Abstract
Immune control of human immunodeficiency virus (HIV) is not restored by highly active antiretroviral therapies (HAART) during chronic infection. We examined the capacity of repeated structured therapeutic interruptions (STI) to restore HIV-specific CD4 and CD8 T-cell responses that controlled virus production. Eleven STI (median duration, 7 days; ranges, 4 to 24 days) were performed in three chronically HIV-infected patients with CD4 counts above 400/mm3 and less than 200 HIV RNA copies/ml after 18 to 21 months of HAART; treatment resumed after 1 week or when virus became detectable. HIV-specific T-cell responses were analyzed by proliferation, gamma interferon (IFN-γ) production, and enzyme-linked immunospot assays. Seven virus rebounds were observed (median, 4,712 HIV-1 RNA copies/ml) with a median of 7 days during which CD4 and CD8 counts did not significantly change. After treatment resumed, the viral load returned below 200 copies/ml within 3 weeks. Significant CD4 T-cell proliferation and IFN-γ production against HIV p24 appeared simultaneously with or even before the virus rebounds in all patients. These CD4 responses lasted for less than 3 weeks and disappeared before therapeutic control of the virus had occurred. Increases in the numbers of HIV-specific CD8 T cells were delayed compared to changes in HIV-specific CD4 T-cell responses. No delay or increase in virus doubling time was observed after repeated STI. Iterative reexposure to HIV during short STI in chronically infected patients only transiently mobilized HIV-specific CD4 T1-helper cells, which might be rapidly altered by virus replication. Such kinetics might explain the failure at delaying subsequent virus rebounds and raises concerns about strategies based on STI to restore durable HIV-specific T-cell responses in chronic HIV infection.
Combined antiretroviral therapy can substantially reconstruct the damaged immune system in human immunodeficiency virus (HIV)-infected patients (1, 10, 17). This major success is, however, limited by the inability of highly active antiretroviral therapy (HAART) to eradicate HIV and restore HIV-specific T-cell immunity. The main immune effector involved in viral control, the HIV-specific CD8 cell response, decreases to resting-memory low levels when plasma viremia becomes undetectable after a successful antiretroviral treatment (2, 5, 13, 14). The loss of HIV-specific stimulation that necessarily follows therapeutic control of HIV might be responsible for such a decline. The CD8 T-cell responses also depend on generating or maintaining high levels of HIV-specific CD4 Th1 T cells, which are detectable only in long-term nonprogressors concurrently with strong immune control of the virus (22; B. Autran, O. Bonduelle, A. Goubar, D. Candotti, H. Agut, P. Debré, et al., submitted for publication). These Th1 cells are, however, partially eliminated early in infection (22) but can be preserved when HAART is initiated at primary infection (16, 22). In chronic disease, however, such responses are usually weak (18) or undetectable and are not restored by HAART (1, 19–21) except in poorly compliant patients (7). These observations led to the proposal of transient structured therapeutic interruptions (STI) to boost the declining T-cell responses to HIV by reexposing the immune system to virus rebounds. Indeed, several consecutive drug “holidays” might enhance the immunological set point and help at controlling virus production when treatment is introduced during primary infection (11, 15). No rebound in HIV-specific CD4 T-helper cells has yet been reported, however, during STI, especially in chronically infected patients. A single scheduled HAART interruption in chronic infection does not affect the efficacy of renewed therapy (12, 23). We also showed that the complete arrest of a triple-drug regimen 1 month after initiation was followed in all cases by a 1-week delay before virus rebound (12). Based on these observations, we performed a prospective pilot study of short-course repeated STI in chronically HIV-infected patients to evaluate whether small bursts of HIV during STI might restimulate in vivo virus-specific CD4 and CD8 T-cell responses and to determine the kinetics of these immune responses and their capacity at delaying subsequent virus rebounds.
MATERIALS AND METHODS
Study design and patients.
HIV-1 chronically infected patients who had been seropositive for 7 to 11 years, were receiving a HAART regimen with 2 nucleoside analogs plus one protease inhibitor or one nonnucleoside reverse transcriptase (RT) inhibitor, and had a CD4 lymphocyte count >400/mm3 and a plasma HIV RNA count of <200 copies/ml for at least 6 months were recruited for the study. All signed an informed-consent form. Based on our previous observation (12), we initially designed a fixed duration of 7 days for STI, which should have led to virus detectability. This schedule was maintained for the first three STI in patient 1, who was the first to be included, and for the first STI in patients 2 and 3. Since virus rebound occurred only once during these five STI, the subsequent discontinuations of therapy were maintained until the virus reached a level above 1,000 copies/ml. Three or four STI were performed for each patient, with an interval of 2 to 3 months between the first and second STI and then an interval of 1 to 2 months between subsequent STI.
Patients were prospectively monitored for CD4 and CD8 cell counts and for plasma viral load every 2 to 3 days during each STI and on days 7, 14, and 28 after reinitiation of treatment. Specific immunologic examinations were done every 7 days during STI and on days 7, 14, and 28 after reinitiation of therapy.
HIV-1 RNA plasma quantification.
Quantification of HIV-1 RNA levels in plasma was performed using the Amplicor Monitor assay (Roche Diagnostics, Basel, Switzerland) with a detection limit of 200 copies in real time. All frozen plasma samples were reanalyzed in batch experiments using the detection limit of 20 copies.
Sequence analysis.
Plasma RNA at viral rebounds was used for sequence analysis of the RT and protease genes. After purification and reverse transcription, a nested PCR with AmpliTaq Gold (Applied Biosystems, Foster City, Calif.) was performed. For the RT gene, primers 5′MJ3 and 3′MJ4 were used for the one-step RT-PCR and primers 5′A35 and 3′NE1-35 were used for the nested PCR (8). For the protease gene, primers 5′eprB and 3′eprB were used for the one-step RT-PCR and primers 5′prB and 3′prB were used for the nested PCR (3). The purified DNA fragments obtained were directly sequenced with the ABI PRISM Dye Terminator cycle-sequencing kit (Applied Biosystems). Sequence products were analyzed with the ABI PRISM 377 automatic sequencing system. Codon changes considered to be mutations were those showing any difference from the HIV-1 consensus B sequence (24).
Statistical analysis.
Rates of viral rebound were calculated using linear regression on log-transformed HIV-1 RNA concentrations in plasma. Doubling times were calculated as ln 2/rate.
Flow cytometry.
Absolute CD4 and CD8 cell counts were obtained in fresh blood samples as previously described (10).
Lymphocyte subpopulations were analysed in whole blood with the following monoclonal antibodies (MAbs): HLA-DR-PE and CD38-PE (Becton-Dickinson, Pont de Claix, France) and CD3-PE, CD4-PECY5, CD8-PECY5, CD45RA-PE, CD62L-FITC, CD45-FITC/CD14-PE, and Ki67-FITC (Immunotech, Marseille-Luminy, France). A total of 5,000 lymphocytes were analyzed by three-color flow cytometry as described previously (10). Intracellular Ki67 expression in CD4+ and CD8+ T cells was analyzed after permeabilization by standard procedures on 20,000 to 100,000 CD4 or CD8 events in a large lymphocyte gate on a forward-scatter and side-scatter plot including blasts; monocytes were excluded by their low-level CD4 expression.
Lymphocyte proliferation assay.
Gradient density separation was used to isolate peripheral-blood mononuclear cells (PBMC). Incubating PBMC with magnetic beads coated with anti-CD8 MAb (Dynabeads; Dynal, Oslo, Norway) negatively selected CD4 T cells, with less than 5% residual CD8+ cells. Total and CD8-depleted PBMC were simultaneously cultured as previously described (10) with p24 (0.25 μg/ml); (Intracell, London, United Kingdom), tuberculin (Statens Serum Institut, Copenhagen, Denmark) or cytomegalovirus (CMV) (Behring, Marburg, Germany) and then labeled with tritiated thymidine (CEA, Saclay, France) on day 6. The stimulation index (SI) was calculated as the ratio of (cells plus stimuli) cpm to (cells plus medium) cpm. Positive antigen-specific responses were defined as those with greater than 3,000 cpm and an SI greater than 3.
In vitro cytokine production assay.
Cell culture supernatants were harvested on day 2 from CD8-depleted PBMC cultured at 106/ml with p24 (0.25 μg/ml) (Intracell) or medium alone and measured by an enzyme immunoassay for IFN-γ (Diaclone, Besançon, France). IFN-γ levels in the control supernatants without antigen were subtracted from the results of specific production. A response above 30 pg/ml was considered positive.
ELISPOT assay for single-cell IFN-γ release using recombinant vaccinia virus constructs or synthetic peptides.
Capture anti-human IFN-γ MAb (Diaclone) was coated on 96-well polyvinylidene difluoride-bottom plates (Millipore, Molsheim, France). The vaccinia virus assay was adapted from Larsson et al. (9). Various recombinant vaccinia viruses encoding either Gag, Pol, Env, or Nef were a kind gift from Transgène (Strasbourg, France). Wild-type vaccinia virus served as negative control. Recombinant vaccinia viruses were added to PBMC at a multiplicity of infection of 5 PFU/cell as previously described (6), after which infected cells were used directly in the ELISPOT assay. Infected or uninfected PBMC were added to triplicate wells at 105 cells per well, and the plates were incubated for 16 to 18 h at 37°C in 5% CO2. The peptide assay was adapted from Dalod et al. (2). PBMC were added to triplicate wells at 1 × 105, 5 ×104, and 1 ×104 cells/well with 10 μg of peptide per ml, phytohemagglutinin (Murex, Paris, France), or medium alone and incubated for 40 h at 37°C in 5% CO2. Twelve published HIV-1 cytotoxic T-lymphooyte epitopes were selected from the Los Alamos HIV molecular immunology database by their HLA class I restriction: HLA-A2: Gag 77–85 (G3), Pol 476–484 (P1); HLA-B44: Gag 306–316 (G4); HLA-B14: Gag 183–191 (G5), Env 586–593 (E4); HLA-A24: Env 584–592 (E1); HLA-B51: Pol 295–302 (P5), Env 557–565 (E3); HLA-A11: Pol 313–321 (P3), Nef 73–82 (N1); and HLA-B35: Gag 254–262 (G7), Pol 311–319 (P4). The synthetic peptides were purchased from Syntem (Nimes, France) or kindly provided by Agence Nationale de la Recherche sur le SIDA.
After the cells were washed, the second biotinylated anti-IFN-γ MAb (Diaclone) was added, followed by streptavidin-alkaline phosphatase conjugate (Amersham, Les Ulis, France) and chromogen substrates (5-bromo-4-chloro-3-indolylphosphate toluidine and nitroblue tetrazolium) (Sigma-Aldrich, St. Quentin, France). Spots were counted under a magnifying glass. The number of peptide-specific T cells, expressed as spot-forming cells (SFC)/106 PBMC, was calculated after subtracting negative control values.
Real-Time PCR detection of IFN-γ.
RNA was extracted from fresh PBMC using RNeasy 96 (Qiagen, Chatsworth, Calif.). A constant amount of target RNA was reverse transcribed with 100 IU of Moloney murine leukemia virus RT (Gibco BRL, Glasgow, Scotland) in the presence of 5 μM per random primer. Real-time quantitative PCR with 1 μl of c-DNA was performed in triplicate using human cytokine IFN-γ pre-developed TaqMan assay reagents (Perkin-Elmer, Applied Biosystems, Foster City, Calif.) as specified by the manufacturer, including controls, on an ABI Prism 7700 sequence detector, which contains a Gene-Amp PCR system 9600 instrument (Perkin-Elmer, Applied Biosystems). A normalization 18S RNA experiment was performed simultaneously for each sample with 18SRNA primer and carboxyfluorescein-labeled 18SRNA probe. CT (threshold) values were calculated for target (IFN-γ CT) and standard (18S RNA CT) genes by determining the point at which the fluorescence exceeded a threshold limit (10 times the standard deviation of the baseline). The results are expressed as ΔCT; expressed as mean (IFN-γ CT) − mean (18SRNA CT).
RESULTS
Virus and CD4 kinetics during STI.
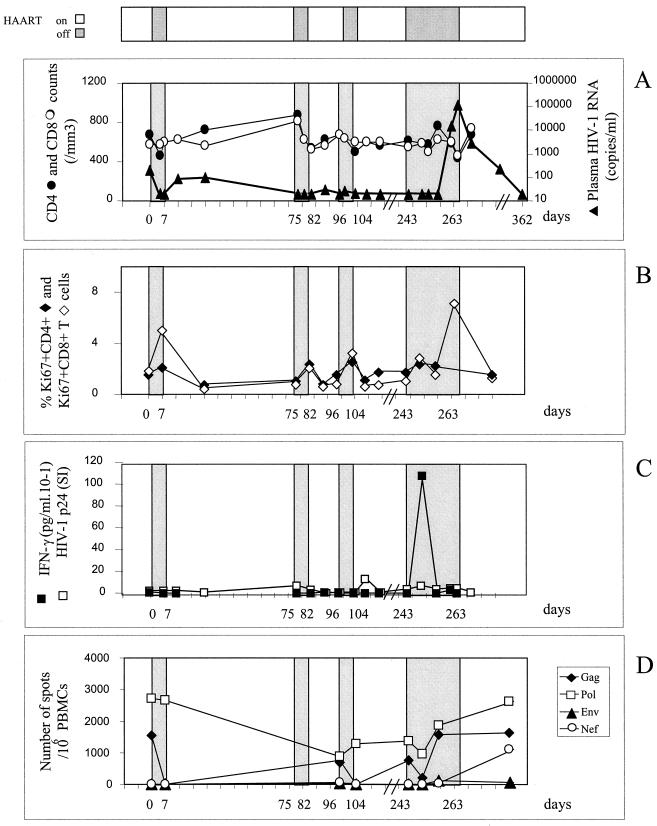
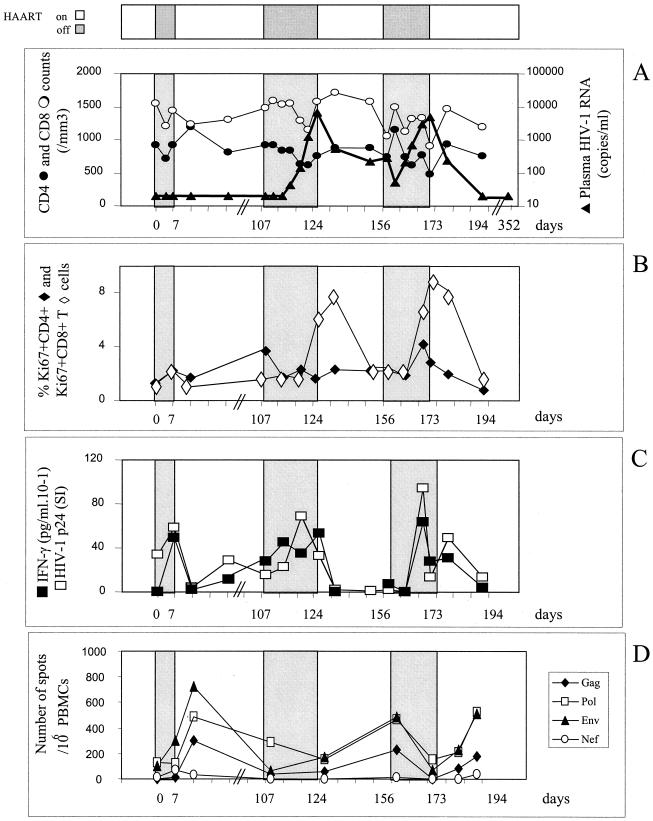
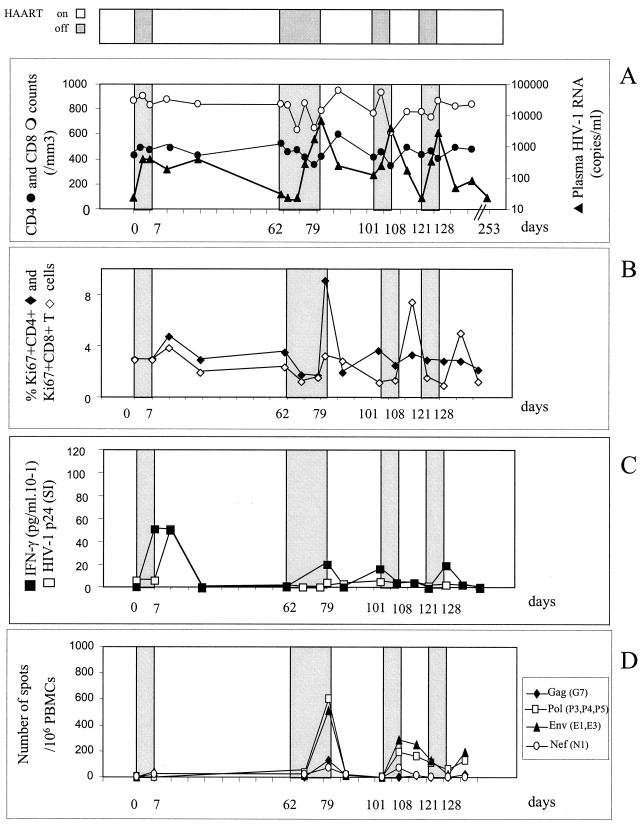
Table 1 summarizes the patients' characteristics. The three HIV-1 chronically infected patients had been seropositive for 7 to 12 years with nadir CD4 levels of 236, 211, and 456/mm3 and maximal viral loads before HAART of 59,000, 48,500, and 73,000 copies/ml. All had been treated with the same line of therapy for 18 to 21 months, which had led to virus control under the threshold of 200 copies/ml for at least the last 6 months and CD4 counts above 400/mm3. Patient 1 (Fig. 1A) did not experience any plasma viral rebound during three STI of 1 week each. The fourth STI thus was continued until the plasma virus level became detectable: on day 21, the viral load reached 15,207 copies/ml, and on day 24 it reached 113,333 copies/ml. For patient 2 (Fig. 2A), the first 7-day STI similarly did not cause his plasma viral load to rebound. The following STI continued until the plasma virus level became detectable. Rebounds occured within 15 and 19 days of the second and third STI, respectively. Viral loads had reached 6,640 and 4,712 copies/ml when treatment resumed. For patient 3 (Fig. 3A), plasma virus levels rebounded slightly, to 399 copies/ml, on day 4 of the first 7-day STI. A second STI continued until the viral load became detectable on day 10 and reached 6,737 copies/ml on day 18. During the next two STI, however, the viral load rapidly increased on day 3, reaching 3,795 and 2,719 copies/ml, and treatment was therefore resumed immediately.
TABLE 1.
Patient characteristics at entry into the studya
| Characteristic | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (yrs) | 46 | 46 | 35 |
| HLA class 1 haplotypes | A2 A24(9)/B51(5) B 62(15) | A2 A32/B12(44) B14/C8 | A11 A24(9)/B35 B51(5)/C4 |
| Risk factor | Homosexual activity | Homosexual activity | Homosexual activity |
| First HIV seropositivity (yr) | 1986 | 1986 | 1991 |
| Maximal viral load before HAART (copies/ml) | 59,000 | 48,500 | 73,000 |
| Nadir CD4 count before HAART (mm−3) | 236 | 456 | 211 |
| Duration of HAART before entry (mo) | 46 | 18 | 21 |
| CD4 count at entry (mm−3) | 674 | 920 | 511 |
| Viral load at entry (copies/ml) | <200 | <200 | <200 |
| Therapy at baseline | Nevirapine + d4T + 3TC | AZT + 3TC + indinavir | AZT + 3TC + indinavir |
d4T, stavudine; 3TC, lamivudine.
FIG. 1.
Patient 1. (A) Effect of intermittent interruptions of antiretroviral therapy on viral load and CD4 or CD8 counts. The HIV-1 RNA level was measured by RT-PCR with a limit of sensitivity of 20 copies per ml. Viral loads are expressed as numbers of HIV-1 RNA copies per milliliter of plasma; CD4 and CD8 counts were analyzed by flow cytometry with fluorescent beads as an internal standard. The values (mean ± standard deviation) in healthy people are as follows: CD4+ cells, 858 ± 260 cells/μl of plasma; CD8+ cells, 482 ± 164/μl plasma. White areas at top of the figure indicate periods of treatment (nevirapine, stavudine, and lamivudine); interruptions are in grey and are also shown in panels A to D. (B) Effect of intermittent interruptions of antiretroviral therapy on Ki67 antigen expression. Ki67 antigen expression was analyzed in CD4+ and CD8+ T cells by flow cytometry after intracellular staining. The values in healthy people are 2.5% ± 0.6% for CD4+ Ki67+ cells and 2% ± 0.6% for CD8+ Ki67+ cells. (C) Effect of intermittent interruptions of antiretroviral therapy on T-helper cell responses to HIV-1 p24 protein. HIV-1 p24-stimulated IFN-γ production by CD8-depleted PBMC was measured by enzyme-linked immunosorbent assay in the 2-day culture supernatants and is expressed as 10−1 picograms per milliliter; T cell proliferative responses against HIV-1 (p24) were measured on CD8-depleted PBMC, and the results are expressed as a stimulation index. (D) Effect of intermittent interruptions of antiretroviral therapy on CD8+ cell responses to HIV-1 protein. The frequency of HIV-specific CD8+ T cells was tested by a recombinant vaccinia virus ELISPOT assay. PBMC were infected with wild-type vaccinia virus or recombinant vaccinia virus Gag, Pol, Env, or Nef, and IFN-γ-producing SFC were enumerated in an 18-h ELISPOT assay. Results are expressed as the number of IFN-γ SFC per 106 PBMC.
FIG. 2.
Patient 2. (A) Effect of intermittent interruptions of antiretroviral therapy on viral load and CD4 or CD8 counts. (B) Effect of intermittent interruptions of antiretroviral therapy on Ki67 antigen expression. (C) Effect of ST1 of therapy on the T-helper cell responses to HIV-1 p24 protein. (D) Effect of ST1 of therapy on the CD8+ cell responses to HIV-1 protein. For further details for all panels, see the legend to Fig. 1.
FIG. 3.
Patient 3. (A) Effect of intermittent interruptions of antiretroviral therapy on viral load and CD4 or CD8 counts. (B) Effect of intermittent interruptions of antiretroviral therapy on Ki67 antigen expression. (C) Effect of ST1 of therapy on the T-helper cell responses to HIV-1 p24 protein. (D) Effect of intermittent interruptions of antiretroviral therapy on the CD8+ cell responses to HIV-1 protein. The frequency of HIV-specific CD8+ T cells was tested by an ELISPOT assay using a panel of peptides according to the HLA of the patient. Results are expressed as the number IFN-γ SFC per 106 PBMC. For further details for all panels, see the legend to Fig. 1.
Overall, 11 STI were analyzed, with seven virus rebounds occurring with a median of 7 days (range, 4 to 21 days) after drug arrest. The median virus doubling time was 1.39 days (range, 1 to 2.25 days). Transient decreases in the numbers of CD4 and CD8 cells were observed when the virus load exceeded 1,000 copies/ml. The median CD4 decrease during the virus rebounds in the three patients was −163/mm3 (range, +14 to −297). Both CD4 and CD8 counts returned to baseline values at end of the study. For all three patients, viral replication decreased below or close to baseline levels within 3 weeks after therapy resumed, except after two STI. This persistence of detectable virus replication did not lead to changes of virus doubling time in either case during the next STI.
No resistance-conferring mutations (24) were observed in the HIV-1 RT and protease genes during the rebounds (data not shown).
Virus replication-induced bursts of T-cell activation.
The consequences of virus rebounds for T-cell activation and differentiation status were evaluated. All patients had normal baseline proportions of both CD4+ and CD8+ cells expressing Ki67+ a cell cycle marker (Fig. 1B, 2B, and 3B). Minimal, if any, increases in the proportion of CD4+ Ki67+ cells were observed except in patient 3. The proportion of CD8+ Ki67+ cells, however, most often increased simultaneously with or a few days after peaks in viral load, to reach 9% of CD8 cells. These levels persisted for 1 week after viral control was reestablished.
Analyzing late CD8+ T-cell activation markers, such as HLA-DR and CD38, yielded similar results, involving slight increases (data not shown) with return to baseline in all cases after each interruption and at the end of the study.
Neither the proportions nor the absolute counts of either naive (CD45RA+ 62L+) or memory (CD45RO+) CD4+ T cells varied significantly (data not shown).
Rapid but transient increase in numbers of HIV-specific CD4+ T cells during virus rebounds.
At baseline, a conventional lymphocyte proliferation assay detected no HIV p24-specific response even when performed with CD8-depleted PBMC, except for patient 2. Similarly cytokine production against HIV-1 p24 was undetectable. In patient 1 (Fig. 1C), the Th1 response to p24 did not increase during the three STI that did not led to virus rebound. The strong virus rebound during the last interruption induced only IFN-γ production but no proliferative response: the p24-specific IFN-γ production increased 1 week after drug arrest and 10 days before virus became detectable. This IFN-γ production, however, rapidly disappeared 1 week before the peak viral load was attained.
In patient 2, both the CD4 T-cell proliferation and IFN-γ production against p24 increased significantly during the first 7-day STI, although no rebound of the viral load in plasma was detectable (Fig. 2C). The peaks in viral load during the subsequent STI were also accompanied by an anti-HIV-1 p24 Th1 response that was mobilized as soon as the virus reached detectability and even 3 days before the increase in viral load during the second STI. The SI and/or IFN-γ production had already decreased by the time of the peak viral load. The HIV-specific Th1 responses became almost undetectable 10 days later, after therapy resumed, whether the viral load was controlled (third STI) or not (second STI). To evaluate whether the CD4 T cells were producing IFN-γ in vivo during these rebounds, we quantified the IFN-γ messengers ex vivo in freshly harvested, unstimulated CD4 T cells by a real-time quantitative RT-PCR method. The levels of these messengers, already detectable when the study started, increased significantly (fourfold) during the STI (data not shown).
In patient 3, HIV-specific CD4-cell proliferative and cytokine responses increased with only a slight virus rebound (400 copies/ml) at the first STI, with an intensity similar to that in patient 2 (Fig. 3C). This HIV-specific Th1 response increased rapidly and persisted for 7 days but disappeared on day 28, after resumption of treatment, when the virus was still detectable in plasma (388 copies/ml). Despite substantial virus rebounds during the following three STI, the proliferative responses remained undetectable while IFN-γ production was stimulated only minimally.
Therefore, the CD4 Th1 cells appeared to be rapidly mobilizable in vivo in all patients with virus rebounds, even though they were undetectable during continuous therapy. Specific IFN-γ production was reinduced in seven STI versus four STI for the CD4 T-cell proliferation. This cytokine response appeared 7 days (median) after drug arrest and hence thus was concurrent with the median delay in the increase of the viral load. The proliferative responses against other recall antigens (tuberculin, CMV, and candidin) did not vary significantly during these 11 STI (data not shown).
Delayed increases of the levels of HIV-specific CD8+ cells during viral rebounds.
Two IFN-γ ELISPOT assays using synthetic peptides and recombinant vaccinia virus allowed us to quantify the CD8 cells specific for HIV. First, a panel of 12 HIV cytotoxic T-lymphocyte epitopes was chosen according to the HLA haplotypes of the patients, with five to seven peptides being tested per patient. No or very small numbers of HIV-specific CD8 cells were detectable at entry for the three patients (data not shown). A moderate increase in HIV-specific CD8 T-cell frequencies was observed only in patient 3 at the time of each rebound in the viral load (Fig. 3D). Since no specific responses to the tested epitopes were detected during STI in patients 1 and 2, we reanalyzed these responses using a recombinant vaccinia virus ELISPOT assay, which ensures a more exhaustive evaluation of HIV-specific CD8 T-cell responses. In fact, very high frequencies of CD8 T cells were detected against Gag and Pol (1,554 and 2,724 SFC/106 cells, respectively) at baseline for patient 1 (Fig. 1D) and during the three STI that did not lead to rebounds in viral loads. No responses to Nef and Env were detected over time. The rebound in viral load during the fourth STI was associated with an increase in the number of Nef-specific T cells (up to 1,100 SFC/106 cells). For patient 2, no or weak specific responses were detected at entry. Each STI induced an increase in the responses to Env, Pol, and Gag, with a range of frequencies from 177 to 724 SFC/106 cells (Fig. 2D). Overall, the increases in the frequencies of HIV-specific CD8 cells were delayed compared to changes in the numbers of HIV-specific CD4 T cells and appeared only when the latter had already diminished in six of seven STI.
DISCUSSION
This study clearly demonstrates that HIV-specific T-helper responses are reinducible during short-course STI in chronically infected patients in whom they were undetectable after 18 months or more of effective antiretroviral therapy.
At study entry, CD4 Th1-cell responses were almost undetectable; they appeared concurrently with rebounds in viral load in the three patients. The HIV-specific IFN-γ production occurred within a median of 7 days after drug arrest, concurrently with the rebound in viral load or even before the virus became detectable. Real-time PCR showed that ex vivo IFN-γ production in the CD4 T cells increased as the viral load rebounded, suggesting that HIV-specific Th1 responses did occur in vivo. This is the first demonstration that Th1 responses to HIV can be boosted after reexposure to the virus even after several years of HIV infection and antiretroviral therapy. However, these responses were weak and lasted for less than 3 weeks after drug arrest. Both proliferation and IFN-γ production vanished in all cases, even before the virus was controlled. Their intensity eventually diminished during subsequent STI. This contrasts with the robust responses to other recall antigens such as CMV or tuberculin, which were unaffected by rebounds in viral load. These data strongly suggest that HIV acts rapidly, preferentially, and directly against the CD4 T cells directed at the virus itself. Finally, subsequent reexposure to virus did not enhance the set point of the HIV-specific Th1 responses, which returned to undetectable levels in all cases at the end of the study.
A recombinant vaccinia virus ELISPOT assay allowed us to quantify the HIV-specific CD8 T cells when they were undetectable by using a panel of peptides representative of the four major HIV proteins (Gag, Pol, Env, and Nef). Some boosting effect was observed for HIV-specific CD8 T cells in the two cases where they were undetectable at baseline. Such boosts were delayed compared to changes in HIV-specific CD4 T-cell responses and were not sustained. The failure to stimulate a strong and durable CD4 Th1 response in these patients during rebounds in viral load might have limited the CD8 T-cell expansion, which in turn might have prevented immune control of the HIV relapses. Indeed, repeated STI in this short pilot study were not accompanied by an increasing delay in the rebound of viral load. These results contrast with those of studies of early treatment discontinuation (11, 15). Recent communications (4, 23) have raised a controversy about whether HIV-specific CD4 Th1 cells could be mobilized after STI in patients treated during established HIV infection. A frequent sampling in our analysis, even limited to three patients, allowed us to describe the respective kinetics of rebounds in viral load and HIV-specific T-cell responses: our data strongly suggest that virus replication leads to a rapid destruction of these HIV-specific Th1 cells and therefore help us understand the contradictions mentioned above.
In conclusion, STI did not harm HIV sensitivity to antiretroviral drugs or CD4 counts in these three chronically infected patients. However, our data demonstrate the limitations of such an STI approach for clinical management, raising concerns about such autovaccination strategies among chronically infected patients. This led us to interrupt our study of short-course STI. Nevertheless, the fine kinetics of HIV-specific CD4 T-cell changes described here contribute to our further understanding of results obtained with longer courses of STI, which might miss these changes. Therapeutic immunization with nonpathogenic vaccines seems an alternative strategy of interest for prolonging the delay to rebounds in viral load during STI without damaging the CD4 T-helper cells specific for HIV.
ACKNOWLEDGMENTS
G. Carcelain and R. Tubiana contributed equally to this work.
We thank N. Jouys, C. Desousa, P. Grenot, C. Robert, and I. Viseux for technical assistance; M. Pauchard for logistical assistance; A. Necker and F. Romagné (Immunotech, Marseilles, France) for kindly providing tetramer reagents; and the patients who participated in this study. The English text was edited by Jo-Ann Cahn.
We thank SIDACTION and the Agence Nationale de Recherche sur le SIDA for financial support.
REFERENCES
- 1.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined anti-retroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Dalod M, Harzic M, Pellegrin I, Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, Deschemin J C, Levy J P, Venet A, Gomard E. Evolution of cytotoxic T lymphocyte responses to human immunodeficiency virus type 1 in patients with symptomatic primary infection receiving antiretroviral triple therapy. J Infect Dis. 1998;178:61–69. doi: 10.1086/515587. [DOI] [PubMed] [Google Scholar]
- 3.Eberle J, Bechowsky B, Rose D, Hauser U, Von der Helm U, Gurtler L, Nitschko H. Resistance of HIV type 1 to proteinase inhibitor RO. AIDS Res Hum Retroviruses. 1995;11:671–676. doi: 10.1089/aid.1995.11.671. [DOI] [PubMed] [Google Scholar]
- 4.Garcia F, Plana M, Ortiz G M, Soriano A, Vidal C, Cruceta A, et al. Structured cyclic antiretroviral therapy interruption in chronic infection may induce immune responses against HIV-1 antigens associated with spontaneous drop in viral load, abstr. LB12. 2000. p. 55. . 7th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 5.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 6.Hadida F, Parrot A, Kieny M P, Sadat-Sowti B, Mayaud C, Debre P, Autran B. Carboxyl-terminal and central regions of human immunodeficiency virus-1 NEF recognized by cytotoxic T cytotoxic T lymphocytes from lymphoid organs. J Clin Investig. 1992;89:53–60. doi: 10.1172/JCI115585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haslett P A J, Nixon D F, Shen Z, Larsson M, Cox W I, Manandhar R, Donahoe S M, Kaplan G. Strong human immunodeficiency virus (HIV)-specific CD4+ T cell responses in a cohort of chronically infected patients are associated with interruptions in anti-HIV chemotherapy. J Infect Dis. 2000;181:1264–1272. doi: 10.1086/315381. [DOI] [PubMed] [Google Scholar]
- 8.Jung M, Agut H, Candotti D, Ingrand D, Katlama C, Huraux J M. Susceptibility of HIV-1 isolates to zidovudine: correlation between widely applicable culture test and PCR analysis. J Acquired Immune Defic Syndr. 1992;5:359–364. [PubMed] [Google Scholar]
- 9.Larsson M, Jin X, Ramratnam B, Ogg G S, Engelmayer J, Demoitie M A, McMichael A J, Cox W I, Steinman R M, Nixon D, Bhardwaj N. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 10.Li T S, Tubiana R, Katlama C, Calvez V, Mohand H A, Autran B. Long lasting recovery in CD4+ T cell function mirrors viral load reduction after highly active anti-retroviral therapy in patients with advanced HIV disease. Lancet. 1998;351:1682–1686. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 11.Lisziewicz L, Rosenberg E, Liebeman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 12.Neumann A U, Tubiana R, Calvez V, Robert C, Li T S, Agut H, Autran B, Katlama C. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. AIDS. 1999;13:677–683. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S I, Cerundalo V, Murley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantification of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 14.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–900. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Cao Y, Hurley A, Moore J P, Ho D D, Markowitz M. HIV-1 specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Investig. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxenius A, Price D A, Easterbrook P J, O'Callaghan C A, Kelleher A D, Whelan J A, Sontag G, Sewell A K, Phillips R E. Early active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pakker N G, Notermans D W, De Boer R J, Roos M T L, De Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T A. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 18.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 19.Plana M, Garcia F, Gallart T, Miro J M, Gatell J M. Lack of T-cell proliferative response to HIV-1 antigens after 1 year of highly active antiretroviral treatment in early HIV-1 disease. Lancet. 1998;352:1194–1195. doi: 10.1016/s0140-6736(05)60532-6. [DOI] [PubMed] [Google Scholar]
- 20.Pontselli O, Kerkhof-Garde S, Notermans D W, Foudraine N A, Roos M T, Klein M R, Danner S A, Lange J M, Miedema F. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldo C R, Jr, Liebman J M, Huang X L, Fan Z, Al-Shboul Q, McMahon D K, Day R D, Riddler S A, Mellors J W. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhacement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–336. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1 specific CD4+ T cell responses associated with control of viremie. Science. 1997;278:1447–1451. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz L, Martinez-Picado J, Romeu J, Parades R, Zayat M K, Marfil S, Negredo E, Tural G, Clotet B. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS. 2000;14:397–403. doi: 10.1097/00002030-200003100-00013. [DOI] [PubMed] [Google Scholar]
- 24.Schinazi R F, Larder B A, Mellors J W. Resistance table mutations in retroviral genes associated with drug resistance: 2000–2001 update. Int Antiviral News. 2000;8:65–92. [Google Scholar]