Abstract
Background
Despite the evidence of beneficial effects of physical activity (PA), people with multiple sclerosis (pwMS) are less physically active than the general population. To increase PA in pwMS, we developed a structured individually tailored PA promotion program which is conducted within clinical practice in a university-based outpatient clinic since 2016. This study serves as retrospective quality control of this program.
Objective
In a retrospective cohort study, we assessed the physical fitness of pwMS and the impact of the program on short- and long-term PA changes and behavioral determinants.
Methods
The program consisted of four appointments each 2–4 weeks apart. Spiroergometric test results of female pwMS were compared to female non-MS controls who underwent a voluntary physical fitness analysis. The short version of the Freiburger questionnaire, self-developed questions and the modified Physical activity screening questionnaire (PASQ) were sent to all participants assessing the PA levels before the program, 3 months after the program (short-term), and at the time of the survey (long-term). Additionally, established questionnaires assessed behavioral determinants before the program and long-term.
Results
A total of 166 participants [mean age 38.32 (± 10.61 SD), mean EDSS 2.30 (±1.29 SD)] and mostly females (63.3%, n = 105) were included in the study and started the program. A total of 136 participants completed the program. Out of these 63.9% (n = 87) answered the questionnaires in 12.38 (±11.34 SD) months after finishing the program. At baseline female pwMS (n = 100) showed a lower physical fitness in comparison to non-MS controls (n = 26) (maximal workload (Watts): 138.86 ± 37.85 vs. 191.73 ± 45.25, p < 0.001; peak oxygen consumption (ml min−1 kg−1): 26.40 ± 7.23 vs. 31.56 ± 10.10, p = 0.020). pwMS were more regularly active in short- (62.1%) and long-term (55.2%) compared to baseline (24.2%, p < 0.001). Among the activated participants, we observed improved internal motivation (p = 0.002) and decreased perception of barriers (p = 0.006) compared to baseline.
Conclusion
PwMS showed a lower physical fitness in comparison to non-MS controls. An individually tailored PA promotion program might improve behavioral determinants and thereby increase short- and long-term PA levels of pwMS.
Keywords: multiple sclerosis, physical activity, training plan, physical fitness assessment, physical activity promotion program
Introduction
People with multiple sclerosis (pwMS) are often less physically active (1) and spend more time in sedentary behavior (2) than the general population. Furthermore, pwMS show decreased physical fitness and muscle strength in comparison to healthy controls (3). Regular physical activity is increasingly regarded as an essential modifiable lifestyle factor in the therapy of pwMS across all disease stages (2–5). Initial studies confirmed that physical activity in pwMS leads to an improvement in physical functions such as muscle strength, endurance, mobility, and balance (6–9). Whereas the impact of physical activity on cognitive functions and disease modification in pwMS is still a matter of discussion (10–15), several studies indicated that physical activity ameliorates MS symptoms and improves quality of life among pwMS (16–18). Based on the beneficial evidence of physical activity, guidelines with physical activity recommendations for pwMS have been provided (5). However, the guidelines are not sufficiently implemented in clinical routine and many pwMS are not aware of the potential of physical activity on their physical fitness symptoms, and quality of life (19) or have difficulties to realize them. Several programs have already shown beneficial effects in promoting PA in pwMS, however, they are often not individually tailored (18, 20–24).
The main factors that influence physical activity behavior are environmental and personal determinants (25, 26). Environmental determinants influencing the physical activity levels in pwMS are, e.g., insufficient patient information about the impact of physical activity in daily clinical routine, lack of possibilities, and hereby a need for more interventions to support pwMS (19, 27–29). Personal determinants include, e.g., self-efficacy, perceived barriers, counterstrategies to overcome barriers, and social support (25, 30). Additionally, disease-related symptoms such as fatigue and motoric impairment also have a substantial impact and can cause uncertainty about the right type of physical activity (27). A detailed assessment of the current physical fitness combined with an individually tailored training recommendation, which takes the above-mentioned various determinants into account and also considers the physical activity preferences of patients might therefore be helpful to promote and increase regular physical activity in pwMS (31).
In 2016, the MS outpatient clinic together with the Center for Athletic Medicine of the University Medical Center Hamburg-Eppendorf (UKE) developed an individualized physical activity promotion program to inform and motivate pwMS performing regular physical activity. The program consisted of a detailed recording of exercise history including the current and past activity behavior as well as personal preferences for being physically active, an assessment of the patient’s physical fitness and an individually tailored recommendation on how to include physical activity in their daily life adapted to the patient’s time allocation, abilities, and preferences.
The aim of this study was to assess the physical fitness of pwMS and analyze the impact of the program on the change in short- and long-term physical activity and behavioral determinants.
Methods
Study design
The study was designed as a retrospective cohort study in pwMS who joined the individualized physical activity promotion program at the MS outpatient clinic and the Center for Athletic Medicine of the UKE following the STROBE guidelines.
Patient recruitment and eligibility criteria for the physical activity promotion program
Patient recruitment for the individualized physical activity promotion program was conducted between April 2016 and December 2019 through leaflets and a subscribed newsletter by the MS outpatient clinic. Furthermore, consecutive patients were asked during their visit in the MS outpatient clinic by the attending doctor whether they would like to participate. Patients of all activity levels were included to avoid bias. Inclusion criteria for the program were: (1) Diagnosis of MS, clinical isolated syndrome (CIS) or radiologically isolated syndrome (RIS) based on McDonald 2010 or 2017 diagnostic criteria (32), (2) a minimum age of 18 years and (3) the ability to perform a bicycle ergometry. Patients were excluded if they had any medical contraindication for physical activity (e.g., severe cardiovascular or orthopedic diseases).
All participants were seen and examined by a neurologist of the MS outpatient clinic before enrollment in the activity program. Demographic data [age, sex, BMI, disease course, year of diagnosis, actual Expanded Disability Status Scale (EDSS) score] (33) were obtained.
Individualized physical activity promotion program
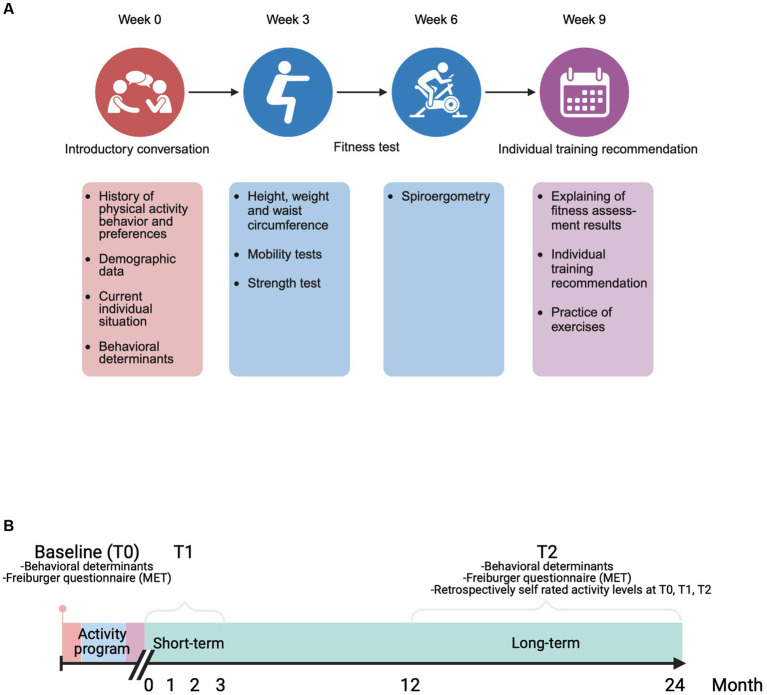
The program consisted of four individual appointments at the Center for Athletic Medicine of the UKE, each 2–4 weeks apart. Every appointment lasted approximately an hour and were conducted by a sports scientist in a 1:1 setting. For the schedule of the program see Figure 1A.
Figure 1.
(A) Schedule of the physical activity promotion program Content and time distance between the four individual appointments. Every appointment lasted an hour and were conducted by a sports scientist in a 1:1 setting. (B) Timelines between the three assessment time points Baseline (at first appointment of the activity program), T1 (within the 3 months after completing the last appointment of the activity program), T2 (at the actual time point of answering the quality control questionnaire). This figure was created with Biorender.
First appointment
The current and previous physical activity habits, personal preferences for physical activity and the current individual situation (e.g., job, family situation, motoric impairment) were recorded. Furthermore, participants received a self-developed questionnaire to assess sociodemographic data, current overall physical activity (short version of the validated Freiburger questionnaire) (34) and behavioral determinants [self-concordance, perceived barriers, barrier management, social support (35–38)] possibly related to long-term adherence to physical activity (see Supplementary material).
Second and third appointment
A comprehensive physical fitness assessment was conducted. After height, weight and waist circumference were measured, different mobility and strength tests (6-min walking test (6MWT), five times sit to stand test (5TSTS), timed 25-Foot Walk test (T25FW), handgrip test (via dynamometer), isometric Muscle Strength Measurement (DIERS myoline professional), S3 body stability test (MFT), a bioelectrical impedance analysis (BIA), ActiGraph wGT3X-BT accelerometer (39–50) were conducted. This was followed by a spiroergometric assessment (51) (see Supplementary material).
Fourth appointment
The results of the physical fitness assessment were explained to the participant. Based on the results of the first three appointments, the sports scientist and the participant set up an individual activity plan for implementation in the everyday life of the participant. The personal advice was based on the MS sport scientists experience. This plan included physical activity recommendations for endurance, strength and balance exercises in an individually adapted type, frequency, duration and intensity. The recommended exercises were shown and practiced together with the participant to guarantee a proper practice. Furthermore, additional recommendations were made to increase lifestyle physical activity (e.g., daily walking instead of going by car).
Patient recruitment for the retrospective analysis of the program
All participants who attended all four appointments of the individualized physical activity promotion program since 2016 were contacted between May 2019 and November 2019 with a questionnaire. The questionnaire was developed based on the results of short telephone interviews with 12 participants of different age, EDSS and baseline physical activity. The questionnaire consisted of 113 items and contained four sections: (1) evaluation of the activity program (see Supplementary material), (2) retrospectively self-rated physical activity level before the activity program (baseline), within the 3 months after completing the last appointment of the activity program (T1 = short-term) and at the actual time point of answering the questionnaires (T2 = long-term) (see Figure 1B and “Methods, Definition, assessment, and validation of self-rated physical activity”), (3) their current overall physical activity assessed with the short version of the Freiburger questionnaire (34) (see Supplementary material), (4) behavioral determinants possibly related to long-term adherence to physical activity (see Supplementary material) (35–38).
Definition, assessment and validation of self-rated physical activity
Following the physical activity guidelines for pwMS (5) “Regular physical activity” was defined as a minimum of 30 min of moderate intensity aerobic activity or strength training on at least 4 days a week. Inactivity was defined as no aerobic or strength at all. Irregular activity was defined as aerobic or strength training between 1 and 3 times per week for 30 min. However, we only provided a definition for “regularly active” in the questionnaire. The terms “irregularly active” and “inactive” were defined at the first appointment in the introductory conversation.
Previous exercise habits at baseline and T1 were assessed retrospectively with self-developed questions asking for the self-assessed physical activity level [“regularly active” (RA), “irregularly active” (IR), “inactive” (IA)] at these concrete points of time.
The current exercise habits at T2 were assessed with the modified physical activity Staging Questionnaire (PASQ) which was used in a previous study of our group (25) and converted into the categories “RA,” “IR” and “IA” (see Supplementary material). By using the PASQ it was possible to ask not only for the physical activity at a specific time point (T2) but also consider the physical activity evolution in the past time period between T1 and T2.
To validate the self-rated physical activity levels (RA, IR, IA) we did a correlation analysis between the self-rated physical activity levels and the metabolic equivalents of task (MET) (52) values at baseline and T2 calculated by the information of the short version of the Freiburger questionnaire (see Supplementary material) and with two objectively measured physical values from the spiroergometry at baseline.
Physical fitness performance analysis compared to non-MS control group
Spiroergometric test results were compared to a control group without a diagnosis of MS or any other neurological disease (“non-MS”), who attended the same spiroergometric test based on a voluntary offer from the Center for Athletic Medicine to determine their physical fitness level. We screened the database for matched non- MS controls but could only find female non-MS controls who were matched to the cohort for age, height and weight. Most of the non-MS controls had orthopedic diseases (n = 21) and were referred by their orthopedist. All other controls (n = 5) were completely healthy recreational athletes who underwent the physical fitness test on their own initiative. All controls with orthopedic conditions were able to execute the spiroergometry without limitations.
Patient consent
The activity program is routinely offered to our patients in the MS outpatient clinic. Since the study served as quality control of the individualized physical activity promotion program at the MS outpatient clinic, no approval by the local ethics committee was considered necessary (certified by the Ethics Committee of the Hamburg Chamber of Physicians) as long as we follow data protection guidelines, i.e., analyzing only pseudonymized data of the cohort. All participants still provided written informed consent.
Statistical analysis
Statistical analysis was performed using SPSS 25.00. Continuous data are described using mean (M), standard deviation (SD), median (MD) and range. Categorical data are presented as absolute and relative frequencies. Summary scores were calculated according to the scoring instructions in the literature (35–38). Missing data were excluded from analysis. Differences in demographic data between the quality control cohort and the non-follow-up cohort were tested using the two-tailed Students t-test for independent samples and Chi-squared Test. Differences in spiroergometric data between the MS-cohort and the non-MS control group were tested using two-tailed Students t-test for independent samples. Correlations of the spiroergometric peak-performance markers and EDSS as well as correlations between spiroergometric peak-performance markers, MET-values and indicated activity levels were calculated using Spearman coefficient. To estimate the magnitude of differences between subgroups we chose a conservative approach. Changes in the physical activity level within the subgroups RA, IR and IA over time were tested using the McNemar Test (53). Differences in behavioral determinants between subgroups were tested using the Kruskal–Wallis-Test. Alpha was set to 0.05 for all tests of significance.
Results
Cohort description
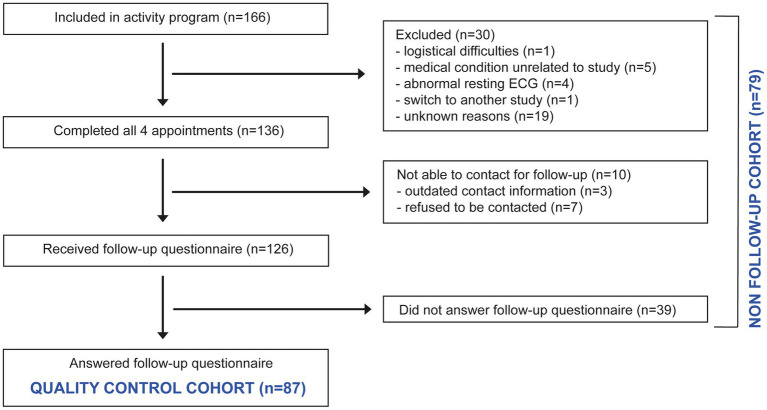
Out of the 166 participants who were included in the study, 30 participants (18.1%) did not participate in all four appointments and were excluded from the quality control study and 10 participants (6.0%) could not be contacted for retrospective quality control. The remaining 126 participants received the questionnaires, of whom 87 (69.1%) participants responded (Quality control cohort, for flow-chart, see Figure 2). There were no significant differences in the demographic, clinical baseline characteristics and additional sociodemographic data (Table 1A) between the quality control (n = 87) and the non-follow-up cohort (n = 79). Furthermore, no significant differences in the baseline physical fitness parameters of the two cohorts were detected (Table 1B).
Figure 2.
Flow-chart of participants in the individualized physical activity promotion program.
Table 1.
Clinical, demographic, sociodemographic (A) and fitness (B) baseline characteristics of the quality control cohort and the non-follow-up cohort.
| (A) | |||
|---|---|---|---|
| Quality control cohort (n = 87) | Non-follow-up cohort (n = 79) | p-value | |
| Age | 39.17 ± 10.55 | 37.63 ± 10.65 | 0.353 |
| Weight | 75.12 ± 17.14 | 78.50 ± 22.37 | 0.277 |
| Height | 174.74 ± 10.04 | 173.32 ± 8.43 | 0.329 |
| BMI | 24.43 ± 4.43 | 26.09 ± 7.03 | 0.074 |
| Disease duration | 5.70 ± 8.85 | 4.56 ± 6.19 | 0.343 |
| EDSS | 2.20 ± 1.32 | 2.34 ± 1.31 | 0.496 |
| Sex (female/male) | 54/33 | 51/28 | 0.750 |
| (62.1/37.9) | (64.6/35.4) | ||
| Type of MS | 0.430 | ||
| RRMS | 77 (87.4) | 64 (81.0) | |
| PPMS | 7 (8) | 11 (13.9) | |
| SPMS | 3 (3.4) | 2 (2.5) | |
| RIS | 1 (1.25) | ||
| CIS | 1 (1.25) | ||
| Marital status | 0.595 | ||
| Married | 32 (36.7) | 21 (26.6) | |
| Partnered | 20 (23) | 17 (21.5) | |
| Single | 26 (29.9) | 24 (30.4) | |
| Divorced | 2(2.3) | 5 (6.3) | |
| Living apart | 1(1.1) | 1 (1.3) | |
| Not available | 6 (6.9) | 11 (13.9) | |
| Children living in the household | 0.542 | ||
| No | 45 (51.7) | 30 (38) | |
| Yes | 32 (36.7) | 29 (36.7) | |
| Not available | 10 (11.5) | 20 (25.3) | |
| School education | 0.336 | ||
| High school degree | 24 (27.6) | 16 (20.3%) | |
| University degree | 39 (44.8%) | 28 (35.4%) | |
| No degree/primary degree | 17 (19.5%) | 23 (29.1%) | |
| Not available | 7(8%) | 12 (15.2) | |
| Employed | 0.171 | ||
| Yes | 74 (85.1) | 59 (74.7) | |
| No | 5 (5.7) | 9 (11.4) | |
| Not available | 8 (9.2) | 11 (13.9) | |
| Smoker | 0.594 | ||
| Yes, currently | 19 (21.8) | 21 (26.6) | |
| No, but in the past | 30 (34.5) | 23 (29.1) | |
| No, never | 32 (36.8) | 24 (30.4) | |
| Not available | 6 (6.9) | 11 (13.9) | |
| EDSS, Expanded Disability Status Scale; BMI, body mass index; MS, multiple sclerosis; RRMS, relapsing remitting multiple sclerosis; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; RIS, Radiologically isolated syndrome; CIS, clinically isolated syndrome. Data given as mean (M) ± standard deviation (SD) for age, disease duration, EDSS and BMI or as total number (n) with percentage (%) for sex, type of MS, marital status, children living in the household, school education, high school degree, employment and smoker status. Statistical analysis was performed by two-tailed student’s t-test for independent samples and Chi-squared Test. | |||
| (B) | |||||
|---|---|---|---|---|---|
| Quality control cohort (n = 87) | Non-follow-up cohort (n = 79) | p-value | |||
| Variables | N | N | |||
| MET | 84 | 26.91 ± 19.71 | 74 | 22.95 ± 20.59 | 0.219 |
| VO2peak kg−1 | 86 | 29.74 ± 7.98 | 70 | 27.44 ± 8.96 | 0.092 |
| Pmax | 86 | 165.73 ± 53.26 | 70 | 151.17 ± 46.77 | 0.075 |
| Lactatepeak | 79 | 6.82 ± 2.33 | 68 | 6.86 ± 2.72 | 0.928 |
| RERpeak | 86 | 1.08 ± 0.06 | 70 | 1.07 ± 0.07 | 0.354 |
| HRmax | 86 | 163.93 ± 20.80 | 70 | 156.74 ± 33.69 | 0.105 |
Performance markers MET, metabolic equivalent of task; VO2peak kg−1 (ml min−1 kg−1), peak oxygen consumtion, Pmax: (Watts) maximal workload, Lactatepeak (mmol l−1); RERmax, maximal peak respiratory exchange ratio; HRmax (beats min−1), maximal heart rate. Data given as mean (M) ± standard deviation (SD). Statistical analysis was performed by two-tailed student’s t-test for independent samples.
PwMS show a reduced physical fitness performance in comparison to non-MS controls
To analyze the physical fitness in our MS cohort, baseline physical fitness was analyzed in all female pwMS of the baseline cohort who performed an ergometry (n = 100), and compared with a female non-MS control group (n = 26) matched for age, weight and height (for baseline demographic data see Table 2A).
Table 2.
Clinical, demographic, sociodemographic (A) and fitness (B) baseline characteristics of the female pwMS and non-MS controls.
| (A) | |||
|---|---|---|---|
| Female pwMS (n = 100) | Female Non-MS Controls (n = 26) | p-value | |
| Age | 38.24 ± 10.78 | 36.65 ± 8.99 | 0.493 |
| Weight | 70.89 ± 14.79 | 65.35 ± 9.31 | 0.072 |
| Height | 169.48 ± 6.54 | 169.04 ± 6.29 | 0.758 |
| BMI | 24.72 ± 5.38 | 22.69 ± 2.69 | 0.065 |
| Disease duration | 7.30 ± 8.53 | n.a | |
| EDSS | 2.28 ± 1.34 | n.a | |
| Sex (female/male) | (100/0) | (26/0) | |
| Type of MS | n.a. | ||
| RRMS | 88 (88) | ||
| PPMS | 8 (8) | ||
| SPMS | 2 (2) | ||
| RIS | 1 (1) | ||
| CIS | 1 (1) | ||
| Marital status | n.a. | ||
| Married | 29 (29) | ||
| Partnered | 27 (27) | ||
| Single | 32 (32) | ||
| Divorced | 4 (4) | ||
| Living apart | 1 (1) | ||
| Not available | 7 (7) | ||
| Children living in the household | n.a. | ||
| No | 47 (47) | ||
| Yes | 35 (35) | ||
| Not available | 18 (18) | ||
| School education | n.a. | ||
| High school degree | 27 (27) | ||
| University degree | 45 (45) | ||
| No degree/primary degree | 21 (21) | ||
| Not available | 7 (7) | ||
| Employed | n.a. | ||
| Yes | 83 (83) | ||
| No | 8 (8) | ||
| Not available | 9 (9) | ||
| Smoker | n.a. | ||
| Yes, currently | 22 (22) | ||
| No, but in the past | 34 (34) | ||
| No, never | 37 (37) | ||
| Not available | 7 (7) |
| (B) | |||||
|---|---|---|---|---|---|
| Female pwMS (n = 100) | Female Non-MS Controls (n = 26) | p-value | |||
| Variables | N | N | |||
| VO2peak kg−1 | 100 | 26.40 ± 7.23 | 26 | 31.56 ± 10.10 | 0.020* |
| Pmax | 100 | 138.86 ± 37.85 | 26 | 191.73 ± 45.25 | < 0.001*** |
| Lactatepeak | 90 | 6.35 ± 2.22 | 26 | 7.44 ± 1.98 | 0.026* |
| RERpeak | 100 | 1.07 ± 0.07 | 26 | 1.17 ± 0.09 | < 0.001*** |
| HRmax | 100 | 157.37 ± 33.75 | 26 | 169.08 ± 17.88 | 0.091 |
Performance markers. Lactatepeak (mmol l−1), VO2peak kg−1 (ml min−1 kg−1), peak oxygen consumtion, Pmax: (Watts) maximal workload; RERmax, maximal peak respiratory exchange ratio; HRmax (beats min−1), maximal heart rate. Data given as mean (M) ± standard deviation (SD); Statistical analysis was performed by two-tailed student’s t-test for independent samples. *p < 0.05, ***p < 0.001.
Female pwMS showed a lower maximal workload (Pmax) (p < 0.001), peak oxygen consumption (VO2peak kg−1) (p = 0.020), peak respiratory exchange ratio (RERpeak) (p < 0.001) and peak blood lactate (Lactatepeak) (p = 0.026) compared to female non-MS controls (Table 2B). Correlation analysis showed that, except of RERpeak, all of the measured performance values of the female MS cohort were negatively associated with the EDSS score (VO2peak kg−1: r = − 0.281, p < 0.01; Pmax: r = − 0.286, p < 0.01; HRmax r = − 0.462, p < 0.01; Lactatepeak r = −0.250, p < 0.05). This indicates that female pwMS show a decreased physical fitness which correlates with the degree of disability measured by EDSS. All additional results of the physical fitness analysis in the MS cohort are provided in Supplementary Tables 1, 2.
The individualized physical activity promotion program increased the physical activity of pwMS
Next, we investigated if the program increased the physical activity level of pwMS. We performed an analysis of the self-rated physical activity levels in the quality control cohort. The mean time between completing the last appointment of the program and answering the questionnaire was 12.38 (±11.34) months.
To validate the self-rated physical activity levels, we analyzed its correlation to the converted MET levels of the short version of the Freiburger questionnaire and two objectively measured assessment parameters during the spiroergometry at baseline. The self-rated activity level at baseline correlated with MET, VO2peak kg−1 and Pmax (MET: r = 0.357, p < 0.01; VO2peak kg−1: r = 0.387, p < 0.01; Pmax r = 0.263, p < 0.05), indicating validity of the self-rated physical activity levels.
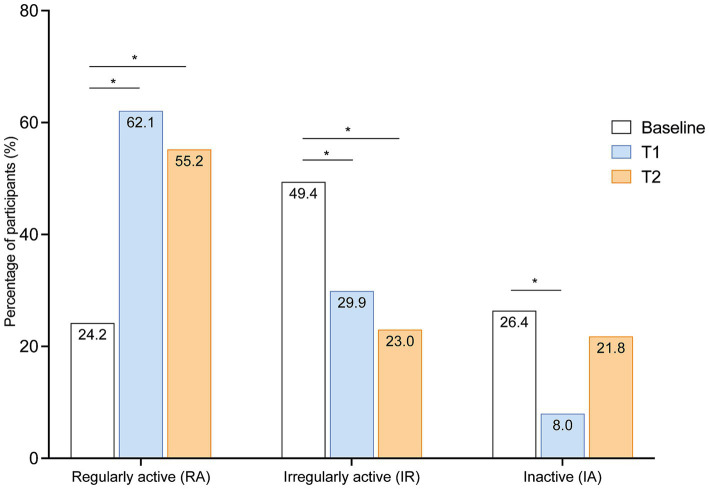
We then compared the self-rated activity levels at baseline, at short-term (T1) and long-term (T2). At baseline most participants (49.4%, n = 43) were irregularly physically active (IR), whereas 24.2% (n = 21) were regularly active (RA) and 26.1% (n = 23) were completely inactive (IA) (Figure 3). In comparison to baseline, the number of participants reporting being regularly active was higher at T1 (62.1%, n = 54; p < 0.001), which persisted until T2 (55.2%, n = 48; p < 0.001, Figure 3). Correspondingly the number of participants reporting being irregularly active was lower at T1 (29.9%, n = 26, p < 0.014) and T2 (23%, n = 20, p < 0.001, Figure 3). At T1, less participants reported being completely inactive than at baseline (8%, n = 7, p < 0.001), however, there was only a trend at T2 in comparison to baseline (21.8%, n = 19, p = 0.523) (Figure 3). These results indicate that the activity program increases physical activity in pwMS in short- and long-term.
Figure 3.
Changes of physical activity levels at short-term and long-term in comparison to baseline. Percentage of different activity levels of pwMS (n = 87) at short- (T1) and long-term (T2) after completing the program in comparison to baseline. Bars show percentage. Statistical analysis was performed by McNemar Test; *p < 0.05.
The individualized physical activity promotion program leads to an increase of physical activity in irregularly active as well as in inactive pwMS
We then further discriminated who of the pwMS maintained their baseline physical activity (“remained regularly active” or “remained irregularly active or inactive”) and who changed their physical activity and became “activated” or “inactivated” at T1 and T2.
Almost all (n = 20, 95.2%) of the participants who were regularly active at baseline (n = 21) also remained regularly active at T1 and T2. Out of the irregularly active or inactive participants at baseline (n = 66) 25.8% (n = 17) remained in this activity stage at T1 and 36.4% (n = 24) at T2, whereas 47 of these participants (71.2%) reported increased physical activity at T1 and 34 participants (51.5%) at T2. In contrast, of the irregularly active and regular active participants at baseline (n = 64), only 3 (4.7%) participants were less physically active at T1 and 9 (14%) at T2 compared to baseline. The increase of participants being activated at T1 or T2 in comparison to participants being inactivated or remaining in their baseline activity stage was statistically significant (T1: p < 0.001; T2: p < 0.001).
Participants who increased their physical activity level at T2 also showed higher MET-values at T2 compared to baseline (p = 0.004) (see Table 3). There was also a trend to higher MET-values at T2 in those who remained regularly active (p = 0.071), suggesting that participants who were already regularly active even increased their frequency and/or intensity of physical activity.
Table 3.
Metabolic equivalents (MET) per week of the different subgroups of the quality control cohort at T2.
| Baseline | T2 | ||||||
|---|---|---|---|---|---|---|---|
| N | Median | Range | N | Median | Range | p-value | |
| Quality control cohort | 84 | 23.90 | 101.5 | 86 | 27.78 | 134.65 | 0.001 |
| Group 1: remained RA | 20 | 35.28 | 94.85 | 19 | 44.40 | 121.72 | 0.071 |
| Group 1: remained IA/I | 23 | 22.78 | 58.91 | 24 | 20.78 | 85.60 | 0.858 |
| Group 2: activated | 32 | 19.22 | 78.0 | 34 | 27.71 | 100.13 | 0.004 |
| Group 3: inactivated | 9 | 23.95 | 42.08 | 9 | 22.05 | 77.34 | 0.859 |
RA, regularly active; IA, irregularly active; I, inactive. N = numbers of available MET values per group at respective timepoint. Data are presented as median and range.
These results indicate that both groups—irregularly active and inactive pwMS - were activated by the activity program. However, as some participants were not activated or only activated in the short-term, we investigated possible reasons. No significant differences were found between subjects of the four subgroups on any of the demographic or clinical baseline characteristics (see Supplementary Table 3). Furthermore, no difference regarding the time period between participation in the activity program and the time of quality control was detected between the four groups (p = 0.791). To evaluate the perception of the activity program by pwMS, an evaluative questionnaire was provided. The activity program was assessed with a mean score of 1.92 ± 0.97 (Likert Scale 1–6) (n = 87). Most participants considered the activity program as a strong (n = 55, 63.2%) or moderate (n = 27, 31%) impulse to engage with physical activity in MS. No differences in the evaluation of the program were found between the four subgroups (see Supplementary Table 4).
The individualized physical activity promotion program increases the intrinsic self-concordance and decreases the perceived barriers in the activated participants
In order to determine whether the physical activity changes of the participants are linked to behavioral determinants, we analyzed differences of behavioral determinants between the four subgroups at baseline and within each group from baseline to T2.
At baseline, there were no differences between the four groups regarding the extrinsic, identified and introjected self-efficacy values and the social support they received. However, the intrinsic self-efficacy, the average rate of perceived barriers and the number of applied counterstrategies to overcome situational barriers differed significantly between the “remaining regularly active” and the other three groups at baseline (Table 4) (only the behavioral determinants with significant group differences at baseline are shown).
Table 4.
Differences of behavioral determinants between the different subgroups of the quality control cohort at baseline.
| Behavioral determinants at baseline | Remained RA (N = 20) | Remained IA/I (N = 24) | Activated (N = 34) | Inactivated (N = 9) | p-value |
|---|---|---|---|---|---|
| Intrinsic Self-concordance |
3.67 (2) | 2.67 (2.67) | 2.67 (3) | 3.0 (1.67) | 0.001 |
| Extrinsic Self-concordance |
1.67 (1.67) | 1.67 (1.33) | 1.67 (2.0) | 1.0 (1.33) | 0.339 |
| Introjected Self-concordance |
2.83 (2.67) | 3.0 (2.67) | 2.67 (2.67) | 2.5 (2.0) | 0.659 |
| Identified Self-concordance |
4.0 (1.0) | 3.67 (1.33) | 4.0 (1.67) | 3.67 (1.33) | 0.054 |
| Barriers | 1.53 (1.2) | 1.97(1.0) | 1.90 (1.4) | 1.95(1.1) | 0.006 |
| Counterstrategies | 11.5 (10.0) | 8.0 (12.0) | 8.0(11.0) | 8.0 (9.0) | 0.025 |
| Social support | 2.07 (2.57) | 2.29 (2.86) | 2.14 (2.14) | 1.86 (2.14) | 0.352 |
RA, regularly active; IA, irregularly active; I, inactive. N = numbers of available behavioral model results per group at respective timepoint. Data are presented as median and range. **p < 0.01, *p < 0.05, p, Kruskal–Wallis-Test.
From baseline to T2, intrinsic self-concordance increased among “activated” participants after attendance of the program (p = 0.002) (Table 5), whereas the extrinsic self-concordance declined in the “remaining regularly active” (p = 0.007). Introjected and identified self-concordance did not change significantly in any group. The average number of perceived barriers declined in the overall group and the “activated” group (p = 0.006). In the “remained regularly active” and the “remained irregularly active/inactive” the average amount of applied counterstrategies decreased from baseline to T2 (p = 0.041 and p = 0.004 respectively). Changes in the received social support from baseline to T2 were not detected in any group (Table 5).
Table 5.
Changes of behavioral determinants intrinsic self-concordance identified self-concordance, introjected self-concordance, extrinsic self-concordance, barriers, counterstrategies and social support of different subgroups of the quality control cohort from baseline to T2.
| Baseline | T2 | ||||||
|---|---|---|---|---|---|---|---|
| N | Median | Range | N | Median | Range | p-value | |
| Intrinsic self-concordance | |||||||
| Quality control cohort | 80 | 3.0 | 3.0 | 84 | 3.0 | 3.0 | 0.139 |
| Group 1: remained RA | 20 | 3.66 | 2 | 19 | 3.33 | 3 | 0.145 |
| Group 1: remained IR/IA | 21 | 2.67 | 2.67 | 23 | 2.33 | 3.0 | 0.550 |
| Group 2: activated | 30 | 2.67 | 3 | 33 | 3.0 | 2.67 | 0.002 ** |
| Group 3: inactivated | 9 | 3.0 | 1.67 | 9 | 2.67 | 1.67 | 0.327 |
| Extrinsic self-concordance | |||||||
| Quality control cohort | 80 | 1.5 | 2.0 | 84 | 1.33 | 3.0 | 0.157 |
| Group 1: remained RA | 20 | 1.67 | 1.67 | 19 | 1.0 | 0.67 | 0.007 ** |
| Group 1: remained IR/IA | 21 | 1.67 | 1.33 | 23 | 1.33 | 2.0 | 1.00 |
| Group 2: activated | 30 | 1.67 | 2.0 | 33 | 1.67 | 3.0 | 0.632 |
| Group 3: inactivated | 9 | 1.0 | 1.33 | 9 | 1.33 | 1.0 | 0.595 |
| Introjected self-concordance | |||||||
| Quality control cohort | 80 | 2.67 | 3.0 | 84 | 2.67 | 3.0 | 0.410 |
| Group 1: remained RA | 20 | 2.83 | 2.67 | 19 | 2.33 | 2.33 | 0.059 |
| Group 1: remained IR/IA | 21 | 3.0 | 2.67 | 23 | 3.0 | 3.0 | 0.674 |
| Group 2: activated | 30 | 2.67 | 2.67 | 33 | 2.67 | 3.0 | 0.082 |
| Group 3: inactivated | 9 | 2.5 | 2.0 | 9 | 3.0 | 2.33 | 0.054 |
| Identified self-concordance | |||||||
| Quality control cohort | 80 | 4.0 | 1.67 | 83 | 4.0 | 2.33 | 0.957 |
| Group 1: remained RA | 20 | 4.0 | 1 | 19 | 4.0 | 1.33 | 0.617 |
| Group 1: remained IR/IA | 21 | 3.67 | 1.33 | 23 | 3.67 | 2.33 | 0.679 |
| Group 2: activated | 30 | 4.0 | 1.67 | 32 | 4.0 | 1.33 | 0.916 |
| Group 3: inactivated | 9 | 3.67 | 1.33 | 9 | 3.67 | 1.0 | 0.589 |
| Barriers | |||||||
| Quality control cohort | 82 | 1.87 | 1.37 | 86 | 1.71 | 1.74 | 0.005 ** |
| Group 1: remained RA | 20 | 1.53 | 1.2 | 19 | 1.47 | 1.0 | 0.073 |
| Group 1: remained IR/IA | 22 | 1.97 | 1.0 | 24 | 1.84 | 1.2 | 0.661 |
| Group 2: activated | 31 | 1.90 | 1.4 | 34 | 1.68 | 1.7 | 0.006 ** |
| Group 3: inactivated | 9 | 1.95 | 1.1 | 9 | 2.05 | 1.1 | 0.484 |
| Counterstrategies | |||||||
| Quality control cohort | 82 | 8.0 | 12.0 | 86 | 8.0 | 15.0 | 0.072 |
| Group 1: remained RA | 20 | 11.5 | 10.0 | 19 | 9.0 | 10.0 | 0.041 * |
| Group 1: remained IR/IA | 22 | 8.0 | 12.0 | 24 | 5.50 | 13.0 | 0.004 ** |
| Group 2: activated | 31 | 8.0 | 11.0 | 34 | 9.0 | 15.0 | 0.061 |
| Group 3: inactivated | 9 | 8.0 | 9.0 | 9 | 7.0 | 10.0 | 0.306 |
| Social support | |||||||
| Quality control cohort | 82 | 2.14 | 2.86 | 87 | 2.29 | 2.86 | 0.742 |
| Group 1: remained RA | 20 | 2.07 | 2.57 | 20 | 2.43 | 2.43 | 0.549 |
| Group 1: remained IR/IA | 22 | 2.29 | 2.86 | 24 | 2.00 | 2.43 | 0.185 |
| Group 2: activated | 31 | 2.14 | 2.14 | 34 | 2.43 | 2.86 | 0.592 |
| Group 3: inactivated | 9 | 1.86 | 2.14 | 9 | 2.0 | 1.86 | 0.635 |
RA, regularly active; IA, irregularly active; I, inactive. N = numbers of available behavioral model results per group at respective timepoint. Data are presented as median and range. **p < 0.01, *p < 0.05, p, McNemar-Test.
Discussion
This study was initiated as a retrospective analysis of our individualized physical activity promotion program for pwMS. PwMS are less physically active than the general population and show reduced physical fitness (3), which was confirmed by our study. Compared to our non-MS control cohort and healthy control cohorts from the literature (43, 44, 54–60) our minor disabled cohort showed lower physical fitness in all assessments.
As the female non-MS control group was matched to the MS-cohort regarding age, weight and height, the difference in the spiroergometric peak performance values cannot be explained by these variables. In addition, the participants of our female MS-group were not able to reach the age and weight adjusted peak performance values predicted for healthy untrained subjects in the literature (58, 59, 61). According to the defined thresholds for HRmax and RERmax (58, 62, 63), we can suppose the majority of our MS-cohort was at least approaching respiratory exhaustion as the mean of these values were above or close to the thresholds. We found an association between EDSS and peak blood lactate levels, Pmax, VO2max and HRmax, which confirms findings from other studies showing a link between the reduced aerobic capacity in pwMS and disease severity (31, 60, 64). Our findings underscore the necessity of establishing programs motivating pwMS to engage in physical activity.
Participation in our program resulted in an overall increase of self-rated physical activity in pwMS with different baseline physical activity levels. While almost all participants who were regularly active at baseline maintained or even increased their physical activity in short and long-term, the percentage of activated participants who were irregularly active or inactive at baseline was higher in short- than in long-term, which could be explained by different aspects. The participants who were already regularly active at baseline possibly benefitted much from the detailed physical assessment results which refined and specialized the training recommendation at baseline, resulting as a fine-tuning of previous training settings. Those participants who were physically inactive or irregularly active at baseline possibly experienced more benefit from general advice and encouragement to be physically active than from detailed physical assessment results. A recent survey study of our research group indicates that self-efficacy and motivation are major health psychological drivers for physical activity (25). The evaluation of the actual activity program showed that the majority of participants perceived the program as an impulse to engage in physical activity, supposing that intention and motivation could be enhanced. As action planning is considered a key behavior change and adherence technique (25, 65) this idea was implemented in our activity program in the form of an individual plan that was provided to each participant. This plan included time schedules, locations and different kinds of recommended physical activities. In the evaluation of the activity program, the provided training recommendations were rated as helpful in regard of “individuality” and “concreteness” so that we assume that the planning of physical activity succeeded. Moreover, our study provides evidence that the activity program leads to changes in self-determined motivational variables (intrinsic self-concordance) (25). This is in line with a small study which could show that an individual exercise intervention could stabilize self-efficacy and increase exercise motivation in pwMS (66). Furthermore, in our study physical activation was associated with a reduction in perceived barriers. The relevance of barriers on long-term physical activity has been shown as well in earlier studies (19, 27, 67).
Exercise adherence remains a major complex issue in the general population as well as in pwMS (11, 68) and the main driving factors are still unclear (69). Behavioral interventions targeting behavioral determinants of physical activity successfully increased physical activity in pwMS (1, 23, 68) and are presumed to have possible long-term effects (25) but data beyond 6 months are largely lacking. In our study we also assessed the desires and needs of participants, which should be considered for refinements of the program to achieve long-term adherence. A frequently mentioned suggestion for improvement was the implementation of follow-up checks of the physical fitness. Studies including follow-up phone calls or the completion of activity logs into physical activity programs have shown beneficial effects (69) and could be implemented in future programs. It is conceivable that a repeated physical assessment could increase motivation to maintain improved physical activity behavior at least until follow-up and thereby increase maintenance self-efficacy. Follow-up interviews could also be helpful to identify possible reasons for decreases in physical activity or could possibly motivate pwMS to resume after a physical activity break (recovery self-efficacy).
Additionally, many participants would welcome a more intense practice of recommended exercises together with an expert at the last appointment. As supervised training generally provides better results than non-supervised training (70), we need to clarify which kind and amount of supervision is needed. Furthermore, many pwMS requested an MS sports group, which would probably have an effect on behavioral determinants such as social support.
There are some limitations in this study, one is the dropout rate. From 166 participants who started the activity program, 30 participants dropped out in the beginning and 49 did not answer the quality control questionnaires. The reasons for study drop-outs can be diverse and can be related to adverse events, study related factors or can lie in the exercise intervention itself (69). In our study, pwMS who dropped out (non-follow-up cohort) showed no difference in baseline characteristics and fitness levels compared to the adherent group (quality control cohort). Thus, it cannot be assumed that the dropouts were less physically active at baseline. Thus, it cannot be assumed that the dropouts were less physically active at baseline. However, people who were activated by the program might have responded more frequently. Further aspects of the activity program, e.g., expenditure of time, could have been a reason for the high dropout rate.
Furthermore, the size of our non-MS control group was small and consisted of only females. However, the spiroergometric performance values of our non-MS-cohort is in line with reference values in the literature (58, 59, 61). Another limitation is that people with a specific interest in the topic, here physical activity, are more motivated to participate which might create a selection bias. Though, in our study participants showed different physical activity levels. Additionally, the analysis was largely based on self-rated levels of physical activity which might be biased by wrong self-perception. However, the self-rated levels at baseline correlated with objectively measured values at baseline. While the few former studies in MS (71, 72) have claimed a good correlation of self-reported and objective PA levels studies in the general PA literature investigating the correlation of self-reported and objectively measured PA and physical fitness are inconsistent and partially demonstrate a low validity of self-reported physical activity.
Furthermore, in perspective of longer follow-ups possibly addressing disease evolution the impact of disease modifying drugs (DMDs) needs to be considered. Information on DMT usage or change was not available in the current study. The goal of this study was not to assess if the individualized activity promotion program improves classical MS outcomes but rather to assess if it improves the physical activity in pwMS which we could show.
In summary, our activity program was perceived as informative, individualized and motivating by pwMS and led to an increased short- and long-term physical activity level. This effect might be based on the increased intrinsic self-concordance and the decrease of perceived barriers. These behavioral determinants are considered as important prerequisites for increasing physical activity in pwMS (68, 70).
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. SCR was supported by an Advanced Clinician–Scientist Fellowship from the BMBF (iSTAR, 01EO2106) and the Hertie Network of Excellence in Clinical Neuroscience of the Gemeinnützige Hertie-Stiftung. We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethikkomission der Ärztekammer Hamburg. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EV: Writing – original draft, Investigation, Formal analysis, Data curation. SP: Writing – review & editing, Data curation. KR-L: Writing – review & editing. KK: Writing – review & editing. KF-S: Data curation, Writing – review & editing. GW: Data curation, Conceptualization, Writing – review & editing. NK: Writing – review & editing. CH: Writing – original draft, Supervision, Project administration, Funding acquisition, Conceptualization, Writing – review & editing. SR: Writing – original draft, Project administration, Conceptualization.
Conflict of interest
CH has received research grants, speaker honoraria and travel grants from Biogen, Celgene, Genzyme, Merck, and Roche, all outside of this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1428712/full#supplementary-material
References
- 1.Motl RW, Dlugonski D, Pilutti LA, Klaren RE. Does the effect of a physical activity behavior intervention vary by characteristics of persons with multiple sclerosis? Int J MS Care. (2014) 17:140903145137009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veldhuijzen van Zanten JJ, Pilutti LA, Duda JL, Motl RW. Sedentary behaviour in people with multiple sclerosis: is it time to stand up against MS? Mult Scler. (2016) 22:1250–6. doi: 10.1177/1352458516644340, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. (2017) 17:185–5. doi: 10.1186/s12883-017-0960-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as medicine in multiple sclerosis—time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. (2019) 19:88. doi: 10.1007/s11910-019-1002-3, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Latimer-Cheung AE, Martin Ginis KA, Hicks AL, Motl RW, Pilutti LA, Duggan M, et al. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch Phys Med Rehabil. (2013) 94:1829–36. doi: 10.1016/j.apmr.2013.05.015, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Gunn H, Markevics S, Haas B, Marsden J, Freeman J. Systematic review: the effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis. Arch Phys Med Rehabil. (2015) 96:1898–912. doi: 10.1016/j.apmr.2015.05.018, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Latimer-Cheung AE, Pilutti LA, Hicks AL, Martin Ginis KA, Fenuta AM, MacKibbon KA, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. (2013) 94:1800–28. doi: 10.1016/j.apmr.2013.04.020, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. (1996) 39:432–41. doi: 10.1002/ana.410390405, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil. (2015) 96:1339–48. doi: 10.1016/j.apmr.2015.02.011, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Kjølhede T, Siemonsen S, Wenzel D, Stellmann JP, Ringgaard S, Pedersen BG, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. (2018) 24:1356–65. doi: 10.1177/1352458517722645, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Briken S, Gold SM, Patra S, Vettorazzi E, Harbs D, Tallner A, et al. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Mult Scler. (2014) 20:382–90. doi: 10.1177/1352458513507358, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Sandroff BM, Motl RW, Scudder MR, DeLuca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. (2016) 26:271–94. doi: 10.1007/s11065-016-9324-2, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Heesen C, Rosenkranz SC. Physical exercise in multiple sclerosis is not just a symptomatic therapy, it has a disease-modifying effect: no. Mult Scler. (2022) 28:861–2. doi: 10.1177/13524585211064968 [DOI] [PubMed] [Google Scholar]
- 14.Dalgas U, Stenager E, Hvid LG. Physical exercise in multiple sclerosis is not just a symptomatic therapy, it has a disease-modifying effect: commentary. Mult Scler. (2022) 28:863–4. doi: 10.1177/13524585211072702 [DOI] [PubMed] [Google Scholar]
- 15.Motl RW, Sandroff BM. Physical exercise in multiple sclerosis is not just a symptomatic therapy: it has a disease-modifying effect-yes. Mult Scler. (2022) 28:859–61. doi: 10.1177/13524585211061651 [DOI] [PubMed] [Google Scholar]
- 16.Torres-Costoso A, Martínez-Vizcaíno V, Reina-Gutiérrez S, Álvarez-Bueno C, Guzmán-Pavón MJ, Pozuelo-Carrascosa DP, et al. Effect of exercise on fatigue in multiple sclerosis: a network Meta-analysis comparing different types of exercise. Arch Phys Med Rehabil. (2022) 103:970–987.e18. doi: 10.1016/j.apmr.2021.08.008, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Herring MP, Fleming KM, Hayes SP, Motl RW, Coote SB. Moderators of exercise effects on depressive symptoms in multiple sclerosis: a meta-regression. Am J Prev Med. (2017) 53:508–18. doi: 10.1016/j.amepre.2017.04.011, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Edwards T, Michelsen AS, Fakolade AO, Dalgas U, Pilutti LA. Exercise training improves participation in persons with multiple sclerosis: a systematic review and meta-analysis. J Sport Health Sci. (2022) 11:393–402. doi: 10.1016/j.jshs.2021.07.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. (2016) 38:1227–42. doi: 10.3109/09638288.2015.1077397, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Flachenecker P, Bures AK, Gawlik A, Weiland AC, Kuld S, Gusowski K, et al. Efficacy of an internet-based program to promote physical activity and exercise after inpatient rehabilitation in persons with multiple sclerosis: a randomized, single-blind, controlled study. Int J Environ Res Public Health. (2020) 17:544. doi: 10.3390/ijerph17124544, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silveira SL, Huynh T, Kidwell A, Sadeghi-Bahmani D, Motl RW. Behavior change techniques in physical activity interventions for multiple sclerosis. Arch Phys Med Rehabil. (2021) 102:1788–800. doi: 10.1016/j.apmr.2021.01.071, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinstein A, Amato MP, Brichetto G, Chataway J, Chiaravalloti ND, Cutter G, et al. Cognitive rehabilitation and aerobic exercise for cognitive impairment in people with progressive multiple sclerosis (CogEx): a randomised, blinded, sham-controlled trial. Lancet Neurol. (2023) 22:912–24. doi: 10.1016/S1474-4422(23)00280-6, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Dlugonski D, Motl RW, Mohr DC, Sandroff BM. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: sustainability and secondary outcomes. Psychol Health Med. (2012) 17:636–51. doi: 10.1080/13548506.2011.652640, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Daniel N, Bruns I, Casey B, Coote S, Daubmann A, Heesen C, et al. "Activity matters was great - I now realize: if I move, I'm fitter.": development and process evaluation of a web-based program for persons with multiple sclerosis. Disabil Rehabil. (2023) 24:1–10. doi: 10.1080/09638288.2023.2269845, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Riemann-Lorenz K, Motl RW, Casey B, Coote S, Daubmann A, Heesen C, et al. Possible determinants of long-term adherence to physical activity in multiple sclerosis-theory-based development of a comprehensive questionnaire and results from a German survey study. Disabil Rehabil. (2020) 43:1–14. [DOI] [PubMed] [Google Scholar]
- 26.Huynh TLT, Silveira SL, Motl RW. Physical activity behavior in persons newly diagnosed with multiple sclerosis: applying the capability - opportunity - motivation - behavior (COM-B) model. Mult Scler Relat Disord. (2023) 69:104432. doi: 10.1016/j.msard.2022.104432, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Ploughman M. Breaking down the barriers to physical activity among people with multiple sclerosis – a narrative review. Phys Ther Rev. (2017) 22:124–32. doi: 10.1080/10833196.2017.1315212 [DOI] [Google Scholar]
- 28.Correale L, Martinis L, Tavazzi E, Pedullà L, Mallucci G, Brichetto G, et al. Barriers to exercise and the role of general practitioner: a cross-sectional survey among people with multiple sclerosis. Front Neurol. (2022) 13:1016143. doi: 10.3389/fneur.2022.1016143, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moumdjian L, Smedal T, Arntzen EC, van der Linden ML, Learmonth Y, Pedullà L, et al. Impact of the COVID-19 pandemic on physical activity and associated technology use in persons with multiple sclerosis: an international RIMS-SIG mobility survey study. Arch Phys Med Rehabil. (2022) 103:2009–15. doi: 10.1016/j.apmr.2022.06.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motl RW, Pekmezi D, Wingo BC. Promotion of physical activity and exercise in multiple sclerosis: importance of behavioral science and theory. Mult Scler J Exp Transl Clin. (2018) 4:2055217318786745. doi: 10.1177/2055217318786745, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heesen C, Romberg A, Gold S, Schulz KH. Physical exercise in multiple sclerosis: supportive care or a putative disease-modifying treatment. Expert Rev Neurother. (2006) 6:347–55. doi: 10.1586/14737175.6.3.347 [DOI] [PubMed] [Google Scholar]
- 32.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 33.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 34.Frey I, Berg A, Grathwohl D, Keul J. Freiburger Fragebogen zur körperlichen Aktivität-Entwicklung. Prüfung und Anwendung Sozial- und Präventivmedizin. (1999) 44:55–64. doi: 10.1007/BF01667127, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Fuchs R, Seelig H. Messung der sport-und bewegungsbezogenen Selbstkonkordanz In: Stiller J, Alfermann D, editors. Zeitschrift für Sportpsychologie. Frankfurt: Hofgrefe Verlag; (2006). 121–39. [Google Scholar]
- 36.Fuchs R., Göhner W., Mahler C., Krämer L., Wanner H., Wehrstein S. (2008). Endbericht zum forschungsvorhaben, aufbau eines körperlich-aktiven lebensstils im kontext der medizinischen rehabilitation: ein motivational-volitionales interventionskonzept. MoVo-LISA Projekt.
- 37.Fuchs R. Psychologie und körperliche Bewegung: Grundlage für theoriegeleitete Interventionen. Göttingen: Verlag für Psychologie, Hofgrefe; (1997). [Google Scholar]
- 38.Krämer LUFR. Barrieren und barrieremanagement im prozess der sportteilnahme. Zeitschrift für Gesundheitspsychologie. (2010) 18:170–82. doi: 10.1026/0943-8149/a000026 [DOI] [Google Scholar]
- 39.Troiano . Wear time validation parameters. (2022) Available at: https://actigraphcorp.force.com/support/s/article/Troiano-2007-Wear-Time-Validation-Parameters
- 40.Colley R, Connor Gorber S, Tremblay MS. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep. (2010) 21:63–9. PMID: [PubMed] [Google Scholar]
- 41.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc. (1998) 30:777–81. doi: 10.1097/00005768-199805000-00021, PMID: [DOI] [PubMed] [Google Scholar]
- 42.ActiGraph . What’s the difference among the cut points available in ActiLife? (2022) Available at: https://actigraphcorp.my.site.com/support/s/article/What-s-the-difference-among-the-Cut-Points-available-in-ActiLife.
- 43.Bohannon RW, Bubela DJ, Magasi SR, Wang YC, Gershon RC. Sit-to-stand test: performance and determinants across the age-span. Isokinet Exerc Sci. (2010) 18:235–40. doi: 10.3233/IES-2010-0389, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilscher H, Gruber D, Lembert S, Raschner C. Auswirkungen von Beeinträchtigungen am Bewegungsapparat auf das Ergebnis des S3-Körperstabilitätstests. Man Med. (2007) 45:409–14. doi: 10.1007/s00337-007-0558-1 [DOI] [Google Scholar]
- 45.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 46.Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. (2012) 18:914–24. doi: 10.1177/1352458512444498 [DOI] [PubMed] [Google Scholar]
- 47.Smith S, Madden AM. Body composition and functional assessment of nutritional status in adults: a narrative review of imaging, impedance, strength and functional techniques. J Hum Nutr Diet. (2016) 29:714–32. doi: 10.1111/jhn.12372 [DOI] [PubMed] [Google Scholar]
- 48.DIERS . Ganzheitliche muskelkraftmessung: DIERS myoline. (2022) Available at: https://diers.eu/de/produkte/muskelkraftmessung/diers-myoline/
- 49.Kyle UG, Bosaeus I, de Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. (2004) 23:1226–43. doi: 10.1016/j.clnu.2004.06.004, PMID: [DOI] [PubMed] [Google Scholar]
- 50.ActiGraph . Actigraph wGT3X-BT. (2022) Available at: https://actigraphcorp.com/actigraph-wgt3x-bt/
- 51.Borg G. (1998). Borg's perceived exertion and pain scales.
- 52.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. (2000) 32:S498–516. doi: 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 53.Pembury Smith MQR, Ruxton GD. Effective use of the McNemar test. Behav Ecol Sociobiol. (2020) 74:133. doi: 10.1007/s00265-020-02916-y [DOI] [Google Scholar]
- 54.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. (1999) 14:270–4. doi: 10.1034/j.1399-3003.1999.14b06.x [DOI] [PubMed] [Google Scholar]
- 55.Phan-Ba R, Pace A, Calay P, Grodent P, Douchamps F, Hyde R, et al. Comparison of the timed 25-foot and the 100-meter walk as performance measures in multiple sclerosis. Neurorehabil Neural Repair. (2011) 25:672–9. doi: 10.1177/1545968310397204, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. (2014) 9:e113637. doi: 10.1371/journal.pone.0113637, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosy-Westphal A, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr. (2006) 30:309–16. doi: 10.1177/0148607106030004309 [DOI] [PubMed] [Google Scholar]
- 58.Rost R. Lehrbuch der sportmedizin. 1st ed. Köln: Deutscher Ärzte-Verlag; (2001). [Google Scholar]
- 59.Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. (2001) 104:1694–740. doi: 10.1161/hc3901.095960 [DOI] [PubMed] [Google Scholar]
- 60.Schlüter K, Maier J, Patra S, Gold SM, Heesen C, Schulz KH. Aberrant peak lactate response in MS. NeuroRehabilitation. (2017) 41:811–22. doi: 10.3233/NRE-172182 [DOI] [PubMed] [Google Scholar]
- 61.Kenney WL, Wilmore JH, Costill DL. Physiology of sport and exercise. 5th ed. Champaign, IL: Human Kinetics; (2012). [Google Scholar]
- 62.Löllgen HEE, Gitt A. Ergometrie. 3rd ed. Heidelberg: Springer Verlag; (2010). [Google Scholar]
- 63.Such U.M.T. Die maximale herzfrequenz. (2010) Available at: https://www.germanjournalsportsmedicine.com/archive/archive-2010/heft-12/die-maximale-herzfrequenz/
- 64.Langeskov-Christensen M, Heine M, Kwakkel G, Dalgas U. Aerobic capacity in persons with multiple sclerosis: a systematic review and meta-analysis. Sports Med. (2015) 45:905–23. doi: 10.1007/s40279-015-0307-x, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Milne S, Orbell S, Sheeran P. Combining motivational and volitional interventions to promote exercise participation: protection motivation theory and implementation intentions. Br J Health Psychol. (2002) 7:163–84. doi: 10.1348/135910702169420, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Geertz W, Dechow AS, Patra S, Heesen C, Gold SM, Schulz KH. Changes of motivational variables in patients with multiple sclerosis in an exercise intervention: associations between physical performance and motivational determinants. Behav Neurol. (2015) 2015:248193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riemann-Lorenz K, Wienert J, Streber R, Motl RW, Coote S, Heesen C. Long-term physical activity in people with multiple sclerosis: exploring expert views on facilitators and barriers. Disabil Rehabil. (2020) 42:3059–71. doi: 10.1080/09638288.2019.1584253, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Heesen C, Bruce J, Gearing R, Moss-Morris R, Weinmann J, Hamalainen P, et al. Adherence to behavioural interventions in multiple sclerosis: follow-up meeting report (AD@MS-2). Mult Scler J Exp Transl Clin. (2015) 1:2055217315585333. doi: 10.1177/2055217315585333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dennett R, Madsen LT, Connolly L, Hosking J, Dalgas U, Freeman J. Adherence and drop-out in randomized controlled trials of exercise interventions in people with multiple sclerosis: a systematic review and meta-analyses. Mult Scler Relat Disord. (2020) 43:102169. doi: 10.1016/j.msard.2020.102169, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Kalb R, Brown TR, Coote S, Costello K, Dalgas U, Garmon E. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler J. (2020) 26:135245852091562. doi: 10.1177/1352458520915629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Commun Health. (2007) 30:144–50. doi: 10.1097/01.FCH.0000264411.20766.0c [DOI] [PubMed] [Google Scholar]
- 72.Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. (2006) 28:1151–6. doi: 10.1080/09638280600551476 [DOI] [PubMed] [Google Scholar]
- 73.Poole DC, Rossiter HB, Brooks GA, Gladden LB. The anaerobic threshold: 50+ years of controversy. J Physiol. (2021) 599:737–67. doi: 10.1113/JP279963, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.