Abstract
Background
The causality between neuroticism, a personality trait characterized by the tendency to experience negative emotions, and female reproductive diseases remains unclear. To provide evidence for the development of effective screening and prevention strategies, this study employed Mendelian randomization (MR) to investigate the causality between neuroticism clusters and female reproductive diseases.
Methods
Instrumental variables were obtained from large-scale genome-wide association studies of populations of European descent involving three neuroticism clusters (depressed affect, worry, sensitivity to environmental stress, and adversity [SESA]) in the Complex Trait Genetics database and six female reproductive diseases (infertility, polycystic ovary syndrome [PCOS], spontaneous abortion, recurrent spontaneous abortion, endometriosis, and uterine fibroids) in the FinnGen database. The bidirectional two-sample MR analysis was conducted using the inverse variance-weighted, weighted median, and MR-Egger methods, whereas the sensitivity analysis was conducted using the Cochran’s Q-test, MR-Egger intercept, and leave-one-out analysis.
Results
In the forward analysis, genetically predicted depressed affect and worry components of neuroticism significantly increased the risk of infertility (depressed affect: odds ratio [OR] = 1.399, 95% confidence interval [CI]: 1.054–1.856, p = 0.020; worry: OR = 1.587, 95% CI: 1.229–2.049, p = 0.000) and endometriosis (depressed affect: OR = 1.611, 95% CI: 1.234–2.102, p = 0.000; worry: OR = 1.812, 95% CI: 1.405–2.338, p = 0.000). Genetically predicted SESA component of neuroticism increased only the risk of endometriosis (OR = 1.524, 95% CI: 1.104–2.103, p = 0.010). In the reverse analysis, genetically predicted PCOS was causally associated with an increased risk of the worry component of neuroticism (Beta = 0.009, 95% CI: 0.003–0.016, p = 0.003).
Conclusions
The MR study showed that the three neuroticism personality clusters had definite causal effects on at least one specific female reproductive disease. Moreover, PCOS may increase the risk of the worry component of neuroticism. This finding suggests the need to screen for specific female reproductive diseases in populations with high neuroticism and assess the psychological status of patients with PCOS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-024-03347-x.
Keywords: Neuroticism personality clusters, Female reproductive diseases, Mendelian randomization, Causality
Background
Neuroticism is a personality trait characterized by a greater tendency to experience negative emotions [1]. Individuals with high neuroticism experience more stressful life events, exaggerated stress responses, and have longer recovery times. Therefore, they tend to have stronger emotional responses, poorer emotional coping skills, and worse emotional experiences [2–4]. Neuroticism is measured using the short form of the Eysenck Personality Questionnaire [5], 12 items in the questionnaire are used to evaluate the neuroticism level, the higher the total score, the higher the neuroticism level. By conducting hierarchical clustering analyses, Nagel et al. [6, 7] identified three genetically distinct neuroticism clusters: “depressed affect”, “worry” and “sensitivity to environmental stress and adversity (SESA)”. More precise causative factors and disease outcomes can be identified by decomposing the complex traits of neuroticism into genetically homogeneous clusters. Increasing evidence suggests that neuroticism is associated with a wide range of adverse health outcomes, including mental disorders [8, 9], cerebrovascular and cardiovascular diseases [10], endocrine disorders [11], and cancer [12]. Neuroticism is a robust and highly heritable personality trait [13], however, some studies have shown that the stability of neuroticism decreases gradually over time [14], and that personality traits can be modified through interventions [15]. This provides the possibility of intervening in neuroticism levels for the prevention and treatment of diseases mediated by it.
With societal development and the advancement of the status of women, female reproductive diseases have received increasing attention. However, the causes of many female reproductive diseases are complex and undefined, while treatment options are limited and mostly symptomatic, placing a heavy burden on global health systems. Therefore, exploring the causes and effective interventions for female reproductive diseases has become a focus of research in recent years. Many studies have provided important evidence of the correlation between neuroticism and female reproductive diseases: Regardless of whether they undergo in vitro fertilization treatment, infertile women have significantly higher neuroticism scores [16], and neuroticism is an independent predictor of depression and anxiety in women who are unable to conceive after In vitro fertilization treatment [17]. Women with polycystic ovary syndrome (PCOS) have higher levels of neuroticism and greater difficulty coping with stress than those without PCOS [18, 19]. Patients with endometriosis have elevated neuroticism, anxiety, and psychiatric morbidity scores relative to healthy individuals [20]. A low neuroticism score was reportedly associated with high natural killer cell activity among women with recurrent spontaneous abortion (RSA), however, an increased pre-conceptual level of natural killer cell activity was more likely to lead to pregnancy loss during the next pregnancy [21]. Although these observational studies have provided ample evidence of the correlation between neuroticism and female reproductive diseases, they have some inevitable defects, such as residual confounding and reverse causality [22]. Therefore, the causality between neuroticism and female reproductive diseases has not been clearly established, and methods free of confounding factors and reverse causation should be used to re-examine these correlations carefully. Thus, further studies are necessary to elucidate the causality between neuroticism and female reproductive diseases.
Mendelian randomization (MR) is an analytical method used to determine causality. The fundamental principle of MR is to use genetic variants that are closely associated with exposure factors as instrumental variables (IVs) to estimate the causality between exposure factors and disease outcomes [23]. Since genetic variants are randomly assigned at conception and cannot be altered by subsequent occurrences of the disease, MR studies are less susceptible to confounding factors and reverse causation. Therefore, here we conducted a two-sample bidirectional MR analysis using publicly available data from the genome-wide association study (GWAS) to explore the potential causality between three neurotic clusters and six common female reproductive diseases (infertility, PCOS, spontaneous abortion [SA], RSA, endometriosis, and uterine fibroids [UF]) in the European population, which can provide insights into the screening and prevention of female reproductive diseases.
Materials and methods
Study design
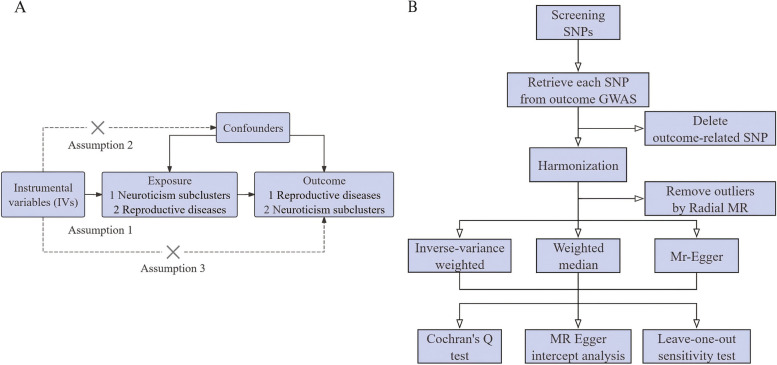
We implemented a bidirectional two-sample MR to investigate the causality between neuroticism clusters and female reproductive diseases. The three basic assumptions of the MR design are shown in Fig. 1A: genetic variations as IVs are strongly associated with the exposure of interest, IVs are not associated with any confounding factors, and IVs affect the risk of outcomes only through the exposure of interest [24]. A flowchart of the current MR design is presented in Fig. 1B. All analyses were conducted using summary-level data from published and publicly available GWAS [6, 7, 25], and all GWAS data were subjected to data cleaning and quality checks before publication.
Fig. 1.
Overview of present study design. A Three basic assumptions of the Mendelian randomization study; B Flow chart of the study design. Abbreviation: GWAS, genome-wide association study; SNP, single nucleotide polymorphism
Data source for exposures and outcomes
In the forward analysis of this study, we chose the neuroticism clusters as the exposures to explore their causal effect on the risk of female reproductive diseases (outcomes); in the reverse analysis, we explored the female reproductive diseases as the exposures to detect their reverse causality with the neuroticism clusters (outcomes).
By applying prospective cohort study data from self-reported British populations in the UK Biobank (https://www.ukbiobank.ac.uk/), two recent GWAS [6, 7] identified three distinct neuroticism clusters from the 12 Eysenck Personality Questionnaire using hierarchical clustering analyses: “depressed affect” (N = 357,957), “worry” (N = 348,219), and “sensitivity to environmental stress and adversity (SESA)” (N = 351,827). The depressed affect cluster was defined by the following four questions: “Do you often feel lonely?”, “Do you ever feel ‘just miserable’ for no reason?”, “Does your mood often go up and down?” and “Do you often feel fed up?”. The worry cluster was defined by the following four questions: “Are you a worrier?”, “Do you suffer from nerves?”, “Would you call yourself a nervous person?” and “Would you call yourself tense or highly stung?”. The SESA cluster was defined by the following three questions: “Are your feelings easily hurt?”, “Do you worry too long after an embarrassing experience?” and “Are you often troubled by feelings of guilt?”. Significant and replicable genetic differences were observed among the three clusters. Genome-wide summary statistics data on three neuroticism clusters (depressed affect, worry, and SESA) were available from the Complex Trait Genetics (CTG) database (https://ctg.cncr.nl/software/summary_statistics/).
Data on female reproductive diseases, including female infertility (13,142 cases, 107,564 controls), PCOS (1,424 cases, 200,581 controls), SA (16,906 cases, 149,622 controls), RSA (571 cases, 107,564 controls), endometriosis (15,088 cases, 107,564 controls), and UF (31,661 cases, 179,209 controls), were accessed from the FinnGen database (https://www.finngen.fi/en/access_results) R9 release on 11 May 2023 [25]. The FinnGen database is a large-scale biomedicine project based on the Finnish population, involving data from the nationwide longitudinal health register collected since 1969 from every resident in Finland.
All GWAS data were derived from the European population, and there was no overlap between participants in the exposure and outcome groups. Detailed information on the exposures and outcomes is provided in the Supplementary Material 1 (Table S1).
Selection of genetic instruments
Progress in the selection of genetic instruments was performed in R software version 4.2.3 (https://www.r-project.org/) with R packages “TwoSample MR (version 0.5.7)” and “Radial MR (version 1.1)”, specific R codes are available in Supplementary Material 2. A meticulous selection process is implemented to select appropriate IVs: (1) To ensure a robust association between the exposure and IVs, we set the standard of “p < 5 × 10–8” for screening single nucleotide polymorphisms (SNPs), this standard is recognized as a genome-wide significant threshold. When fewer than three SNPs were screened based on this standard, the p-value would be amplified to 5 × 10–6, which was widely used in previous studies [26, 27]. When there were fewer than three SNPs, an MR analysis was not conducted. (2) To obtain independent IVs, we selected SNPs with the linkage disequilibrium (LD) r2 < 0.001 in the 10,000-kb region, this standard could be considered the most rigorous cutoff to mitigate LD. (3) To prevent bias from weak IVs, we selected SNPs with F-statistic > 10 as the IVs [28]. F-statistics were calculated using the formula , where N indicates the sample size of the exposure, k indicates the number of IVs, and for each SNP, k is 1. R2 indicates the proportion of variance in the exposure of interest explained by a given SNP and was calculated using the formula , where β indicates the genetic effect of SNP on the exposure, EAF is the effect allele frequency, SE is the standard error, and N indicates sample size [29, 30]. (4) To ensure that IVs were not related to outcomes, SNPs associated with outcomes at genome-wide significance were removed. (5) To ensure that the effect of an SNP on the exposure and the outcome corresponded to the same allele, data harmonization was performed to unify allelic direction and remove palindromic SNPs (allele frequency > 0.42, which is the default parameter of “harmonize_data” function in “TwoSample MR” package). (6) To identify and remove outliers, MR-radial was implemented before each MR analysis [31].
After rigorous SNP filtering (Supplementary Material 1: Tables S2–S4), the number of IVs varied from 33 to 47 for the neuroticism clusters (Supplementary Material 1: Table S4). Due to the limited number of SNPs reaching p < 5 × 10–8 in some female reproductive diseases (infertility, PCOS, and SA), a relaxed p threshold (5 × 10–6) was used to screen IVs in these diseases. Since the number of SNPs screened for RSA remained less than three, even according to p < 5 × 10–6, MR analysis of the causality of RSA on neuroticism clusters was not performed. The number of IVs varied from 10 to 45 for the remaining female reproductive diseases (Supplementary Material 1: Table S4). The F-statistic values for all IVs used in this study were > 10, indicating their high quality and reliability.
MR analysis
The MR analysis was performed using the “TwoSample MR” package in R software, specific R codes are available in Supplementary Material 2. The data required for the analysis are available in Supplementary Material 3. The threshold for a significant difference was set at p < 0.05. Three models were used to perform this bidirectional MR study: inverse variance-weighted (IVW), weighted median (WM), and MR-Egger regression models. The IVW method can integrate the Wald ratio estimates for each SNP to produce a composite effect estimate, which assumes that all IVs are valid with no horizontal pleiotropy or heterogeneity, therefore, it has higher statistical power [32]. Compared to the fixed-effects IVW method, the random-effects IVW method can obtain a more conservative causal estimate, accounting for the uncertainty due to pleiotropy. Therefore, the random-effects IVW method was regarded as the primary estimator for identifying significant causality. The WM and MR-Egger methods were used for the complementary analysis of IVW. The WM model can provide a consistent estimate of causality when at least 50% of the IVs are effective [33]. The MR-Egger regression model can still provide an unbiased estimate, even if all SNPs included in the selection are invalid [34]. Therefore, these methods provide more robust estimates over a wider range.
Sensitivity analysis
Sensitivity analysis was performed using the “TwoSample MR” package in the R software, specific R codes are available in Supplementary Material 2. The data required for the analysis are available in Supplementary Material 3. Three analytical methods were applied for the sensitivity analysis. Cochran’s Q-test was used to detect heterogeneity among SNPs, and a value of p > 0.05 indicated no significant heterogeneity [35]. MR-Egger intercept analysis was used to detect pleiotropy, the value of the MR-Egger intercept analysis was p > 0.05, indicating the absence of horizontal pleiotropy [36]. Leave-one-out (LOO) analysis was used to recalculate and visualize the overall effect in the IVW analysis by sequentially omitting each SNP, and assess whether the observed causality depends on any individual SNP [37]. The results were typically considered robust if the results of all three analyses were negative.
Results
Causality of neuroticism clusters on female reproductive diseases
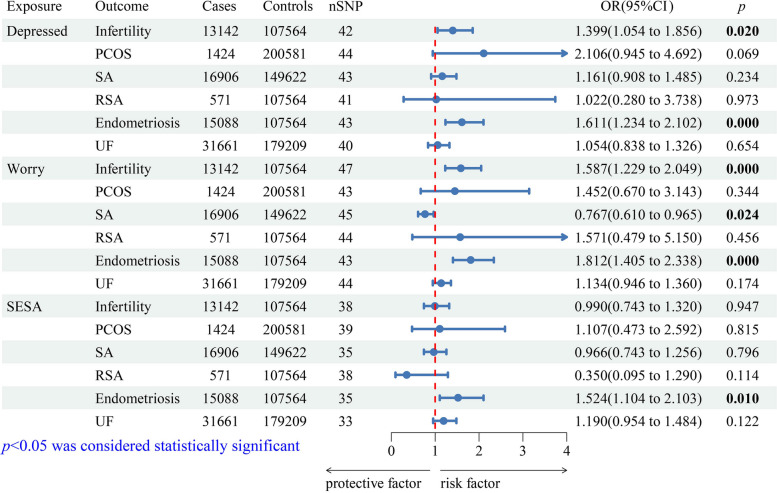
The forward MR analysis was as follows: In primary estimates (IVW method), genetically predicted depressed affect cluster could significantly increase the risk of infertility (odds ratio (OR) = 1.399, 95% confidence interval (CI): 1.054–1.856, p = 0.020) and endometriosis (OR = 1.611, 95% CI: 1.234–2.102, p = 0.000) (Fig. 2). Similar to the depressed affect cluster, the genetically proxied worry cluster was positively associated with infertility (OR = 1.587, 95% CI: 1.229–2.049, p = 0.000) and endometriosis (OR = 1.812, 95% CI: 1.405–2.338, p = 0.000) (Fig. 2). Genetically predicted SESA cluster only increased the risk of endometriosis (OR = 1.524, 95% CI: 1.104–2.103, p = 0.010) (Fig. 2). Corresponding complementary analyses are shown in Supplementary Material 1 (Table S5), in which causalities similar to those of the IVW method were demonstrated in the WM model but not in the MR-Egger model. In addition, the IVW method detected a statistically significant protective effect of the worry cluster on SA (OR = 0.767; 95% CI, 0.610–0.965; p = 0.024) (Fig. 2), whereas no significant causality was observed in the complementary analyses (Supplementary Material 1: Table S5). Thus, there was no convincing evidence to support causality between any other neuroticism cluster and female reproductive diseases (Fig. 2; Supplementary Material 1: Table S5).
Fig. 2.
Primary analyses (IVW method) of the causal effect of neuroticism clusters on female reproductive diseases. Mendelian randomization estimates are presented as OR with corresponding 95% CI, which provide an estimate of the relative outcome risk caused by each standard-deviation increase in exposure. Abbreviation: CI, confidence interval; OR, odds ratio; PCOS, polycystic ovary syndrome; RSA, recurrent spontaneous abortion; SA, spontaneous abortion; SESA, sensitivity to environmental stress and adversity; SNP, single nucleotide polymorphism; UF, uterine fibroids
In the sensitivity analysis, Cochran’s Q test did not detect any evidence of heterogeneity in the forward analysis (Table 1). The MR-Egger intercept test revealed pleiotropy in the analysis between the SESA cluster and PCOS but not in any other analysis (Table 1). The LOO analysis indicated that a few specific SNPs had a potential impact on the IVW result between the depressed affect cluster and PCOS, whereas other specific SNPs had a potential impact on the IVW result between the worry cluster and SA, suggesting that the protective effect of genetically predicted worry cluster on SA was not robust (Supplementary Material 1: Figure S1). In addition, the LOO analysis suggested that the other estimation results were robust (Supplementary Material 1: Figure S1).
Table 1.
Heterogeneity and horizontal pleiotropy for genetically causal effect of neuroticism clusters on female reproductive diseases
| Exposure | Outcome | Horizontal pleiotropy | Heterogeneity (IVW method) | |||
|---|---|---|---|---|---|---|
| MR Egger_intercept | pval | Cochran’s Q | Q_pval | |||
| Depressed | Infertility | -0.005 | 0.622 | 25.092 | 0.976 | |
| PCOS | -0.029 | 0.421 | 32.736 | 0.872 | ||
| SA | -0.006 | 0.541 | 34.221 | 0.798 | ||
| RSA | -0.011 | 0.844 | 20.891 | 0.995 | ||
| Endometriosis | 0.004 | 0.677 | 31.548 | 0.880 | ||
| UF | 0.000 | 0.993 | 50.397 | 0.104 | ||
| Worry | Infertility | -0.010 | 0.384 | 30.927 | 0.957 | |
| PCOS | -0.052 | 0.136 | 37.599 | 0.664 | ||
| SA | 0.005 | 0.653 | 41.305 | 0.588 | ||
| RSA | -0.038 | 0.482 | 24.074 | 0.991 | ||
| Endometriosis | 0.017 | 0.134 | 26.905 | 0.966 | ||
| UF | 0.008 | 0.332 | 31.511 | 0.903 | ||
| SESA | Infertility | -0.019 | 0.139 | 24.357 | 0.945 | |
| PCOS | -0.091 | 0.016 | 41.264 | 0.330 | ||
| SA | -0.005 | 0.700 | 34.727 | 0.433 | ||
| RSA | -0.100 | 0.090 | 27.086 | 0.884 | ||
| Endometriosis | 0.008 | 0.599 | 44.516 | 0.107 | ||
| UF | -0.005 | 0.652 | 34.933 | 0.330 | ||
Abbreviation: IVW Inverse variance-weighted, PCOS Polycystic ovary syndrome, RSA Recurrent spontaneous abortion, SA Spontaneous abortion, SESA Sensitivity to environmental stress and adversity, UF Uterine fibroids
Therefore, in the forward MR analysis, genetically predicted depressed affect and worry components of neuroticism were associated with higher risks of infertility and endometriosis, while genetically predicted SESA cluster increased the risk of endometriosis.
Causality of female reproductive diseases on neuroticism clusters
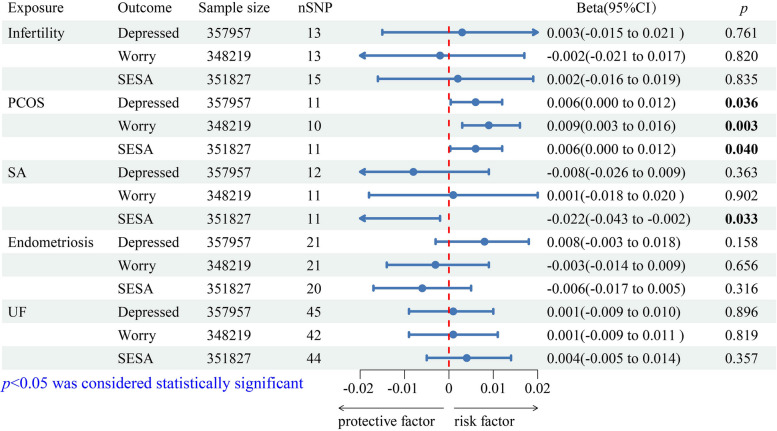
In the reverse MR analysis, the IVW method detected that genetically predicted PCOS could increase the risk of the three neuroticism clusters (depressed affect: Beta = 0.006, 95% CI: 0.000–0.012, p = 0.036; worry: Beta = 0.009, 95% CI: 0.003–0.016, p = 0.003; SESA: Beta = 0.006, 95% CI: 0.000–0.012, p = 0.040) (Fig. 3), while the IVW method also detected a significant protective effect of SA on the SESA cluster (Beta = -0.022, 95% CI: -0.043– -0.002, p = 0.033) (Fig. 3). The WM and MR-Egger methods yielded directionally consistent results in the aforementioned analyses (Supplementary Material 1: Table S6). No causality was detected between other female reproductive diseases and the neuroticism clusters (Fig. 3; Supplementary Material 1: Table S6).
Fig. 3.
Primary analyses (IVW method) of the causal effect of female reproductive diseases on neuroticism clusters. Mendelian randomization estimates are presented as Beta values with the corresponding 95% CI, which provide an estimate of the relative outcome risk caused by exposure. Abbreviation: CI, confidence interval; PCOS, polycystic ovary syndrome; SA, spontaneous abortion; SESA, sensitivity to environmental stress and adversity; SNP, single nucleotide polymorphism; UF, uterine fibroids
In the sensitivity analysis, heterogeneity and horizontal pleiotropy were not detected in any of the MR results (Table 2). The LOO analysis revealed that the causality between PCOS and worry cluster was the only robust analysis of the causalities mentioned above (Supplementary Material 1: Figure S2).
Table 2.
Heterogeneity and horizontal pleiotropy for genetically causal effect of female reproductive diseases on neuroticism clusters
| Exposure | Outcome | Horizontal pleiotropy | Heterogeneity (IVW method) | ||
|---|---|---|---|---|---|
| MR Egger_intercept | pval | Cochran’s Q | Q_pval | ||
| Infertility | Depressed | 0.002 | 0.241 | 11.084 | 0.522 |
| Worry | 0.001 | 0.620 | 10.989 | 0.530 | |
| SESA | 0.003 | 0.070 | 14.554 | 0.409 | |
| PCOS | Depressed | 0.001 | 0.736 | 10.255 | 0.418 |
| Worry | 0.001 | 0.756 | 6.673 | 0.671 | |
| SESA | -0.001 | 0.748 | 5.281 | 0.872 | |
| SA | Depressed | -0.002 | 0.278 | 7.792 | 0.732 |
| Worry | -0.002 | 0.440 | 4.108 | 0.942 | |
| SESA | -0.002 | 0.352 | 7.879 | 0.641 | |
| Endometriosis | Depressed | -0.004 | 0.067 | 22.399 | 0.319 |
| Worry | 0.000 | 0.800 | 16.498 | 0.685 | |
| SESA | -0.002 | 0.322 | 16.213 | 0.643 | |
| UF | Depressed | 0.000 | 0.586 | 42.116 | 0.553 |
| Worry | 0.000 | 0.870 | 41.226 | 0.461 | |
| SESA | 0.001 | 0.312 | 38.284 | 0.676 | |
Abbreviation: IVW Inverse variance-weighted, PCOS Polycystic ovary syndrome, SA Spontaneous abortion, SESA Sensitivity to environmental stress and adversity, UF Uterine fibroids
Therefore, in the reverse analysis, we detected only robust causality in which genetically predicted PCOS could increase the risk of worry cluster.
Discussion
In the current study, a bidirectional two-sample MR study was conducted to assess causality between neuroticism personality clusters and female reproductive diseases. The findings of the forward analysis supported that genetically predicted depressed affect and worry components of neuroticism could significantly increase the risk of infertility and endometriosis, while genetically predicted SESA cluster could increase the risk of endometriosis. In the reverse analysis, genetically predicted PCOS was causally associated with an increased risk of the worry component of personality. These results provide further evidence to clarify the causality between the components of neuroticism and female reproductive diseases.
The forward analysis provided strong evidence that the neuroticism clusters were causally linked to a higher risk of infertility and endometriosis. Previous studies consistently demonstrated a correlation between neuroticism and these conditions: infertile women exhibit higher levels of neuroticism than their male partners [16, 38, 39]. High neuroticism levels predicted pregnancy failure post-treatment and increased anxiety and depression scores after follow-up [40]. Similarly, patients with endometriosis exhibit higher levels of neuroticism, introversion, and anxiety personality traits [20]. However, most previous studies were cross-sectional and could not establish causality between neuroticism and female reproductive diseases. This study used the MR analysis to confirm that neuroticism is a risk factor for infertility and endometriosis. The underlying mechanism may involve abnormal activity in the hypothalamic–pituitary–adrenal (HPA) and sympathetic nervous system (SNS) axes. Owing to the rapid arousal and slow suppression of emotions in individuals with high neuroticism [41], they are likely to experience more chronic or severe stress, leading to HPA and SNS dysregulation [42, 43]. Stress-related biomarkers such as corticotropin-releasing hormone (CRH), cortisol, and catecholamines may play an important role in the development of infertility and endometriosis.
The sensitive response to stressful events in individuals with high neuroticism triggers overactivation of the HPA axis, leading to increased CRH and cortisol secretion. This phenomenon disrupts ovulation by interfering with the pulsatile release of gonadotropin-releasing hormone and inhibiting ovarian steroidogenesis, resulting in infertility [44, 45]. Excessive cortisol also suppresses immune cell function [46], allowing endometrial cells carried by the retrograde menstrual flow to escape immune surveillance and form lesions in the peritoneal cavity [47], thereby causing endometriosis. Similarly, when individuals with high neuroticism experience stressful events, the SNS axis becomes overactivated, subsequently, the impulses reach the adrenal medulla, causing a surge in catecholamine secretion. Excess catecholamines impair follicular quality and endometrial decidualization [48, 49], thereby contributing to infertility. Similar to cortisol, excess catecholamines suppress immune cells, facilitating the implantation of ectopic endometrial cells into the peritoneal cavity [46, 47]. In addition, excess catecholamines promote angiogenesis and cellular proliferation in the ectopic endometrium [50], thereby advancing endometriosis. However, the effect of neuroticism on HPA and SNS axes activities remains controversial. Several studies have suggested that high neuroticism leads to the hyperactivation of HPA and SNS axes [51–56], others argue that no such associations exist [57–59], and some even claim that it suppresses these axes [60–62]. Factors such as heterogeneity of neuroticism, selection of observational indicators, and confounder control may have influenced the conclusions [63]. Additionally, long-term chronic stress exposure may trigger human adaptive strategies to cope with chaotic environments, resulting in the HPA and SNS axes blunting in individuals with high neuroticism, thereby preventing persistent endocrine and cardiovascular responses [64, 65]. Thus, the HPA and SNS axes were gradually desensitized from an overactivated state to an inhibited state over time, leading to differences in the findings. However, diminished cortisol function induced by HPA axis inhibition may also suppress the immune system, which could trigger a pro-inflammatory state [66], similarly contributing to the progression of infertility and endometriosis [67]. In summary, further investigations are required to determine whether HPA and SNS axes abnormalities mediate the association between neuroticism and these diseases.
In the reverse analysis, we found that genetically predicted PCOS increased the risk of the worry component of neuroticism. Several studies have confirmed that patients with PCOS exhibit more negative personality traits: In addition to higher levels of neuroticism, these patients demonstrate greater emotionality and fear, increased anger and anxiety symptoms, and lower openness to experience and conscientiousness scores [19, 68]. However, the causality of these associations remains unclear. This study demonstrated that PCOS leads to an increased risk of the worry cluster of neuroticism. We attributed these possible causes to changes in the physical features caused by PCOS. PCOS induces more significant changes in appearance than other female reproductive diseases, such as obesity, acne, and hirsuteness. These changes result in women with PCOS experiencing significantly lower confidence or satisfaction with their bodies and a greater tendency to develop perceptions of low self-esteem and feelings of lack of attractiveness. Therefore, they are more likely to avoid social interactions, thus affecting the normal establishment of social and intimate relationships [69, 70]. In some cultures, having children after marriage is an important part of a woman’s social values, and infertility caused by PCOS can weaken the couple’s relationship and threaten the continuity of marriage [71, 72]. These factors lead to greater social pressure and less social support for women with PCOS, both of which have been recognized as contributors to high levels of neuroticism [73]. Another possible explanation is that blood glucose levels mediate PCOS triggering high levels of neuroticism. Some studies have shown that women with PCOS suffer from a much higher prevalence of reactive hypoglycemia than healthy women [74], which triggers neuroticism-like manifestations such as anger, anxiety, and moodiness [75, 76]. However, in this study, we only found a robust result that PCOS was causally related to the higher risk of worry component of neuroticism; the results for the depressed affect and SESA clusters were not robust. In fact, most studies confirmed that PCOS is closely related to various abnormal psychological states such as depression, anxiety, and decreased self-esteem [77, 78]. However, some psychological disorders share similar symptoms and are difficult to differentiate, and some women with PCOS lack a professional diagnosis of the psychological disorder. Therefore, more distinct studies should be conducted on women with PCOS for a single state of neuroticism or psychological disorders.
Elucidating the causality between neuroticism clusters and female reproductive diseases has important clinical implications. For women with high neuroticism identified through screening, it is important to address their neuroticism with therapies such as positive psychology exercises. In addition, more attention should be focused on their reproductive health. Early screening and intervention can minimize the risk and progression of infertility and endometriosis. Similarly, PCOS is prone to inducing neuroticism, and more attention should be focused on the psychological status of patients with PCOS during treatment and therapy. Focusing on the causality between neuroticism clusters and female reproductive diseases can help break the vicious circle and prevent the occurrence of comorbidities.
Our study has the following strengths: To the best of our knowledge, it is the first MR analysis to explore the causality between genetically distinct neuroticism clusters and female reproductive diseases. Meanwhile, more precise causative factors and disease outcomes can be identified by dividing the complex traits of neuroticism into genetically homogeneous clusters. Finally, we applied a bidirectional two-sample MR design to reduce residual confounding and mitigate the reverse causality of observational studies.
This study has several limitations. First, it included only individuals of European ancestry, thereby reducing the generalizability of its findings. Second, the reproductive diseases we studied were female-specific. However, no sex-specific analysis has been reported for genetically distinct neuroticism clusters, resulting in potential bias. Moreover, due to the limited number of SNPs reaching genome-wide significance in some female reproductive diseases, a relaxed p threshold was used for these diseases. Finally, the Beta value for the positive result obtained in the reverse analysis was only 0.009, possibly due to the insufficient sample size of the selected GWAS databases. The result still require validation in a future study with a larger sample size.
Conclusions
Through a series of procedures including genetic instrument selection, MR analysis, and sensitivity analysis, our study found that the neuroticism personality clusters, including depressed affect, worry, and SESA, exerted definite causal effects on at least one specific female reproductive disease (infertility or endometriosis) to differing degrees. Genetically predicted PCOS may increase the risk of the worry component of neuroticism. This finding suggests the need to screen for specific female reproductive diseases in populations with high neuroticism and assess the psychological status of patients with PCOS.
Supplementary Information
Acknowledgements
We acknowledge the investigators and participants of the original GWAS. We are grateful for the GWAS sharing of the summary data used in this study.
Abbreviations
- CRH
Corticotropin-releasing hormone
- GWAS
Genome-wide association study
- HPA
Hypothalamic–pituitary–adrenal
- IVs
Instrumental variables
- IVW
Inverse variance-weighted
- LOO
Leave-one-out
- MR
Mendelian randomization
- PCOS
Polycystic ovary syndrome
- RSA
Recurrent spontaneous abortion
- SA
Spontaneous abortion
- SESA
Sensitivity to environmental stress and adversity
- SNP
Single nucleotide polymorphism
- SNS
Sympathetic nervous system
- UF
Uterine fibroids
- WM
Weighted median
Authors’ contributions
XK and FJ conceived and designed the study. GT, YQ, and DL performed the data analysis. XK prepared the first draft of the manuscript. FJ and JS performed the subsequent amendments. XK revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.
Availability of data and materials
All data used in this study are publicly available, and the original studies are cited. All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Digman JM. Higher-order factors of the Big Five. J Pers Soc Psychol. 1997;73(6):1246–56. 10.1037/0022-3514.73.6.1246 [DOI] [PubMed] [Google Scholar]
- 2.Canli T. Toward a neurogenetic theory of neuroticism. Ann N Y Acad Sci. 2008;1129:153–74. 10.1196/annals.1417.022 [DOI] [PubMed] [Google Scholar]
- 3.Lahey BB. Public health significance of neuroticism. Am Psychol. 2009;64(4):241–56. 10.1037/a0015309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. J Abnorm Psychol. 1994;103(1):18–31. 10.1037/0021-843X.103.1.18 [DOI] [PubMed] [Google Scholar]
- 5.Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality Individ Differ. 1985;6(1):21–9. 10.1016/0191-8869(85)90026-1 [DOI] [Google Scholar]
- 6.Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet. 2018;50(7):920–7. 10.1038/s41588-018-0151-7 [DOI] [PubMed] [Google Scholar]
- 7.Nagel M, Speed D, van der Sluis S, Østergaard SD. Genome-wide association study of the sensitivity to environmental stress and adversity neuroticism cluster. Acta Psychiatr Scand. 2020;141(5):476–8. 10.1111/acps.13155 [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150(8):1139–48. 10.1176/ajp.150.8.1139 [DOI] [PubMed] [Google Scholar]
- 9.Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103(1):103–16. 10.1037/0021-843X.103.1.103 [DOI] [PubMed] [Google Scholar]
- 10.Jokela M, Pulkki-Råback L, Elovainio M, Kivimäki M. Personality traits as risk factors for stroke and coronary heart disease mortality: pooled analysis of three cohort studies. J Behav Med. 2014;37(5):881–9. 10.1007/s10865-013-9548-z [DOI] [PubMed] [Google Scholar]
- 11.Goodwin RD, Cox BJ, Clara I. Neuroticism and physical disorders among adults in the community: results from the National Comorbidity Survey. J Behav Med. 2006;29(3):229–38. 10.1007/s10865-006-9048-5 [DOI] [PubMed] [Google Scholar]
- 12.Niedzwiedz CL, Robb KA, Katikireddi SV, Pell JP, Smith DJ. Depressive symptoms, neuroticism, and participation in breast and cervical cancer screening: Cross-sectional and prospective evidence from UK Biobank. Psychooncology. 2020;29(2):381–8. 10.1002/pon.5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vukasović T, Bratko D. Heritability of personality: A meta-analysis of behavior genetic studies. Psychol Bull. 2015;141(4):769–85. 10.1037/bul0000017 [DOI] [PubMed] [Google Scholar]
- 14.Ormel J, Rijsdijk FV. Continuing change in neuroticism during adulthood—structural modelling of a 16-year, 5-wave community study. Personality Individ Differ. 2000;28(3):461–78. 10.1016/S0191-8869(99)00112-9 [DOI] [Google Scholar]
- 15.Roberts BW, Luo J, Briley DA, Chow PI, Su R, Hill PL. A systematic review of personality trait change through intervention. Psychol Bull. 2017;143(2):117–41. 10.1037/bul0000088 [DOI] [PubMed] [Google Scholar]
- 16.Edelmann RJ, Connolly KJ. Gender differences in response to infertility and infertility investigations: Real or illusory. Br J Health Psychol. 2000;5(4):365–75. 10.1348/135910700168982 [DOI] [Google Scholar]
- 17.Volgsten H, Ekselius L, Poromaa IS, Svanberg AS. Personality traits associated with depressive and anxiety disorders in infertile women and men undergoing in vitro fertilization treatment. Acta Obstet Gynecol Scand. 2010;89(1):27–34. 10.3109/00016340903447396 [DOI] [PubMed] [Google Scholar]
- 18.Cesta CE, Kuja-Halkola R, Lehto K, Iliadou AN, Landén M. Polycystic ovary syndrome, personality, and depression: A twin study. Psychoneuroendocrinology. 2017;85:63–8. 10.1016/j.psyneuen.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 19.Barry JA, Hardiman PJ, Saxby BK, Kuczmierczyk A. Testosterone and mood dysfunction in women with polycystic ovarian syndrome compared to subfertile controls. J Psychosom Obstet Gynaecol. 2011;32(2):104–11. 10.3109/0167482X.2011.568129 [DOI] [PubMed] [Google Scholar]
- 20.Low WY, Edelmann RJ, Sutton C. A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins. J Psychosom Res. 1993;37(2):111–6. 10.1016/0022-3999(93)90077-S [DOI] [PubMed] [Google Scholar]
- 21.Hori S, Nakano Y, Furukawa TA, Ogasawara M, Katano K, Aoki K, et al. Psychosocial factors regulating natural-killer cell activity in recurrent spontaneous abortions. Am J Reprod Immunol. 2000;44(5):299–302. 10.1111/j.8755-8920.2000.440509.x [DOI] [PubMed] [Google Scholar]
- 22.Boyko EJ. Observational research–opportunities and limitations. J Diabetes Complications. 2013;27(6):642–8. 10.1016/j.jdiacomp.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richmond RC, Davey Smith G. Mendelian randomization: Concepts and scope. Cold Spring Harb Perspect Med. 2022;12(1):a040501. [DOI] [PMC free article] [PubMed]
- 24.Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511. 10.1093/ije/dyv071 [DOI] [PubMed] [Google Scholar]
- 25.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18. 10.1038/s41586-022-05473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X, Wu TQ, Bian Y, Chen L, Feng X. Psychological distress and uterine fibroids: a bidirectional two-sample mendelian randomization study. BMC Womens Health. 2024;24(1):351. 10.1186/s12905-024-03196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Wang J, Hong H, Feng X, Zhang X, Song J. The association between diets and periodontitis: a bidirectional two-sample Mendelian randomization study. Front Genet. 2024;15:1398101. 10.3389/fgene.2024.1398101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26(5):2333–55. 10.1177/0962280215597579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Mi S, Zhu J, Jin W, Li Y, Wang T, et al. No causal association between adiponectin and the risk of rheumatoid arthritis: a Mendelian randomization study. Front Genet. 2021;12:670282. 10.3389/fgene.2021.670282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dan YL, Wang P, Cheng Z, Wu Q, Wang XR, Wang DG, et al. Circulating adiponectin levels and systemic lupus erythematosus: a two-sample Mendelian randomization study. Rheumatology (Oxford). 2021;60(2):940–6. 10.1093/rheumatology/keaa506 [DOI] [PubMed] [Google Scholar]
- 31.Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47(4):1264–78. 10.1093/ije/dyy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. 2013;178(7):1177–84. 10.1093/aje/kwt084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40. 10.1002/sim.6522 [DOI] [PubMed] [Google Scholar]
- 36.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemani G, Bowden J, Davey SG. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195-r208. 10.1093/hmg/ddy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bargiel-Matusiewicz K, Kroemeke A. Personality traits and coping styles in women with Mayer-Rokitansky-Küster-Hauser syndrome. Arch Med Sci. 2015;11(6):1244–9. 10.5114/aoms.2015.56350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coşkuner Potur D, Onat G, Doğan MY. An evaluation of the relationship between violence exposure status and personality characteristics among infertile women. Health Care Women Int. 2019;40(11):1135–48. 10.1080/07399332.2019.1622704 [DOI] [PubMed] [Google Scholar]
- 40.Verhaak CM, Smeenk JM, Evers AW, van Minnen A, Kremer JA, Kraaimaat FW. Predicting emotional response to unsuccessful fertility treatment: a prospective study. J Behav Med. 2005;28(2):181–90. 10.1007/s10865-005-3667-0 [DOI] [PubMed] [Google Scholar]
- 41.Suls J, Martin R. The daily life of the garden-variety neurotic: reactivity, stressor exposure, mood spillover, and maladaptive coping. J Pers. 2005;73(6):1485–509. 10.1111/j.1467-6494.2005.00356.x [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, Zhou R, Oei TP, Wang Q, Zhao Y, Liu Y. Variation in the stress response between high- and low-neuroticism female undergraduates across the menstrual cycle. Stress. 2013;16(5):503–9. 10.3109/10253890.2013.797958 [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Banda G, Chellew K, Fornes J, Perez G, Servera M, Evans P. Neuroticism and cortisol: pinning down an expected effect. Int J Psychophysiol. 2014;91(2):132–8. 10.1016/j.ijpsycho.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 44.Joseph DN, Whirledge S. Stress and the HPA Axis: balancing homeostasis and fertility. Int J Mol Sci. 2017;18(10):2224. [DOI] [PMC free article] [PubMed]
- 45.Ghizzoni L, Mastorakos G, Vottero A, Barreca A, Furlini M, Cesarone A, et al. Corticotropin-releasing hormone (CRH) inhibits steroid biosynthesis by cultured human granulosa-lutein cells in a CRH and interleukin-1 receptor-mediated fashion. Endocrinology. 1997;138(11):4806–11. 10.1210/endo.138.11.5474 [DOI] [PubMed] [Google Scholar]
- 46.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25. 10.1016/S1470-2045(04)01597-9 [DOI] [PubMed] [Google Scholar]
- 47.Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. 2018;17(10):945–55. 10.1016/j.autrev.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 48.Li XH, Ma YG, Geng LH, Qin L, Hu H, Li SW. Baseline psychological stress and ovarian norepinephrine levels negatively affect the outcome of in vitro fertilisation. Gynecol Endocrinol. 2011;27(3):139–43. 10.3109/09513590.2010.501871 [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Kong S, Guo C, Wang J, Lu J, Jiang R, et al. An exaggerated epinephrine-adrenergic receptor signaling impairs uterine decidualization in mice. Reprod Toxicol. 2019;90:109–17. 10.1016/j.reprotox.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 50.Long Q, Liu X, Qi Q, Guo SW. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor β2. Hum Reprod. 2016;31(11):2506–19. 10.1093/humrep/dew237 [DOI] [PubMed] [Google Scholar]
- 51.Yoshino A, Kimura Y, Yoshida T, Takahashi Y, Nomura S. Relationships between temperament dimensions in personality and unconscious emotional responses. Biol Psychiatry. 2005;57(1):1–6. 10.1016/j.biopsych.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 52.Nater UM, Hoppmann C, Klumb PL. Neuroticism and conscientiousness are associated with cortisol diurnal profiles in adults–role of positive and negative affect. Psychoneuroendocrinology. 2010;35(10):1573–7. 10.1016/j.psyneuen.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 53.Roger D, Najarian B. The relationship between emotional rumination and cortisol secretion under stress. Pers Individ Diff. 1998;24(4):531–8.
- 54.Zobel A, Barkow K, Schulze-Rauschenbach S, Von Widdern O, Metten M, Pfeiffer U, et al. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic-pituitary-adrenocortical system in healthy volunteers. Acta Psychiatr Scand. 2004;109(5):392–9. 10.1111/j.1600-0447.2004.00313.x [DOI] [PubMed] [Google Scholar]
- 55.Norris CJ, Larsen JT, Cacioppo JT. Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology. 2007;44(5):823–6. 10.1111/j.1469-8986.2007.00551.x [DOI] [PubMed] [Google Scholar]
- 56.Wilson GD, Kumari V, Gray JA, Corr PJ. The role of neuroticism in startle reactions to fearful and disgusting stimuli. Personality Individ Differ. 2000;29(6):1077–82. 10.1016/S0191-8869(99)00255-X [DOI] [Google Scholar]
- 57.van Santen A, Vreeburg SA, Van der Does AJ, Spinhoven P, Zitman FG, Penninx BW. Psychological traits and the cortisol awakening response: results from the Netherlands Study of Depression and Anxiety. Psychoneuroendocrinology. 2011;36(2):240–8. 10.1016/j.psyneuen.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 58.McCleery JM, Goodwin GM. High and low neuroticism predict different cortisol responses to the combined dexamethasone–CRH test. Biol Psychiatry. 2001;49(5):410–5. 10.1016/S0006-3223(00)01056-8 [DOI] [PubMed] [Google Scholar]
- 59.Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134(6):829–85. 10.1037/a0013342 [DOI] [PubMed] [Google Scholar]
- 60.LeBlanc J, Ducharme MB. Influence of personality traits on plasma levels of cortisol and cholesterol. Physiol Behav. 2005;84(5):677–80. 10.1016/j.physbeh.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 61.Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31(7):1583–91. 10.1038/sj.npp.1301012 [DOI] [PubMed] [Google Scholar]
- 62.Di Simplicio M, Costoloni G, Western D, Hanson B, Taggart P, Harmer CJ. Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med. 2012;42(8):1775–83. 10.1017/S0033291711002479 [DOI] [PubMed] [Google Scholar]
- 63.Ormel J, Bastiaansen A, Riese H, Bos EH, Servaas M, Ellenbogen M, et al. The biological and psychological basis of neuroticism: current status and future directions. Neurosci Biobehav Rev. 2013;37(1):59–72. 10.1016/j.neubiorev.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 64.Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30(3):376–89. 10.1016/j.neubiorev.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 65.Poppelaars ES, Klackl J, Pletzer B, Wilhelm FH, Jonas E. Social-evaluative threat: Stress response stages and influences of biological sex and neuroticism. Psychoneuroendocrinology. 2019;109:104378. 10.1016/j.psyneuen.2019.104378 [DOI] [PubMed] [Google Scholar]
- 66.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32–47. 10.1038/mp.2014.163 [DOI] [PubMed] [Google Scholar]
- 67.Ortiz R, Gemmill JAL, Sinaii N, Stegmann B, Khachikyan I, Chrousos G, et al. Hypothalamic-pituitary-adrenal axis responses in women with endometriosis-related chronic pelvic pain. Reprod Sci. 2020;27(10):1839–47. 10.1007/s43032-020-00201-x [DOI] [PubMed] [Google Scholar]
- 68.Urban W, Nizioł A, Pytlewski A, Zaborowska Ł, Dadański E, Rutkowski K, et al. Polycystic ovary syndrome: personality and temperamental characteristics. J Obstet Gynaecol Can. 2022;44(7):813–8. 10.1016/j.jogc.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 69.Fliegner M, Richter-Appelt H, Krupp K, Brunner F. Sexual function and socio-sexual difficulties in women with Polycystic Ovary Syndrome (PCOS). Geburtshilfe Frauenheilkd. 2019;79(5):498–509. 10.1055/a-0828-7901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alkheyr Z, Murad M, Das P, Aljenaee K, Kamel C, Hajji SA, et al. Self-esteem and body image satisfaction in women with PCOS in the Middle East: Cross-sectional social media study. PLoS ONE. 2024;19(4):e0301707. 10.1371/journal.pone.0301707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.H AL, Szatkowski L, Gibson J, Fiaschi L, Bains M. Psychosocial impacts of infertility among omani women with polycystic ovarian syndrome: a qualitative study. Int J Fertil Steril. 2023;17(2):107–14. [DOI] [PMC free article] [PubMed]
- 72.Schmid J, Kirchengast S, Vytiska-Binstorfer E, Huber J. Infertility caused by PCOS–health-related quality of life among Austrian and Moslem immigrant women in Austria. Hum Reprod. 2004;19(10):2251–7. 10.1093/humrep/deh432 [DOI] [PubMed] [Google Scholar]
- 73.Xue K, Gao B, Chen F, Wang M, Cheng J, Zhang B, et al. Covariation of preadult environmental exposures, adult brain imaging phenotypes, and adult personality traits. Mol Psychiatry. 2023;28(11):4853–66. [DOI] [PubMed]
- 74.Mumm H, Altinok ML, Henriksen JE, Ravn P, Glintborg D, Andersen M. Prevalence and possible mechanisms of reactive hypoglycemia in polycystic ovary syndrome. Hum Reprod. 2016;31(5):1105–12. 10.1093/humrep/dew046 [DOI] [PubMed] [Google Scholar]
- 75.McCrimmon RJ, Ewing FM, Frier BM, Deary IJ. Anger state during acute insulin-induced hypoglycaemia. Physiol Behav. 1999;67(1):35–9. 10.1016/S0031-9384(99)00036-0 [DOI] [PubMed] [Google Scholar]
- 76.McCrimmon RJ, Frier BM, Deary IJ. Appraisal of mood and personality during hypoglycaemia in human subjects. Physiol Behav. 1999;67(1):27–33. 10.1016/S0031-9384(99)00035-9 [DOI] [PubMed] [Google Scholar]
- 77.Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ. Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychol Med. 2019;49(9):1510–20. 10.1017/S0033291718002076 [DOI] [PubMed] [Google Scholar]
- 78.de Niet JE, de Koning CM, Pastoor H, Duivenvoorden HJ, Valkenburg O, Ramakers MJ, et al. Psychological well-being and sexarche in women with polycystic ovary syndrome. Hum Reprod. 2010;25(6):1497–503. 10.1093/humrep/deq068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are publicly available, and the original studies are cited. All data generated or analysed during this study are included in this published article and its supplementary information files.