Abstract
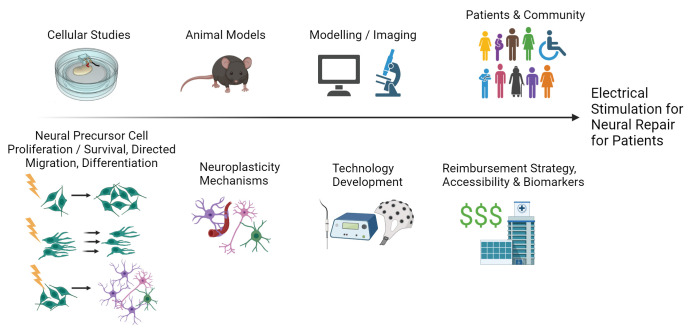
Visual Abstract
Significance Statement
A multidisciplinary and international convergent working group (neural stem cell biology, functional electrical stimulation, materials engineering, electrical engineering, neurosurgery, neurology, biomedical device, and commercialization) met in Canada to create a call to action for Electrical Stimulation for Neural Repair. Electrical stimulation, in the form of deep brain stimulation (DBS), is an approved treatment for various neurological disorders such as essential tremor, Parkinson's disease, and epilepsy. Here, DBS works through disrupting neural circuits; however, electrical stimulation may also effectively promote neural repair due to the activation of electrosensitive resident neural stem cells. Activating neural stem cells has great promise for enhancing neuroplasticity to treat damaged brains. To realize this therapy's potential, multidisciplinary experts met to identify barriers, gaps, and next steps.
Introduction
Neurological disorders are a leading cause of death and disabilities worldwide and represent an enormous public health challenge (Feigin and Vos, 2019). A conservative estimate of the global financial burden is more than two trillion US dollars/year, and these costs are expected to double by 2030. For those afflicted, they have a decreased quality of life, and their dependence on others places a heavy burden on our society and healthcare networks. The major neurological disorders contributing to the overall burden are stroke and dementias (including Alzheimer's disease; Feigin et al., 2020). There are presently no cures and limited treatment options for stroke or neurodegenerative diseases. There is a clear need and incentive for therapeutic interventions to promote neural repair and functional recovery.
Neural stem cells and their progeny (together termed neural precursor cells, NPCs) reside within well-delineated niches in the central nervous system (CNS). These resident cells are self-renewing and can differentiate into the different cell types of the brain, which has spurred interest in the design of strategies to harness NPC potential for promoting neural repair (Reynolds and Weiss, 1992; Iwasa et al., 2020; Purvis et al., 2020). Modulating NPC behavior has been correlated to functional recovery in models of brain injury using drugs and small molecules (R. L. Zhang et al., 2006, 2012; Sachewsky et al., 2014; Tang et al., 2023). NPCs are electrosensitive cells affording the opportunity to use electrical stimulation as a prospective repair strategy (Ariza et al., 2010). Electrical stimulation is a compelling treatment option because of the spatial and temporal precision afforded by its application, particularly when applied invasively. Furthermore, electric fields are also present endogenously and are important during development and wound healing. These endogenous fields influence NPC behavior within the mature CNS such as migration along the rostral migratory stream and in the corpus callosum (Cao et al., 2013; Iwasa et al., 2019). Electric field application can increase NPC proliferation, direct migration, and modulate differentiation (Babona-Pilipos et al., 2011; Chang et al., 2011).
Electrical stimulation is widely used as a therapeutic treatment. In deep brain stimulation (DBS), implanted electrodes commonly deliver electrical stimulation to targeted regions such as the basal ganglia and thalamus to treat movement disorders, epilepsy, and pain. New indications for DBS such as obsessive–compulsive disorder, depression, Alzheimer's disease, and obesity are emerging (Lozano et al., 2019; Jakobs et al., 2020; Hitti et al., 2023). Electrical stimulation to the brain can also be delivered noninvasively through transcranial electrical stimulation to treat neurological disorders (e.g., depression; Bhattacharya et al., 2022). Electrical stimulation is delivered to the spinal cord for pain and to peripheral nerves (termed functional electrical stimulation, FES) for rehabilitation after stroke or spinal cord injury. These forms for electrical stimulation work and the parameters (duration, intensity) vary depending on the disease/injury. We propose that electrical stimulation can be a novel, safe, and effective strategy to activate resident NPCs and lead to neural regeneration and improved functional outcomes.
To bring an electrical stimulation-mediated treatment option for neural repair from cells to patients, a multidisciplinary approach is necessary. The mechanism of NPC activation needs to be elucidated to determine the appropriate stimulation paradigm. Electrodes and stimulators for animal model testing and with specifications for NPC activation need to be designed. Furthermore, the technologies and stimulation paradigms need to be designed with patient needs, usability, and marketability in mind. This would involve considerations of benefits and limitations of invasive and noninvasive stimulation as well as using wearable technologies. To address these prerequisites, multidisciplinary experts met for a Mini-Symposium and Workshop held in February 2023 in Canada to establish the state of the field, challenges, and directions. All participants were volunteers and included internationally recognized experts in their respective fields. There were early career investigators and patient-facing practitioners within and outside of Canada. Here, we aim to generate excitement for the field while presenting relevant background and illustrating the main challenges and next steps to bringing electrical stimulation for neural repair as a treatment to fruition.
Mechanism of Neural Repair Using Electrical Stimulation to Activate Neural Stem Cells
Neural stem cells are found during development, adulthood, and throughout aging (Negredo et al., 2020). These self-renewing, multipotent cells can be isolated from along the entire neuraxis, where they are found in highly specified and restricted niches including the periventricular region lining the ventricles. In the brain, NPCs are migratory cells that proliferate and contribute to olfactory bulb neurogenesis. In vitro, individual neural stem cells give rise to NPC colonies of cells, termed neurospheres. This forms the basis of the simple and robust neurosphere assay used to assess the size of the NPC pool. Following injury alone, NPCs can be “activated,” meaning there is an increase in neural stem cell proliferation, survival, migration, and neurogenesis (Arvidsson et al., 2002; Li et al., 2010; Faiz et al., 2015), albeit limited. While this injury-induced activation is not sufficient for neural regeneration and functional recovery, the administration of small molecules, drugs, and growth factors to enhance NPC activation is correlated with improved tissue and functional outcomes (C. Zhang et al., 2010; Jeffers et al., 2014; Dadwal et al., 2015). We look to enhance resident NPC activation using electrical stimulation. Indeed, electrical stimulation has been shown to increase NPC proliferation, direct migration, and modulate differentiation both in vitro and in vivo in rodents (Feng et al., 2017; Sefton et al., 2020).
Studies have also shown that electrical stimulation can lead to functional recovery and neurogenesis (Balseanu et al., 2020). The correlation between functional benefit and NPC activation in a disease model has been demonstrated in a number of settings (Dadwal et al., 2015; Battistini et al., 2023; Gilbert et al., 2023), and knock-out models of neural stem cells have provided evidence to support the relationship between neural stem cell activation and improved outcomes (Williamson et al., 2023). Indeed, work is still needed to solidify the direct link between neural stem cell activation via electrical stimulation and functional recovery, and rigorous preclinical injury model experiments required for these studies are ongoing.
Ongoing studies that deliver electrical stimulation after an injury (e.g., stroke) and compare with injured mice with implants and no stimulation can establish the effectiveness of the treatment using behavior tasks before and after injury. Once functional recovery is observed, NPC kinetics (proliferation, survival, migration, and differentiation into mature neural cells) can be examined using in vitro, ex vivo, in vivo, and in silico methodologies (Meng et al., 2011; Sefton et al., 2020; Zimmerman et al., 2021). Two aspects of these studies are critical for interpretation. First, it is important to perform these studies in transgenic mouse models that enable lineage tracking of endogenous NPCs (Albright et al., 2016). Labeling the endogenous NPCs prior to injury will confirm that electrical stimulation of the NPCs leads to their activation and contribution to tissue repair. Important studies employing inducible NPC ablation in transgenic mice followed by electrical stimulation will be needed to definitively demonstrate that NPC activation is necessary and/or sufficient for the recovery.
The stimulation parameters (strength, frequency, duration, waveform shape) and the brain region stimulated can affect the extent of NPC migration and proliferation (Lau et al., 2023). Optimized parameters and the timing of the intervention after injury or during a disease progression are also critical factors for successful treatments (Dromerick et al., 2021). Additionally, observation on the effect of age and sex on NPC response to electrical stimulation is important as environmental factors affect NPC response as well (Ahmed et al., 2020).
Concomitant with optimizing NPC activation, our studies need to address fundamental biological questions to understand how the electrical stimulation-mediated repair is affecting the cells and circuitry to support neuroplasticity. For example, DBS has been demonstrated to disrupt neural circuits (Milosevic et al., 2018); how will the stimulation designed for NPC activation affect aspects of neuroplasticity such as axon regeneration, synaptic connection, modified neural circuits, glial cells, and changes in brain vasculature or the blood–brain barrier? Technical and experimental expertise in wet labs using animal studies, state-of-the-art imaging, and recording technologies and modeling can help elucidate the answers to these questions.
Developing Electrical Stimulation Devices and Therapies for Neuroregeneration
Research labs and clinics regularly use stimulators and electrodes for DBS, transcranial stimulation, and transcutaneous stimulation as well as employ electrodes for recordings. The current tools are not customized for examining and optimizing the effects of electrical stimulation on NPCs to investigate the questions posed above. Multidisciplinary efforts to acquire an understanding of the limitations of stimulators (electrical engineering) and electrodes (device design/materials engineering) is needed alongside the development of intervention. There are several main considerations/challenges with respect to stimulator and electrode design, development, and fabrication: (1) safety, generating charge-balanced stimulation that does not cause tissue damage over long-term, continuous stimulation delivery; (2) performance, generating the waveform with optimized parameters and delivering these in real-world settings with noise, varying tissue contact impedances, and other confounders; (3) determining electrode placement and the number of the electrodes needed to deliver electrical stimulation most conducive to activating endogenous NPCs; (4) shape and insulation of the electrodes to produce desirable electric fields in vivo; (5) miniaturization, allowing devices to be used in small animal models during freely behaving experiments and eventually enable the translation to implantable and potentially wearable devices; and (6) powering, having a safe, reliable, and lightweight power source that can remain in or on the body for prolonged periods of time without causing damage to the surrounding tissue (e.g., wireless power systems or battery housing systems that prevent leakage) that is critical (de Haas et al., 2012; Shepherd et al., 2018; Alpaugh et al., 2019). Validation experiments are essential for testing the technology in preclinical animal models prior to human application to determine whether these considerations have been addressed appropriately (e.g., consistent results in activating NPCs, no changes in behavior while wearing the stimulator, no tissue damage).
For NPC activation, the implanted electrodes need to stimulate the endogenous neural stem cell niche, which comprises the periventricular region of the forebrain. These stimulating electrodes could resemble DBS electrodes; hence considering existing DBS electrodes designs, stimulation patterns, and postmortem tissue responses is relevant (Cagnan et al., 2019; Evers and Lowery, 2021). However, certain characteristics will likely need adjustment for NPC activation. For example, current treatment of progressive diseases such as Parkinson's disease dictates ongoing/continuous stimulation (although adaptive DBS is being investigated to consider how a patient's symptoms and needs can fluctuate throughout the day; van Wijk et al., 2023). It may be different in injury models, such as stroke, where a permanent implant may not be required. One could envision implantation of the device early after injury with delivery of the stimulus while the patient is receiving physical and occupational therapy treatments or implantation in the subacute or chronic phases poststroke to enhance NPC activation (Boese et al., 2018). In injury models that are not progressive diseases, it may serve to remove the stimulator and the electrode. In that embodiment of the stimulation technology, it may be beneficial to have electrodes and cabling that degrades over time to allow for the effective removal of the implanted device (Shim et al., 2021). Alternatively, stimulation could be applied noninvasively. This would solve the challenge of removing the stimulator and electrodes; however, modeling and analysis would be required to accurately determine spatial and temporal control of the electrical stimulation in targeted regions. Stroke represents an enormous clinical population making these considerations important engineering goals for system design and implementation.
To enhance repair in various neurological disorders, electrical stimulation can be provided in combination with drug delivery, cell transplantation, and/or rehabilitation. Drug delivery and/or cell transplantation along with electrical stimulation could be achieved through multimodal electrodes. For example, electrodes could be designed to deliver electrical stimulation and provide immunomodulatory and/or neuroprotective compounds through controlled release of drugs such as brain-derived neurotrophic factor and short interfering RNAs (Iwasa et al., 2020; Barman and Jhunjhunwala, 2024). Alternatively, or in addition, electrical stimulation could be combined with NPC transplantation (which has been used in clinical trials and shown promise; Leone et al., 2023). Electrical stimulation may improve functional recovery by improving cell survival which is invariably low following transplantation (Tejeda et al., 2022; Leone et al., 2023). Electrical stimulation could be delivered to the brain in conjunction with rehabilitation treatments to support neuroplasticity, integration of surviving cells in the neurocircuitry, and/or cell survival (Corbett et al., 2015). This potential is highlighted by studies in the peripheral nervous system, whereby FES combined with rehabilitation leads to functional improvements through neuroplasticity (Marquez-Chin and Popovic, 2020). Many electrical stimulation combinatorial strategies are possible, and synergies between these strategies may augment the extent of recovery for individuals with neurological disorders.
Clinical Relevance—Biomarkers, Therapy Adoption, and Reimbursement
To test the success of the therapy and adjust the therapy for human application, we need relevant biomarkers as measures of success. In preclinical models, behavioral outcomes and brain tissue analyses are used to evaluate success, but in humans the outcome measures are more limited. Potential biomarkers could be related to brain imaging (e.g., diffusion tensor imaging for corticospinal tract analysis; Findlater et al., 2019; Moura et al., 2019), neural recordings (e.g., local field potential changes similar to what is used for adaptive DBS; Vissani et al., 2020), electromyography recordings (e.g., changes in muscle synergies; Irastorza-Landa et al., 2021), or motion sensors (e.g., changes in ranges of motion; Derungs et al., 2020). As digital health technologies such as wearable devices become more common, they can serve as an accessible alternative to record data and aid in the identification of functional and sensitive digital biomarkers, ultimately guiding the optimization of the stimulation parameters (i.e., timing and dosing) of the therapy (Pathak et al., 2021). If a technology is wearable, it may be easier for underserved populations (e.g., remote communities, previously discriminated against communities) to access and participate in therapy development. It is important to have a diverse group of end-users participate in the process as each will have usability suggestions and different needs (Levine et al., 2020; Adler et al., 2023), leading to improved therapy relevance for a greater number of patients.
To bring this therapy from bench to bedside, all aspects of the therapy implementation need to be considered, and above all, the therapy adoption and reimbursement strategies need to be considered early in the development phase. Efforts to demonstrate that this therapy modality produces significant and clinically meaningful impact on patients and their family members are the most clinically relevant outcomes; however, the cost-effectiveness of continuing with the present treatment modalities versus this proposed intervention must be carefully assessed. If a clear business model shows functional improvements for patients and captures savings for a specific territory's healthcare system, then discussions and negotiation around reimbursement in targeted regions for technology deployment are warranted (Stieglitz, 2020; Smuck et al., 2021). A therapy is only worth pursuing if it positively impacts patient outcomes. Therefore, the research team has to engage regulatory, ethics, and business people as soon as the therapy shows clinical promise.
Next steps
The use of electrical stimulation to activate NPCs with the objective of developing next-generation neuroregenerative treatments and technologies is very attractive. Electrical stimulation has been used for over six decades in rehabilitation, in cardiac pacemakers and hearing aids, and more recently for DBS systems. This is a mature technology that can be relatively easily adopted to promote neuroregenerative processes based on resident NPC activation. However, even though this technology is mature and available, it will require considerable effort from various disciplines to make it relevant and appropriate for injury/disease applications. We will need neurobiologists and stem cell biologists, neural engineers, material and manufacturing experts, electrical engineers, data scientists, neurosurgeons, neurologists, regulatory experts, and commercialization experts to move this field forward. We will also need the patient, caregiver, and community groups to define the unmet need as well. Each group brings their unique perspective, expertise, and ideas and can identify the promise and the gaps that need to be addressed before this technology becomes a reality. We identified the multidisciplinary team of people to bring to the conversation and engage in meaningful discussion (Table 1).
Table 1.
Research areas to target and people to consult to develop electrical stimulation for neural repair
| Research areas to target | People to bring to the conversation |
|---|---|
|
|
To address the challenges, we need to establish a hub to educate the community and attract new public and private partners to engage in developing electrical stimulation tools for neuroregeneration. The hub will promote collaborations within themes and across disciplines to engage and learn to speak each other's languages, build teams, connect with physician–scientists, and ensure research endeavors address relevant questions and challenges. This would include a mix of local, regional, national, and international members. The knowledge and strategies from different regions would be helpful in referencing and considering potential healthcare strategies, for example. The established hub can address our long-term call to action (>5 years):
Bring an electrical stimulation regenerative medicine therapy from cells to patients and provide a model for broad application to diverse neural disorders.
Understand the cellular response and mechanism to electrical stimulation for NPC activation through high-resolution imaging technologies and in vitro, ex vivo, and in vivo methodologies.
Identify appropriate target biomarkers to more accurately select stimulation parameters and device configurations (hardware and software) and enable the monitoring of patient improvements.
Enable long-term monitoring of the patients, their devices, and biomarkers, as a means to enable continuous system and patient experience improvement with the device and the therapy. For example, the stimulation process could be controlled in a closed-loop configuration in real time to accelerate recovery and minimize side effects.
Develop standardized and interchangeable electrical stimulators (e.g., open stimulator platforms that could be used off-label for a range of different research uses), electrodes, and monitoring devices for the neural repair field allowing faster deployment of this regenerative medicine technology to patient populations. This will allow for the creation of a neuromodulation “hub” using similar and compatible tools benefiting diverse clinical populations and facilitating rapid interdisciplinary partnerships, knowledge exchange, technology development, and safe clinical deployment.
We believe that the use of electrical stimulation as a means to engage resident NPCs with the objective of developing next-generation neuromodulation treatments and technologies is the future of neuroregenerative medicine. This position paper can be leveraged with policymakers, regulators, funding agencies, and the public. We are looking for like-minded people in academia, industry, healthcare, NGOs, and government to help us move this field forward with the goal making these technologies available to our patients within the next 5–10 years.
Synthesis
Reviewing Editor: Jennifer Dulin, Texas A&M University
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Matthew Hogan.
Reviewer #1:
In this opinion piece, the authors highlight critical thrust areas for the advancement of electrical stimulation targeting nascent neural stem cells as a potential therapy for central nervous system disorders. The outlined approach may have relevance to multiple disorders and impact a wide population should it prove effective.
Critically, there is little information on the overall effectiveness of the strategy itself. The authors highlight that it is necessary to understand whether electrical stimulation is a valid strategy for improving function, however this unmet need is limited to a single sentence. In the opinion of the reviewer, the most critical barrier to advancing the field is a direct demonstration of functional benefit in a preclinical disease model. While two studies demonstrated a correlation between functional benefit and electrical stimulation in a disease model, it is critical to link the "activation" of nascent stem cells to a functional benefit directly. The authors should expand on this unmet need and highlight strategies to address necessary rigorous gain and loss of function experiments.
Further, there is a missed opportunity to address cell-replacement therapeutic strategies. Lessons learned in the context of nascent stem cell activation may reveal potential for synergy/cross-pollination with stem cell graft-based interventions and vice-versa.
In general, the call-to-action outlines major sticking points in advancing the outlined therapeutic strategy, however it fails to highlight the importance of rigor. More discussion of essential and missing pre-clinical modeling experiments is necessary. Still, the authors nicely describe other hurdles necessary to advance this therapeutic approach and present an informative wholistic summary.
References
- Adler G, Taylor D, Rashid U, Olsen S, Brooks T, Terry G, Niazi IK, Signal N (2023) A brain computer interface neuromodulatory device for stroke rehabilitation: iterative user-centered design approach. JMIR Rehabil Assist Technol 10:e49702. 10.2196/49702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Iwasa SN, Poloni L, Ahlfors J-E, Yip C, Popovic MR, Morshead CM (2020) Substrate-dependent galvanotaxis of directly reprogrammed human neural precursor cells. Bioelectricity 2:229–237. 10.1089/bioe.2019.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright JE, Stojkovska I, Rahman AA, Brown CJ, Morrison BE (2016) Nestin-positive/SOX2− negative cells mediate adult neurogenesis of nigral dopaminergic neurons in mice. Neurosci Lett 615:50–54. 10.1016/j.neulet.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpaugh M, Saint-Pierre M, Dubois M, Aubé B, Arsenault D, Kriz J, Cicchetti A, Cicchetti F (2019) A novel wireless brain stimulation device for long-term use in freely moving mice. Sci Rep 9:6444. 10.1038/s41598-019-42910-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza CA, Fleury AT, Tormos CJ, Petruk V, Chawla S, Oh J, Sakaguchi DS, Mallapragada SK (2010) The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev Rep 6:585–600. 10.1007/s12015-010-9171-0 [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- Babona-Pilipos R, Droujinine IA, Popovic MR, Morshead CM (2011) Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS One 6:e23808. 10.1371/journal.pone.0023808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balseanu AT, Grigore M, Pinosanu L-R, Slevin M, Hermann DM, Glavan D, Popa-Wagner A (2020) Electric stimulation of neurogenesis improves behavioral recovery after focal ischemia in aged rats. Front Neurosci 14:732. 10.3389/fnins.2020.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SR, Jhunjhunwala S (2024) Electrical stimulation for immunomodulation. ACS Omega 9:52–66. 10.1021/acsomega.3c06696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistini JI, Mastrorilli V, Nicolis di Robilant V, Saraulli D, Marinelli S, Farioli Vecchioli S (2023) Role of running-activated neural stem cells in the anatomical and functional recovery after traumatic brain injury in p21 knock-out mice. Int J Mol Sci 24:2911. 10.3390/ijms24032911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Mrudula K, Sreepada SS, Sathyaprabha TN, Pal PK, Chen R, Udupa K (2022) An overview of noninvasive brain stimulation: basic principles and clinical applications. Can J Neurol Sci 49:479–492. 10.1017/cjn.2021.158 [DOI] [PubMed] [Google Scholar]
- Boese AC, Le Q-SE, Pham D, Hamblin MH, Lee J-P (2018) Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res Ther 9:154. 10.1186/s13287-018-0913-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnan H, Denison T, McIntyre C, Brown P (2019) Emerging technologies for improved deep brain stimulation. Nat Biotechnol 37:1024–1033. 10.1038/s41587-019-0244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah E, Zhao M (2013) Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep 14:184–190. 10.1038/embor.2012.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-A, Kim JW, Kim JA, Lee SE, Kim S, Suh WH, Kim H-S, Kwon S, Kim SJ, Suh Y-H (2011) Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS One 6:e18738. 10.1371/journal.pone.0018738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Jeffers M, Nguemeni C, Gomez-Smith M, Livingston-Thomas J (2015) Lost in translation: rethinking approaches to stroke recovery. Prog Brain Res 218:413–434. 10.1016/bs.pbr.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Dadwal P, Mahmud N, Sinai L, Azimi A, Fatt M, Wondisford FE, Miller FD, Morshead CM (2015) Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Reports 5:166–173. 10.1016/j.stemcr.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas R, Struikmans R, van der Plasse G, van Kerkhof L, Brakkee JH, Kas MJH, Westenberg HGM (2012) Wireless implantable micro-stimulation device for high frequency bilateral deep brain stimulation in freely moving mice. J Neurosci Methods 209:113–119. 10.1016/j.jneumeth.2012.05.028 [DOI] [PubMed] [Google Scholar]
- Derungs A, Schuster-Amft C, Amft O (2020) Wearable motion sensors and digital biomarkers in stroke rehabilitation. Curr Direct Biomed Eng 6:229–232. 10.1515/cdbme-2020-3058 [DOI] [Google Scholar]
- Dromerick AW, et al. (2021) Critical period after stroke study (CPASS): a phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc Natl Acad Sci U S A 118:e2026676118. 10.1073/pnas.2026676118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers J, Lowery M (2021) The active electrode in the living brain: the response of the brain parenchyma to chronically implanted deep brain stimulation electrodes. Oper Neurosurg 20:131–140. 10.1093/ons/opaa326 [DOI] [PubMed] [Google Scholar]
- Faiz M, Sachewsky N, Gascón S, Bang KWA, Morshead CM, Nagy A (2015) Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell 17:624–634. 10.1016/j.stem.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Feigin VL, Vos T (2019) Global burden of neurological disorders: from global burden of disease estimates to actions. Neuroepidemiology 52:1–2. 10.1159/000495197 [DOI] [PubMed] [Google Scholar]
- Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C (2020) The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 19:255–265. 10.1016/S1474-4422(19)30411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J-F, Jing L, Zhang L, Jiang J-Y, Russell M, Lyeth BG, Nolta JA, Zhao M (2017) Electrical guidance of human stem cells in the rat brain. Stem Cell Reports 9:177–189. 10.1016/j.stemcr.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlater SE, Hawe RL, Mazerolle EL, Al Sultan AS, Cassidy JM, Scott SH, Pike GB, Dukelow SP (2019) Comparing CST lesion metrics as biomarkers for recovery of motor and proprioceptive impairments after stroke. Neurorehabil Neural Repair 33:848–861. 10.1177/1545968319868714 [DOI] [PubMed] [Google Scholar]
- Gilbert EAB, Livingston J, Kehtari T, Garcia-Flores E, Morshead CM (2023) Metformin improves functional outcomes, activates neural precursor cells and modulates microglia in a sex-dependent manner after spinal cord injury. Stem Cells Transl Med 12:415–428. 10.1093/stcltm/szad030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, et al. (2023) Future directions in psychiatric neurosurgery: proceedings of the 2022 American Society for Stereotactic and Functional Neurosurgery meeting on surgical neuromodulation for psychiatric disorders. Brain Stimul 16:867–878. 10.1016/j.brs.2023.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irastorza-Landa N, García-Cossio E, Sarasola-Sanz A, Brötz D, Birbaumer N, Ramos-Murguialday A (2021) Functional synergy recruitment index as a reliable biomarker of motor function and recovery in chronic stroke patients. J Neural Eng 18:046061. 10.1088/1741-2552/abe244 [DOI] [PubMed] [Google Scholar]
- Iwasa SN, Rashidi A, Sefton E, Liu NX, Popovic MR, Morshead CM (2019) Charge-balanced electrical stimulation can modulate neural precursor cell migration in the presence of endogenous electric fields in mouse brains. eNeuro 6:ENEURO.0382-19.2019. 10.1523/ENEURO.0382-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa SN, Shi HH, Hong SH, Chen T, Marquez-Chin M, Iorio-Morin C, Kalia SK, Popovic MR, Naguib HE, Morshead CM (2020) Novel electrode designs for neurostimulation in regenerative medicine: activation of stem cells. Bioelectricity 2:348–361. 10.1089/bioe.2020.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs M, Lee DJ, Lozano AM (2020) Modifying the progression of Alzheimer's and Parkinson's disease with deep brain stimulation. Neuropharmacology 171:107860. 10.1016/j.neuropharm.2019.107860 [DOI] [PubMed] [Google Scholar]
- Jeffers MS, Hoyles A, Morshead C, Corbett D (2014) Epidermal growth factor and erythropoietin infusion accelerate functional recovery in combination with rehabilitation. Stroke 45:1856–1858. 10.1161/STROKEAHA.114.005464 [DOI] [PubMed] [Google Scholar]
- Lau KSK, Chen T, Iwasa SN, Volpatti M, Popovic MR, Morshead CM (2023) A novel ex vivo assay to define charge-balanced electrical stimulation parameters for neural precursor cell activation in vivo. Brain Res 1804:148263. 10.1016/j.brainres.2023.148263 [DOI] [PubMed] [Google Scholar]
- Leone MA, et al. (2023) Phase I clinical trial of intracerebroventricular transplantation of allogeneic neural stem cells in people with progressive multiple sclerosis. Cell Stem Cell 30:1597–1609.e8. 10.1016/j.stem.2023.11.001 [DOI] [PubMed] [Google Scholar]
- Levine DA, Duncan PW, Nguyen-Huynh MN, Ogedegbe OG (2020) Interventions targeting racial/ethnic disparities in stroke prevention and treatment. Stroke 51:3425–3432. 10.1161/STROKEAHA.120.030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA (2010) Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58:1610–1619. 10.1002/glia.21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, et al. (2019) Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15:148–160. 10.1038/s41582-018-0128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Chin C, Popovic MR (2020) Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: a review. Biomed Eng Online 19:34. 10.1186/s12938-020-00773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Arocena M, Penninger J, Gage FH, Zhao M, Song B (2011) PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol 227:210–217. 10.1016/j.expneurol.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic L, Kalia SK, Hodaie M, Lozano AM, Fasano A, Popovic MR, Hutchison WD (2018) Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson's disease. Brain 141:177–190. 10.1093/brain/awx296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura LM, Luccas R, de Paiva JPQ, Amaro E Jr, Leemans A, Leite CDC, Otaduy MCG, Conforto AB (2019) Diffusion tensor imaging biomarkers to predict motor outcomes in stroke: a narrative review. Front Neurol 10:445. 10.3389/fneur.2019.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negredo PN, Yeo RW, Brunet A (2020) Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 27:202–223. 10.1016/j.stem.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak YJ, et al. (2021) Digital health integration with neuromodulation therapies: the future of patient centric innovation in neuromodulation. Front Digit Health 3:618959. 10.3389/fdgth.2021.618959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis EM, O’Donnell JC, Chen HI, Cullen DK (2020) Tissue engineering and biomaterial strategies to elicit endogenous neuronal replacement in the brain. Front Neurol 11:344. 10.3389/fneur.2020.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710. 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- Sachewsky N, Hunt J, Cooke MJ, Azimi A, Zarin T, Miu C, Shoichet MS, Morshead CM (2014) Cyclosporin A enhances neural precursor cell survival in mice through a calcineurin-independent pathway. Dis Model Mech 7:953–961. 10.1242/dmm.014480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton E, Iwasa SN, Morrison T, Naguib HE, Popovic MR, Morshead CM (2020) Electric field application in vivo regulates neural precursor cell behaviour in the adult mammalian forebrain. eNeuro 7:ENEURO.0273-20.2020. 10.1523/ENEURO.0273-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Villalobos J, Burns O, Nayagam DAX (2018) The development of neural stimulators: a review of preclinical safety and efficacy studies. J Neural Eng 15:041004. 10.1088/1741-2552/aac43c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J-S, Rogers JA, Kang S-K (2021) Physically transient electronic materials and devices. Mater Sci Eng R Rep 145:100624. 10.1016/j.mser.2021.100624 [DOI] [Google Scholar]
- Smuck M, Odonkor CA, Wilt JK, Schmidt N, Swiernik MA (2021) The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digit Med 4:45. 10.1038/s41746-021-00418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz T (2020) Of man and mice: translational research in neurotechnology. Neuron 105:12–15. 10.1016/j.neuron.2019.11.030 [DOI] [PubMed] [Google Scholar]
- Tang H, Li Y, Tang W, Zhu J, Parker GC, Zhang JH (2023) Endogenous neural stem cell–induced neurogenesis after ischemic stroke: processes for brain repair and perspectives. Transl Stroke Res 14:297–303. 10.1007/s12975-022-01078-5 [DOI] [PubMed] [Google Scholar]
- Tejeda G, Ciciriello AJ, Dumont CM (2022) Biomaterial strategies to bolster neural stem cell-mediated repair of the central nervous system. Cells Tissues Organs 211:655–669. 10.1159/000515351 [DOI] [PubMed] [Google Scholar]
- van Wijk BCM, de Bie RMA, Beudel M (2023) A systematic review of local field potential physiomarkers in Parkinson’s disease: from clinical correlations to adaptive deep brain stimulation algorithms. J Neurol 270:1162–1177. 10.1007/s00415-022-11388-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissani M, Isaias IU, Mazzoni A (2020) Deep brain stimulation: a review of the open neural engineering challenges. J Neural Eng 17:051002. 10.1088/1741-2552/abb581 [DOI] [PubMed] [Google Scholar]
- Williamson MR, et al. (2023) Subventricular zone cytogenesis provides trophic support for neural repair in a mouse model of stroke. Nat Commun 14:6341. 10.1038/s41467-023-42138-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, et al. (2010) Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res 88:3275–3281. 10.1002/jnr.22495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M (2006) Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res 83:1213–1219. 10.1002/jnr.20813 [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, Lu M, Zhang ZG (2012) Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One 7:e48141. 10.1371/journal.pone.0048141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J, Budde K, Arbeiter N, Molina F, Storch A, Uhrmacher AM, van Rienen U (2021) Using a digital twin of an electrical stimulation device to monitor and control the electrical stimulation of cells in vitro. Front Bioeng Biotechnol 9:765516. 10.3389/fbioe.2021.765516 [DOI] [PMC free article] [PubMed] [Google Scholar]