Abstract
Background
Point-of-care testing (POCT) is commonly used in epidemiological surveys due to its various advantages, such as portability and immediate test results. The CardioChek® PA analyser 3-in-1 lipid panel measures total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol. This study tested the reliability and diagnostic accuracy of the CardioChek® PA analyser using a 3-in-1 lipid panel.
Methods
A cross-sectional study design with quota sampling was used. A total of 203 respondents aged 18 years and above from a research centre in the Ministry of Health, Malaysia, were recruited. Venous blood was sent to the laboratory and tested with Siemens Atellica CH, while a POCT analyser was used for capillary blood measurements. Intraclass coefficient correlation (ICC) analysis was employed to determine the agreement between capillary and venous blood parameters. The diagnostic performance of the evaluated tests was evaluated using STATA version 12.
Results
The agreement between capillary and laboratory venous blood was moderate (0.64–0.67) for TC and HDL, good (0.75) for LDL and excellent (0.91) for TG). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were as follows: TC, 57.1%, 94.3%, 92.3% and 64.8%; TG, 76.0%, 100%, 100%, and 96.6%; HDL, 96.2%, 83.2%, 47.2% and 99.3%; and LDL, 81.0%, 100%, 100% and 68.3%, respectively.
Conclusions
The CardioChek® PA analyser showed acceptable diagnostic accuracy for screening high-risk individuals more often in places where laboratories are inaccessible. It could also be used in clinical settings where patients would benefit from swift treatment decisions.
Keywords: Epidemiological survey, CardioChek® PA, Point-of-care testing (POCT), Diagnostic accuracy, Cholesterol, Lipid
Introduction
Hyperlipidaemia is one of the risk factors contributing to cardiovascular diseases (CVD) [1]. Total cholesterol (TC) is the total amount of cholesterol in your blood based on your High-Density Lipoprotein Cholesterol (HDL-C), Low-Density Lipoprotein Cholesterol (LDL-C), and triglycerides (TG) numbers [2]. Atherosclerotic CVD is the acute and chronic clinical manifestation of a progressive pathogenic process initiated by inflammatory responses to dyslipidaemia [3].Dyslipidaemia is a pressing issue in Malaysia. According to a review of global trends in the epidemiology of dyslipidaemia, among the top 10 countries with the highest age-standardized mean non-HDL-C levels in 2018 for men (number 1 country) and women (number 2) was Malaysia [4]. On the other hand, the National Health and Morbidity Survey (NHMS) 2019 Malaysia reported [5], the overall prevalence of elevated blood cholesterol (≥ 5.2 mmol/l) was 38.1%, while the NHMS 2015 reported, among those who were diagnosed with hypercholesterolaemia, only 19.2% were aware of their hypercholesterolaemia status. Just 12.7% (95% CI: 12.4–13.1) of individuals who were cognizant of their condition were undergoing treatment, and among them, merely 53.7% (95% CI: 50.1–57.2) had effectively managed their cholesterol levels. [6]. A prospective cohort study of more than 8000 men and women aged 35 years and older showed that TC levels were significantly associated with myocardial infarction in males [7]. Cholesterol testing, encompassing all its parameters, is essential for assessing cardiovascular risk in both individuals and populations. A retrospective cohort study conducted among the Chinese population revealed that a higher LDL: HDL ratio was associated with an increased risk of prediabetes, especially among women, individuals with a family history of diabetes, younger people, and non-obese individuals [8].
Point-of-care testing (POCT) produces rapid test results, allowing for informed and timely clinical decisions. It provides patients with greater convenience and access to health services, thereby enhancing clinical outcomes [9]. POCT provides innovative solutions for detecting and managing non-communicable diseases (NCDs), as well as chronic, acute, and infectious diseases. It is particularly beneficial in family practices, indigenous medical services, community health facilities, rural and remote areas, and developing countries where healthcare services are frequently isolated from pathology laboratories [10, 11]. These advantages are especially useful when used on a larger scale, such as in epidemiological surveys where laboratories are situated at far distances. While many national surveys use venous blood to diagnose dyslipidaemia [12, 13], it can be an obstacle for low to middle income countries like Malaysia where housing is situated in remote areas although POCTs have usually higher per test cost and risk of poor quality control [14].
Understanding cholesterol levels in national studies like the NHMS is vital for shaping public health programs and targeting preventive measures. POCT for TC, TG, HDL, and LDL facilitates easy population studies, enhancing dyslipidaemia treatment in primary care. The focus could be shifted to non-HDL cholesterol management given the superiority of non-HDL-C in cardiovascular risk prediction [15]. Despite POCT devices’ portability, ensuring their results are accurate and comparable to laboratory analysis remains a challenge [16]. Cholesterol POCT technology became widely available around the year 2000 but was initially ineffective and cumbersome. Over time, the devices have become more portable, compact, and capable of integrating with data servers [17]. Nevertheless, POCT analysers need to be periodically evaluated for reliability to ensure accurate results. Agreement between devices and between the readings of capillary blood and laboratory venous blood (routine using) should be compared. This study aimed to evaluate the performance of the CardioChek® PA 3-in-1 lipid panel analyser in measuring blood cholesterol levels by comparing it to a reference value from the laboratory.
Methodology
Study design
A cross-sectional study design using a quota sampling design was used in this study.
Ethical statement
This study was reviewed and approved by the Medical Ethics and Research Committee, Ministry of Health Malaysia (NMRR ID-22-00833-K1A). All the information obtained in this survey was kept and handled in a confidential manner. The respondents were given a patient information sheet (PIS) in the Malay or English language to read prior to providing consent. Any clarifications about the study were addressed by the study team. Written consent was obtained from all eligible respondents.
Population
The respondents involved in this study were staff from a research institute under the Ministry of Health, Malaysia, aged 18 years and older. The invitation to participate in this study was extended to all staff. The exclusion criteria for this study were pregnancy and within six weeks postnatal period, known anaemia or any blood disorders, and not physically or mentally fit to answer the questionnaire or for blood collection. This is because a higher haemoglobin concentration is associated with an unfavourable lipoprotein particle profile [18], whereas hypocholesterolaemia accompanies anaemias with high-erythropoietic activity [19]The intraclass correlation coefficient (ICC) test was used to calculate the sample size [20, 21], with a minimum acceptable reliability of 0.85 and an expected reliability of 0.90. The optimum sample size (n) was 169. The estimation for lysed blood samples was 20%, which made the minimum sample required 203.
Data collection process
The data were collected from September to October 2022. All respondents were required to answer several questions on sociodemographic information, including information about existing medical conditions. Unique identification identifiers were assigned to each respondent, and the identity of the respondent was blinded to the researchers. Respondents were required to fast for at least eight hours prior to blood collection. Capillary and venous blood was collected from all respondents by trained phlebotomists, nurses, and doctors. Approximately 40 µL of capillary blood was withdrawn to measure blood cholesterol levels using the POCT. A total of 3 mL of venous blood was collected via aseptic technique into a plain tube for determination of the fasting lipid profile (FLP). Siemens Atellica CH (Siemens Healthcare Sdn Bhd) was used to measure the FLP in the laboratory, while LDL-C was calculated using the Friedewald equation – LDL-C (mmol/L) = TC -HDL- TG/2.2 [22]. Table 1 shows the analytical measurement range (AMR) of Siemens Atellica CH and CardioChek® PA for all three parameters. All laboratory staff performing the FLP assays were blinded to the clinical characteristics of the subjects. Enzymatic cholesterol oxidase, esterase, peroxidase were the assays used by the laboratory to test the fasting serum lipid. The serum tubes that were used were manufactured by Greiner Bio-One. The blood samples were collected by the laboratory personal right after the blood withdrawal was done. The samples were transported in cooler boxes to the laboratory. Blood results from the laboratory and the POCT were recorded in the data collection form and Microsoft Excel worksheet. Simultaneously, the respondents were informed of the results, and appropriate recommendations and referrals were given to the nearest clinics by medical doctors on the research team.
Table 1.
Analytical measurement range (AMR) of Siemens Atellica CH and CardioChek® PA
| Analytical measurement range (AMR) (mmol/L) | TC | HDL-C | TG |
|---|---|---|---|
| Siemens Atellica CH | 0.65–16.01 | 0.13–5.18 | 0.17–11.30 |
| CardioChek® PA | 2.59–10.36 | 0.39–2.59 | 0.57–5.65 |
CardioChek® PA 3-in-1 lipid panel
The POCT device used in this study was the CardioChek® PA 3-in-1 lipid panel, which measures all cholesterol parameters (total cholesterol, triglycerides, and high-density lipoprotein cholesterol [HDL]) using a single strip and calculates LDL values for in less than 2 min. It is battery-operated, handheld, and portable and can be used to test capillary and venous blood. A sample volume of 40 µL was required for a test to be performed successfully. PTS Diagnostics (USA) provided the CardioChek 3-in-1 lipid panels in kind, while existing CardioChek® PA devices were utilized.
Calibration and quality control (QC)
Calibration and QC were performed to ensure the reliability and consistency of the assay results. For capillary blood, the POCT device was calibrated, and QC was run daily before use to check its performance. Control solutions from the manufacturer were used for QC of the device. For venous cholesterol, all blood samples were sent to the laboratory for analysis. The selected laboratories fulfilled the requirements for complete calibration and QC, and all the details were requested by our research team. The control intervals and limits should be adapted to each laboratory’s individual requirements.
Data analysis
The sociodemographic characteristics of the respondents and the mean and standard deviation of the TC, TG, HDL and LDL were described using descriptive analyses. Diagnostic accuracy, namely sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each of the lipid profile parameters, was computed using standard formulas, where: sensitivity = true positives (TP) / [true positives + false negatives (FN)], specificity = true negatives (TN) / [TN + false positives (FP)], PPV = TP/(TP + FP), and NPV = TN/(TN + FN). The data analyses up to this part were performed using STATA version 12 (StataCorp, TX, US).
Subsequently, the MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium) was used to perform the agreement analyses between the index and reference tests for each of the parameters. First, the Passing-Bablok regression was performed. This is a non-parametric test that is more suitable compared to simple linear regression as it has no assumptions of the distribution of the test results and their measurement errors. The linearity between the results of the index and reference tests were established and the regression model was validated if the modified cumulative sum (Cusum) test was not significantly different from a linear model (p > 0.05). Systematic difference between the tests was significant, if the 95% CI of the intercept A did not contain 0, and proportional difference (difference changes as the test value increases in magnitude) was significant, if the 95%CI of the slope B did not contain 1 [23, 24]. Next, the Bland-Altman limits of agreement was plotted with the mean value of the index and reference tests on the x-axis, and the difference between both tests as percentage (%D) (index value – reference value/mean of index and reference value X 100%) on the y-axis. Significant systematic difference was present if the mean %D and its 95%CI did not include the line of equality (difference = 0). Proportional difference was present if the regression line of differences was not aligned with the horizontal line of the mean %D. Finally, both the index and the reference tests were considered to be in agreement with each other, if the ± 1.96 standard deviation of the %D, or the limits of agreement (LoA) and their 95%CI did not exceed the lines of maximum allowed difference [25], which was set by the US National Cholesterol Education Program (NCEP) at ± 5% for TC, ± 4% for TG, ± 5% for HDL, and ± 3% for LDL [16].
The ICC using the two-way mixed model and average measures [21] was used to calculate the agreement between the capillary blood results and the laboratory results. Based on the ICC estimate, values less than 0.5, between 0.5 and less than 0.75, between 0.75 and less than 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent agreement, respectively [26, 27]. A significance level of 0.05 was utilised to assess statistical significance in the analysis. Diagnostic accuracy to assess sensitivity and specificity was assessed using the same software.
Diagnostic values for blood cholesterol levels
According to the Malaysian Clinical Practice Guidelines for the Management of Dyslipidaemia, hypercholesterolaemia is defined as a total cholesterol level equal to or greater than 5.2 mmol/L. Additionally, specific criteria for diagnosing dyslipidaemia include HDL levels less than 1.0 mmol/L for males and less than 1.2 mmol/L for females, triglyceride (TG) levels exceeding 1.7 mmol/L, and LDL levels surpassing 2.6 mmol/ [28].
Results
The data of a total of 196 samples were analysed. The sociodemographic characteristics of the sample is shown in Table 2 while mean values from the POCT and lab is shown in Table 3. Passing-Bablok regression and Bland-Altman plots in Figs. 1 and 2 show a graphical representation of the results obtained.
Table 2.
Sociodemographic data of the respondents in the study (n = 196)
| Characteristics | % (n) |
|---|---|
| Sex | |
| Male | 25.0 (49) |
| Female | 75.0 (147) |
| Age | |
| 18–39 years | 57.1 (112) |
| 40–59 years | 36.7 (72) |
| 60 years and above | 6.12 (12) |
| Ethnicity | |
| Malay | 57.1 (112) |
| Chinese | 4.6 (9) |
| Indian | 35.2 (69) |
| Bumiputera Sabah & Sarawak | 3.0 (6) |
| Smoking status | |
| Smoker | 4.6 (9) |
| Nonsmoker | 95.4 (187) |
| Diabetes | |
| Yes | 9.2 (18) |
| No | 90.8 (178) |
| Hypertension | |
| Yes | 10.2 (20) |
| No | 89.8 (176) |
| Hypercholesterolaemia | |
| Yes | 8.7 (17) |
| No | 91.3 (179) |
| Cardiovascular disease | |
| Yes | 2.0 (4) |
| No | 98.0 (192) |
Table 3.
Mean values measured by a CardioChek® PA analyser for all parameters
| Total Cholesterol | Triglycerides | HDL | LDL | |||||
|---|---|---|---|---|---|---|---|---|
| POCT | Lab | POCT | Lab | POCT | Lab | POCT | Lab | |
| Mean ± SD (mmol/L) | 4.68 ± 1.14 | 5.27 ± 1.01 | 1.16 ± 0.63 | 1.25 ± 0.87 | 1.27 ± 0.29 | 1.47 ± 0.36 | 2.89 ± 1.03 | 3.26 ± 0.88 |
| Minimum | 2.58 | 3.0 | 0.56 | 0.3 | 0.69 | 0.8 | 0.50 | 1.3 |
| Maximum | 8.30 | 7.8 | 5.66 | 8.0 | 2.35 | 3.7 | 6.33 | 5.6 |
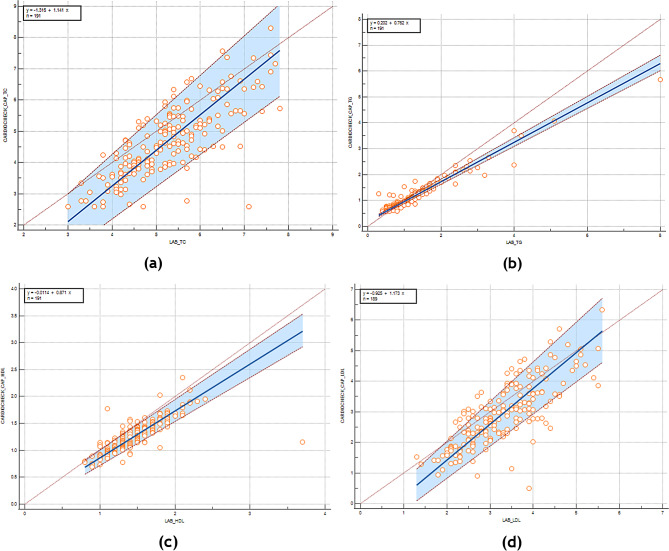
Fig. 1.
Passing-Bablock regression plots comparing the POCT and laboratory measurements for TC, TG, HDL-C and LDL-C. The diagonal line that extends from the bottom left towards the upper right of the plot is the line of perfect agreement between both index and reference tests. The regression line of differences that best fits the data is indicated by bold blue line, and the dashed red lines on both sides, its 95%CI. (a) Total Cholestrol (b) Triglycerides (c) HDL-C (d) LDL-c
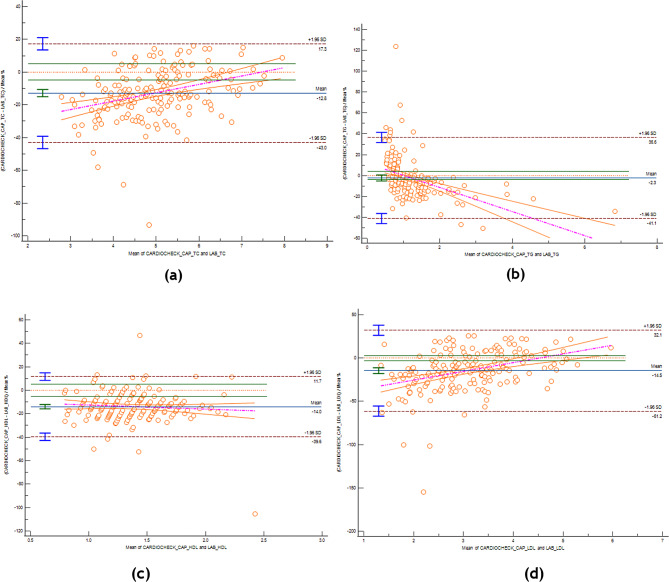
Fig. 2.
Bland-Altman plot comparison of the POCT and laboratory TC, TG, HDL-C and LDL-C measurements. Red dashed horizontal line – line of equality (difference = 0), green horizontal lines on both sides – lines of maximum allowed difference (± X% for Y parameter, e.g. ±5% for total cholesterol), blue horizontal line with green upper and lower limit lines on its extreme left – mean percent differences and its 95%CI, brown dashed horizontal lines with blue upper and lower limit lines on its extreme left – the respective upper and lower limits of agreement and their 95%CI, purple dashed line and orange lines on its sides – the regression line differences that best fits the data and its 95%CI. (a) Total Cholestrol (b) Triglycerides (c) HDL-C (d) LDL-c
Total cholesterol
There was a linear relationship between the TC value of the index and reference tests (Cusum test p = 0.96). Both Passing-Bablok regression and Bland-Altman plot indicated the presence of systematic and proportional differences. The mean %D was − 12.82% (95%CI -15.01, -10.62). The lower and upper LoA were beyond the ± 5% maximum allowed difference, at -42.96% (95%CI -46.72, -39.20) and 17.34% (95%CI 13.58, 21.09), respectively, indicating that the index test disagree with the reference test, mainly by underestimation. As there was a proportional difference, this underestimation was more obvious when the magnitude of the tests value was lower.
Triglycerides
There was a linear relationship between the TG value of the index and reference tests (Cusum test p = 0.78). Systematic difference was significant only on the Passing-Bablok regression, while the mean %D on the Bland-Altman plot, -2.27% (95%CI -5.11, 0.56), included the line of equality. This underestimation was small due to the significant proportional difference on both the regression and the plot, so much so that there was only one account of overestimation when the mean value of both tests exceeds 1.5 on the x-axis. The lower and upper LoA were beyond the ± 4% maximum allowed difference, at -41.14% (95%CI -45.99, -36.30) and 36.59% (95%CI 31.75, 41.44), respectively, indicating that the index test disagree with the reference test.
High-density lipoproteins
There was a linear relationship between the HDL value of the index and reference tests (Cusum test p = 0.84). Proportional difference was present on the Passing-Bablok regression only, while systematic difference was present only on the Bland-Altman plot. The mean %D was − 13.97% (95%CI -15.84, -12.10). The lower and upper LoA were beyond the ± 5% maximum allowed difference, at -39.63% (95%CI -42.83, -36.43) and 11.69% (95%CI 8.49, 14.89), respectively, indicating that the index test disagree with the reference test, mainly by underestimation that remained constant across the magnitude of the tests value.
Low-density lipoproteins
There was a linear relationship between the LDL value of the index and reference tests (Cusum test p = 0.65). Both Passing-Bablok regression and Bland-Altman plot indicated the presence of systematic and proportional differences. The mean %D was − 14.54% (95%CI -17.96, -11.13). The lower and upper LoA were beyond the ± 3% maximum allowed difference, at -61.23% (95%CI -67.09, -55.38) and 32.15% (95%CI 26.29, 38.00), respectively, indicating that the index test disagree with the reference test, mainly by underestimation, which was more prominent when the magnitude of the tests value was lower.
The sensitivity ranged from 57.1 to 96.2%, with the lowest value occurring for TC and the highest value occurring for HDL. The specificity ranged from 83.2 to 100.0%, with the lowest value occurring for HDL and the highest value occurring for 100.0% for TG and LDL. The PPV ranged from 47.2 to 100.0%, with the lowest value occurring for HDL and the highest values occurring for LDL and TG. The NPV ranged from 64.8 to 96.6%, with the lowest value occurring for TC and the highest value occurring for TG (Table 4).
Table 4.
Diagnostic performance of the CardioChek® PA analyser
| Capillary vs. Lab | Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
Positive predictive value (PPV) (95% CI) |
Negative predictive value (NPV) (95% CI) |
|---|---|---|---|---|
| Total cholesterol (TC) |
60/105 57.1% (47.11–66.76) |
83/88 94.3% (87.24–98.13) |
60/65 92.3% (82.95–97.46) |
83/128 64.8% (55.91–73.07) |
| Triglycerides (TG) |
19/25 76.0% (54.87–90.64) |
168/168 100.0% (97.83–100.0) |
19/19 100.0% (82.35–100.0) |
168/174 96.6% (92.65–98.72) |
| High-density lipoprotein (HDL) cholesterol |
25/26 96.2% (80.36–99.90) |
139/167 83.2% (76.7–88.6) |
25/53 47.2% (33.30-61.36) |
139/140 99.3% (96.08–99.98) |
| Low-density lipoprotein (LDL) cholesterol |
111/137 81.0% (73.44–87.21) |
56/56 100.0% (93.62–100.0) |
111/111 100.0% (96.73–100.0) |
56/82 68.3% (57.08–78.13) |
Agreement between capillary and venous blood parameters according to the CardioChek® PA analyser 3-in-1 lipid panel was TC: 0.67 (95% CI 0.26, 0.81, p < 0.001); TG: 0.91 (95% CI 0.88, 0.94, p < 0.001); HDL: 0.64 (95% CI 0.20, 0.82, p < 0.001); and LDL: 0.75 (95% CI 0.55, 0.85, p < 0.001). The CardioChek® PA analyser 3-in-1 lipid panel showed excellent agreement for TG and good agreement for LDL, with moderate agreement for TC and HDL between capillary and venous blood (Table 5).
Table 5.
Agreement using intraclass coefficient correlation (ICC) (n = 193)
| Capillary versus Lab | Agreement | p value |
|---|---|---|
| Total cholesterol (TC) | 0.67 (0.26, 0.81) | < 0.001 |
| Triglycerides (TG) | 0.91 (0.88, 0.94) | < 0.001 |
| High density lipoprotein (HDL) cholesterol | 0.64 (0.20, 0.82) | < 0.001 |
| Low density lipoprotein (LDL) cholesterol | 0.75 (0.55, 0.85) | < 0.001 |
Discussion
The data from this study showed excellent agreement for TG, good agreement for LDL, and moderate agreement for TC and HDL. According to Ferreira et al., 2015, the correlation between fingerstick and venous parameters was extremely high for HDL-C (r = 0.953) and TG (r = 0.953) and good for TC (r = 0.879) [29]. This study and the previous one are comparable in that the correlation for TG was strong, while it was lower for the other measures. In a study comparing two POCT devices, namely, the Cholestech LDX and CardioChek PA devices, the ICC for TG obtained from the CardioChek PA device was above 0.75, while the ICCs for TC, HDL, and LDL were less than 0.75 [30]. These findings are very similar to those obtained from this study. In a study done in 2012, the sensitivity and specificity of the CardioChek® PA using a single lipid panel (TC only) was reported as 62.7% and 76.1% respectively [31]. These results when compared to our study was slightly higher for sensitivity and significantly higher for specificity. The same study also reported PPV and NPV to be 76.4% and 62.4% respectively [31], which is lower compared to our study. This may be due to the use of different lipid panels; single and 3-in-1 lipid panels.
The CardioChek® PA 3-in-1 lipid panel analyser has been well studied and compared to other similar POCTs. In a study performed among a small sample of 3 normolipidemic patients, comparable imprecision between the CardioChek® PA and the Elemark™ was found [32]. The CardioChek® PA analyser has been used since 2011 in nationwide surveys conducted by the Institute for Public Health [33]. This device is also mentioned in the WHO STEPwise approach to noncommunicable disease risk factor surveillance as a tool for measuring blood cholesterol, including HDL, LDL and TG levels [34]. According to the Centers for Disease Control and Prevention (CDC) United States, CardioChek PA met the National Cholesterol Education Programme (NCEP)’s recommended test protocols and guidelines and is certified by the Cholesterol Reference Method Laboratory Network (CRMLN), which uses rigorously standardized reference methods set by the CDC to certify the manufacturers of diagnostic products measuring TC, HDL, and LDL [35].
While POCTs are not suitable to be used for clinical judgement unlike laboratory tests, it still has a place in epidemiological surveys. The use of POCTs is indeed valuable in screening and monitoring diseases such as diabetes and hypercholesterolaemia. The general population should be encouraged to use POCTs as an initial screening tool. The user-friendly design of the system allows patients to independently track their blood lipid levels in response to lifestyle modifications, such as dietary changes, increased physical activity, or other therapeutic approaches [36]. In a study assessing the feasibility, acceptability, and efficacy of POCT and quantitative CVD risk assessment for improving guideline-recommended statin use in high-risk adults for primary prevention, the outcomes showed that 83% of the participants engaged in a discussion about CVD risk with their primary care physician (PCP). Of those individuals, 47% received a statin recommendation from their PCP, while 29% were provided with a new statin prescription during the PCP visit. As a result, participants expressed a high level of satisfaction with the intervention. [37]. A separate study aimed at assessing patient satisfaction with POCT in a general practice setting indicated that patients exhibited higher levels of satisfaction and confidence with this testing method. Moreover, they perceived POCT as a means of strengthening their rapport with their general practitioner (GP) and found it motivating in terms of enhancing their ability to manage their medical condition more effectively [38]. Importantly, while POCT offers many advantages, there may also be limitations, such as the need for proper training, quality control, and potential limitations in the range of tests that can be performed using POCT devices. Careful planning and validation of POCT methods should be part of the survey design to ensure the accuracy and reliability of the data collected. Although the CardioChek® PA 3-in-1 lipid panel analyser is a well-established POCT device, to our knowledge, this is the first independent study to evaluate its diagnostic performance in Malaysia.
Though device performance often matches laboratory data in validation studies, significant differences between POCT and lab results in field conditions raise concerns. [39, 40]. Variations in POCT cholesterol readings may be due to operational factors, reflecting the difficulty in running simple but sensitive technology under field conditions, where the operators are often nontechnical personnel and technical support may not be immediately available. Furthermore, this highlights the need for rigorous quality control measures to detect any deviation from the expected trend [29, 41].
A limitation of this study is the mismatch between the ethnic composition of the sample and Malaysia’s demographic distribution. Nonetheless, respondents included individuals with underlying conditions like diabetes and hypertension, providing valuable insights. Some of these individuals were also healthy with no known underlying comorbidities. There were also more females than males in this study, which does not represent the population in Malaysia. This could have been due to the quota sampling method that was used, which introduced sampling bias, as not everyone had an equal chance of being selected. Another limitation of quota sampling is that it may not always accurately represent the true prevalence or distribution of characteristics within the population. The total sample for this study fell short from the minimum sample required mainly due to having insufficient blood sample.
Conclusion
The CardioChek® PA analyser showed acceptable (excellent agreement for TG, good agreement for LDL, and moderate agreement for TC and HDL) diagnostic accuracy for the purpose of screening high-risk individuals more so in places where labs are inaccessible. It could also be used in clinical settings where patients would benefit from swift treatment decisions. However, the device should not be used as a substitute for certified laboratory methods in the diagnosis of dyslipidaemia but can be used for fieldwork.
Acknowledgements
The author would like to thank the Director General of Health Malaysia for permission to publish this article. We also thank PTS Diagnostics IN, USA, for in-kind donations of the CardioChek® PA 3-in-1 lipid panel strips. We appreciate all the data collectors and research assistants who contributed to this study.
Author contributions
TG, TA wrote the main manuscript text; TG, CZ, HA performed analysis of the data; TG, TA, HM, WKS, NL, KK, HI contributed to the concept of the study; NA contributed to the quality control and methodology of the study; MF was the overall reviewer and editor.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was granted by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID 22-00833-K1A). Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO). Noncommunicable diseases [Internet]. 2021 [cited 2022 Feb 6]. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- 2.Centers for Disease Control and Prevention (CDC). Cholesterol Home [Internet]. 2023. https://www.cdc.gov/cholesterol/about.htm
- 3.Wengrofsky P, Lee JMA. Dyslipidemia and Its Role in the Pathogenesis of Atherosclerotic Cardiovascular Disease: Implications for Evaluation and Targets for Treatment of Dyslipidemia Based on Recent Guidelines. In: SI M, editor. IntechOpen; 2019. https://www.intechopen.com/chapters/6672510.5772/intechopen.85772
- 4.Liu T, Zhao D, Qi Y. Global trends in the Epidemiology and Management of Dyslipidemia. J Clin Med. 2022;11:21. 10.3390/jcm11216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute for Public Health. National Health and Morbidity Survey (NHMS). 2019: NCDs - Non-Communicable Diseases: Risk Factors and other Health Problems [Internet]. Vol. 1, Institute for Public Health, National Institutes of Health (NIH), Ministry of Health Malaysia. 2019. https://iku.moh.gov.my/images/IKU/Document/REPORT/NHMS2019/Report_NHMS2019-NCD_v2.pdf
- 6.Mat Rifin H, Robert Lourdes TG, Abdul Majid NL, Abd Hamid HA, Rodzlan Hasani WS, Ling MY, et al. Hypercholesterolemia Prevalence, awareness, treatment and control among adults in Malaysia: the 2015 National Health and Morbidity Survey, Malaysia. Glob J Health Sci. 2018;10(7):11. 10.5539/gjhs.v10n7p11 [DOI] [Google Scholar]
- 7.Hedayatnia M, Asadi Z, Zare-feyzabadi R, Yaghooti-khorasani M, Ghazizadeh H, Ghaffarian-zirak R et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. 2020;1–11. [DOI] [PMC free article] [PubMed]
- 8.Kuang M, Peng N, Qiu J, Zhong Y, Zou Y, Sheng G. Association of LDL:HDL ratio with prediabetes risk: a longitudinal observational study based on Chinese adults. Lipids Health Dis. 2022;21(1):1–11. 10.1186/s12944-022-01655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gencer B, Marston NA, Im KA, Cannon CP, Sever P, Keech A et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet [Internet]. 2020;396(10263):1637–43. 10.1016/S0140-6736(20)32332-1 [DOI] [PMC free article] [PubMed]
- 10.Chen H, Liu K, Li Z, Wang P. Point of care testing for infectious diseases. Clin Chim Acta [Internet]. 2019;493(March):138–47. 10.1016/j.cca.2019.03.008 [DOI] [PMC free article] [PubMed]
- 11.Malcolm S, Cadet J, Crompton L, DeGennaro V. A model for point of care testing for non-communicable disease diagnosis in resource-limited countries. Glob Heal Epidemiol Genomics. 2019;4:e7. 10.1017/gheg.2019.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SJ, Kwon OD, Kim KS. Prevalence, awareness, treatment, and control of dyslipidemia among diabetes mellitus patients and predictors of optimal dyslipidemia control: results from the Korea National Health and Nutrition Examination Survey. Lipids Health Dis. 2021;20(1):1–10. 10.1186/s12944-021-01455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang FL, Xing YQ, Wu YH, Liu HY, Luo Y, Sun MS, et al. The prevalence, awareness, treatment, and control of dyslipidemia in northeast China: a population-based cross-sectional survey. Lipids Health Dis. 2017;16(1):1–13. 10.1186/s12944-016-0392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigl BH, Neogi T, McGuire H. Point-of-Care Diagnostics in Low-Resource settings and their impact on care in the age of the noncommunicable and chronic Disease Epidemic. J Lab Autom. 2014;19(3):248–57. 10.1177/2211068213515246 [DOI] [PubMed] [Google Scholar]
- 15.Su X, Kong Y, Peng D. Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis. 2019;18(1):1–7. 10.1186/s12944-019-1080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park PH, Chege P, Hagedorn IC, Kwena A, Bloomfield GS, Pastakia SD. Assessing the accuracy of a point-of-care analyzer for hyperlipidaemia in western Kenya. Trop Med Int Heal. 2016;21(3):437–44. 10.1111/tmi.12653 [DOI] [PubMed] [Google Scholar]
- 17.Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. Point-of-care testing for the analysis of lipid panels: primary care diagnostic technology update. Br J Gen Pract. 2012;62(596):2011–3. 10.3399/bjgp12X630241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hämäläinen P, Saltevo J, Kautiainen H, Mäntyselkä P, Vanhala M. Hemoglobin level and lipoprotein particle size. Lipids Health Dis. 2018;17(1):2–7. 10.1186/s12944-018-0655-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalev H, Kapelushnik J, Moser A, Knobler H, Tamary H. Hypocholesterolemia in chronic anemias with increased erythropoietic activity. Am J Hematol. 2007;82(3):199–202. 10.1002/ajh.20804 [DOI] [PubMed] [Google Scholar]
- 20.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med [Internet]. 1998;17(1):101–110. 10.1002/(sici)1097-0258(19980115)17:1%3C101::aid-sim727%3E3.0.co;2-e. [DOI] [PubMed]
- 21.Sainani KL. Reliability statistics. PM R. 2017;9(6):622–8. 10.1016/j.pmrj.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 22.Martins J, Steyn N, Rossouw HM, Pillay TS. Best practice for LDL-cholesterol: when and how to calculate. J Clin Pathol. 2023;76(3):145–52. 10.1136/jcp-2022-208480 [DOI] [PubMed] [Google Scholar]
- 23.Sui J, Lin Z, Azizpour S, Chen F, Gaur S, Keene K et al. Clinical evaluation of a fully electronic microfluidic white blood cell analyzer. PLoS One [Internet]. 2024;19(1 January):1–13. 10.1371/journal.pone.0296344 [DOI] [PMC free article] [PubMed]
- 24.MedCalc Software Ltd. [Internet]. 2024 [cited 2024 Feb 6]. https://www.medcalc.org/manual/passing-bablok-regression.php
- 25.MedCalc S. Ltd. 2024.
- 26.Koo TK, Li MY. A Guideline of selecting and reporting Intraclass correlation coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X. On statistical measures for data quality evaluation. J Geogr Inf Syst. 2020;12(03):178–87. [Google Scholar]
- 28.Ministry of Health Malaysia. Clinical Practice Guidelines Management of Dyslipidemia 2023 6th Edition. 2023.
- 29.Ferreira CE dos, França S, Correr CN, Zucker CJ, Andriolo ML, Scartezini A. M. Clinical correlation between a point-of-care testing system and laboratory automation for lipid profile. Clin Chim Acta [Internet]. 2015;446:263–6. 10.1016/j.cca.2015.04.036 [DOI] [PubMed]
- 30.Dale RA, Jensen LH, Krantz MJ. Comparison of two point-of-care lipid analyzers for use in global cardiovascular risk assessments. Ann Pharmacother. 2008;42(5):633–9. 10.1345/aph.1K688 [DOI] [PubMed] [Google Scholar]
- 31.Noor Ani A, Ummi Nadiah Y, Noor Azah D, Hamizatul Akmal AH. Sensitivity and specificity of CardioChek® PA in detecting individuals with abnormal cholesterol and glucose level. Int J Biomed. 2012;2(2):132–5. [Google Scholar]
- 32.Bolodeoku J, Pinkney S. Imprecision Evaluation of Self-Monitoring of Blood Cholesterol (SMBC) Handheld Point of Care Testing Devices: Elemark™ and Cardiochek PA. Ann Clin Lab Res [Internet]. 2019;7(1):290. http://www.aclr.com.es/
- 33.Institute for Public Health. National Health and Morbidity Survey (NHMS) 2011: NCDs - Non-Communicable Diseases: Risk Factors and other Health Problems. 2011.
- 34.World Health Organization (WHO). The WHO STEPwise approach to noncommunicable disease risk factor surveillance. 2017. [DOI] [PMC free article] [PubMed]
- 35.Centers for Disease Control and Prevention. Cholesterol Reference Method Laboratory Network [Internet]. 2023 [cited 2023 Nov 29]. https://www.cdc.gov/labstandards/csp/crmln.html
- 36.Rapi S, Bazzini C, Tozzetti C, Sbolci V, Modesti PA. Point-of-care testing of cholesterol and triglycerides for epidemiologic studies: evaluation of the multicare-in system. Transl Res [Internet]. 2009;153(2):71–6. 10.1016/j.trsl.2008.11.010 [DOI] [PubMed]
- 37.Karmali KN, Brown T, Sanchez T, Long T, Persell SD. Point-of-care testing to promote cardiovascular disease risk assessment: a proof of concept study. Prev Med Rep. 2017;7:136–9. 10.1016/j.pmedr.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laurence CO, Gialamas A, Bubner T, Yelland L, Willson K, Ryan P, et al. Patient satisfaction with point-of-care testing in general practice. Br J Gen Pract. 2010;60(572):166–71. 10.3399/bjgp10X483508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xavier HT, Ruiz RM, Kencis L, Melone G, Costa W, Fraga RF, et al. Clinical correlation between the point-of-care testing method and the traditional clinical laboratory diagnosis in the measure of the lipid profile in patients seen in medical offices. J Bras Patol E Med Lab. 2016;52(6):387–90. [Google Scholar]
- 40.Whitehead SJ, Ford C, Gama R. A combined laboratory and field evaluation of the Cholestech LDX and CardioChek PA point-of-care testing lipid and glucose analysers. Ann Clin Biochem. 2014;51(1):54–67. 10.1177/0004563213482890 [DOI] [PubMed] [Google Scholar]
- 41.Matteucci E, Della Bartola L, Rossi L, Pellegrini G, Giampietro O. Improving CardioCheck PA analytical performance: three-year study. Clin Chem Lab Med. 2014;52(9):1291–6. 10.1515/cclm-2013-1084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.