Abstract
BACKGROUND
Around 40% of stroke survivor develop spasticity. Plantar flexors (PF) muscles are often affected, with severe functional impairment. The treatment of choice is botulinum toxin type A (BoNT-A) combined with adjuvant treatments. The temporary pharmacological effect implies periodic reassessment and reinjection. These long-term chronic programs require monitoring the functional impact of each cycle and the clinical evolution in relation to aging and repeated interventions.
AIM
Evaluating changes of functional level in patients with post-stroke spasticity treated with BoNT-A by assessing the long-term maintenance of the therapeutic efficacy.
DESIGN
Retrospective longitudinal observational study.
SETTING
Outpatients.
POPULATION
Chronic stroke survivors undergoing BoNT-A treatment and subsequent intensive rehabilitation (10 sessions in a day-hospital regime).
METHODS
Medical records of the enrolled patients were consulted. The primary endpoint was the change in PF spasticity by at least 1 point on the Modified Ashworth Scale (MAS) at each cycle. Secondary endpoints were the assessment of possible trends in gait parameters (Six Minute Walking Test [6MWT]; Timed Up and Go [TUG], and 10 Meters Walking Test [10mWT]) pre- and post-injection and at each cycle.
RESULTS
Thirty-six patients were enrolled. A reduction of at least one MAS point for PF was recorded after each cycle in all subjects. A time-dependent reduction in the proportion of patients reporting an improvement higher than the minimal clinically important difference (MCID) in 6MWT and 10mWT was observed. In the case of TUG, this data kept stable at all cycles. A one-point increase in the basal functional ambulation classification (FAC) score resulted in a reduction in the probability of having a TUG improvement greater than the MCID. The opposite correlation was found for 6MWT and 10mWT.
CONCLUSIONS
With the proposed treatment, the clinical significance TUG improvement remains constant throughout repeated cycles and the proportion of patients with improvement in 6MWT and 10mWT tends to decline over time.
The predictive value of basal FAC on the functional variables expected improvement may provide a potential treatment targeting tool.
CLINICAL REHABILITATION IMPACT
These results may deliver prognostic indication allowing an optimized integration of different post-BoNT-A rehabilitation approaches, agreeing with current evidence. Adequate monitoring and treatment protocols are crucial for the stability of functional level and may prevent excessive fluctuations.
Key words: Muscle spasticity, Botulinum toxins, Health care outcomes assessment, Follow-up, Rehabilitation
Stroke incidence varies between 15 and 40 cases per 10,000 people per year in the general population. The occurrence is age dependent, leading to the 75% of cases happening in subjects over 65 years old.1
Stroke is the second cause of death globally (third in industrialized countries) and the leading cause of chronic disability. Every year around 5 million people in the world, corresponding to one third of those affected by strokes, experience a moderate to severe disability.2
Most importantly, the prevalence of “chronic stroke” increased by 27% in high income countries.3 This translates into a growth in stroke survivors by 84% and stroke-related deaths by 26% globally, with an increase in disability-adjusted life years (DALY).4
It is interesting to note that in the last 15 years the 5-year relapse rate and the relapse-free survival have not undergone further improvement and that relapses are in most cases with the same pathogenesis of the first event.5 This could imply a suboptimal use of secondary prevention strategies.
A key element is the assessment of an adequate functional prognosis for stroke survivors. Many evaluation scales showed a prognostic value on the functional outcome of these patients.6-8 Most importantly, rehabilitation intervention demonstrated to positively modify the prognostic outcome in all functional areas compromised by stroke, however the level of evidence is often low or undetermined due to the scarcity of comparable randomized clinical trials.9
A declining but still high incidence of stroke, a growing survival rate in post-acute and the general aging of the population, cause a considerable impact on global health. In particular, we are increasingly witnessing the saturation of “conventional” therapeutic and rehabilitation resources. Therefore, the adoption of appropriate long-term monitoring protocols aimed to the optimization of secondary prevention and to the maintenance of the functional status plays a crucial role.
Although it is shared that the recovery of motor and cognitive functions is more striking in the first 3-6 months after the acute event, the condition of chronic disability does not imply the achievement of a “functional plateau.”10-12 In fact, a significant gradient of modifiability of the disability level is maintained, splitting the concept of chronicity and stability and implicitly introducing the idea of aging as an additional element.10 In this context it seems even more necessary to assess the role of rehabilitation, which should be specific on each phase after stroke, and provide monitoring and corrective protocols of modifiable risk factors.
One of the main complications after a stroke is spasticity. Around 43% of stroke survivors develop spasticity13 within 12 months from the acute event,14 while in chronic phase the prevalence reaches 97%. This phenomenon negatively affects disability, impacting on all the domains of the International Classification of Function, Disability and Health (ICF).15
Plantar flexor muscles (PF) are among the most commonly involved in the case of post stroke spasticity (PSS) giving the clinical presentation of the spastic equinovarus foot (SEF). This condition often leads to impaired foot-ground interaction, reduced ankle dorsiflexion and foot clearance, abnormal compensatory movements, altered load progression with increased energy expenditure and higher fall risk.16, 17
The treatment of choice for focal spasticity is botulinum toxin type A (BoNT-A).18 The pharmacological effect lasts about 4 to 6 months in the reduction of spasticity and proved to have a high safety and efficacy also in the improvement of pain, walking speed and walking pattern19 and in the increase of the quality of life.20-23 Notably, the pharmacological approach needs to be necessarily implemented in a context of a multimodal approach, including adjuvant treatments focused on patients’ specific needs,24-26 providing an integrated treatment for spasticity.
Given the temporary and reversible effect of BoNT-A, the condition of chronicity and the increase in survival and life expectancy after the acute event, it is necessary to structure follow-up protocols based on periodic clinical reassessment, the possible repetition of BoNT-A treatment and the association with post-injection rehabilitation procedures aimed to optimize the therapeutic effect.
As mentioned, the main determinant for the therapeutic approach is the functional relevance of spasticity. Therefore, it is necessary to set up evaluation protocols that can be used in normal clinical practice to monitor the trend over time of specific functional characteristics.
The assessment of functional capacity is fundamental both as a diagnostic and as a prognostic tool aimed to modulate the rehabilitation approach according to disability and aging, and to collect epidemiological data intended to improve the evidence based therapeutic proposal. In addition, periodic measurements may optimize treatment compliance, often affected by the cognitive and communication deficits that may prevent the adequate report of patients’ needs.27
Typically, in stroke survivors, aerobic endurance is measured with the Six-Minute Walking Test (6MWT), walking speed is measured with the Ten-Meter Walking Test (10mWT)28 and agility, lower limb strength, balance and fall risk estimation are assessed with the Timed Up-and-Go test (TUG).29
These assessments, used as daily clinical practice, can provide crucial information about the functional status. Walking speed is closely related to the functional level of walking30 and can also be used for classification purposes of the quality of life. Indeed, changes in gait speed seem to correlate with global changes in the disability level.31, 32 Similarly, TUG threshold values could classify functional independence in walking: beyond 20 s the increased fall risk suggests the need of assistance in walking.27
Additionally, the level of walking autonomy can be determined with the functional ambulation classification (FAC).33 In fact, in the chronic phase, FAC allows to categorize patients according to the degree of walking independence, and also correlates with the level of muscle fibroadipose degeneration in PF, which is typical of stroke survivors.34
On these bases, it is essential to assess the impact of aging and of a long-term chronic treatment on specific functional outcomes. Properly knowing the effect of therapeutic strategies and aging on the long term, would help to create monitoring, prevention and treatment protocols increasingly focused on individual specific needs.
As stated above, current literature is quite rich in providing updated evidence about the role of botulinum toxin in stroke survivors. However, studies about the functional impact of a long term and repeated treatment are still scant. In fact, few authors focused on the maintenance and the possible trend of functional parameters over time, in relation to cyclic BoNT-A injections and researches mainly aimed to assess BoNT-A safety35 or efficacy on shorter term.36 Furthermore, a correlation between the therapeutic effect entity and the basal functional condition is yet to be studied.
This study aims to evaluate the efficacy maintenance of the periodic and long term BoNT-A treatment, associated with post-injection rehabilitation, in reducing spasticity at the level of PF muscles, and in the preservation of the functional level in stroke survivors with PSS.
The primary endpoint is the change in PF spastic hypertonia by at least 1 point on the modified Ashworth scale (MAS) at every treatment cycle.
The secondary endpoints include the evaluation of the overtime trend of the outcome variables modification throughout each cycle. Specifically, 6MWT, TUG and 10mWT variations at each cycle will be mapped through time and their statistical and clinical significance will be assessed. In addition, the influence of the basal functional walking status (FAC) on the probability to have a variation greater than the minimal clinically important difference (MCID) in said parameters will be assessed.
Materials and methods
Retrospective longitudinal observational cohort study set in the Physical and Rehabilitative Medicine Unit of Maggiore della Carità University Hospital in Novara, Italy. The subjects included in the trial were recruited among chronic stoke survivors already addressed to our center for the periodical clinical re-evaluation and treatment with BoNT-A.
Study participation was subject to the presence of a clinical indication to perform BoNT-A injection followed by a rehabilitation treatment cycle in a day-hospital regime (10 sessions, 90 minutes each).
The inclusion criteria were age older than 18 years old, unilateral ischemic or hemorrhagic stroke (documented with clinical examination and neuroradiological findings), presence of spasticity at plantar flexor muscles at least of grade 2 of the modified Ashworth scale (MAS), history of at least 8 cycles of integrated spasticity treatment (BoNT-A + adjunctive treatment).
The exclusion criteria were inability to walk before the acute event, the presence of severe cognitive impairment, concomitant musculoskeletal, neurological or cardiopulmonary alterations that could interfere with clinical results, the presence of skin lesions that could contraindicate BoNT-A treatment, previous functional surgery (myotendinous elongation, neurotomy) on PF.
Patients were evaluated, according to normal clinical practice in our Centre, before every BoNT-A treatment and one month after injection, at the conclusion of the 10-sessions treatment cycle and at the peak of the pharmacological effect. Injections were performed by trained physicians using ultrasound guide. The subsequent rehabilitation treatment was delivered by expert physical therapists.
Eight consecutive treatment cycles were considered for each patient, for a total of 16 evaluative sessions and an overall period of 4 years. For each assessment MAS, FAC, 6MWT, 10mWT and TUG were collected. The MCID considered is 34.4 m for the 6MWT,37 0.16 m/s for the 10mWT38 and the minimal detectable change (MDC) considered for TUG is 2.9 s.27
Descriptive statistics were used to summarize the characteristics of the population. Specifically, categorical variables were reported as absolute and percentage frequencies while numerical variables such as mean and standard deviation or median, Q1 and Q3 if not normally distributed. The mean values of pre- and post-treatment 6MWT, 10mWT and TUG were calculated to evaluate the temporal trend. The same procedure was applied to the average difference in pre-post treatment. Linear models for repeated measures were applied to evaluate the trend of the outcome variables. For each index, univariate and multivariate models were performed considering pre-and post-treatment values and their difference as response variables, and time as an independent variable. For multivariate models, patient characteristics identified at baseline were considered. For each variable, the clinically significant response rate was also calculated at every cycle, expressed as a percentage of patients with change in outcome values greater than specific MCID. For spasticity assessment, MAS variation was calculated.
The study was designed and conducted in accordance with international and national ethical standards on biomedical research with human beings; in particular: the Helsinki Declaration, the Standards of Good Clinical Practice (ICH/GCP) of the European Union, the Convention on Human Rights and Biomedicine and the Italian Codes of Ethics of Health Professions. The recruited subjects were presented with specific information regarding the type of study, the purposes, the procedures, the collection and processing of data, the possible benefits of the research and the absence of specific risks, being an observational study. The patients and their possible trusted persons were able to freely express their consent and had the possibility to revoke it at any time.
The trial was approved by the local Ethics Committee of Novara with the registration number CE031/2022 and by the Competent Authority (Maggiore della Carità University Hospital) with the protocol number 016.156. The approval was issued on 11/03/2022.
Data availability
The data ownership is held by the Maggiore della Carità University Hospital in Novara.
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Results
Thirty-six patients completed the study. For every subject, eight consecutive treatment cycles were considered, for a total of 16 assessment sessions and 4 years of study period. The descriptive statistics of the demographic variables is shown in Table I.
Table I. —Demographic characteristics and descriptive statistics (N.=36).
| Variable | Value |
|---|---|
| Sex | |
| Female | 17 (47.22%) |
| Male | 19 (52.78%) |
| Hemiplegic side | |
| Right | 15 (41.67%) |
| Left | 21 (58.33%) |
| Toxin | |
| Onabotulinumtoxin A | 29 (81.82%) |
| Abobotulinumtoxin A | 7 (18.18%) |
| Age at event | 56.72±10.93 |
| Years of chronic disability | 8.33 (3.44%) |
| Median FAC | 3 (2-4) |
FAC: functional ambulation classification.
For every subject a reduction of at least one point of MAS at PF muscles was observed after each treatment. Slight variations of BoNT-A dose on triceps surae were occasionally recorded.
Each gait parameter (6MWT, TUG, 10mWT) tends to improve after treatment at each cycle considered.
The mean pre-treatment values of 6MWT and post-treatment values of 6MWT, TUG and 10mWT keep stable over time, while the pre-treatment values of TUG and 10mWT tend to vary without specific trends (Table II, III).
Table II. —Beta, standard error and P value of the time variable derived from the multivariable model of the 6MWT, TUG and 10mWT variables measured before treatment.
| Timepoint | 6MWT | TUG | 10mWT | |||
|---|---|---|---|---|---|---|
| Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value | |
| T0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| T1 | 13.92 (10.63) | 0.1999 | -2.08 (0.63) | 0.0024* | 0.05 (0.03) | 0.1443 |
| T2 | 16.75 (10.26) | 0.1128 | -1.43 (0.80) | 0.0837 | 0.06 (0.04) | 0.1204 |
| T3 | 19.67 (11.51) | 0.0975 | -2.28 (0.97) | 0.0252* | 0.06 (0.04) | 0.1442 |
| T4 | 13.36 (11.84) | 0.2677 | -1.69 (1.29) | 0.2016 | 0.08 (0.04) | 0.0465* |
| T5 | 7.86 (10.25) | 0.4489 | -2.70 (1.49) | 0.0797 | 0.09 (0.04) | 0.0297* |
| T6 | 5.89 (12.80) | 0.6486 | -2.69 (1.62) | 0.1067 | 0.07 (0.04) | 0.1074 |
| T7 | 4.36 (11.57) | 0.7089 | -3.1 (1.65) | 0.0697 | 0.09 (0.04) | 0.0629 |
| T8 | 6.00 (11.66) | 0.6106 | -3.36 (1.58) | 0.0420* | 0.11 (0.04) | 0.0102* |
6MWT: 6-Minute Walking Test; TUG: Timed Up-and-Go; 10mWT: 10-Meter Walking Test; beta: mean change in value of the dependent variable in relation to change of the independent variable; SE: standard error. *Statistically significant values.
Table III. —Beta, standard error and P value of the time variable derived from the multivariable model of the 6MWT, TUG and 10mWT variables measured after treatment.
| Timepoint | 6MWT | TUG | 10mWT | |||
|---|---|---|---|---|---|---|
| Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value | |
| T0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| T1 | 3.54 (7.38) | 0.6347 | -1.07 (0.41) | 0.0136* | -0.02 (0.02) | 0.2541 |
| T2 | -2.64 (10.76) | 0.8078 | -0.36 (0.64) | 0.5808 | -0.03 (0.03) | 0.3289 |
| T3 | 5.11 (10.57) | 0.6322 | -1.40 (0.71) | 0.0568 | 0.02 (0.03) | 0.6223 |
| T4 | 2.67 (12.31) | 0.8299 | -0.87 (1.16) | 0.4616 | -0.01 (0.03) | 0.7970 |
| T5 | -10.83 (10.11) | 0.2921 | -1.52 (1.40) | 0.2851 | 0.01 (0.03) | 0.7636 |
| T6 | -6.33 (13.79) | 0.6492 | -2.06 (1.54) | 0.1909 | 0.00 (0.03) | 0.9500 |
| T7 | -10.25 (11.16) | 0.3653 | -2.52 (1.48) | 0.0992 | 0.00 (0.03) | 0.8907 |
| T8 | -9.97 (11.09) | 0.3754 | -2.63 (1.49) | 0.0860 | 0.04 (0.03) | 0.2127 |
6MWT: 6-Minute Walking Test; TUG: Timed Up-and-Go; 10mWT: 10-Meter Walking Test; beta: mean change in value of the dependent variable in relation to change of the independent variable; SE: standard error. *Statistically significant value.
Considering the mean values of post-pre score variations and the respective difference from the reference value at T0, the statistical significance of the 6MWT changes appears to fluctuate in relation to the reference value. Otherwise, the improvement in TUG was significantly stable over time (with an exception of T2). Finally, the mean increase in gait speed (10mWT) appears to be significantly higher than at T0 in the following evaluations except at T3 and T8, where the difference is equivalent to the reference value. The results are shown in Table IV.
Table IV. —Beta, standard error and P value of the time variable derived from the multivariable model of the 6MWT, TUG and 10mWT variations after treatment.
| Timepoint | 6MWT | TUG | 10mWT | |||
|---|---|---|---|---|---|---|
| Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value | |
| T0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| T1 | -10.38 (8.39) | 0.2255 | 1.01 (0.5) | 0.0538 | -0.07 (0.03) | 0.0248* |
| T2 | -19.39 (9.12) | 0.0415* | 1.07 (0.47) | 0.0282* | -0.09 (0.04) | 0.0170* |
| T3 | -14.56 (8.79) | 0.1078 | 0.88 (0.58) | 0.1403 | -0.04 (0.03) | 0.1305 |
| T4 | -10.69 (9.32) | 0.2600 | 0.82 (0.6) | 0.1811 | -0.09 (0.03) | 0.0060* |
| T5 | -18.69 (8.13) | 0.0284* | 1.18 (0.58) | 0.0501 | -0.09 (0.03) | 0.0131* |
| T6 | -12.22 (8.56) | 0.1634 | 0.64 (0.71) | 0.3753 | -0.07 (0.03) | 0.0284* |
| T7 | -14.61 (8.36) | 0.0903 | 0.58 (0.71) | 0.4223 | -0.08 (0.03) | 0.0157* |
| T8 | -15.97 (7.74) | 0.0476* | 0.72 (0.63) | 0.2587 | -0.07 (0.03) | 0.0625 |
6MWT: 6-Minute Walking Test; TUG: Timed Up-and-Go; 10mWT: 10-Meter Walking Test; beta: mean change in value of the dependent variable in relation to change of the independent variable; SE: standard error. *Statistically significant values.
Considering the MCID, there is a time-dependent reduction of the number of patients reporting a clinically significant improvement of 6MWT and 10mWT through time. For TUG, the proportion of subjects with clinically significant change in score keeps stable at all cycles (except T2) (Table V, Figure 1).
Table V. —Absolute number and percentage of subjects with values greater than or equal to the MCID in each cycle for TUG, 6MWT and 10MWT.
| Timepoint | 6MWT ≥34.4 m | TUG ≤-2.9 s | 10mWT ≥0.16 m/s | |||
|---|---|---|---|---|---|---|
| N. | % | N. | % | N. | % | |
| 0 | 11 | 30.56% | 12 | 33.33% | 6 | 16.67% |
| T0 | 7 | 19.44% | 10 | 27.78% | 3 | 8.33% |
| T1 | 8 | 22.22% | 5 | 13.89% | 2 | 5.56% |
| T2 | 7 | 19.44% | 9 | 25.00% | 8 | 22.22% |
| T3 | 5 | 13.89% | 9 | 25.00% | 1 | 2.78% |
| T4 | 4 | 11.11% | 10 | 27.78% | 0 | 0.00% |
| T5 | 7 | 19.44% | 10 | 27.78% | 1 | 2.78% |
| T6 | 5 | 13.89% | 10 | 27.78% | 1 | 2.78% |
| T7 | 5 | 13.89% | 9 | 25.00% | 1 | 2.78% |
| T8 | ||||||
6MWT: 6-Minute Walking Test; TUG: Timed Up-and-Go; 10mWT: 10-Meter Walking Test; MCID: minimal clinically important difference.
Figure 1.
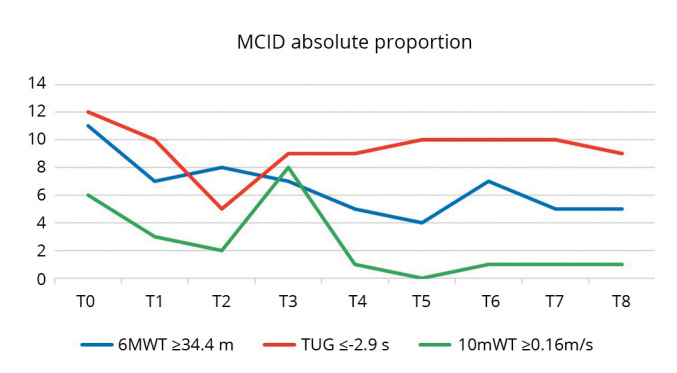
—Graphical trend of the MCID share of the variables of interest at each cycle. The number of patients with improvement greater than or equal to the MCID (ordinate) in the case of 6MWT and 10mWT tends to decrease over time with no specific trend. In the case of TUG, the proportion of subjects with clinically significant changes remains constant over time with an isolated decrease at T2. 6MWT: 6-Minute Walking Test; TUG: Timed Up-and-Go; 10mWT: 10-Meter Walking Test; MCID: minimal clinically important difference.
Finally, FAC appears to be significantly associated with pre and post values and the variation of each outcome variable: a one-point increase in the FAC score resulted in a 39% reduction in the probability of having a TUG improvement greater than the MCID, and in a 72% and 86% increase in the probability of a 6MWT and 10mWT improvement, respectively (Table VI).
Table VI. —Repeated-measures model assessing the presence of a trend in the proportion of subjects with changes ≥MCID adjusted for FAC.
| Variable | 6MWT ≥34.4 m | TUG ≤-2.9 s | 10mWT ≥0.16 m/s | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| FAC | 1.725 | (1.121-2.654) | 0.609 | (0.456-0.814) | 1.862 | (1.434-2.417) |
6MWT: 6-Minute Walking Test; TUG: Timed Up-and-Go; 10mWT: 10-Meter Walking Test; FAC: functional ambulation classification; OR: odds ratio; CI confidence interval.
Discussion
The results reconfirm the effectiveness of BoNT-A in reducing focal spasticity of PF muscles in stroke survivors by at least one point on the MAS scale at each treatment cycle. This data is consistent with the current scientific evidence about the efficacy of botulinum toxin24, 26 and supports the maintenance of the therapeutic effect even after repeated injections.
Concerning chronic patients with a defined spasticity pattern and clinical presentation, a similar treatment schedule is expected at every follow-up. Anyway, slight variations of doses were detected in some treatment cycles. In the case of the target muscles considered in this study, these minor entity adjustments should be noted in the context of a periodic re-evaluation and possible inoculation fine-tuning. In fact, the choice of the therapeutic dose, and similarly of the pharmaceutical form and dilution, rely on clinical examination based on spasticity severity, muscle size, muscle selection and the predicted treatment goal. Also, the frequent need of a multifocal treatment addressed to numerous target sites should be considered. These variables may lead to a dynamic and goal-oriented treatment adaptation.
Additionally, the use of MAS as an indicator of BoNT-A efficacy is surely common but also debated due to its intrinsic limitation. Anyway, its implementation both in clinical and in research settings is validated by the drug data sheet in which MAS reduction is the primary outcome measure to assess BoNT-A effect and therefore the most common evaluation to assess spasticity with significant effectiveness.39, 40 Furthermore, being this a retrospective study, the presented results concern past medical records in which MAS was the most implemented and consistently used evaluation. This choice relies also on the familiarity of physicians on this scale, which is easy and reliable to deploy in a clinical context.
With regard to functional parameters, the average scores of 6MWT, TUG and 10mWT in the entire population deliver an improvement in each variable accordingly with each cycle. This finding support the already known therapeutic efficacy of BoNT-A in improving walking speed.19, 41 Regarding the other gait variables such as endurance and the characteristics assessed by TUG, the current scientific evidence is less strong in attributing to BoNT-A treatment a univocally significant role.42, 43 Nevertheless, the results obtained may support a positive effect of the proposed treatment on every outcome measure, with variable significance.
It is interesting to note that the average pre-treatment values of 6MWT and post treatment values of 6MWT, TUG and 10mWT keep substantially stable through time. These results suggest a preservation of “basal” endurance over the years and a stable post-treatment functional result over time, in relation to periodic follow-up. In contrast, the oscillation of the pre-treatment values of TUG and 10mWT do not allow a clear interpretation of these results.
Considering the average change of the variables after every treatment compared to the reference value at T0, the TUG appears to improve steadily over time and the 10mWT shows significantly better changes compared to the reference except for T3 and T8 where it appears to be overlapping to T0. In the case of walking speed, our results are consistent with the data reported by Esquenazi et al. on a larger sample size19 in which a statistically significant and sustained improvement of gait speed (alongside cadence and step length) was described in patients undergoing repeated treatment sessions with botulinum toxin to the lower limb. An interesting feature is the inclusion in the present work of eight treatment cycles, compared to the four reported by Esquenazi et al. This element may add further evidence about the implications of a chronic long-term follow-up program. On the contrary, the average changes in 6MWT do not show a clear trend, with a significant reduction in the endurance improvement compared to T0 observed at T2, T5 and T8 which does not allow a clear interpretation of the results. Even in this case our findings are coherent with current literature. In particular, a review by Santamato et al.43 described how endurance is more prone to change after BoNT-A treatment in a population of subacute stroke survivors, meanwhile in chronic patients, such as in the present study, the 6MWT improvement may not reach clinical significance. Similarly, Cofré Lizama et al.42 underlined the necessity to consider the impact of BoNT-A injections beyond single variable modifications but in a multiparametric assessment.
Obviously, it is necessary to consider the clinical significance of these variations.
It was observed a reduction over time in the proportion of patients reporting a clinically significant increase in endurance, i.e. an increase of at least 34.4 m at 6MWT.37 The number of subjects with a variation greater than MCID goes from 11 at T0 to 5 at T8, without following a specific time trend. Similarly, also with regard to the 10mWT, patients with variation of gait speed higher than 0.16 m/s38 drop from 6 at T0 to 1 at T8 with important fluctuations in the cycles in between. Interestingly, considering the TUG the number of subjects with an improvement of at least 2.9 s27 keeps stable over time, with a maximum of 12 at T0 and a constant of 9-10 patients at subsequent cycles, excepting T2.
What emerges from the interpretation of these results is that the percentage of patients significantly responding to treatment decreases over time for endurance and gait speed and additionally it regards a relatively low number compared to the total population. In the case of TUG, the significant stability of the number of patients with an improvement greater than the MCID supports the effect of the rehabilitation treatment considered in this study.
Based on these findings it is possible to recognize a significant impact of the integrated treatment protocol, in particular with regard to the level of overall walking motility measured by the TUG (agility, balance and reduction of falling risk). In accordance with current evidence, these characteristics appear more easily “trainable” through short and periodic post-injection rehabilitation cycles, preserving a modifiability comparable to baseline even after years from the acute event. Similarly, walking speed has also demonstrated a consistent gradient of modifiability.19 However, the significance despite being statistically relevant, has an uncertain clinically relevant meaning.41 In contrast, endurance is more susceptible to long-term maintenance aerobic exercise with mild to moderate intensity, which proved to be highly effective in preserving aerobic capacity.44
A further analysis can be made about the influence of the level of independence in walking, measured with the FAC scale, on the probability to have a change at least equivalent to the MCID in the outcome variables. With a one-point increase in FAC, a 39% reduction of the probability of TUG improvement is observed, implying a greater modifiability of the degree of motility in patients with lower level of walking independence through the proposed treatment. This trend is reversed for the 6MWT and 10mWT, where a higher FAC score is correlated with a higher probability to have a clinically significant change in endurance and walking speed of 72% and 86%, respectively.
In particular, patients with FAC≤3 reported greater TUG modifiability, while subjects with FAC>3 experienced a higher gradient of improvement of 6MWT and 10mWT.
Based on these findings, FAC can be used as a prognostic indicator of the effectiveness of the proposed rehabilitation integrated treatment. In fact, given the different susceptibility of the outcome variables to clinically significant changes based on the different level of walking independence, it would be possible to structure the intervention by focusing resources on the aspects with a higher modifiability. Accordingly, it is necessary to implement in-hospital treatment with maintenance programs aimed at the correct preservation of the “basal” functional state.
Taking inspiration from these results, patients with less walking independence (FAC≤3) should be given intensive treatment aimed to train agility, postural passages and direction changes which are moreover crucial in patients with low FAC values and high risk of falling. In the case of these patients, the goal setting concerns mostly basic aspects of daily living according to the ICF.15 Conversely, patients with a higher functional level could have a major benefit from a treatment focused on increasing walking speed and endurance aiming to a higher functional performance and more complex and integrated activities. The necessity of a personalized post injection treatment tailored on specific needs and outcomes is in fact of fundamental importance for the effectiveness of rehabilitation.45 In fact, it should be noted that the presented results require to be considered as a consequence of a multimodal take in charge of patients and not only as a mere pharmacological effect of BoNT-A injection. The real added value of this protocol lies in the integration of botulinum toxin and subsequent rehabilitation treatment set on patients’ specific needs, exploiting the operating window opened by the pharmacological reduction of spasticity.
The need to be met is twofold. On the one hand the progressive increase in patients with clinical indication to undergo a rehabilitation treatment,3 on the other hand the need to implement the current management that presents gaps in terms of effectiveness on the maintenance of functional status. In this perspective, it is crucial to bridge the intervals between intensive treatment cycles with strategies that are both effective and efficient.
A viable solution consists in the adoption of guided self-rehabilitation protocols. After BoNT-A injection could be introduced a session of specific patients and caregivers’ training carried out by an experienced therapist. Subsequently, the use of operating pamphlets and home treatment diaries may allow a better engagement. The goal achievement can then be monitored at the periodic follow-up. In this operating model there is the stipulation of a real contract between the physician and patient that encourages therapeutic adherence.46
An additional option is the creation of disability-specific Adapted Physical Activity (APA) programs. According to this model, health personnel deliver the treatment in the context of small groups of people, selected on the basis of a common diagnosis and functional level. This approach may optimize resources and increase patient engagement through the social value of the context. This approach has a supplementary role in regard to the conventional treatment, and showed efficacy both on functional aspects and as a secondary prevention tool.47
Limitations of the study
The authors are aware of the limitations of this study.
First of all, the sample size is relatively small, although similar studies about the topic of repeated chronic treatment with BoNT-A involve populations with similar numerosity.48, 49 Nevertheless, the presence of oscillations with non-univocal interpretation in the trend of outcome variables might be mitigated with a larger sample.
In addition, the inclusion of other functional level assessments could furtherly increase the clinical significance of the study.
Furthermore, the use of MAS to assess spasticity has surely well-known limitations. It was chosen based on its demonstrated intra- and inter-rater reliability39, 40 and on the parameters of BoNT-A validation reported in the drug data sheet. Most importantly, being this a retrospective study, we included the most deployed spasticity scale in medical records.
Finally, the sample is quite heterogeneous about the disability severity and the type of post-injection treatment. However, this limitation is typical of studies based on similar populations where the expected outcomes and the preset goals may significantly differ from patient to patient and therefore also the treatment proposal.
Conclusions
Botulinum toxin is reconfirmed as an effective tool in reducing spasticity in plant flexor muscles, even after multiple treatment cycles.
The integrated post-injection treatment is a key element for therapeutic efficacy and for the maintenance of a more stable functional level over time.
The clinical significance of the agility and balance improvement and fall risk reduction (TUG) remains constant throughout repeated cycles. In contrast, the proportion of patients with improvement in endurance (6MWT) and walking speed (10mWT) after treatment tends to decline over time.
In this context, the baseline level of walking independence (FAC) plays a key role, which is directly related to clinically significant increases in endurance and speed and inversely related to changes in TUG, constituting a potential treatment prescription criterion.
The programming of adequate monitoring and treatment protocols is decisive and can allow the maintenance of an adequate and stable functional level avoiding excessive fluctuations and deflections of the performance status.
It is necessary to integrate inpatient treatment with strategies based on self-treatment and adapted physical activity, which, in accordance with the current evidence, act on different and complementary functional areas.
Further studies are needed to deepen these aspects and outline high-yield integrated protocols.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Simmons CA, Poupore N, Nathaniel TI. Age Stratification and Stroke Severity in the Telestroke Network. J Clin Med 2023;12:1519. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36836054&dopt=Abstract 10.3390/jcm12041519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Società Italiana di Neurologia. Schede di patologia - Ictus; 2024 [Internet]. Available from: https://www.neuro.it/web/eventi/NEURO/patologia.cfm?p=ictus [cited 2024, Jun 5].
- 3.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24449944&dopt=Abstract 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Disability-adjusted life years (DALYs); 2024 [Internet]. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/158 [cited 2024, Jun 5].
- 5.Flach C, Muruet W, Wolfe CD, Bhalla A, Douiri A. Risk and Secondary Prevention of Stroke Recurrence: A Population-Base Cohort Study. Stroke 2020;51:2435–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32646337&dopt=Abstract 10.1161/STROKEAHA.120.028992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry 1990;53:576–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2391521&dopt=Abstract 10.1136/jnnp.53.7.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linee Guida SPREAD VIII edizione – isa-aii. Accessed September 3, 2023. https://isa-aii.com/linee-guida-spread-viii-edizione/
- 8.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016;47:e98–169. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27145936&dopt=Abstract 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 9.Hankey GJ. Stroke. Lancet 2017;389:641–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27637676&dopt=Abstract 10.1016/S0140-6736(16)30962-X [DOI] [PubMed] [Google Scholar]
- 10.Kwakkel G, Buma FE, Selzer ME. Understanding the mechanisms underlying recovery after stroke. In: Selzer ME, Clarke S, Cohen LG, Kwakkel G, Miller RH, eds. Textbook of Neural Repair and Rehabilitation. Second edition. Cambridge University Press; 2014. p. 7-24. [Google Scholar]
- 11.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693–702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21571152&dopt=Abstract 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 12.Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front Hum Neurosci 2016;10:442. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27679565&dopt=Abstract 10.3389/fnhum.2016.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Francisco GE, Rymer WZ. A New Definition of Poststroke Spasticity and the Interference of Spasticity With Motor Recovery From Acute to Chronic Stages. Neurorehabil Neural Repair 2021;35:601–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33978513&dopt=Abstract 10.1177/15459683211011214 [DOI] [PubMed] [Google Scholar]
- 14.Dorňák T, Justanová M, Konvalinková R, Říha M, Mužík J, Hoskovcová M, et al. Prevalence and evolution of spasticity in patients suffering from first-ever stroke with carotid origin: a prospective, longitudinal study. Eur J Neurol 2019;26:880–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30623522&dopt=Abstract 10.1111/ene.13902 [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos HM, de Oliveira LC, Bonifácio SR, et al. Use of the International Classification of Functioning, Disability and Health (ICF) to expand and standardize the assessment of quality-of-life following a stroke: proposal for the use of codes and qualifiers. Disabil Rehabil 2022;44:7449–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34752176&dopt=Abstract 10.1080/09638288.2021.1995055 [DOI] [PubMed] [Google Scholar]
- 16.Harlaar J, Becher JG, Snijders CJ, Lankhorst GJ. Passive stiffness characteristics of ankle plantar flexors in hemiplegia. Clin Biomech (Bristol, Avon) 2000;15:261–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10675667&dopt=Abstract 10.1016/S0268-0033(99)00069-8 [DOI] [PubMed] [Google Scholar]
- 17.Deltombe T, Wautier D, De Cloedt P, Fostier M, Gustin T. Assessment and treatment of spastic equinovarus foot after stroke: guidance from the Mont-Godinne interdisciplinary group. J Rehabil Med 2017;49:461–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28451697&dopt=Abstract https://doi.org/ 10.2340/16501977-2226 [DOI] [PubMed] [Google Scholar]
- 18.Wissel J, Ward AB, Erztgaard P, Bensmail D, Hecht MJ, Lejeune TM, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med 2009;41:13–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19197564&dopt=Abstract https://doi.org/ 10.2340/16501977-0303 [DOI] [PubMed] [Google Scholar]
- 19.Esquenazi A, Brashear A, Deltombe T, Rudzinska-Bar M, Krawczyk M, Skoromets A, et al. The Effect of Repeated abobotulinumtoxinA (Dysport®) Injections on Walking Velocity in Persons with Spastic Hemiparesis Caused by Stroke or Traumatic Brain Injury. PM R 2021;13:488–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32741133&dopt=Abstract 10.1002/pmrj.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roncoroni LP, Weiss D, Hieber L, Sturm J, Börtlein A, Mayr I, et al. Health-Related Quality of Life Outcomes from Botulinum Toxin Treatment in Spasticity. Toxins (Basel) 2020;12:292. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32375388&dopt=Abstract 10.3390/toxins12050292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta Gupta A, Visvanathan R, Cameron I, Koblar SA, Howell S, Wilson D. Efficacy of botulinum toxin in modifying spasticity to improve walking and quality of life in post-stroke lower limb spasticity - a randomized double-blind placebo controlled study. BMC Neurol 2019;19:96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31078139&dopt=Abstract 10.1186/s12883-019-1325-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta AD, Chu WH, Howell S, Chakraborty S, Koblar S, Visvanathan R, et al. A systematic review: efficacy of botulinum toxin in walking and quality of life in post-stroke lower limb spasticity. Syst Rev 2018;7:1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29304876&dopt=Abstract 10.1186/s13643-017-0670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baricich A, Battaglia M, Cuneo D, Cosenza L, Millevolte M, Cosma M, et al. Clinical efficacy of botulinum toxin type A in patients with traumatic brain injury, spinal cord injury, or multiple sclerosis: an observational longitudinal study. Front Neurol 2023;14:1133390. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37090974&dopt=Abstract 10.3389/fneur.2023.1133390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baricich A, Bertoni M, Santamato A, Osio M, Gasperini G, Picelli A, et al. Collaborative Working Group . Adjunctive treatment and BoNT-A for post-stroke spasticity: are we really focusing on the patient-centered goals? Front Neurol 2023;14:1134691. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36970525&dopt=Abstract 10.3389/fneur.2023.1134691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picelli A, Santamato A, Chemello E, Cinone N, Cisari C, Gandolfi M, et al. Adjuvant treatments associated with botulinum toxin injection for managing spasticity: an overview of the literature. Ann Phys Rehabil Med 2019;62:291–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30219307&dopt=Abstract 10.1016/j.rehab.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Baricich A, Wein T, Cinone N, Bertoni M, Picelli A, Chisari C, et al. BoNT-A for Post-Stroke Spasticity: Guidance on Unmet Clinical Needs from a Delphi Panel Approach. Toxins (Basel) 2021;13:236. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33805988&dopt=Abstract 10.3390/toxins13040236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med 2005;37:75–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15788341&dopt=Abstract 10.1080/16501970410017215 [DOI] [PubMed] [Google Scholar]
- 28.Cheng DK, Nelson M, Brooks D, Salbach NM. Validation of stroke-specific protocols for the 10-meter walk test and 6-minute walk test conducted using 15-meter and 30-meter walkways. Top Stroke Rehabil 2020;27:251–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31752634&dopt=Abstract 10.1080/10749357.2019.1691815 [DOI] [PubMed] [Google Scholar]
- 29.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil 2005;86:1641–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16084820&dopt=Abstract 10.1016/j.apmr.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26:982–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7762050&dopt=Abstract 10.1161/01.STR.26.6.982 [DOI] [PubMed] [Google Scholar]
- 31.Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, et al. Improvements in speed-based gait classifications are meaningful. Stroke 2007;38:2096–100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17510461&dopt=Abstract 10.1161/STROKEAHA.106.475921 [DOI] [PubMed] [Google Scholar]
- 32.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair 2008;22:672–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18971382&dopt=Abstract 10.1177/1545968308318837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil 2007;88:1314–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17908575&dopt=Abstract 10.1016/j.apmr.2007.06.764 [DOI] [PubMed] [Google Scholar]
- 34.Battaglia M, Cosenza L, Scotti L, Bertoni M, Polverelli M, Loro A, et al. Triceps Surae Muscle Characteristics in Spastic Hemiparetic Stroke Survivors Treated with Botulinum Toxin Type A: Clinical Implications from Ultrasonographic Evaluation. Toxins (Basel) 2021;13:889. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34941726&dopt=Abstract 10.3390/toxins13120889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santamato A, Panza F, Intiso D, Baricich A, Picelli A, Smania N, et al. Long-term safety of repeated high doses of incobotulinumtoxinA injections for the treatment of upper and lower limb spasticity after stroke. J Neurol Sci 2017;378:182–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28566161&dopt=Abstract 10.1016/j.jns.2017.04.052 [DOI] [PubMed] [Google Scholar]
- 36.Gracies JM, Esquenazi A, Brashear A, Banach M, Kocer S, Jech R, et al. ; International AbobotulinumtoxinA Adult Lower Limb Spasticity Study Group. Efficacy and safety of abobotulinumtoxinA in spastic lower limb: randomized trial and extension. Neurology 2017;89:2245–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29093068&dopt=Abstract 10.1212/WNL.0000000000004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang A, Eng JJ, Rand D. Relationship between perceived and measured changes in walking after stroke. J Neurol Phys Ther 2012;36:115–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22850336&dopt=Abstract 10.1097/NPT.0b013e318262dbd0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Locomotor Experience Applied Post Stroke (LEAPS) Investigative Team . Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther 2010;90:196–208. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20022995&dopt=Abstract 10.2522/ptj.20090079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3809245&dopt=Abstract 10.1093/ptj/67.2.206 [DOI] [PubMed] [Google Scholar]
- 40.Meseguer-Henarejos AB, Sánchez-Meca J, López-Pina JA, Carles-Hernández R. Inter- and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med 2018;54:576–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28901119&dopt=Abstract 10.23736/S1973-9087.17.04796-7 [DOI] [PubMed] [Google Scholar]
- 41.Foley N, Murie-Fernandez M, Speechley M, Salter K, Sequeira K, Teasell R. Does the treatment of spastic equinovarus deformity following stroke with botulinum toxin increase gait velocity? A systematic review and meta-analysis. Eur J Neurol 2010;17:1419–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20491885&dopt=Abstract 10.1111/j.1468-1331.2010.03084.x [DOI] [PubMed] [Google Scholar]
- 42.Cofré Lizama LE, Khan F, Galea MP. Beyond speed: gait changes after botulinum toxin injections in chronic stroke survivors (a systematic review). Gait Posture 2019;70:389–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30974394&dopt=Abstract 10.1016/j.gaitpost.2019.03.035 [DOI] [PubMed] [Google Scholar]
- 43.Santamato A, Cinone N, Panza F, Letizia S, Santoro L, Lozupone M, et al. Botulinum Toxin Type A for the Treatment of Lower Limb Spasticity after Stroke. Drugs 2019;79:143–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30623347&dopt=Abstract 10.1007/s40265-018-1042-z [DOI] [PubMed] [Google Scholar]
- 44.Lamberti N, Straudi S, Malagoni AM, Argirò M, Felisatti M, Nardini E, et al. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: a pilot randomized controlled study. Eur J Phys Rehabil Med 2017;53:228–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27626795&dopt=Abstract 10.23736/S1973-9087.16.04322-7 [DOI] [PubMed] [Google Scholar]
- 45.Fujita K, Kobayashi Y, Hitosugi M, Nomura T, Nishida T, Tsushima Y, et al. Factors Influencing Gait Velocity Improvement Following Botulinum Toxin Injection for Spasticity of the Plantar Flexors in Patients with Stroke. Prog Rehabil Med 2020;5:20200024. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33033774&dopt=Abstract 10.2490/prm.20200024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gracies JM, Francisco GE, Jech R, Khatkova S, Rios CD, Maisonobe P, ENGAGE Study Group . Guided Self-rehabilitation Contracts Combined With AbobotulinumtoxinA in Adults With Spastic Paresis. J Neurol Phys Ther 2021;45:203–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34039905&dopt=Abstract 10.1097/NPT.0000000000000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belfiore P, Miele A, Gallè F, Liguori G. Adapted physical activity and stroke: a systematic review. J Sports Med Phys Fitness 2018;58:1867–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29072029&dopt=Abstract 10.23736/S0022-4707.17.07749-0 [DOI] [PubMed] [Google Scholar]
- 48.Hara T, Abo M, Hara H, Sasaki N, Yamada N, Niimi M, et al. The Effect of Repeated Botulinum Toxin A Therapy Combined with Intensive Rehabilitation on Lower Limb Spasticity in Post-Stroke Patients. Toxins (Basel) 2018;10:349. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30200281&dopt=Abstract 10.3390/toxins10090349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakheit AM, Fedorova NV, Skoromets AA, Timerbaeva SL, Bhakta BB, Coxon L. The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles. J Neurol Neurosurg Psychiatry 2004;75:1558–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15489387&dopt=Abstract 10.1136/jnnp.2003.035139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data ownership is held by the Maggiore della Carità University Hospital in Novara.
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.


