Robot-assisted therapy (RAT) (including robot and electromechanical devices), to date, seems to be a valid option to improve upper limb (UL) impairment due to stroke. RAT showed promising benefits in the UL functioning but with limitations due to quality assessment of the evidence affecting the interpretation.1, 2 Despite this, results of the experimental investigations have been translated into clinical indications, and in the last decade RAT has been included in the international guidelines (GLs). However, these are new rehabilitation approaches and the provided indications are changing quickly.
In the context of the CICERONE Italian Consensus Conference on RAT in neurorehabilitation, a previously published review3 synthesized the RAT recommendations reported in the international GLs, finding a general consensus in the use of robotic technologies to improve UL motor function, and in particular strength, applying RAT alone or as an add-on to usual rehabilitation care. Despite this, across the eight included GLs, a lack of recommendations according to patients’ characteristics and timing of intervention remains unsolved. Since RAT is rapidly spreading but still highly debated, in the present review, we aimed to update the GLs’ recommendations on using RAT for UL rehabilitation in stroke, highlighting differences and current recommendations.
The updated search was conducted by two blinded reviewers (A.M.C. and A.P.) according to SPIDER tool strategy and methodological procedures from the previously published work.3 We included GLs written in English and referred to the use of robots for UL rehabilitation in adults with stroke.
Each selected GL was checked by the two blinded reviewers for achieving its contents and main characteristics. These include year, edition, country, national/international recommendations contained, and textual descriptive synthesis of recommendations used to analyze the scope, context and consistency of the founded guidelines. The methodological quality of the included GLs was appraised using the AGREE-II instrument by three independent and blinded raters (D.M., G.L., and M.P.). As recommended by AGREE II, two domains (Applicability and Overall) were prioritized, and a quality threshold (>70%) for those domains was used, according to Hoffmann-Eßer4 (for complete methodology, see Morone et al.3).
Five out of eight updates of previous GLs and four new GLs were selected (see Supplementary Digital Material 1: Supplementary Table I). The Scottish Intercollegiate Guidelines Network and the Royal College of Physicians converged in the National Clinical Guideline for Stroke for the UK and Ireland.5 Similarly, the Stroke Foundation of New Zealand and Stroke Foundation became the Australian and New Zealand Living Clinical Guidelines for Stroke Management.6 The Canadian Stroke and Best Practice provided a new live GL that is currently refreshed online.7 Regarding new GLs, Japan,8 Brazil,9 South Korea,10 and the UK (NICE)11 released the new GLs containing RAT.
Moreover, the European Stroke Organization (ESO)12 and the World Stroke Organization (WOS)13 provided a synthesis of recommendations based on an overview of international GLs, including a session for robotics for UL recovery. It is worth noticing the broader distribution of RAT inclusion in GLs worldwide, from Europe to Americas, from Eastern Asia to Australia, proving the great attention this topic is gaining worldwide. The complete results can be found in Supplementary Table I.
Nine out of ten GLs supported using RAT to recover UL functioning, especially activity and participation, for patients with moderate-to-severe impairment. RAT is often suggested as an add-on to other treatments, taking advantage of the opportunity to increase intensity through repetition or to extend the treatment time.3, 5, 7 Specifically, RAT allows task-oriented training, boosting engagement via increased biofeedback, repetition, and intensity. The newly included GLs show a moderate to high level of evidence/recommendation in favour of RAT.
Differently, The NICE GLs does not recommend the use of RAT for UL recovery, providing a detailed committee’s discussion and interpretation of the evidence-based on four main-analysis: 1) outcomes that matter most; 2) quality of the evidence; 3) benefits and harms; and 4) cost effectiveness and resource use.
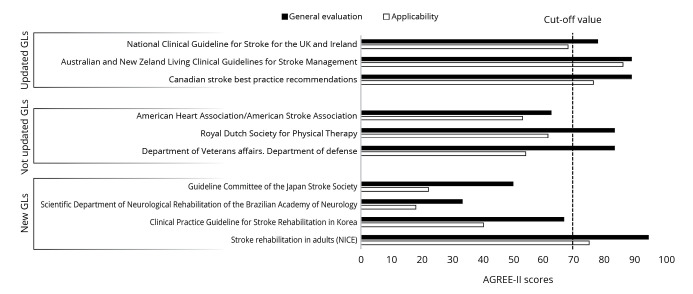
The evaluation of updated and new GLs performed with AGREE II showed a general positive score in four out of seven GLs (>70%). Three GLs presented positive results in both general and applicability domains. The updates of previously published GLs presented a higher score with respect to newly issued GLs, and only NICE guidelines exceeded the quality threshold among new GLs (Figure 1).
Figure 1.
—Bar plot of the general evaluation and applicability of the AGREE-II scores. GLs: guidelines.
This updated review on RAT for UL rehabilitation in adults with stroke includes four new and three updated GLs. Nine out of ten GLs recommended using RAT to recover UL function. Unfortunately, there are still generic recommendations about using robotics in these GLs.
In contrast, with a sufficient level of declared confidence, NICE committee provided recommendations against the use of RAT. The negative final judgment is imputable to three factors: 1) the limited evidence about the RAT’s impact on quality of life; 2) the risk of bias due to financial support of robot-farm; 3) the higher maintenance cost with respect to availability in a limited subgroup of patients. The different recommendations could be linked to the use of different studies at the basis of the different GLs. Probably, a recent RCT,14 the largest ever published, might have contributed to shift the judgment in NICE GL. Some issues have been addressed on this trial, i.e., variable time from stroke to baseline and treatment dosage, and undefined usual care received by control group; and they should be taken into account for the interpretation of results. The disagreement with the other GLs deserves careful consideration, and the presented negative factors need to be examined in future studies. Moreover, the comparison of judgements across the international GLs would be desirable to reach a univocal recommendation, since better economic outcome than conventional therapy was reported, especially for patients with severe disability.15 However, since the limited number of cost-effectiveness studies, the relatively small sample size and the variability among resources reduce the generalizability of conclusions, larger high-quality investigations are needed. Despite the debate on economic sustainability versus effectiveness is still ongoing, cost-effectiveness analyses could make it possible to better assess the opportunity to introduce robotics into the healthcare systems.
One of main problems emerging from all the guidelines is the lack of reporting guidance on clinical protocols (i.e. different dose, intensity, and frequency, according to individuals’ characteristics). Furthermore, only three out of ten GLs clearly suggest RAT as an add-on to conventional rehabilitation, while the other seven do not specify the use of RAT alone or in addition to other treatments. Performing robotic therapy in addition to conventional therapy, or as a substitute will be one of the important knots to be solved also in relation to the phase of use of robotic therapy (early subacute, late subacute, chronic).
It is worth noticing that more detailed GLs focus on the functional classification of patients that could benefit from this treatment (for example, moderate to severe impairment, unilateral or bilateral involvement) or focus on the different endpoints of the RAT (i.e. motor functions and/or ADL functions).
The results of this update suggested that RAT is a valid approach to improve motor functions, activities, and participation in people with moderate and severe UL impairment post-stroke. This final observation is in line with the ESO and WOS recommendations. Indeed, ESO GLs strongly recommended RAT as an adjunct to conventional therapy to increase arm activity,12 and WOS synthesizing global GLs suggests that “robotic interfaces [...] can be used as adjunct tools to other rehabilitation therapies” as advanced approaches.13 The discordant opinion and the parameters proposed by the NICE committee, needs to be considered in further investigation and in the next updates of other GLs to clarify the future of UL robotic rehabilitation in stroke population.
Supplementary Digital Material 1
Supplementary Table I
Characteristics of included clinical practice guidelines of past search with correspondent update and new GLs.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2018;9:CD006876. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30175845&dopt=Abstract 10.1002/14651858.CD006876.pub5 [DOI] [PMC free article] [PubMed]
- 2.Straudi S, Baluardo L, Arienti C, Bozzolan M, Lazzarini SG, Agostini M, et al. ; Working group upper limb “CICERONE” Italian Consensus Conference on Robotic in Neurorehabilitation. Effectiveness of robot-assisted arm therapy in stroke rehabilitation: an overview of systematic reviews. NeuroRehabilitation 2022;51:559–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36530097&dopt=Abstract 10.3233/NRE-220027 [DOI] [PubMed] [Google Scholar]
- 3.Morone G, Palomba A, Martino Cinnera A, Agostini M, Aprile I, Arienti C, et al. ; “CICERONE” Italian Consensus Conference on Robotic in Neurorehabilitation. Systematic review of guidelines to identify recommendations for upper limb robotic rehabilitation after stroke. Eur J Phys Rehabil Med 2021;57:238–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33491943&dopt=Abstract 10.23736/S1973-9087.21.06625-9 [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann-Eßer W, Siering U, Neugebauer EA, Lampert U, Eikermann M. Systematic review of current guideline appraisals performed with the Appraisal of Guidelines for Research & Evaluation II instrument-a third of AGREE II users apply a cut-off for guideline quality. J Clin Epidemiol 2018;95:120–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29288133&dopt=Abstract 10.1016/j.jclinepi.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 5.National Clinical Guideline for Stroke for the UK and Ireland [Internet]. Available from: https://www.strokeguideline.org/ [cited 2024; May 15].
- 6.Stroke Foundation. Living Clinical Guidelines for Stroke Management [Internet]. Available from: https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management [cited 2024; May 15].
- 7.Canadian Stroke Best Practice. Stroke Best Practices [Internet]. Available from: https://www.strokebestpractices.ca/ [cited 2024; May 15].
- 8.Miyamoto S, Ogasawara K, Kuroda S, Itabashi R, Toyoda K, Itoh Y, et al. Committee for Stroke Guideline 2021, the Japan Stroke Society. Japan Stroke Society Guideline 2021 for the Treatment of Stroke. Int J Stroke 2022;17:1039–49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35443847&dopt=Abstract 10.1177/17474930221090347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minelli C, Luvizutto GJ, Cacho RO, Neves LO, Magalhães SC, Pedatella MT, et al. Brazilian practice guidelines for stroke rehabilitation: part II. Arq Neuropsiquiatr 2022;80:741–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36254447&dopt=Abstract 10.1590/0004-282x-anp-2021-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DY, Ryu B, Oh BM, Kim DY, Kim DS, Kim DY, et al. KSNR Stroke CPG Writing Group . Clinical Practice Guideline for Stroke Rehabilitation in Korea-Part 1: Rehabilitation for Motor Function (2022). Brain Neurorehabil 2023;16:e18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37554256&dopt=Abstract 10.12786/bn.2023.16.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NICE. National Institute for Health and Care Excellence. Stroke rehabilitation in adults. NICE guideline [NG236] [Internet]. Available from: https://www.nice.org.uk/guidance/ng236 [cited 2024, Jun 6].
- 12.Kwakkel G, Stinear C, Essers B, Munoz-Novoa M, Branscheidt M, Cabanas-Valdés R, et al. Motor rehabilitation after stroke: european Stroke Organisation (ESO) consensus-based definition and guiding framework. Eur Stroke J 2023;8:880–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37548025&dopt=Abstract 10.1177/23969873231191304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mead GE, Sposato LA, Sampaio Silva G, Yperzeele L, Wu S, Kutlubaev M, et al. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int J Stroke 2023;18:499–531. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36725717&dopt=Abstract 10.1177/17474930231156753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019;394:51–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31128926&dopt=Abstract 10.1016/S0140-6736(19)31055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo K, Stephenson M, Lockwood C. The economic cost of robotic rehabilitation for adult stroke patients: a systematic review. JBI Database Syst Rev Implement Reports 2019;17:520–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30973526&dopt=Abstract 10.11124/JBISRIR-2017-003896 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Characteristics of included clinical practice guidelines of past search with correspondent update and new GLs.