Abstract
Background
Oral cancers, which include tumors of the oral cavity, salivary glands, and pharynx, are becoming increasingly prevalent worldwide. Squamous cell carcinoma accounts for over 90% of malignant oral lesions, with oral squamous cell carcinoma (OSCC) being notably common in the Indian subcontinent and other regions of Asia. This is especially true in South-Central Asia, including Sri Lanka, where it is particularly prevalent among men. This study aims to evaluate the levels of Vascular Endothelial Growth Factor-A (VEGF-A) and Cytokeratin-19 (CK-19) mRNAs in whole blood as a potential method for the early detection of OSCC.
Methods
The study included 40 patients (each from OSCC, Oral Submucous Fibrosis (OSF), Oral Leukoplakia (OLK), Oral Lichen Planus (OLP), and 10 healthy controls. The expression levels of VEGF-A and CK-19 mRNAs were measured from extracellular RNA extracted from whole blood samples using real-time reverse transcription polymerase chain reaction (RT-PCR) with sequence-specific primers. Receiver operating characteristic (ROC) curve analysis was used to evaluate the effectiveness of these biomarkers in detecting OSCC.
Results
The results demonstrated a significant increase in blood transcripts of the candidate mRNAs CK-19 and VEGF-A in patients with OSCC, OSF, OLK, and OLP. The Wilcoxon signed-rank test revealed a p-value of 0.002 for each specific comparison between diseased patients and healthy controls (i.e., OSCC vs. HC, OSF vs. HC, OLP vs. HC, OLK vs. HC) for both CK-19 and VEGF-A. When these two biomarkers were used together, they provided a 60% predictive probability for patients with OSCC (p = 0.023).
Conclusion
This study highlights the efficacy of blood mRNA transcriptome diagnostics in detecting OSCC. This innovative clinical approach has the potential to be a robust, efficient, and reliable tool for early cancer detection. Blood-based transcriptomes could be further explored for their effectiveness in various health contexts and for routine health monitoring.
Keywords: Oral cancer, Oral potentially malignant disorders, Polymerase chain reaction, Blood, Early detection, mRNA
Introduction
As the sixth most prevalent cancer worldwide, oral and oropharyngeal malignancies are a growing health issue [1–3]. South Asian countries have the highest frequency of oral cancer in men, accounting for 25% of all new cases of cancer [3]. Oral cancer has a 50% 5-year survival rate [1, 3]. OSCC accounts for 95% of oral cavity malignancies, while smoking, betel quid chewing, and excessive alcohol use are major causes [1]. Because alcohol, tobacco, and areca nut use persist despite public awareness and educational campaigns, primary prevention alone has failed [3–5]. The oral cavity’s direct accessibility aids early identification of OSCCs and Oral potentially malignant disorders (OPMDs), yet 60% of OSCCs in Sri Lanka are detected at advanced clinical stages [2, 6]. Late OSCC presentation has poor prognosis and lower rates of survival due to local, regional and distant metastases [3, 6]. The proportion of patients with advanced-stage OSCC has remained stable for 40 years, and despite a large portion of the population receiving regular oral health checks and dental care, survival rates have not improved [3].
VEGF, also known as VEGF-A, is a protein originally purified from a tumor-derived fluid, recognized for its vascular permeability activity [7]. A few years later, a protein with angiogenic activity was independently purified and named VEGF [8]. Molecular cloning subsequently revealed that these two proteins were identical, encoded by a single gene [9]. The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, PlGF (placental growth factor), VEGF-E (Orf-VEGF), and Trimeresurus flavoviridis svVEGF. Except for the latter two members, five VEGF family genes are present in mammalian genomes, including humans [10]. VEGF-A serves multiple functions, including promoting angiogenesis, increasing vascular permeability, and stimulating cell migration in macrophage lineage and endothelial cells. Recently, anti–VEGF-VEGFR drugs, such as anti–VEGF-A neutralizing antibodies and multikinase inhibitors, have been developed and are widely used for treating major solid tumors [11, 12]. Elevated levels of VEGF mRNA or protein have been found to be associated with aggressive progression and poor prognosis in several forms of cancers, such as pancreatic, gastric, colorectal, and breast carcinomas [13–17]. The potential of VEGF protein or mRNA as a predictive marker for oral cancer progression and prognosis is a promising prospect. However, the specific role of VEGF-A in the pathophysiology of oral cancer remains to be clarified [18].
Cytokeratins (CK19) belong to the Keratin family of intermediate filament proteins, consisting of 20 polypeptides. The family mentioned is crucial for maintaining the structural integrity of epithelial cells and serves as a highly effective indicator for tumors [19, 20]. The primary roles of these indicators are to facilitate signal transduction, respond to stress, induce apoptosis, maintain cell integrity, and promote cellular proliferation [21, 22]. Nevertheless, Cytokeratins are subject to intricate regulation, which involves posttranslational modifications of amino acids and interactions with associated proteins [21]. Malignant epithelial cells contain wild-type filamentous polypeptides [23].
CK19 is found in the periderm and epithelial cells of the kidney and the organs of the digestive systema (including the pancreas and gallbladder) [24]. CK19 mRNA has been detected in various cancers, including hepatocellular carcinoma, cholangiocellular carcinoma, colorectal neoplasm, nonmedullary thyroid carcinoma, untreated early-stage cervical carcinoma, breast cancer, and lung cancers [25]. This study aims to (a) evaluate the expression levels of CK-19 mRNAs and VEGF-A in blood specimens of patients with active OSCC lesions, OPMDs (such as oral submucous fibrosis (OSF), oral leukoplakia (OLK), and oral lichen planus (OLP), and healthy controls, and (b) compare the histopathological grade of OSCC with the obtained VEGF-A expression findings.
Methods
Sample collection and preparation
This study was conducted in accordance with the Helsinki Declaration of 1964 (as amended in October 2013 by the World Medical Association General Assembly), at the Dental Teaching Hospital of the University of Peradeniya, Sri Lanka between March, 2023 to October 2023. The study was ethically approved by Ethical Review Committee of the Faculty of Dental Sciences, University of Peradeniya under the reference number ERC/FDS/UOP/E/2021/14, and all patients provided written informed consent prior to participation in this study. Further, participation was informed, anonymous, and voluntary. Individuals under 18 years of age and those physically or mentally unable to respond to the data collection tools were excluded. Eligible participants were adults over 18 years without any physical or mental incapacities, newly diagnosed, and had not undergone any prior interventions such as chemotherapy, radiation, surgery, or alternative therapies. None of the patients or study controls had a medical history of cancer, hepatitis, HIV infection, immunodeficiency, or autoimmune disorders. Control participants were meticulously matched with the experimental group by age and sex. Healthy controls were selected following a detailed oral cavity examination by a specialist in oral medicine, ensuring the absence of any pathological lesions. All participants provided informed consent by signing an official form.
The criteria for diagnosing the oral lesions in our study involved both histopathological confirmation and clinical examination. OSCC was diagnosed through histopathological examination, which confirmed malignant epithelial cells exhibiting features such as keratinization, cellular atypia, and invasion of surrounding tissues, supported by clinical findings consistent with malignant lesions. OLK was identified by the presence of white plaques or patches on the oral mucosa that could not be classified as any other condition clinically or pathologically; histopathological examination was done to assess the dysplastic changes and rule out malignant transformation. OLP was diagnosed based on clinical examination, which revealed bilateral white reticular or lace-like patterns on the buccal mucosa, tongue, or gingiva, and confirmed by histopathological examination showing characteristic features like band-like lymphocytic infiltrate at the epithelial-connective tissue interface and degeneration of the basal cell layer. OSF was diagnosed through clinical examination indicating progressive fibrosis of the submucosal tissues, leading to restricted mouth opening and blanching of the oral mucosa; histopathological examination confirmed the presence of dense collagenous connective tissue with reduced vascularity and chronic inflammatory infiltrate. The demographic and clinical information such as sex, age, drinking and smoking habits, clinical history, treatments, and instances of recurrence were recorded. To ensure confidentiality, all patient identifiers were encoded.
We calculated the sample size using G*Power 3.1 with an F test ANOVA: Fixed effects, omnibus, one-way. At a significance level (α) of 0.05 and power (1-β) of 0.90, with an effect size (f) of 0.6, the required sample size was determined to be 50. These 50 participants were then distributed among the groups for oral cancer, OLK, OLP, OSF, and controls.
A total of 2 mL of blood was withdrawn in EDTA tubes from healthy donors (n = 10), patients with OSCC (n = 10), OLK (n = 10), OLP (n = 10), and OSF (n = 10). 1 mL of human whole blood was mixed with 5 mL of Buffer EL in an appropriately sized tube/falcon tube (15 mL). The sample was incubated for 10–15 min on ice and mixed by vertexing briefly 2 times during incubation. Afterward, the mixture was centrifuged at 400 x g for 10 min at 4 °C, completely removed, and the supernatant was discarded. 2 mL of Buffer EL was added to the cell pellet and resuspended the cells by vertexing briefly. Again, centrifuged the mixture at 400 x g for 10 min at 4 °C, and completely removed and discarded supernatant. 600 µL of Buffer RLT was added to pelleted leukocytes and vortexed well, followed by samples stored at − 70 °C until complete RNA extraction.
RNA isolation
Upon thawing the cell pellets, total RNA extraction was performed according to the manufacturer’s instructions of the Qiagen QIAamp RNA Blood Kit ®. The QIAamp RNA Blood Mini Kit streamlines the extraction of RNA from the blood via a rapid spin-column method. It begins by selectively lysing red blood cells and collecting white cells through centrifugation. These white cells are then lysed under potent denaturing conditions that swiftly deactivate RNases. Following this, the sample is homogenized using the QIAshredder spin column and subsequently applied to the QIAamp spin column. Here, total RNA attaches to the QIAamp membrane while impurities are eliminated through a series of washes, resulting in pure RNA that can be eluted in 30–100 µl of RNase-free water (included with the kit). This pure RNA is then ready for direct utilization in various downstream applications. The extracted RNA’s purity and quantity were assessed via spectrophotometric analysis employing a NanoDrop spectrophotometer, focusing on the A260/A280 ratio.
cDNA synthesis
The total RNA was reverse transcribed using GoScript™ Reverse Transcriptase from Promega Corporation, Madison, Wisconsin, United States, following the manufacturer’s guidelines. Subsequently, the samples were stored at -20 °C for further analysis.
Real-time RT-PCR
Real-time RT-PCR analysis of VEGF-A, CK-19, and GAPDH was conducted on 50 study participants, with each sample analyzed in triplicate. The PCR amplification was carried out in a 10 µL reaction mixture, comprising a final concentration of 10 µmol for both sense and antisense primers, 2 µL of cDNA, and combined with 10 µL of GoTaq® qPCR Master Mix (Promega Corporation, Madison, USA). Quantification was performed using the QuantStudioTM 6 Flex Real-Time PCR Instrument from Thermo Fisher Scientific. The initial cDNA/RNA amount for a specific template was deduced from a previously established standard curve [26]. The sequences and specifications of the PCR primer set (in the 5’-3’ direction) were as follows (Table 1). The relative expression of each specific product was calculated by 2-ΔΔCT (CT = fluorescence threshold value; ΔCT = CT of the target gene - CT of the reference gene (GAPDH); ΔΔCT = ΔCT of the oral cancer/OPMD sample - ΔCT of the calibrator sample).
Table 1.
Specification of the primers used in real-time RT-PCR [27]
| Parameter | CK19-mRNA | VEGF-A | GAPDH |
|---|---|---|---|
| F primer | TCCGAACCAAGTTTGAGAC | ATCACGAAGTGGTGAAGTTC | TGAAGGTCGGAGTCAACGGATTTGGT |
| Length of primer | 19 | 20 | 26 |
| R primer | AATCCACCTCCACACTGA | TGCTGTAGGAAGCTCATCTC | CATGTGGGCCATGAGGTCCACCAC |
| Length of primer | 18 | 20 | 24 |
| Length of proliferation piece | 222 | 265 | 929 |
| Optimum annealing temperature | 58.4 oC | 60 oC | 60 oC |
Evaluation
Statistical analyses and diagram creation were conducted using GraphPad Prism 4.0 software. The Wilcoxon Signed Rank test was employed for statistical assessment to compare each diseased vs. control group. To appraise the biomarker’s efficacy in distinguishing between tumor and control samples in individuals’ whole blood without relying on a subjective threshold, a Receiver Operating Characteristic (ROC) curve was generated. This curve illustrates the relationship between sensitivity (true positives) and 1-specificity (false positives), treating each recorded value as a potential cutoff point. The Area Under the Curve (AUC) was computed to evaluate the overall discriminatory capability of the marker. A biomarker with limited discriminatory value would exhibit a ROC curve closely mirroring the diagonal line, resulting in an AUC value of around 0.5. In contrast, a dependable discriminatory test would shift the ROC curve toward the upper left corner, yielding an AUC value approaching 1.0.
Results
The study cohort comprised 50 individuals, 36 were men, and 14 were women, indicating a slight preference for men. The study included 10 patients diagnosed with OSCC (4 with well-differentiated OSCC, 3 with moderately differentiated OSCC and 3 with poorly differentiated OSCC), 10 with OLP, 10 with OLK displaying varying degrees of dysplasia (2 with keratosis with moderate epithelial dysplasia, 4 with keratosis with mild epithelial dysplasia, 2 with keratosis without dysplasia, and, 2 with verrucous hyperplasia), 10 with OSF displaying diverse degrees of dysplasia (2 with mild epithelial dysplasia, 1 with OSF (Advanced stage) and 7 with no dysplasia), and ten meticulously matched healthy control subjects (Table 2).
Table 2.
Clinicopathological characteristics of the patients in the study (n = 50)
| Parameters | Study population (n = 50) | ||||
|---|---|---|---|---|---|
| OSCC | OLK | OSF | OLP | HC | |
| Total | 10 | 10 | 10 | 10 | 10 |
| Age (years) | |||||
| Median range | 56(37–77) | 47(27–59) | 43(28–68) | 48(18–64) | 37(24–68) |
| Gender | |||||
|
Male Female |
8 2 |
9 1 |
8 2 |
4 6 |
7 3 |
| Past medical history/ drug history | |||||
|
Yes No |
4 6 |
5 5 |
4 6 |
2 8 |
0 10 |
| Areca nut chewing | |||||
|
Yes No |
8 2 |
7 3 |
10 0 |
5 5 |
0 10 |
| Smoking history | |||||
|
Yes No |
3 7 |
6 4 |
5 5 |
2 8 |
0 10 |
| Alcohol consumption | |||||
|
Yes No |
8 2 |
3 7 |
7 3 |
2 8 |
3 7 |
Upon examining the associations between VEGF-A and CK-19 levels and the clinicopathological characteristics of OSCC and OPMD patients, no significant association was identified for age, gender, past medical history, betel quid chewing habit, smoking habit, and alcohol consumption. However, the following associations were detected between the presence of OSCC/OPMD and the age of the patients (Pearson correlation = − 0.351; p = 0.012), presence of OPMD/OSCC vs. drug history of the individual (Pearson correlation = − 0.033; p = 0.018), presence of OPMD/OSCC vs. betel chewing (Pearson correlation = − 0.612; p = 0.000), presence of OPMD/OSCC vs. alcohol consumption (Pearson correlation = − 0.284; p = 0.046), gender vs. smoking (Pearson correlation = − 0.428; p = 0.002), gender vs. alcohol consumption (Pearson correlation = − 0.586; p = 0.000), age vs. drug history (Pearson correlation = − 0.111; p = 0.047) and age vs. past medical history (Pearson correlation = 0.303; p = 0.033).
Since the dataset was not normally distributed, we employed the Spearman Correlation test to evaluate the monotonic relationship between the variables without assuming a linear connection. The p-values for each marker indicate whether there was no statistically significant correlation between their concentrations and dysplasia severity, categorized as follows: 0 = no dysplasia, 1 = mild dysplasia, 2 = moderate dysplasia, and 3 = invasive cancer. The Spearman correlation coefficient for VEGF-A and dysplasia severity was 0.096, with a p-value of 0.506, while for CK-19, the correlation coefficient was 0.003, with a p-value of 0.983.
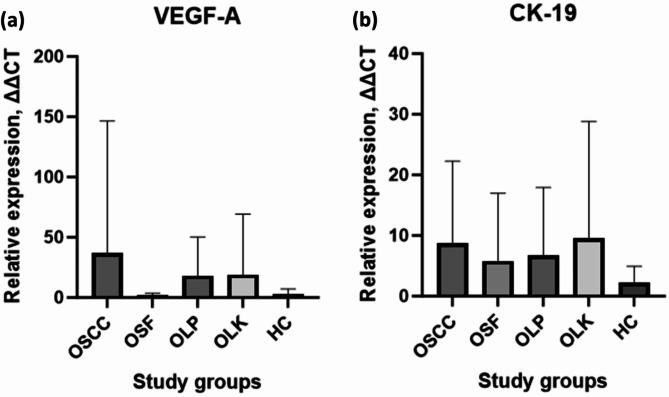
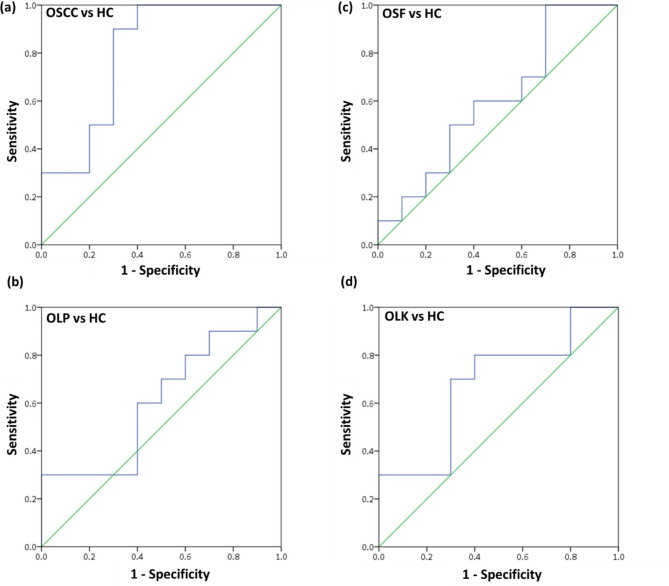
The results affirmed a notable increase in blood transcripts of the 2 candidate mRNAs CK-19 and VEGF-A—in patients with OSCC, OSF, OLK, and OLP. Wilcoxon signed-rank test showed p = 0.002 for each specific comparison: diseased versus controls (i.e., OSCC versus HC, OSF versus HC, OLP versus HC, OLK versus HC) for CK-19 and VEGF. These genes were stratified into two tiers based on the magnitude of elevation: high up-regulated mRNA, encompassing VEGF-A (36.73-fold-OSCC; 1.84-fold-OSF; 19.00-fold-OLK; 18.17-fold-OLP; 3.01-fold-HC); lower up-regulated mRNAs, including CK-19 (8.721-fold-OSCC; 5.800-fold-OSF; 6.706-fold-OLP; 9.505-fold-OLK; 2.249-fold-HC) (Fig. 1). After conducting binary logistic regression analysis, predictive probability values were collected and used to create ROC curves (Fig. 2). The ROC curves were subsequently utilized to calculate the area under the curves (AUC) for the prediction probability of each biomarker, as specified in Table 3.
Fig. 1.
Circulating levels of VEGF-A and CK-19 (a) The box-plot diagram shows VEGF-A mRNA expression levels (b) The box-plot graph illustrates the Cytokeratin (CK-19) mRNA expression levels
Fig. 2.
Receiver operating characteristics (ROC) curve analysis using blood VEGF-A and CK-19 levels in blood in OSCC, OSF, OLP, and OLK patients compared with controls. (a) ROC curve of OSCC vs. HC (b) ROC curve of OLP vs. HC (c) ROC curve of OSF vs. HC (d) ROC curve of OLK vs. HC
Table 3.
Areas under the curve (AUC) of the four combined biomarkers
| Predictive probabilities | Sensitivity (%) |
Specificity (%) |
Cut of probability (%) | Area | Asymptotic sig. | Std Error | Asymptotic 95% C.I. Intervals | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| OSCC | 60 | 70 | 50.64 | 0.8 | 0.023* | 0.105 | 0.595 | 1.000 |
| OSF | 60 | 50 | 50.96 | 0.6 | 0.45 | 0.13 | 0.345 | 0.855 |
| OLP | 30 | 90 | 50.99 | 0.61 | 0.406 | 0.13 | 0.355 | 0.865 |
| OLK | 30 | 70 | 49.27 | 0.68 | 0.174 | 0.124 | 0.437 | 0.923 |
Discussion
Angiogenesis plays a crucial role in the initiation, development, and metastasis of tumors, with VEGF recognized as a potent angiogenic factor driving the formation of new blood vessels during tumor growth [28, 29]. VEGF is known to specifically stimulate the proliferation of vascular endothelial cells. Elevated expression of VEGF was not only observed in the tissue of OSCC but also had a notable impact on serum levels. In a recent study led by Edirisinghe et al., a direct correlation was observed between the expression of the VEGF-A gene, serum levels, tissue VEGFR-2 levels, and the tumor’s stage and grade. These results provide significant evidence endorsing the potential of VEGF-A as a biomarker for the early detection of OSCC [30].
The initial investigation led by Folkman et al. showcased VEGF’s angiogenic capabilities in pre-malignant lesions [31]. This research highlighted the stimulation of blood vessel formation as cells progressed from excessive growth to the development of abnormal tissue. Few studies have explored angiogenesis in the progression of clinically diagnosed OLK towards malignancy [32]. The increase in angiogenesis is evident during the transition from OLK to dysplasia and, ultimately, to OSCC. In OSCC, a study by Gandolfo et al. revealed heightened VEGF expression in leukoplakia, contributing to blood vessel formation beneath the epithelium [32]. Moreover, VEGF expression was significantly higher in leukoplakia with dysplastic alterations compared to those without dysplasia. Specifically, in the context of oral leukoplakia, the expression of VEGF-A can be utilized to assess the malignant evolution of tongue OLK-OSCCs [32]. These investigations collectively indicate that VEGF holds promise as a valuable indicator for diagnosing the malignant transformation of oral leukoplakia.
In a study conducted by Rai et al., the investigation aimed to establish a link between VEGF gene polymorphism and OSF [33]. The results provided compelling evidence indicating a significant correlation between the VEGF − 460 C/T gene polymorphism and OSF in patients. Notably, the observation of polymorphism in advanced stages underscores the importance of VEGF as a biomarker in both the malignant transformation and progression of OSF phases [33]. The progression of OPMDs to malignancy has been associated with the downregulation of E-cadherin and the upregulation of VEGF [34]. The expression of VEGF increased consistently as the disease advanced from normal to higher stages of oral epithelial dysplasia and eventually to cancer. However, in the present study the level of VEGF-A level was downregulated compared to the healthy controls.
Sharma et al. observed a notable rise in vascularity during the early stages of OSF. As OSF advanced, there was a discernible down-regulation in VEGF expression [35]. These findings imply the potential for symptom improvement in oral fibrosis through vascular reconstruction [35].
OLP presents as a persistent inflammatory condition with an autoimmune inflammatory origin [36]. Additionally, Hazzaa et al.‘s investigation uncovered notable immune expression of VEGF in the connective tissue of OLP compared to the control group [37], a finding consistent with Salem et al.‘s results [38]. Moreover, Tao et al. bolstered these findings by illustrating a strong correlation between VEGF immune expression, angiogenesis, and OLP lesions, in contrast to the control group [39]. It’s worth mentioning that immune cells like macrophages, along with resident cells like fibroblasts, release lymph-angiogenic factors, specifically VEGF-C and VEGF-A, in inflamed tissues, highlighting the crucial role of angiogenesis and lymph angiogenesis in OLP development. Furthermore, these discoveries suggest a significant link between angiogenesis and the diverse clinical manifestations of OLP lesions, providing fresh insights into underlying mechanisms and potential treatment strategies [37].
CK19 is a member of the broad category of intermediate filament proteins identified as the Keratin family, comprising approximately 20 polypeptides. The primary role of this family is to uphold the integrity of epithelial cells. These proteins serve as crucial indicators for the diagnosis and management of tumors [40]. The involvement of CK19 has been linked to the migration and infiltration of cancer cells, potentially contributing to the development of distant metastasis through its facilitation of extracellular breakdown and cell motility [41]. A recent study conducted by Rashid et al. revealed that in the patients’ group, 19 individuals showed positive CK19 markers, yielding a sensitivity of 53%. In contrast, the normal group had a total of eight positive cases out of 36 [27]. A statistical analysis employing a two-sample binomial test demonstrated a significant difference in the number of positive instances between the two groups (P-value = 0.011). The researchers concluded that these findings establish a diagnostic screening tool for the early detection of OSCC in its initial stages. To enhance the reliability of the results, it is recommended to carry out further research with larger sample sizes [27].
There is limited literature regarding studies on CK19 gene expression in OPMD. However, several studies utilizing immunohistochemistry have been conducted, shedding light on the involvement of CK-19 in OPMDs. Investigating the role of CKs in OLP has the potential to enhance the understanding and differential diagnosis of this condition. Researchers analyzed the expression of CK10, CK13, CK14, and CK19 in OLP cases, considering their significant expression in the healthy oral mucous membrane [42, 43]. The expression pattern of CK-19 in the healthy nonkeratinized oral mucous membrane shows inconsistencies: Vander Velden described the heterogeneous expression of CK-19 in both the basal and suprabasal regions of the epithelial cells [44]. Referring to a study by Maeda et al., flow cytometry analysis of OLP tissues revealed six specimens with consistent staining in the basal layer, four areas without staining, and three areas showing no staining at all [45]. Boisnic et al. reported positive staining of keratin 19 in multiple basal regions [42]. The research illustrated that as OLP damage progresses, keratinized epithelial cell layers may eventually express CK-19 [46].
OLK serves as an early indicator of oral epithelial malignancy, demanding a thorough investigation to comprehend molecular changes during its transformation and prevent its progression. Multiple molecular-biological and clinicopathological studies have enhanced our understanding of cytokeratin expression alterations in cancer biology [47]. Intermediate filaments (IFs) play a crucial role in the biological features and developmental stages of epithelial organ cells. In the basal layers of basal cells, Ck 5 and Ck 14 signify stratified epithelium associated with cell proliferation [48]. The intermediate layers of keratinizing stratified epithelium express CKs 1 and 10, indicating cellular differentiation [48]. Conversely, glandular epithelia exhibit distinct CKs 8/18 and 19. Alterations in precancerous oral epithelium may be connected to the expression of CKs 8/18 and 19 in the suprabasal layer [47].
In a study conducted by Fillies et al., immunohistochemical staining was utilized to demonstrate a significantly higher expression of CK19 in cases of epithelial transformation. The research observed a statistically significant difference in expression levels between OLK and OSCC (p < 0.01), as well as between OLK and oral lichenoid dysplasia (OLD) (p = 0.021). Similar findings are well-documented in the existing literature, where Ck 19 expression is frequently noted in dysplastic lesions [49, 50] and is primarily considered an indicator of impaired epithelial differentiation [51].
Cytokeratin fragment antigen 21 − 1 (CYFRA21-1), resulting from caspase-3 action on CK-19, has been detected in the serum and saliva of patients with fibrosis, including OSF, and those with malignancy [52]. The decrease in CK-19 and the appearance of CYFRA21-1 in OSF are likely linked to stromal hypoxia-induced activation of caspase-3 and − 9 [53, 54]. In OSF, where CK-19 is reduced, its elevation is observed in OED [52, 55]. Moreover, CK-19 exhibits a gradual increase with advancing grades of OED and OSCC [55, 56]. Its expression is particularly elevated at the invasive edge, serving as an independent indicator of unfavorable prognosis [56, 57]. Although the mechanism underlying changes in CK-19 expression during the malignant transformation of OSF remains unexplored, the heightened CK-19 expression in OED, OSCC, and malignant OSF could be associated with increased matrix stiffness due to progressive matrix cross-linking. The augmented stiffness in OSF mucosa is evidenced by a thickened basement membrane, subepithelial fibrosis, and elevated collagen density [58]. Therefore, studies with substantial sample sizes are imperative to elucidate the role of CK-19 in both OSCC and OPMD.
In our study, no significant difference was observed in the intensity levels of VEGF-A and CK-19 among OSCC, OSF, OLP, and OLK unless considered in the context of disease vs. healthy groups, such as OSCC vs. HC, OSF vs. HC, OLP vs. HC, and OLK vs. HC. However, when combined, these two markers demonstrated effectiveness in detecting OSCC alone with relatively high sensitivity and specificity (refer to Table 3) (p = 0.023). Therefore, our study suggests that CK-19 and VEGF do not play a significant role independently in the transformation of OPMD to OSCC. Even though, these findings indicate significant overexpression of these genes in diseased states compared to healthy controls, suggesting their potential role in the pathogenesis of these conditions. Further gene expression studies on different cytokeratin’s and VEGF individually are warranted to explore broader diagnostic applications in the future, thereby enhancing their diagnostic and prognostic implications in early detection of oral cancer and OPMD.
Acknowledgements
We are grateful to the staff of the Diagnostic Clinic and the Oral Maxillofacial Clinic in the Department of Oral Medicine at the Faculty of Dental Sciences, University of Peradeniya, as well as to other colleagues in Oral Surgery and Oral Medicine who referred patients for this study.
Abbreviations
- AUC
Area Under the Curve
- CYFRA21-1
Cytokeratin fragment antigen 21 − 1
- CK-19
Cytokeratin-19
- OLK
Oral Leukoplakia
- OLP
Oral Lichen Planus
- OLD
Oral lichenoid dysplasia
- OPMDs
Oral potentially malignant disorders
- OSCC
Oral squamous cell carcinoma
- OSF
Oral Submucous Fibrosis
- RT-PCR
Real-time reverse transcription polymerase chain reaction
- ROC
Receiver operating characteristic
- VEGF-A
Vascular Endothelial Growth Factor-A
Author contributions
Study conception and design: KS, TANM, and RJ; Data analysis and interpretation: KS, TANM; Writing original manuscript: KS; Writing reviewing and editing manuscript: KS, TANM, NUJ, CUG, KKK, and RJ; Supervision: TANM, NUJ, JR, CUG, UP, BR, KKK, and RJ; Publication funding acquisition: KKK; All authors have read and approved the manuscript.
Funding
This research was supported by funding from the National Research Council of Sri Lanka (Grant number 22031), University Research Grants from Sri Jayewardenepura University (Grant number FMS/Q-12/2021), and a University Research Grant from the University of Peradeniya (D/F/CROC/URG/2022/29/D/CL/07).
Data availability
The authors confirm that the data underpinning the findings of this study can be obtained from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The study was conducted at the Dental Teaching Hospital, University of Peradeniya, Sri Lanka, following approval from the Ethical Review Committee of the Faculty of Dental Sciences, University of Peradeniya (protocol number: ERC/FDS/UOP/E/2021/14). All patients provided written informed consent prior to participation in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papanakou S, Nikitakis N, Sklavounou-Andrikopoulou A. Epidemiology, etiology and prevention of oral cancer. Archives Hellenic Medicine/Arheia Ellenikes Iatrikes. 2013;30(5).
- 2.Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Thomas G, Anju G, Mathew B. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013;49(4):314–21. 10.1016/j.oraloncology.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–16. 10.1016/j.oraloncology.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Ferlay JS, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: IARC CancerBase. Globocan. 2012;2013(1):11. [DOI] [PubMed] [Google Scholar]
- 5.Psoter WJ, Morse DE, Sánchez-Ayendez M, Vélez Vega CM, Aguilar ML, Buxó-Martinez CJ, Psoter JA, Kerr AR, Lane CM, Scaringi VJ, Elias A. Increasing opportunistic oral cancer screening examinations: findings from focus groups with general dentists in Puerto Rico. J Cancer Educ. 2015;30:277–83. 10.1007/s13187-014-0679-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocklehurst P, Kujan O, O’Malley L, Ogden GR, Shepherd S, Glenny AM. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Reviews. 2013(11). [DOI] [PMC free article] [PubMed]
- 7.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–5. 10.1126/science.6823562 [DOI] [PubMed] [Google Scholar]
- 8.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–9. 10.1126/science.2479986 [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–74. 10.1038/nature04483 [DOI] [PubMed] [Google Scholar]
- 10.Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, De Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci. 1997;94(14):7192–7. [DOI] [PMC free article] [PubMed]
- 11.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N. Bevacizumab plus Irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 12.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 13.Deng YT, Chen HM, Cheng SJ, Chiang CP, Kuo MY. Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: modulation by curcumin. Oral Oncol. 2009;45(9):e99–105. 10.1016/j.oraloncology.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin cancer Research: Official J Am Association Cancer Res. 1996;2(10):1679–84. [PubMed] [Google Scholar]
- 15.Yu JX, Zhang XT, Liao YQ, Zhang QY, Chen H, Lin M, Kumar S. Relationship between expression of CD105 and growth factors in malignant tumors of gastrointestinal tract and its significance. World J Gastroenterology: WJG. 2003;9(12):2866. 10.3748/wjg.v9.i12.2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79(9):1553–63. 10.1038/sj.bjc.6690248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berns EM, Klijn JG, Look MP, Grebenchtchikov N, Vossen R, Peters H, Geurts-Moespot A, Portengen H, van Staveren IL, Meijer-van Gelder ME, Bakker B. Combined vascular endothelial growth factor and TP53 status predicts poor response to tamoxifen therapy in estrogen receptor-positive advanced breast cancer. Clin Cancer Res. 2003;9(4):1253–8. [PubMed] [Google Scholar]
- 18.Dissanayaka DW, Wijeratne KM, Amarasinghe KA, Jayasinghe RD, Jayasooriya PR, Mendis BR, Lombardi T. A preliminary study on early detection of oral Cancer with opportunistic screening: insights from Dental surgeons in Sri Lanka. Cancers. 2023;15(23):5511. 10.3390/cancers15235511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jou YJ, Lin CD, Lai CH, Chen CH, Kao JY, Chen SY, Tsai MH, Huang SH, Lin CW. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal Chim Acta. 2010;681(1–2):41–8. 10.1016/j.aca.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 20.Moshref BN. Expression of CK19-mRNA and CEA-mRNA biomarkers in pleural fluid of patients with non-small cell lung cancer.
- 21.Alix-Panabières C, Vendrell JP, Slijper M, Pellé O, Barbotte E, Mercier G, Jacot W, Fabbro M, Pantel K. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. 2009;11:1–0. 10.1186/bcr2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olszewska E, Sudhoff H. Comparative cytokeratin distribution patterns in cholesteatoma epithelium. Histol Histopathol. 2007. [DOI] [PubMed]
- 23.Oloomi M, Yardehnavi N, Bouzari S, Moazzezy N. Non-coding CK19 RNA in peripheral blood and tissue of breast cancer patients. Acta Medica Iranica. 2013:75–86. [PubMed]
- 24.Leelawat K, Narong S, Udomchaiprasertkul W, Wannaprasert J, Treepongkaruna SA, Subwongcharoen S. Ratanashu-Ek T. Prognostic relevance of circulating CK19 mRNA in advanced malignant biliary tract diseases. World J Gastroenterology: WJG. 2012;18(2):175. 10.3748/wjg.v18.i2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremmer F, Ströbel P, Jarry H, Strecker J, Gaisa N, Strauß A, Schweyer S, Radzun HJ, Behnes CL. CK19 is a sensitive marker for yolk sac tumours of the testis. Diagn Pathol. 2015;10:1–7. 10.1186/s13000-015-0243-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30(6):503–12. 10.1016/S0301-472X(02)00806-8 [DOI] [PubMed] [Google Scholar]
- 27.Rashid F, Naji T, Mohamadnia A, Bahrami N. mRNA biomarkers for detection of oral squamous cell cancer. Asian Pac J Cancer Care. 2018;3(1):1. 10.31557/apjcc.2018.3.1.1 [DOI] [Google Scholar]
- 28.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–4. 10.1038/362841a0 [DOI] [PubMed] [Google Scholar]
- 30.Edirisinghe ST, Weerasekera M, De Silva DK, Devmini MT, Pathmaperuma S, Wijesinghe GK, Nisansala T, Maddumage A, Huzaini H, Rich AM, De Silva H. Vascular endothelial growth factor A (VEGF-A) and vascular endothelial growth factor receptor 2 (VEGFR-2) as potential biomarkers for oral squamous cell carcinoma: a Sri Lankan study. Asian Pac J cancer Prevention: APJCP. 2023;24(1):267. 10.31557/APJCP.2023.24.1.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61. 10.1038/339058a0 [DOI] [PubMed] [Google Scholar]
- 32.Gandolfo M, Keszler A, Lanfranchi H, Itoiz ME. Increased subepithelial vascularization and VEGF expression reveal potentially malignant changes in human oral mucosa lesions. Oral surgery, oral medicine, oral Pathology, oral Radiology, and endodontology. 2011;111(4):486–93. [DOI] [PubMed]
- 33.Rai DV, Guttal KS, Kulkarni BB, Hiremath S, Burde KN. Vascular endothelial growth factor (VEGF) gene polymorphism in oral submucous fibrosis subjects-A preliminary study. Asian J Med Sci. 2016;7(5):10–6. 10.3126/ajms.v7i5.15064 [DOI] [Google Scholar]
- 34.Sharada P, Swaminathan U, Nagamalini BR, Kumar KV, Ashwini BK, Lavanya VL. Coalition of E-cadherin and vascular endothelial growth factor expression in predicting malignant transformation in common oral potentially malignant disorders. J Oral Maxillofacial Pathol. 2018;22(1):40–7. 10.4103/jomfp.JOMFP_13_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma E, Tyagi N, Gupta V, Narwal A, Vij H, Lakhnotra D. Role of angiogenesis in oral submucous fibrosis using vascular endothelial growth factor and CD34: an immunohistochemical study. Indian J Dent Res. 2019;30(5):755–62. 10.4103/ijdr.IJDR_186_17 [DOI] [PubMed] [Google Scholar]
- 36.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: an update on pathogenesis and treatment. J oral Maxillofacial Pathol. 2011;15(2):127–32. 10.4103/0973-029X.84474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazzaa HH, El Shiekh MA, Abdelgawad N, Gouda OM, Kamal NM. Correlation of VEGF and MMP-2 levels in oral lichen planus: an in vivo immunohistochemical study. J oral Biology Craniofac Res. 2020;10(4):747–52. 10.1016/j.jobcr.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salem SA, Aly DG, Youssef NS. Immunohistochemical assessment of angiogenesis and vascular endothelial growth factor expression in cutaneous lichen planus: relation to the degree of inflammation. Eur J Dermatology. 2011;21(2):197–202. 10.1684/ejd.2011.1221 [DOI] [PubMed] [Google Scholar]
- 39.Tao X, Huang Y, Li R, Qing R, Ma L, Rhodus NL, Cheng B. Assessment of local angiogenesis and vascular endothelial growth factor in the patients with atrophic-erosive and reticular oral lichen planus. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2007;103(5):661-9. [DOI] [PubMed]
- 40.Jou YJ, Lin CD, Lai CH, Tang CH, Huang SH, Tsai MH, Chen SY, Kao JY, Lin CW. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clin Chim Acta. 2011;412(15–16):1357–65. 10.1016/j.cca.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 41.Lee YM, Song DE, Kim TY, Sung TY, Yoon JH, Chung KW, Hong SJ. Risk factors for distant metastasis in patients with minimally invasive follicular thyroid carcinoma. PLoS ONE. 2016;11(5):e0155489. 10.1371/journal.pone.0155489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boisnic S, Ouhayoun JP, Branchet MC, Frances C, Beranger JY, Le Charpentier Y, Szpirglas H. Alteration of cytokeratin expression in oral lichen planus. Oral surgery, oral medicine, oral Pathology. Oral Radiol Endodontology. 1995;79(2):207–15. [DOI] [PubMed] [Google Scholar]
- 43.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. 10.1016/0092-8674(82)90400-7 [DOI] [PubMed] [Google Scholar]
- 44.van der Velden LA, Manni JJ, Ramaekers FC, Kuijpers W. Expression of intermediate filament proteins in benign lesions of the oral mucosa. European archives of oto-rhino-laryngology. 1999;256:514–9. [DOI] [PubMed]
- 45.Maeda H, Reibel J, Holmstrup P. Keratin staining pattern in clinically normal and diseased oral mucosa of lichen planus patients. Eur J Oral Sci. 1994;102(4):210–5. 10.1111/j.1600-0722.1994.tb01182.x [DOI] [PubMed] [Google Scholar]
- 46.Jacques CM, Pereira AL, Maia V, Cuzzi T, Ramos-e-Silva M. Expression of cytokeratins 10, 13, 14 and 19 in oral lichen planus. J Oral Sci. 2009;51(3):355–65. 10.2334/josnusd.51.355 [DOI] [PubMed] [Google Scholar]
- 47.Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep. 2007;18(3):639–43. [PubMed] [Google Scholar]
- 48.Presland RB, Jurevic RJ. Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J Dent Educ. 2002;66(4):564–74. 10.1002/j.0022-0337.2002.66.4.tb03536.x [DOI] [PubMed] [Google Scholar]
- 49.Reibel J. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Reviews Oral Biology Med. 2003;14(1):47–62. 10.1177/154411130301400105 [DOI] [PubMed] [Google Scholar]
- 50.Takeda T, Sugihara K, Hirayama Y, Hirano M, Tanuma JI, Semba I. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J oral Pathol Med. 2006;35(6):369–75. 10.1111/j.1600-0714.2006.00444.x [DOI] [PubMed] [Google Scholar]
- 51.Su L, Morgan PR, Lane EB. Keratin 14 and 19 expression in normal, dysplastic and malignant oral epithelia. A study using in situ hybridization and immunohistochemistry. J oral Pathol Med. 1996;25(6):293–301. 10.1111/j.1600-0714.1996.tb00265.x [DOI] [PubMed] [Google Scholar]
- 52.Rajkumar K, Ramya R, Nandhini G, Rajashree P, Ramesh Kumar A, Nirmala Anandan S. Salivary and serum level of CYFRA 21-1 in oral precancer and oral squamous cell carcinoma. Oral Dis. 2015;21(1):90–6. 10.1111/odi.12216 [DOI] [PubMed] [Google Scholar]
- 53.Garnier P, Prigent-Tessier A, Van Hoecke M, Bertrand N, Demougeot C, Sordet O, Swanson RA, Marie C, Beley A. Hypoxia induces caspase‐9 and caspase‐3 activation without neuronal death in gerbil brains. Eur J Neurosci. 2004;20(4):937–46. 10.1111/j.1460-9568.2004.03551.x [DOI] [PubMed] [Google Scholar]
- 54.Veeravarmal V, Austin RD, Siddavaram N, Thiruneelakandan S, Nassar MH. Caspase-3 expression in normal oral epithelium, oral submucous fibrosis and oral squamous cell carcinoma. J Oral Maxillofacial Pathol. 2016;20(3):445–52. 10.4103/0973-029X.190947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safadi RA, Musleh AS, Al-Khateeb TH, Hamasha AA. Analysis of immunohistochemical expression of k19 in oral epithelial dysplasia and oral squamous cell carcinoma using color deconvolution-image analysis method. Head Neck Pathol. 2010;4:282–9. 10.1007/s12105-010-0210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khanom R, Sakamoto K, Kumar Pal S, Shimada Y, Morita KI, Omura K, Miki Y, Yamaguchi A. Expression of basal cell keratin 15 and keratin 19 in oral squamous neoplasms represents diverse pathophysiologies. [DOI] [PubMed]
- 57.Ernst J, Ikenberg K, Apel B, Schumann DM, Huber G, Studer G, Rordorf T, Riesterer O, Rössle M, Korol D, Bredell MG. Expression of CK19 is an independent predictor of negative outcome for patients with squamous cell carcinoma of the tongue. Oncotarget. 2016;7(46):76151. 10.18632/oncotarget.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das RK, Anura A, Pal M, Bag S, Majumdar S, Barui A, Chakraborty C, Ray AK, Sengupta S, Paul RR, Chatterjee J. Epithelio-mesenchymal transitional attributes in oral sub-mucous fibrosis. Exp Mol Pathol. 2013;95(3):259–69. 10.1016/j.yexmp.2013.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data underpinning the findings of this study can be obtained from the corresponding author upon request.