Abstract
Background
In Ethiopia, 79 million people live in soil transmitted helminths endemic areas. The Ethiopia established a National goal to eradicate STH transmission by 2025. To meet that goal, it is imperative that data is acquired on community helminth infection risk. This study examined the prevalence of STH and risk factors for infection in vegetable farmers working on Akaki River Bank, Addis Ababa, Ethiopia.
Methods
A cross-sectional study was conducted between November 7, 2022, and June 2023. A stratified random sampling was used to select farming households. Two hundred and sixteen farmers were enrolled in the study. Data on socio-demographic, WASH, wastewater irrigation related factors were collected by trained data collectors using a structured questionnaire. Kato-Katz concentration was utilized to detect STH. The data were entered using EpiData 3.1 and analyzed with Stata 14.0, using p-values less than 0.05 to identify significant factors. Logistic regression was used to identify independent risk factors for infection.
Results
The prevalence of STH was 22.2% (95% CI = 13.6-27.9%), with Ascaris lumbricoides being the most common (11.1%), followed by hookworm (7.4%), and Trichuris trichiura (3.7%). Low income levels (AOR = 1.85, 95% CI = 1.25–5.99), lack of handwashing before eating (AOR = 2.25, 95% CI = 1.58 − 11.3), absence of fingernails cleanliness (AOR = 1.97, 95% CI = 1.74–39.5), not wearing shoes at work (AOR = 3.4, 95% CI = 2.98–82.2), touching the face with dirty hands (AOR = 2.9, 95% CI = 0.68–28.2), washing vegetables with irrigation wastewater (AOR = 2.1, 95% CI = 1.95–45.2), and not wearing protective clothing during farming activities (AOR = 2.99, 95% CI = 1.58 − 22.4) were the significant risk factors for infection with STH.
Conclusion
Of the farming communities examined in this study, one of the five was found to be infected with soil transmitted helminth. This research has shown clear risk factors for STH infection including: lack of personal hygiene practices, insufficient sanitation access, and limited use of protective equipment. To achieve the national goal, there is a need for farming communities to understand preventative risks of infection, improve WASH (Water access, sanitation and hygine) practices, WASH access, protective equipment, and health education.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09704-3.
Keywords: STH, Irrigation, Wastewater, Farmers, Akaki river
Introduction
Soil transmitted helminths (STH) infections are widespread, affecting more than one point five billion people globally [1]. The most common STH species are Ascaris lumbricoides, hookworms (Ancylostoma duodenale and Necator americanus), and Trichuris trichiura [2]. These infections are most prevalent in low-income countries, particularly in tropical and subtropical regions with poor sanitation and hygiene practices. The World Health Organisation (WHO) estimates that over 568 million school-age children live in areas where STH is intensively transmitted, with the highest burden of disease found in sub-Saharan Africa, Southeast Asia, and Latin America [1&2].
In many urban and peri-urban African countries, including Ethiopia, untreated wastewater is released into water bodies, subsequently used for irrigation [3]. This practice is a major public health concern, especially where access to safe water and adequate sanitation is limited. Irrigation with untreated or inadequately treated wastewater can lead to contamination of soil and vegetables with STH eggs and larvae [2]. Contamination increases the prevalence of helminth infection among farmers and nearby communities [4]. STH infections can cause health issues, including anemia, malnutrition, impaired cognitive development, and reduced physical fitness [5].
Ethiopia is a populous, low-income African country with a high prevalence of soil-transmitted helminth infections. Around 79 million people live in helminth-endemic areas [6]. As a result, Ethiopia has set a national target of eliminating STH transmission by 2025, meeting the new WHO goals of “achieving and maintaining elimination of STH morbidity in pre-school children by 2030 and reducing moderate and heavy intensity STH infections in pre-school and school-age children to less than 2%” [6]. In order to prevent infection, farmers who are also at risk of infection must be included in the existing target population to increase the impact of the interventions and eliminate STH as a public health concern.
The Akaki River is one of the primary rivers that passes through Addis Ababa and it is frequently used for socioeconomic development purposes. Urban and peri-urban farmers use this river to cultivate vegetables along the river banks, supplying the City with fresh vegetables. Previous, studies have reported microbiological and parasite contamination in Akaki river water, vegetables, and soil [7–11], trace metal distribution in river sediments [12–14], and heavy metal contamination of vegetables [15]. Studies have also looked at combined hazards and health risks from vegetables irrigated from the Akaki river [16], physicochemical parameters in river water [17], as well as farmers’ perception of irrigation river water quality and health risk [17, 18], despite adequate legislation, there are significant issues with management of sewage with industries and municipal administrators failing to manage waste water treatment [16, 18–20]. To date, no study that have investigated the risk factors for the prevalence of STH infection. To National goals communities infected with STH or at risk infection, need to be identified. This study therefor aims to investigate the prevalence of infection and risk factors for infection in communities using wastewater irrigation farming for vegetables along the Akaki River Bank, Addis Ababa.
Materials and methods
Study area
A study was conducted on the Akaki River bank in Addis Ababa, Ethiopia. The Akaki River is formed by the confluence of two rivers, the Little and Great Akaki. The river flows in to Aba-Samuel reservoir and forms part of the larger Awash River catchment, which flows into the eastern parts of Oromia [20].
Historically the river was much cleaner in the early days of the foundation of the city. However, the situation has changed over time as a consequence of industrialization, urbanization and population growth. According to a study by Mekonnen in 2020, excessive amounts of organic, chemical, and microbiological contaminants, including human excreta, were found in the Akaki river [21]. Farmers unfortunately, use untreated wastewater from the Akaki river directly for vegetable irrigation. The use of the polluted river for irrigation in the city is not the farmers’ choice; rather, it is what is available to maintain the economic livelihood of poor farm households. Polluted irrigation water is easily available and requires minimal artificial fertilizer for crop growth, therefore providing a reasonable income source for many producers, allowing them to supply fresh vegetables at low prices to the nearby cities.
Addis Ababa comprises both modern and slum areas with most of the irrigated farmland located in slum areas, in the three sub-cities of Nifas Silk Lafto, Bole, and Akaki Kality. According to population projections for 2023, the estimated population in Nifas Silk Lafto is 455,500, Bole is 445,013, and Akaki Kality is 260,967 [22].
In these areas, 33% of households share a toilet with more than six households. Additionally, 35% of the generated solid and liquid waste is never collected and is dumped in nearby rivers, and 71% of households lack adequate sanitation facilities [23]. Municipally piped drinking water supplied to 91 and 100% of household in Nifas Silk Lafto and Bole, but 0–60% households in Akaki as of 2017. There are 96 health centres, 11 public hospitals, and 28 private hospitals in Addis Ababa. Currently, the ninety-six health centres serve 11 districts in Addis Ababa, and the bureau aims to have at least one health centres in each sub-cities [22].
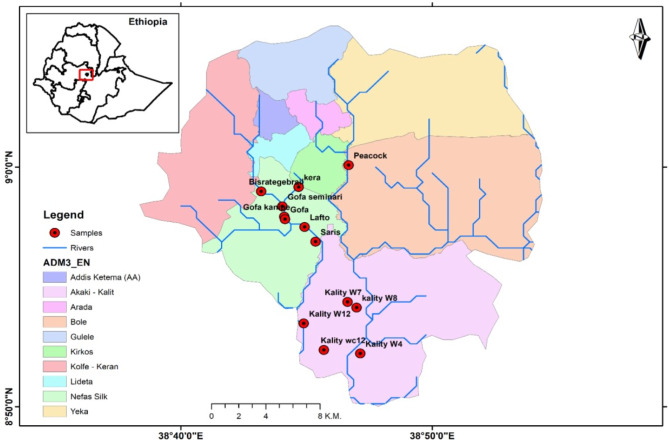
The study area included seven vegetable farming sites known locally as Bisrategabriel, Gofa, Lafto, Saries, and Kera, which were irrigated with the little Akaki River, Peacock-Urael, and Akaki-Kality irrigated by the Great Akaki River (Fig. 1). The majority of farmers on Akaki-Kality, farm sites use diesel motor pumps to extract wastewater straight from the river and transfer it to the farm via connected plastic pipes. Farmers used sandbags and coarse stones to build traditional weirs at other farming locations [17].
Fig. 1.
Map of the study area along the Akaki River bank in Addis Ababa, Ethiopia [8]
Study design
A community-based cross-sectional study was conducted from November 7, 2022 to June 2023 to determine the prevalence and risk factors of soil transmitted helminths among wastewater irrigation vegetable farmers in Akaki Rivers Bank, Addis Ababa.
Population
Source population
The study source population consisted of Akakir River bank vegetable farmers who cultivate vegetables using the contaminated river water for irrigation along the River bank in Addis Ababa.
Study population
The study population included of wastewater irrigated vegetable farmers. Sampled purposively from those willing to participate, along the Akakir River bank in the city. Farmer households near the farmland were considered in order to determine the cross-contamination of STH from the farmlands to the households.
Inclusion and exclusion criteria
Inclusion criteria
Wastewater farmers recruited into the study met the following inclusion criteria: - (a) Farmers who have a sub-city identification card and have lived in the area for more than 10 years. (b) Those who use the Akaki River for irrigation; - (c) Farmers engaged in growing vegetables only. A ten-year timeline was established based on the country’s land patent policy, which is required for farmers to obtain a land patent. Farmers who have obtained patents are legally entitled to rent or cultivate land. This assures that the farmers had a legal right to cultivate the land and had a long-standing connection to the area. Those with land patents were able to participate as they had a legal right to farm and could therefore participate in the study without fear.
Exclusion criteria
The following exclusion criteria were considered: - (a) Daily labourers: they work on the farm but do not reside near the farmland, which made collecting household data challenging. Furthermore, because they do not have a permanent farm to cultivate and are mobile, the research population may not be representative of the local farming community being examined in this study. (b) Farmers who used more than one river water source for irrigation were also excluded as this would make source tracking of helminths difficult.
Sample size
The sample size was determined using the single population proportion formula N = Z2P(1-P)/D2 [24]. By considering a prevalence of 18% in a study conducted by Zeynudin A et al., in 2022 [25], a marginal error of 5%, a 95% confidence interval, and 10% non-response rates. Hence, the calculated sample size was 250. Where Z = 95% confidence interval, P = Estimated prevalence rate, D = Marginal of sampling error, N = Total study population.
Sampling technique
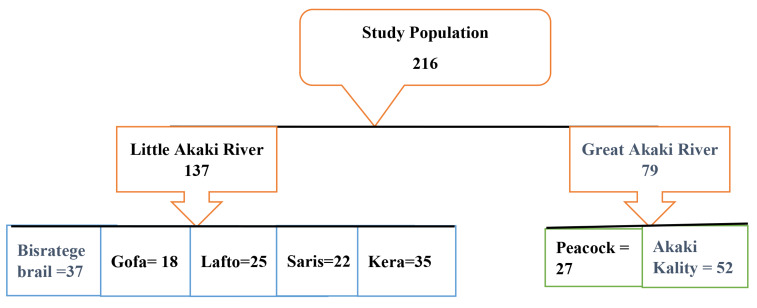
A stratified random sampling method was utilized. Based on the inclusion criteria, 52 households were involved to participate in the study. The sample size for each district was determined through proportionate allocation to the seven study sites. Out of a potential study population of 250 farmers, 216 were willing to participate and were enrolled in the study (Fig. 2).
Fig. 2.
Proportional allocation of study population along Akakir River Addis Ababa, Ethiopia
Data collection
A household socioeconomic and farm baseline survey was conducted using a structured questionnaire, along with interviews and observations. The questionnaire was first prepared in English and then translated into the local language (Amharic and Afan Oromo) and back translated to English by an anonymous translator to see the consistency of the two versions. Before commencing the study, a pilot study was conducted on 5% of the calculated sample size at a nearby farming site that was not included in the study to test and validate proposed questionnaire. Amendments were subsequently made to the data collection tool as per the finding of the pre-test.
The prevalence of STH was assessed using a health diary and self-reporting sheet. Data collectors were trained by the principal investigator to ensure standardization of data collection at different sites. Completed questionnaires were collected at the end of each working day, organized, and handed over to the researcher.
Stool sample collection and examination
A clean, leak-proof, screw-cap stool cup with a unique identifier was used to gather a 2gm stool sample from each study participant. The collected specimens were brought to the laboratory of EPHI. According to WHO standards, a Kato-Katz smear was prepared for each sample and examined microscopically to identify helminth eggs and quantify the intensity of infections based on fecal egg count [2]. The Kato-Katz slides were made as soon as the stool specimen arrived in the laboratory.
Data quality management
Training was given to data collectors on techniques of structured questionnaire collation and general approaches to communicate study population. The accuracy of the laboratory results was controlled by correctly labelling, calibrating equipment, and following standard operating procedures. The laboratory data collection sheet was used to collect lab data, which was then quickly entered into EpiData version 3.1. Errors were corrected by revisiting the original questionnaire and responses. Microsoft Excel® was used to construct figure.
Data analysis
The data were statistically analysed with STATA Version 14.0. The explanatory factors were classified into three major categories: socio-demographic, WASH and farming practices. To determine the association between helminth infection and various factors, multivariable analyses were performed. In the multivariable analysis, potential confounders were controlled to examine the association between occupational exposure factors and helminths. Multi-collinearity of variables was assessed using the variance inflation factor (VIF). Variables with p < 0.05 in the AOR analysis were considered statistically significant and independently associated factors with helminths.
Results
The socio‑demographic characteristics of the study participants
A total of 216 farmers using waste water for irrigation participated in the study with an 86% response rate. Table 1 depicts the socio-demographic factors of the wastewater irrigation farming community. The highest proportion of homes examined was in Akaki district 52 (24.1%), followed by Bisrategebrail 37 (17.1%), and Kera 35 (16.2%). Eighty-three (38.4%) of the study participants were between the ages of 41 and 50, and 163 (75.5%) were male. More than half of the respondents 116 (53.7%) had no formal education. The family size statistics showed that 136 (63%) of families had 4–6 members and live in dwellings with 3–4 rooms-house. Moreover, 78 (36.1%) households’ income was between 3001 and 5000 ETB per month (Table 1).
Table 1.
Socio-demographic characteristics of wastewater irrigation farming community along Akaki River bank Addis Ababa, Ethiopia, 2023 (n = 216)
| Factors | Categories | Frequency (%) |
|---|---|---|
| Districts | Bisrategebrail | 37 (17.1) |
| Gofa | 18 (8.3) | |
| Lafto | 25 (11.6) | |
| Saris | 22 (10.2) | |
| Kera | 35 (16.2) | |
| Peacock | 27 (12.5) | |
| Akaki | 52 (24.1) | |
| Age | < 30 | 15 (6.9) |
| 31–40 | 50 (23.2) | |
| 41–50 | 83 (38.4) | |
| > 50 | 68 (31.5) | |
| Sex | Male | 163 (75.5) |
| Female | 53 (24.5) | |
| Education | Primary education | 86 (39.8) |
| Secondary and above | 14 (6.5) | |
| No formal education | 116 (53.7) | |
| Family Size | 1–3 | 34 (15.7) |
| 4–6 | 136 (63) | |
| > 6 | 46 (21.3) | |
| Number of rooms | 1–2 | 85(39.4) |
| 3–4 | 96(44.4) | |
| > 4 | 35(16.2) | |
| Income (ETB) | < 1000 | 39(18.1) |
| 1001–3000 | 46(21.3) | |
| 3001–5000 | 78(36.1) | |
| > 5000 | 53(24.5) |
Socio-demographic factors
Female farmers had a significantly higher prevalence of STH than males (39.6% vs. 16.5%,). They were 1.55 times more vulnerable for STH infection (OR: 1.55, 95% CI = 0.78–2.20). Participants above 50 years of age were 1.85 times more likely to be STH-positive than those under 50 years of age, (OR: 1.85, 95% CI = 1.15–2.91).
Education was one of the most significant predisposing factors of the prevalence of STH as, of the 86 primary education attended farmers, only 15.1% were positive for STH. Farmers who had not attained formal education had 1.62 times odds of STH (OR: 1.62, 95% CI = 0.25–1.78). Living in relatively cramped homes with only 1–2 rooms was also associated with greater odds of STH infection (OR: 1.51, 95% CI = 0.56–2.48). Prevalence of STH infection in families with more than six family members was 1.72 times higher than in families having four to six family members (OR: 1.72, 95% CI = 0.94–2.89). The majority of households with monthly incomes of less than 1000 Ethiopian Birr (ETB) were infected with STH at 84.6%. Income under 1000 ETB carried 2.91 times the risks of STH (OR: 2.91,95% CI = 1.33–3.66) as compared to the highest income group above 3000 ETB. Therefore, in bivariate analysis sex, older age, illiteracy, large families, packed housing, and low income were significantly associated with STH prevalence (Table 2).
Table 2.
Prevalence of STH disease in relation to socio-demographic factors, among wastewater irrigation farming area of Akaki River bank, Addis Ababa, Ethiopia, 2023. (n = 216)
| Factors | Categories | STH Prevalence | Total (%) | Crude ORa (95% CI)b |
p-value | |
|---|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | |||||
| Districts | Bisrategebrail | 11(29.7) | 26 (70.3) | 37 (17.1) | 1.92 (1.22–2.05) | 0.008 |
| Gofa | 2 (11.1) | 16 (88.9) | 18 (8.3) | 1 | ||
| Lafto | 4 (16) | 21 (84) | 25 (11.6) | 1.41 (0.95–1.98) | 0.445 | |
| Saris | 2 (9.1) | 20 (90.9) | 22 (10.2) | 1.32 (0.98–1.79) | 0.498 | |
| Kera | 7 (20) | 28 (80) | 35 (16.2) | 1.70 (1.25–2.32) | 0.019 | |
| Peacock | 5 (18.5) | 22 (81.5) | 27 (12.5) | 1.65 (1.20–2.22) | 0.007 | |
| Akaki | 17 (32.7) | 35 (67.3) | 52 (24.1) | 1.98 (1.37–2.09) | ≤ 0.001 | |
| Age | < 30 | 3(20) | 12 (80) | 15 (6.94) | 1 | |
| 31–40 | 28 (56) | 22 (44) | 50 (23.1) | 1.52 (1.28–1.99) | 0.460 | |
| 41–50 | 49 (59.1) | 34 (41) | 83 (38.4) | 1.76 (0.96–1.77) | 0.010 | |
| > 50 | 43 (63.2) | 25 (36.8) | 68 (31.5) | 1.85 (1.15–2.91) | ≤ 0.001 | |
| Sex | Male | 27 (16.5) | 136 (83.4) | 163 (75.5) | 1 | |
| Female | 21 (39.6) | 32 (60.4) | 53 (24.5) | 1.55 (0.78–2.20) | 0.020 | |
| Education | Primary | 13 (15.1) | 73 (84.9) | 86 (39.8) | 0.84 (0.35–1.09) | |
| Secondary& above | 5 (35.7) | 9 (64.3) | 14 (6.5) | 1 | ||
| No formal education | 34 (29.3) | 82 (70.7) | 116 (53.7) | 1.62(0.25–1.78) | ≤ 0.001 | |
| Family Size | 1–3 | 5 (14.7) | 29 (85.3) | 34 (15.7) | 1 | |
| 4–6 | 24 (17.6) | 112 (82.3) | 136 (63) | 1.58 (1.32–2.49) | 0.300 | |
| > 6 | 19 (41.3) | 27 (58.7) | 46 (21.3) | 1.72 (0.94–2.89) | ≤ 0.001 | |
| Number of rooms | 1–2 | 23 (27.1) | 62 (72.9) | 85(39.3) | 1.51 (0.56–2.48) | 0.200 |
| 3–4 | 19 (19.8) | 77 (80.2) | 96(44.4) | 1.26 (0.77–1.87) | 0.041 | |
| > 4 | 6 (17.1) | 29 (82.8) | 35(16.2) | 1 | ||
| Income (ETB) | < 1000 | 33 (84.6) | 6 (15.4) | 39(18.1) | 2.91 (1.33–3.66) | 0.001 |
| 1001–3000 | 8 (17.4) | 38 (82.6) | 46(21.3) | 1.6 (0.68–1.83) | 0.004 | |
| 3001–5000 | 4 (5.1) | 74 (94.9) | 78(36.1) | 0.3 (0.09–1.41) | 0.073 | |
| > 5000 | 3 (5.7) | 50 (94.3) | 53(24.5) | 1 | ||
aOdd Ratio
b95% Confidence Interval
Prevalence soil-transmitted helminth infection
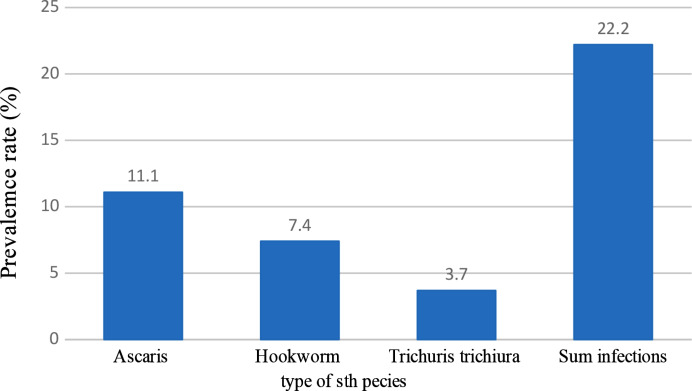
The overall prevalence of soil-transmitted helminth infection among farmers using wastewater for irrigation was 22.2% (48/216) (Fig. 3). As indicated in Fig. 3, the most common STH parasite identified was Ascaris lumbricoides, 11.1% (24/216), followed by hookworm 7.4% (16/216) and Trichuris trichiura 3.7% (8/216).
Fig. 3.
- Helminth infections in farmers’ stools along Akaki river bank Addis Ababa, Ethiopia, 2023
Water, sanitation, and hygiene (WASH) factors
The study identified several risk factors significantly associated with soil-transmitted helminths prevalence among farming communities. The absence of water near the toilet (OR: 2.0; 95% CI = 1.3–3.2) and not using soap for handwashing after using the toilet (OR: 2.42; 95% CI = 0.4–4.4) contributed to a higher likelihood existence of soil transmitted helminthiasis. Defecating outside the toilet (OR: 2.12; 95% CI = 1.3–5.8) and on the farm (OR 6.10; 95% CI = 3.9–7.3) were also high-risk factors for STH infection in the bivariable logistic regression (Table 3).
Table 3.
Prevalence of STH in relation to WASH factors, among wastewater irrigation farming area of Akaki River bank, Addis Ababa, Ethiopia, 2023. (n = 216)
| Factors | Categories | STH Prevalence | Total (%) | Crude ORa (95% CI)b |
p-value | |
|---|---|---|---|---|---|---|
| Positive n (%) |
Negative n (%) | |||||
| Water availability near the toilet | No | 97 (68.8) | 44 (31.2) | 141 (65.3) | 2.0 (1.3–3.2) | 0.010 |
| Yes | 4 (5.4) | 71 (94.7) | 75 (34.7) | 1 | ||
| Use of soap after toilet use | No | 136 (74.3) | 47 (25.7) | 183 (84.7) | 2.42 (0.4–4.4) | 0.020 |
| Yes | 1 (3.0) | 32 (96.9) | 33 (15.3) | 1 | ||
| Share toilet with other households | No | 7 (19.4) | 29 (80.6) | 36 (16.7) | 1 | |
| Yes | 139 (77.2) | 41 (22.7) | 180 (83.3) | 1.21 (0.7–3.5) | 0.661 | |
| Defecation outside toilet | No | 27 (15) | 153 (85) | 180 (83.3) | 1 | |
| Yes | 21 (58.3) | 15 (41.7) | 36 (16.7) | 2.12 (1.3–5.8) | ≤ 0.001 | |
| Defecate in the farm | No | 26 (15.4) | 143 (84.6) | 169 (78.2) | 1 | |
| Yes | 25 (53.2) | 22 (46.8) | 47 (21.8) | 6.10 (3.9–7.3) | ≤ 0.001 | |
| Hand washing before feeding | No | 50 (52.1) | 46 (47.9) | 96 (44.4) | 3.91 (2.5–5.4) | ≤ 0.001 |
| Yes | 1 (0.8) | 118 (99.2) | 119 (55.0) | 1 | ||
| Bathing after farm work | No | 129 (79.6) | 33 (20.4) | 162 (75) | 1.82 (1.4–2.6) | 0.002 |
| Yes | 8 (23.5) | 26 (76.5) | 34 (15.7) | 1 | ||
| Regular hand washing after work with soap | No | 51 (52.6) | 46 (47.4) | 97 (44.9) | 8.90(2.5–9.6) | ≤ 0.001 |
| Yes | 1 (0.8) | 117 (99.1) | 118 (54.6) | 1 | ||
| On-site hand washing after work | No | 51 (61.4) | 32 (38.5) | 83 (38.4) | 1.96(0.9–2.3) | ≤ 0.001 |
| Yes | 15 (11.4) | 117 (88.6) | 132 (61.1) | 1 | ||
| Fingernails | No | 59 (59.6) | 106 (40.4) | 165 (76.4) | 1.98 (1.5–2.3) | 0.005 |
| Yes | 7 (14.9) | 40 (85.1) | 47(21.7) | 1 | ||
| Always wash feet after work | No | 63 (69.2) | 28 (30.8) | 91 (42.1) | 2.91 (1.2–3.5) | 0.008 |
| Yes | 19 (15.2) | 106 (84.8) | 125 (57.9) | 1 | ||
| Wear Boots/shoes at work | No | 115 (73.7) | 41 (26.3) | 156 (72.2) | 11.2 (2.3 -5 2.1) | 0.004 |
| Yes | 7 (11.7) | 53 (88.3) | 60 (27.8) | 1 | ||
The majority of the farming communities’ hand washing practices were deplorable. Handwashing could prevent numerous types of intestinal diseases. More than half of the farmers who did not wash their hands or who did not use soap before feeding tested positive for STH. Those farmers practicing on-site hand washing after work, on the other hand, had 88.6% tested negative. The STH infection prevalence was 3.91 times more likely among those who did not wash their hands before eating (OR: 3.91, 95% CI = 2.5–5.4). Farmers who did not practice hand-washing with soap were 8.90 times more vulnerable to STH infection (OR: 8.90, 95% CI = 2.5–9.6). The odds of STH were also 1.96 times higher among those who did not practice on-site hand washing after work in the bivariable logistic regression (OR: 1.96, 95% CI = 0.9–2.3) (Table 3).
Similarly, in the bivariable logistic regression the study discovered that among 47 farmers who cleansed their fingernails regularly, only 7 tested positive for STH. However, those who engaged in wastewater farming and did not frequently clean their fingernails had 1.98 times risk of acquiring STH infection than those cleaned their fingernails (OR: 1.98, 95% CI = 1.5–2.3). A significant portion of study participants (75%) did not have a culture of bathing after work, had risks of STH infection (OR: 1.82, 95% CI = 1.4–2.6) (Table 3).
Furthermore, farmers who wore protective boots while working in the fields (27.8% of participants) had a lower prevalence of STH infection. 88% of those who wore boots tested negative for STH. Additionally, farmers who did not consistently clean their feet after farm work had 2.91 times had higher the odds of STH infection in the bivariable logistic regression (OR: 2.91, 95% CI = 1.2–3.5) (Table 3).
STH infection was substantially much more prevalent in wastewater farmers with poor hygiene, practice open defecation, and lack occupational precautions. So, these factors appear to play important roles in STH transmission among the farming communities.
Farming practices factors
The findings show that diverse risky ways of farming had a significant association with a prevalence of STH disease. Walking barefoot on the farm during irrigation (OR: 6.4, 95% CI = 1.6–25.5), hand contamination with soil and wastewater (OR: 20.4, 95% CI = 2.9-203.5), and touching the face with filthy hands (OR: 34.6, 95% CI = 8.14–147.6) all enhance the risk of STH transmission through skin and oral-fecal routes (Table 4). Working cloth contaminated with wastewater (OR: 1.77, 95% CI = 0.19–3.01) and wearing them at home (OR: 4.43, 95% CI = 1.8–10.8) led to transmission to residential surroundings. Washing vegetables with irrigation wastewater (OR:15.1, 95% CI = 5.05–47.3) and eating fallen food (OR:10.2, 95% CI = 1.92–52.8) are perils that increase food-related transmission. Exposures through drinking water at farm and chewing chat at farm were not significantly associated with STH prevalence. Lack of personal protection equipment during farming activities increases exposure risk (OR: 1.99, 95% CI = 0.49–5.52). The result indicated the urgent need to improve agricultural hygiene and deploy barrier measures in order to lower parasitic disease burden (Table 4).
Table 4.
Prevalence of STH in relation to farming practice factors, among wastewater irrigation farming area of Akaki River bank, Addis Ababa, Ethiopia, 2023 (n = 216)
| Factors | Categories | STH Prevalence | Total (%) | Crude ORa (95% CI)b |
p-value | |
|---|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | |||||
| Walking through the farm during irrigation barefoot | No | 9(17) | 44(83) | 53(24.5) | 1 | |
| Yes | 145(88.9) | 18(11) | 163(75.5) | 6.4(1.6–25.5) | 0.006 | |
| Hand contamination with soil and irrigation wastewater | No | 3(20) | 12(80) | 15(6.9) | 1 | |
| Yes | 172(85.6) | 29(14.2) | 201(93.0) | 20.4(2.9-203.5) | 0.002 | |
| Touch face with dirty hand | No | 21(20.3) | 82(79.6) | 103(47.7) | 1 | |
| Yes | 108(95.5) | 5(4.4) | 113(52.3) | 34.6(8.14–147.6) | ≤ 0.001 | |
| Cloth Contamination with the wastewater | No | 2(3.8) | 50(96.1) | 52(24.07) | 1 | |
| Yes | 85(51.8) | 79(48.1) | 164(75.9) | 1.77(0.19–3.01) | 0.005 | |
| Use working clothes at home | No | 6(6.4) | 88(93.6) | 94(43.5) | 1 | |
| Yes | 76(62.3) | 46(37.7) | 122(56.5) | 4.43(1.8–10.8) | ≤ 0.001 | |
| Bring unwashed farm tools to home | No | 12(41.4) | 17(58.6) | 29(13.4) | 1 | |
| Yes | 94(50.3) | 93(49.7) | 187(86.5) | 0.93(0.39–94.9) | 0.021 | |
| Wash vegetable with irrigation water | No | 50(44.2) | 63(55.6) | 113(52.3) | 1 | |
| Yes | 97(94.2) | 6(5.8) | 103(47.7) | 15.1(5.05–47.3) | ≤ 0.001 | |
| Drink water at farm | No | 1(9) | 10(90.9) | 11(5.09) | 1 | |
| Yes | 100(48.8) | 105(51.2) | 205(94.9) | 0.48(0.11–2.05) | 0.476 | |
| Eat food fall ground in farm | No | 15(28.3) | 38(71.7) | 53(24.5) | 1 | |
| Yes | 143(87.7) | 20(12.3) | 163(75.5) | 10.2(1.92–52.8) | 0.005 | |
| Chew chat at farm | No | 2(8.7) | 21(91.3) | 23(10.7) | 1 | |
| Yes | 29(15) | 164(85) | 193(89.4) | 1.4(0.38–5.103) | 0.810 | |
| Wearing protective wears during farming activities | No | 199(95.7) | 9(4.3) | 208(96.3) | 1.99(0.49–5.52) | ≤ 0.001 |
| Yes | 2(25) | 6(75) | 8(3.7) | 1 | ||
Predisposing factors of soil transmitted helminth among wastewater irrigation farmers
In the final multivariable regression, three models (M1: socio-demographic, M2: water, sanitation, and hygiene, M3: farming practices factors) were tested to identify the independent predisposing factors for STH infection that remained significant after controlling for potential confounders. In the fourth model, seven predisposing factors were independently associated with STH infection. Income less than 1000 ETB was strongly associated with STH infection, (AOR = 1.85; 95% CI = 1.25–5.99) compared to income greater than 1000ETB. Poor sanitation access and hygiene practices, such as no handwashing before feeding (AOR = 2.25; 95% CI = 1.58–11.3), absence of finger nails cleaning (AOR = 1.97; 95% CI = 0.56–39.5) and touching face with dirty hand (AOR = 2.9; 95% CI = 0.68–28.2) were significantly associated with STH infection.
Agricultural exposures like not wearing shoes during farming activities (AOR = 3.4; 95% CI = 1.02–5.43), washing vegetables with irrigation wastewater (AOR = 2.1; 95% CI = 1.95–45.2), and lack of protective wear during farming activities (AOR = 2.99; 95% CI = 1.58–22.4), increased the odds of STH prevalence (Table 5).
Table 5.
Factors associated with STH infection among wastewater irrigation farming, Akaki River bank, Addis Ababa, Ethiopia, 2023
| Predisposing factors | Categories | M1, AOR*(95%CI) |
M2, AOR*(95%CI) |
M3, AOR*(95%CI) |
M4, AOR*(95% CI) |
|---|---|---|---|---|---|
| Number of rooms | 1–2 |
1.25 (0.85–2.31** |
1.18 (0.28–2.31) |
||
| > 4 | 1 | 1 | |||
| Income | < 1000 |
3.85 (1.56–5.96) ** |
1.85 (1.25–5.99)** |
||
| > 5000 | 1 | 1 | |||
| Water availability near toilet | No |
4.5 (2.5–15.7) ** |
1.86 (1.52–11.3) |
||
| Yes | 1 | 1 | |||
| Defecation outside toilet | No | 1 | 1 | ||
| Yes |
5.6 (1.4–38.2)** |
1.65 (1.29–9.56) |
|||
| Hand washing before feeding | No |
6.8 (1.7–12.3) ** |
2.25 (1.58–11.3) ** |
||
| Yes | 1 | 1 | |||
| On-site hand washing after work | No |
8.2 (3.5–19.8) ** |
1.95 (1.29–98.2) |
||
| Yes | 1 | 1 | |||
| Finger nails cleanness | No |
10.3 (8.1–22.6) ** |
1.97 (0.56–39.5) ** |
||
| Yes | 1 | 1 | |||
| Wear Boots/shoes at work | No |
11.2 (9.4–38.2) ** |
3.4 (1.02–5.43) ** |
||
| Yes | 1 | 1 | |||
| Touch face with dirty hand | No | 1 | 1 | ||
| Yes | 10.5(6.8–68.1)** |
2.9 (0.68–28.2) ** |
|||
| Use working clothes at home | No | 1 | 1 | ||
| Yes |
1.79 (0.9–2.88) ** |
0.35 (0.09–1.62) |
|||
| Wash vegetable with irrigation water | No | 1 | 1 | ||
| Yes |
1.99 (0.25–36.5)** |
2.1 (1.95–45.2)** |
|||
| Wearing protective wears during farming activities | No |
2.98 (1.56–17.5) ** |
2.99 (1.58–22.4) ** |
||
| Yes | 1 | 1 |
*Adjusted odds ratio
**p < 0.05
Discussion
Soil-transmitted helminth infections are one of the leading causes of morbidity in Ethiopia [5]. The national pooled prevalence of intestinal helminth infection was 33.35% (95% CI = 0.288–0.378) [9], with the pooled prevalence in Addis Ababa was 35.5% (95% CI = -0.109-0.799) [9]. According to the findings of this study, the prevalence of STH infection among wastewater irrigating farmer households in Akaki River was 22.22% (95% CI = 13.6 − 27.9%). This is consistent with the community-based study prevalence reported in Bibugn, Northwest Ethiopia 20.9% (95% CI = 0.179–0.243) [26], and peri-urban areas in Jimma town, Ethiopia, 18.1% (95% CI = 0.146–0.221) [25]. In other research reported in Kogi state, Nigeria, 34.2% (95% CI = 0.162–0.390) [27], a study conducted in Kampala, Uganda, urban farmers have the greatest prevalence 76% (95% CI = 0.693–0.827) [28]. The disparity in prevalence rates of STH infection is due to, differences in examination procedures, geographical locations, sample sizes, and individual factors favouring difference prevalence of infection to occur.
After controlling for all STH predictors, low-income wastewater farmers were 1.85 times more likely to get STH infection than the reference group (AOR = 1.85%; 95% CI = 1.25–5.99). The findings were comparable to research conducted in peri-urban regions in Jimma town, Ethiopia, (AOR = 2.7%; 95% CI = 1.31–5.50) [25], in Semarang, Central Java, Indonesia, (AOR = 2.1%; 95% CI = 1.77–2.58) [29], and in rural Malaysia, (AOR = 1.77%; 95% CI = 1.32–2.66) [30]. This shows that poor income likely exacerbates STH transmission via interrelated pathways that include nutrition, sanitation, housing inadequacies, and impediments to preventative measures. Income is a significant distal driver of proximal risk factor.
A systematic review and meta-analysis highlighted the critical importance of adequate water, sanitation, and hygiene (WASH) access in reducing the likelihood of STH infections [10, 30, 31]. According to the findings, adequate WASH access can reduce the risk of having these diseases by 33–70% [30]. However, in this study’s farming community lack of adequate WASH facilities and practices have exacerbated the STH problem. The farming environment had been badly degraded, with solid trash dumped carelessly along farmland edges [17]. Furthermore, the irrigation water used for vegetable growth was contaminated with toilet waste, which posed a major STH risk [8]. Inadequate handwashing, as well as a lack of access to clean water and sanitation, created an ideal breeding environment ground for the STH parasites to thrive and multiply [30].
Other studies, have demonstrated the existence of several intestinal pathogens in wastewater used to irrigate and wash vegetables [7, 28, 32, 33]. Vegetables can be contaminated with STH at numerous phases, including during growth, harvest, post-harvest handling, and distribution [20]. In this study washing vegetables with irrigated wastewater had higher risk factor for the high prevalence of STH among the farmers. This contamination extends beyond the farmlands, creating a severe threat to homes, with children being especially vulnerable. Children may come into contact with polluted vegetables brought home, or objects that have been contaminated with such vegetables, and subsequently handling potentially pathogen contaminated objects. Furthermore, the usual practice of consuming vegetables without sufficient cleaning during food preparation, as well as eating vegetables raw or undercooked, exposes people of all age groups to pathogenic STHs.
Studies have shown the increased risk of wastewater-associated diseases for farm workers and their families when suitable protective equipment is not used [10, 34, 35]. The majority of the farming community in this survey neglected the usage of protective equipment, claiming cost as the primary reason for their decision. However, given the characteristic of risks linked with their working environment, which puts them at a higher risk of STH infections, wearing suitable protective equipment while on the farm is necessary as risk of exposure to STH is high. Because of the nature of their occupation; farmers spend prolonged periods in contact with contaminated soil and wastewater, which increases the risk of accidental and dermal intake of STH pathogen. Failure to wear proper protective apparel not only jeopardizes the health of farm workers, but also puts family members at risk for STH transmission.
A major strength of this study was the inclusion of home visits to evaluate farmer participants’ household and socioeconomic conditions related to their helminth infection status. Conducting direct observations of sanitation infrastructure, hygiene practices, and living conditions at farmers’ homes allowed for a more comprehensive assessment of infection prevalence compared to reliance on survey data alone. Triangulating the survey results with findings from the home visits enhanced the validity of the factors associated with parasitic prevalence identified in this analysis. Furthermore, this study established valuable baseline data on the prevalence and risk factors for intestinal parasites in this occupational group. The availability of this baseline data enables future longitudinal monitoring of trends in parasitic infection patterns following any interventions or behaviour changes, providing a means to evaluate the impact of control measures in this population over time.
This study had some shortcomings that provide an opportunity for future research. The cross-sectional design offers prevalence estimates at a specific point in time but does not characterize potential seasonal fluctuations in STH infection patterns among farmers over the year. Future studies should incorporate, seasonal fluctuation, infection intensity categories, and species-level identification into the epidemiological assessment.
Conclusion
The prevalence of soil-transmitted helminth infections among wastewater irrigation vegetable producers was high in the study nearly one out of five farmers. Predisposing factors like low-income, not washing hands before feeding, absence fingernail hygiene, touching the face with dirty hand, washing vegetables with irrigation wastewater, not wear Boots/shoes at work and not wearing protective wears during farming activities were significantly associated with STH infection.
Based on the findings, wastewater farmers are a high-risk community, if left untreated, can serve as a critical STH source of reinfection even for schoolchildren completing deworming programs. Unless the intestinal parasite prevention strategies consider these segments of the population, it will be difficult to meet Ethiopia’s 2025 goal of eliminating STH transmission. To reduce the burden of STH the implementation of periodic community-wide deworming is warranted. Based on WHO guidelines, the recommended medications include a single 400 mg oral dose of albendazole or 500 mg dose of mebendazole administered every 6 months to at-risk groups. Ensuring adequate treatment coverage and medication supply will be crucial steps in controlling STH transmission in these exposed communities.
Deworming activities on a large scale, together with hygiene and sanitation education, are critical for at-risk farmers and farming households. Moreover, investing in educational programs for farmers has the potential to increase food safety and quality. Enhanced monitoring and periodic STH assessments among agricultural workers by health authorities are required to improve the quality of life for this vulnerable demographic. For long-term STH reduction in these occupational communities, an integrated control approach addressing deworming, WASH access, protective equipment, and educational needs is required.
Operational definitions
- Farmers
Those communities cultivate vegetables along the Akaki River bank.
- Wastewater
Untreated Little and Great Akaki River.
- Permanent
Farmers have lived along the Akaki River bank for more than ten years.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Water Security and Sustainable Development Hub for their valued comments on the manuscript and to the staff of the EPHI Laboratory for their assistance. We also wish to acknowledge the fieldwork sustenance of development agents at various sub-city administrative areas of Addis Ababa.
Abbreviations
- STH
Soil-Transmitted Helminths
- EPHI
Ethiopia Public Health Institute
Author contributions
BKG conceived and designed the study, carried out information collection and laboratory examination, and finalized the writing of the manuscript. BM, SK, and ET designed the study and revised the manuscript. ML, CL, and BKD revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Water Security and Sustainable Development Hub, which is funded by the UK Research and Innovation’s Global Challenges Research Fund (GCRF), Grant no.: ES/S008179/1.
Data availability
Data presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethical considerations
Ethical agreement for the study procedure was obtained from Institutional Review Board Committee belonging to the College of Natural and Computational Sciences of Addis Ababa University. An official approval letter was gained from each urban agriculture and health sub-city administration. Written agreement was obtained from participants after the purpose and details of the study were explained to all participants. Results were kept confidential. No other tests were done on the collected stool sample other than those mentioned in the data collection part.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Soil-transmitted helminth infections. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminthinfections. Accessed 6 Nov 2023.
- 2.Isaac Dennis. Detection and quantification of soil-transmitted helminths in environmental samples: a review of current state-of-the-art and future perspectives. Acta Trop. 2017;169:187–201. 10.1016/j.actatropica.2017.02.014. 10.1016/j.actatropica.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 3.Amoah ID, Reddy P, Seidu R, Stenström TA. Concentration of soil-transmitted helminth eggs in sludge from South Africa and Senegal: a probabilistic estimation of infection risks associated with agricultural application. J Environ Manage. 2018;206:1020–7. 10.1016/j.jenvman.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adu-Gyasi D, Asante KP, Frempong MT, Gyasi DK, Iddrisu LF, Ankrah L, et al. Epidemiology of soil transmitted Helminth infections in the middle-belt of Ghana, Africa. Parasite Epidemiol Control. 2018;3(3). 10.1016/j.parepi.2018.e00071. [DOI] [PMC free article] [PubMed]
- 5.Leta GT, Mekete K, Wuletaw Y, Gebretsadik A, Sime H, Mekasha S. National mapping of soil – transmitted helminth and schistosome infections in Ethiopia. Parasite Vectors. 2020;13(437):1–13. 10.1186/s13071-020-04317-6. 10.1186/s13071-020-04317-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddren R, Phillips A, Ower A, Landeryou T, Mengistu B, Anjulo U. Soil-transmitted helminths and schistosome infections in Ethiopia: a systematic review of progress in their control over the past 20 years. Parasites Vectors. 2021;14(1):1–15. 10.1186/s13071-021-04600-0. 10.1186/s13071-021-04600-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali AS, Gari SR, Goodson ML, Walsh CL, Dessie BK, Ambelu A. The impact of wastewater-irrigated urban agriculture on microbial quality of drinking water at household level in Addis Ababa, Ethiopia. Urban Water J. 2023;00(00):1–12. 10.1080/1573062X.2023.2253215. 10.1080/1573062X.2023.2253215 [DOI] [Google Scholar]
- 8.Gurmassa BK, Gari SR, Solomon ET, Goodson ML, Walsh CL, Dessie BK, et al. Distribution of helminth eggs in environmental and stool samples of farming households along Akaki River in Addis Ababa, Ethiopia. Trop Med Health. 2023;51(67). 10.1186/s41182-023-00558-0. [DOI] [PMC free article] [PubMed]
- 9.Liyih M, Damtie D, Tegen D. Prevalence and associated risk factors of human intestinal helminths parasitic infections in Ethiopia: a systematic review and Meta-analysis. Sci World J. 2022;2022:8–10. 10.1155/2022/3905963. 10.1155/2022/3905963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitore WA, Ali MM, Yoseph A, Mangesha AE, Debiso AT. Prevalence of soil-transmitted helminths and its association with water, sanitation, hygiene among schoolchildren and barriers for schools’ level prevention in technology villages of Hawassa University: mixed design. PLoS ONE. 2020;15(9):1–18. 10.1371/journal.pone.0239557. 10.1371/journal.pone.0239557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mekuria DM, Kassegne AB, Asfaw SL. Little Akaki River sediment enrichment with heavy metals, pollution load and potential ecological risks in downstream. Cent Ethiopia Environ Syst Res. 2020;9(1). 10.1186/s40068-020-00188-z.
- 12.Akele ML, Kelderman P, Koning CW, Irvine K. Trace metal distributions in the sediments of the Little Akaki River, Addis Ababa, Ethiopia. Environ Monit Assess. 2016;188. 10.1007/s10661-016-5387-z. [DOI] [PubMed]
- 13.Kassegne AB, Esho TB, Okonkwo JO, Asfaw SL. Distribution and ecological risk assessment of trace metals in surface sediments from Akaki River catchment and Aba Samuel reservoir, Central Ethiopia. Environ Syst Res. 2018;7(1). 10.1186/s40068-018-0127-8.
- 14.Aschale M, Yilma SM, Kelly-Quinn, Dereje H. Assessment of potentially toxic elements in vegetables grown along Akaki River in Addis Ababa and potential health implications. Food Sci Qual Manage. 2015;40:42–53. [Google Scholar]
- 15.Aschale M, Sileshi Y, Kelly-Quinn M. Health risk assessment of potentially toxic elements via consumption of vegetables irrigated with polluted river water in Addis Ababa, Ethiopia. Environ Syst Res. 2019;8(1). 10.1186/s40068-019-0157-x.
- 16.Dessie BK, Aschale M, Assegide E, Alamirew T, Walsh CL, Zeleke G. Pollution challenges and consequences of the Akaki catchment, Upper Awash Basin, Ethiopia: Evidence for policy reform and action. World Water Policy. 2024;10(1). doi.org/10. 10 02/wwp2.12169
- 17.Tadesse Animaw Sinshaw. Understanding the situation of wastewater irrigation in community based irrigation schemes the case of Akaki catchment, Ethiopia. 2011. (Unpublished).
- 18.Woldetsadik D, Drechsel P, Keraita B, Itanna F, Gebrekidan H. Farmers’ perceptions on irrigation water contamination, health risks and risk management measures in prominent wastewater-irrigated vegetable farming sites of Addis Ababa, Ethiopia. Environ Syst Decis. 2018;38(1):52–64. 10.1007/s10669-017-9665-2. 10.1007/s10669-017-9665-2 [DOI] [Google Scholar]
- 19.Zinabu E, Kelderman P, van der Kwast J, Irvine K. Impacts and policy implications of metals effluent discharge into Rivers within industrial zones: a sub-saharan perspective from Ethiopia. Environ Manage. 2018;61(4):700–15. 10.1007/s00267-017-0970-9. 10.1007/s00267-017-0970-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woldetsadik D, Drechsel P, Keraita B, Itanna F, Erko B, Gebrekidan H. Microbiological quality of lettuce (Lactuca sativa) irrigated with wastewater in Addis Ababa, Ethiopia and effect of green salads washing methods. Int J Food Contam. 2017;4(3). 10.1186/s40550-017-0048-8.
- 21.Mekonnen ET, Temesgen SA, Wu Z. An overview of water pollution status in Ethiopia with a particular emphasis on Akaki River: a review an overview of water pollution status in Ethiopia with a particular emphasis on Akaki River. Ethiop j Public Health nur. 2020;3(2):12–128. 10.20372/ejphn.v3i2.87. 10.20372/ejphn.v3i2.87 [DOI] [Google Scholar]
- 22.Federal Democratic Republic Of Ethiopia. the population of the sub-cities of Addis Ababa, 2007 census, 2022 projection Ethiopia. 2023. http://www.statsethiopia.gov.et/wp-content/uploads/2020/08/Population-Projection_Weredas Accessed 12 Nov 2023.
- 23.Yonas T, Assefa, Mukand S, Babel JS. Development of a generic domestic Water Security Index, and its application in Addis Ababa. Ethiopia MDPI. 2019;1. 10.3390/w11010037.
- 24.Van Belle G, Fisher LD, Heagerty PJ, Lumley T. Biostatistics: a methodology for the health sciences. 2004 (519).
- 25.Zeynudin A, Degefa T, Tesfaye M, Suleman S, Yesuf EA, Hajikelil Z. Prevalence and intensity of soil-transmitted helminth infections and associated risk factors among household heads living in the peri-urban areas of Jimma town, Oromia, Ethiopia: a community-based cross-sectional study. PLoS ONE. 2022;17(9):1–17. 10.1371/journal.pone.0274702.27. 10.1371/journal.pone.0274702.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goshu A, Alemu G, Ayehu A. Prevalence and intensity of soil-transmitted helminths and associated factors among adolescents and adults in Bibugn Woreda, Northwest Ethiopia: a community-based cross-sectional study. J Trop Med. 2021;2021:1–9. 10.1155/2021/7043881. 10.1155/2021/7043881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anunobi JT, Okoye IC, Aguzie IO, Ndukwe YE, Okpasuo OJ. Risk of soil-transmitted helminthiasis among agrarian communities of Kogi state, Nigeria. Ann Glob Heal. 2019;85(1):1–13. 10.5334/aogh.2563. 10.5334/aogh.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuhrimann S, Winkler MS, Kabatereine NB, Tukahebwa EM, Halage AA. Rutebemberwa. Risk of intestinal parasitic infections in people with different exposures to Wastewater and Fecal Sludge in Kampala, Uganda: a cross-sectional study. PLoS Negl Trop Dis. 2016;10(3):1–19. 10.1371/journal.pntd.0004469. 10.1371/journal.pntd.0004469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurscheid J, Laksono B, Park MJ, Clements ACA, Sadler R, McCarthy JS, et al. Epidemiology of soil-transmitted helminth infections in semarang, central java, Indonesia. PLoS Negl Trop Dis. 2020;14(12):1–17. 10.1371/journal.pntd.0008907. 10.1371/journal.pntd.0008907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, Sanitation, Hygiene, and soil-transmitted Helminth infection: a systematic review and Meta-analysis. PLoS Med. 2014;11(3). 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed]
- 31.Destaw Damtie B, Sitotaw, Haileyesus Mekuriaw. Prevalence of intestinal parasitic infections and related risk factors among Jawi primary school children, Jawi town. North-west Ethiopia. 2019;19:341. 10.1186/s12879-019-3971-x. 10.1186/s12879-019-3971-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weldesilassie AB, Boelee E, Drechsel PAY. Wastewater use in crop production in peri-urban areas of Addis Ababa : impacts on health in farm households. Environ Dev Econ. 2011;16(01):25–49. 10.1017/S1355770X1000029X. 10.1017/S1355770X1000029X [DOI] [Google Scholar]
- 33.Mohammed SA, Vantsawa PA, Haroon AA, Dikwa KB, Bature AM, Dari BS. Prevalence and transmission of soil transmitted Helminths among Farmers Living along the Metropolitan Section of River Kaduna, Nigeria. J Adv Microbiol. 2021;21(12):88–97. 10.9734/jamb/2021/v21i1230417. 10.9734/jamb/2021/v21i1230417 [DOI] [Google Scholar]
- 34.Aemiro A, Menkir S, Tegen D, Tola G. Prevalence of soil-transmitted helminthes and Associated Risk factors among people of Ethiopia: a systematic review and Meta-analysis. 2022;26(15). 10.1177/11786337211055437 [DOI] [PMC free article] [PubMed]
- 35.Alemu Y, Degefa T, Bajiro M, Teshome G. Prevalence and intensity of soil-transmitted helminths infection among individuals in model and non-model households, South West Ethiopia: a comparative cross-sectional community based study. PLoS ONE. 2022;17(1):1–14. 10.1371/journal.pone.0276137. 10.1371/journal.pone.0276137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this study are available from the corresponding author upon reasonable request.