exists substantial difference between women and men APOE ε4 carriers, which become evident only after subtyping based on multi-dimensional multi-modal features. The results with PP subtype (poor prognosis of MCI to AD conversion) are consistent with previous studies [33–35] that reported the higher incidence of AD in women in their sixties carrying one or two APOE ε4 alleles compared to their male counterparts. Across subtypes, converters/reverters were mostly women. On the contrary, more men than women were stable during the observation period across each subtype. In the GP population, more women (30.15%) were obese than men (14.49%). On poor-prognosis (PP) level, diabetes and cardiovascular conditions showed higher prevalence in males than their respective female counterparts. Clinical attention to these factors for male MCI subjects in PP subtype, may be warranted. The existing facts like APOE ε4 risk factor for women, cardiovascular factors for men were restricted to the sub-populations (subtypes) of MCI subjects, and the necessary preventive care should be considered in these respective subgroups. Findings from this study demonstrate that gender-based subtyping strategy is able to separate gender-wise patients with MCI into groups with different clinical outcomes or prognosis with both high sensitivity and high specificity.
One potentially important application for such gender-specific stratification is in clinical trials for drugs aimed at preventing or slowing the progression of AD. The number of participants required to detect improvement in such a trial will be greater the less the decline in cognitive function. Therefore, starting trials with patients in the PP group or the GP group, but not the BP group, will allow study of fewer patients for a definitive answer, and therefore fewer research participants at risk. Moreover, the less the variability in the outcome measures, the fewer participants are required, which also argues for stratification of participants. Given the remarkable number of non-productive and expensive clinical trials for AD and for gender-differences in AD, it is critical to conserve the patient population as well as to obtain definitive results in minimum time. Stratification could assist in this goal.
Some limitations of the current study are recognized. First, our current study is based on the ADNI database. Although ADNI is designed to develop clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of AD and is one of the largest databases for AD research containing multiple types of AD-related data, including MRI, PET, APOE, age, and blood biomarkers, it is not a population-based study. Our findings need to be validated in other independent cohorts but current availability of such multi-modality databases with both MCI and AD subjects are limited. Second, longitudinal follow up changes were not considered due to the lack of information on follow-up changes with comorbidities and behavioral factors. The gender-based heterogeneity in patients with MCI may evolve over time as features including comorbidities, brain volumetric data, cognitive measurements, plasma biomarkers, and behavioral factors will change over time. This study showed significant gender-specific heterogeneity in MCI patients with baseline characteristics; however, it will be important to examine how it evolves over time. Third, as our study used multilevel features and which often partially correlate with each other, for example, age with comorbidities, cognitive functions and brain images, genotypes with biomarkers and brain images. In future, we will consider such relations while stratifying the patients. Fourth limitation of this study was lack of consideration of competing risks or the possibility that a subject might convert to another type of dementia, such as dementia with Lewy bodies or frontotemporal dementia. Public databases that include multimodal data such as comorbidities, MRI volumes of patients with dementia with Lewy bodies or frontotemporal dementia are currently unavailable.
Conclusion
We performed gender-specific multi-modal subtype analysis of the MCI population from the ADNI database utilizing AD-relevant biomarkers. These results highlight the differences and importance of considering the gender-based stratification analysis of MCI with distinct disease prognosis. Both female and male stratifications provided three subtypes with significantly different trajectories. The subtype-wise comparison (for example, poor-prognosis subtype in women versus poor-prognosis subtype in men) for gender differences showed different behaviors having significantly different means for MRI volumetric, cognitive test-related, plasma features, and by the proportion of comorbidities. Also, there were substantial gender differences in the proportions of participants who reverted to normal function, remained stable, or converted to AD. As many of AD risk factors, comorbidities and disease prognoses are gender based, both stratifications and subsequent clusters identified by the gender-specific multi-modal subtyping strategy may yield additional new treatment targets for risk modification and allowing a more personalized strategy for AD prevention, diagnosis, treatment, and management intervention.
Table 3.
Distribution of features in female-GP and male-GP subtypes
| Feature | Female-GP N = 63 | Male-GP N = 69 | Significance |
|---|---|---|---|
| Age (y, mean ± SD) | 71.16 ± 7.79 | 73.31 ± 6.48 | NS |
| Education (y, mean ± SD) | 15.30 ± 2.61 | 16.79 ± 2.65 | * |
| Family History, n (%) | 35 (55.55) | 45 (65.21) | NS |
| APOE ε4 (% of >=1 allele) | 27 (42.86) | 39 (56.52) | NS |
|
| |||
| The MRI volumetric features (volumes in mm3, mean ± SD) | |||
| Ventricles | 0.022 ± 0.013 | 0.029 ± 0.010 | * |
| Hippocampus | 0.004 ± 7e-04 | 0.004 ± 6e-04 | * |
| Middle temporal gyrus | 0.013 ± 0.001 | 0.013 ± 0.001 | * |
| Entorhinal | 0.002 ± 4e-04 | 0.002 ± 4e-04 | NS |
| Fusiform | 0.012 ± 0.001 | 0.011 ± 0.001 | NS |
|
| |||
| Cognitive tests (scores, mean ± SD) | |||
| ADAS11 | 8.01 ± 2.53 | 9.83 ± 2.81 | * |
| ADAS13 | 13.93 ± 4.20 | 15.56 ± 4.30 | * |
| CDRSB | 1.22 ± 0.70 | 1.19 ± 0.64 | NS |
| FAQ | 1.15 ± 1.88 | 1.14 ± 1.33 | NS |
| MMSE | 28.30 ± 1.67 | 27.95 ± 1.62 | NS |
| RAVLT immediate | 37.30 ± 6.49 | 33.34 ± 7.36 | * |
| RAVLT learning | 4.93 ± 2.26 | 4.36 ± 2.29 | NS |
| RAVLT forgetting | 5.74 ± 2.81 | 5.20 ± 2.48 | NS |
| RAVLT % forgetting | 60.99 ± 27.83 | 61.70 ± 26.30 | NS |
|
| |||
| Plasma biomarkers (pg/mL, mean ± SD) | |||
| Nfl | 42.27 ± 17.52 | 39.54 ± 16.12 | NS |
| P-tau181 | 19.56 ± 11.10 | 20.82 ± 11.74 | NS |
|
| |||
| Lifestyle factors, n (%) | |||
| Alcoholic | 0 (0) | 2 (2.89) | NS |
| Smoking | 25 (39.68) | 29 (42.02) | NS |
|
| |||
| Comorbidities, n (%) | |||
| Hypertension | 33 (52.38) | 40 (57.97) | NS |
| Diabetes | 9 (14.28) | 11 (15.94) | NS |
| Cholesterol | 31 (49.2) | 44 (63.76) | NS |
| Depression | 19 (30.15) | 14 (20.29) | NS |
| Obesity | 19 (30.15) | 10 (14.49) | * |
| Cardiovascular | 48 (76.19) | 51 (73.91) | NS |
| Hearing Problems | 5 (7.93) | 26 (37.68) | * |
| TBI | 1 (1.58) | 5 (7.24) | NS |
GP, good prognosis; APOE, Apolipoprotein; ADAS, Alzheimer’s Disease Assessment Scale, CDRSB, Clinical Dementia Rating scale Sum of Boxes; FAQ, Functional Assessment Questionnaire; MMSE, Mini-Mental State Examination; RAVLT, Rey’s Auditory Verbal Learning Test; Nfl, neurofilament light; P-tau181, tau phosphorylated at threonine-181; TBI, traumatic brain injury; NS, not significant. MRI volumes presented as a fraction of intracranial volume.
p < 0.05,
p < 0.01, NS p > 0.05.
ACKNOWLEDGMENTS
We thank the Alzheimer’s Disease Neuroimaging Initiative (ADNI) for generously sharing clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of Alzheimer’s disease.
We acknowledge support from National Institute on Aging (grants nos. AG057557, AG061388, AG062272, AG076649), the Clinical and Translational Science Collaborative (CTSC) of Cleveland (grant no. TR002548).
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (https://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0600r2).
REFERENCES
- [1].Petersen RC (2000) Mild cognitive impairment: Transition between aging and Alzheimer’s disease. Neurologia 15, 93–101. [PubMed] [Google Scholar]
- [2].Mitchell AJ, Shiri-Feshki M (2008) Temporal trends in the long-term risk of progression of mild cognitive impairment: A pooled analysis. J Neurol Neurosurg Psychiatry 79, 1386–1391. [DOI] [PubMed] [Google Scholar]
- [3].DeCarli C (2003) Mild cognitive impairment: Prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2, 15–21. [DOI] [PubMed] [Google Scholar]
- [4].Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF (2015) Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology 85, 331–338. [DOI] [PubMed] [Google Scholar]
- [5].Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA (2013) Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med 29, 873–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Santiago JA, Potashkin JA (2021) The impact of disease comorbidities in Alzheimer’s disease. Front Aging Neurosci 13, 631770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oveisgharan S, Buchman AS, Yu L, Farfel J, Hachinski V, Gaiteri C, De Jager PL, Schneider JA, Bennett DA (2018) APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology 90, e2127–e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G (2006) Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment. Neurol Res 28, 625–629. [DOI] [PubMed] [Google Scholar]
- [9].Ma F, Wu T, Miao R, Xiao YY, Zhang W, Huang G (2015) Conversion of mild cognitive impairment to dementia among subjects with diabetes: A population-based study of incidence and risk factors with five years of follow-up. J Alzheimers Dis 43, 1441–1449. [DOI] [PubMed] [Google Scholar]
- [10].Zhou S, Zhou R, Zhong T, Li R, Tan J, Zhou H (2014) Association of smoking and alcohol drinking with dementia risk among elderly men in China. Curr Alzheimer Res 11, 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA (2001) Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol 153, 132–136. [DOI] [PubMed] [Google Scholar]
- [12].Snyder HM, Asthana S, Bain L, Brinton R, Craft S, Dubal DB, Espeland MA, Gatz M, Mielke MM, Raber J, Rapp PR, Yaffe K, Carrillo MC (2016) Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement 12, 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K (2008) Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry 79, 979–84. [DOI] [PubMed] [Google Scholar]
- [14].Burke SL, Hu T, Fava NM, Li T, Rodriguez MJ, Schuldiner KL, Burgess A, Laird A (2019) Sex differences in the development of mild cognitive impairment and probable Alzheimer’s disease as predicted by hippocampal volume or white matter hyperintensities. J Women Aging 31, 140–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D’Agostino RB (1997) Lifetime risk of dementia and Alzheimer’s disease: The impact of mortality on risk estimates in the Framingham Study. Neurology 49, 1498–1504. [DOI] [PubMed] [Google Scholar]
- [16].Holland D, Desikan RS, Dale AM, McEvoy LK, Alzheimer’s Disease Neuroimaging Initiative (2013) Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am J Neuroradiol 34, 2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, Alzheimer’s Disease Neuroimaging Initiative (2015) Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 1), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gamberger D, Lavrač N, Srivatsa S, Tanzi RE, Doraiswamy PM (2017) Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Sci Rep 7, 6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ezzati A, Zammit AR, Habeck C, Hall CB, Lipton RB, Alzheimer’s Disease Neuroimaging Initiative (2020) Detecting biological heterogeneity patterns in ADNI amnestic mild cognitive impairment based on volumetric MRI. Brain Imaging Behav 14, 1792–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Devanand DP, Bansal R, Liu J, Hao X, Pradhaban G, Peterson BS (2012) MRI hippocampal and entorhinal cortex mapping in predicting conversion to Alzheimer’s disease. Neuroimage 60, 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Katabathula S, Wang Q, Xu R (2021) Predict Alzheimer’s disease using hippocampus MRI data: A lightweight 3D deep convolutional network model with visual and global shape representations. Alzheimers Res Ther 13, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE, Alzheimer’s Disease Neuroimaging Initiative (2011) Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch Gen Psychiatry 68, 961–969. [DOI] [PubMed] [Google Scholar]
- [23].Korolev IO, Symonds LL, Bozoki AC, Alzheimer’s Disease Neuroimaging Initiative (2016) Predicting progression from mild cognitive impairment to Alzheimer’s dementia using clinical, MRI, and plasma biomarkers via probabilistic pattern classification. PLoS One 11, e0138866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang YL, Chen J, Du ZL, Weng H, Zhang Y, Li R, Jia Z, Sun M, Jiang J, Wang FZ, Xu J, Alzheimer’s Disease Neuroimaging Initiative (2021) Plasma p-tau181 level predicts neurodegeneration and progression to Alzheimer’s dementia: A longitudinal study. Front Neurol 12, 695696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H (2018) Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther 10, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jorgensen M, Hunt L (1996) Mixture model clustering of data sets with categorical and continuous variables. Proc Conf ISIS 96, 375–384. [Google Scholar]
- [27].Marbac M, Sedki M, Patin T (2020) Variable selection for mixed data clustering: Application in human population genomics. J Classif 37, 124–142. [Google Scholar]
- [28].Katabathula S, Davis PB, Xu R (2022) Comorbidity-driven multi-modal subtype analysis in mild cognitive impairment of Alzheimer’s disease. Alzheimers Dement. doi: 10.1002/alz.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17, 2815–2834. [DOI] [PubMed] [Google Scholar]
- [30].Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50, 163–170. [PubMed] [Google Scholar]
- [31].Beinhoff U, Tumani H, Brettschneider J, Bittner D, Riepe MW (2008) Gender-specificities in Alzheimer’s disease and mild cognitive impairment. J Neurol 255, 117–122. [DOI] [PubMed] [Google Scholar]
- [32].Perlaki G, Orsi G, Plozer E, Altbacker A, Darnai G, Nagy SA, Horvath R, Toth A, Doczi T, Kovacs N, Bogner P, Schwarcz A, Janszky J (2014) Are there any gender differences in the hippocampus volume after head-size correction? A volumetric and voxel-based morphometric study. Neurosci Lett 570, 119–123. [DOI] [PubMed] [Google Scholar]
- [33].Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative Investigators (2014) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC Jr, Roses AD, Pericak-Vance MA, Small GW, Haines JL (1995) The apolipoprotein E E4 allele and sex-specific risk of Alzheimer’s disease. JAMA 273, 373–374. [PubMed] [Google Scholar]
- [35].Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD (1996) Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: A possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 58, 803. [PMC free article] [PubMed] [Google Scholar]


