Abstract
Background
Periodontitis is a dental disease characterized by inflammation of periodontal tissues and loss of the periodontal ligaments and alveolar bone. Exosomes are a class of extracellular vesicles that are involved in a variety of diseases by releasing active substances. In this study, we aimed to investigate the effect and mechanism of exosomes from M2 polarized macrophages (M2-exos) on osteogenic differentiation in human periodontal ligament stem cells (hPDLSCs).
Methods
M2-exos were isolated from IL-4-induced RAW264.7 cells (M2 macrophages) and then treated on hPDLSCs. Osteogenic differentiation was assessed by alkaline phosphatase (ALP) staining, alizarin red S (ARS) staining, measurement of osteogenic differentiation-related genes and proteins, and inflammation was evaluated by measuring the levels of inflammatory factors. The mechanism of M2-exo was confirmed through qPCR, western blot, ALP and ARS staining.
Results
Results suggested that M2-exo improved osteogenic differentiation and inhibited inflammation in LPS-induced hPDLSCs. CXCL12 expression was elevated in M2 macrophages, but decreased in LPS-induced hPDLSCs. Moreover, the effect of M2-exo on osteogenic differentiation and inflammation in LPS-induced hPDLSCs was reversed by CXCL12 knockdown.
Conclusion
We demonstrated that M2-exo facilitated osteogenic differentiation and suppressed inflammation in LPS-induced hPDLSCs through promotion of CXCL12 expression. These results suggested the potential of M2-exo in the treatment of periodontitis, which may provide a new theoretical basis for M2-exo treatment of periodontitis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-04831-4.
Keywords: Periodontitis, Macrophage, Exosome, CXCL12, Osteogenic differentiation
Introduction
Periodontitis is a common dental disease, which mainly results from the destruction of periodontal tissue by bacteria in dental plaque. Periodontitis is characterized by inflammation of the periodontal soft tissues and progressive loss of the periodontal ligaments and alveolar bone, which in severe cases can affect chewing and digestion [1]. Conventional treatment of periodontitis focuses on suppressing inflammation but can not regenerate periodontal tissue. Therefore, tissue regeneration and bone reconstruction have become the new directions of periodontitis treatment. Human periodontal ligament stem cells (hPDLSCs) have multidirectional differentiation potential and are considered a potentially applicable cell source for periodontal regenerative therapy [2, 3]. Numerous studies have explored the role of hPDLSCs in periodontal restoration, and demonstrated that it may enable periodontal tissue regeneration and repair by promoting osteogenic differentiation [4, 5]. These studies demonstrate the potential of hPDLSCs and lay the foundation for regenerative treatments for periodontitis.
Exosomes are extracellular vesicles with diameters in the range of 40–160 nm that can be secreted by almost all types of cells [6, 7]. Exosomes exist naturally in body fluids and contain proteins, mRNA, microRNA and other substances, which mediate long-range signal transduction through cell-to-cell communication [8]. Furthermore, exosomes play a role as intercellular communication mediators due to the release of a variety of active substances, and are involved in regulating the pathological and physiological processes of many diseases, including periodontitis [9]. Nakao et al. [10]. reported that xosomes from TNF-α-treated human gingiva-derived mesenchymal stem cells inhibits inflammation in periodontitis, and exhibited anti-osteoclastogenic activity because of exosomal miR-1260b targets to Wnt5a-mediated RANKL pathway. Dental pulp stem cell-derived exosomes promotes the proliferation, migration and osteogenesis of PDLSCs in in vitro simulated periodontitis environment and reduces inflammation by inhibiting IL-6/JAK2/STAT3 signaling pathway [11]. Notably, these studies suggest the potential of exosomes on promoting formation of periodontal tissue and inhibiting inflammation, and demonstrated that exosomes polarize macrophages from the M1 phenotype to the M2 phenotype. Macrophages are immune cells that play an important role in the regulation of chronic inflammation and various diseases, which can be divided into two subpopulations, classically activated or M1 macrophages and substitutively activated or M2 macrophages [12]. M1 macrophages are proinflammatory cells that can be polarized by LPS and release proinflammatory factors. Inversely, M2 macrophages have anti-inflammatory and immunomodulatory effects and can be polarized by Th2 cytokines and release anti-inflammatory factors [13]. Interestingly, a recent study revealed that exosomes from M2 polarized macrophages (M2-exos) reduces alveolar bone resorption in mice with periodontitis by activating IL-10/IL-10R pathway [14]. Moreover, Chen et al. [15]. constructed engineered M2-exos loading with melatonin, and demonstrated it improves the osteogenic and cementogenic differentiation capacity in inflammatory hPDLSCs by reducing excessive endoplasmic reticulum stress and unfolded protein response. These evidences suggest that M2-exo is effective on promoting bone formation and suppressing inflammation in periodontitis. However, studies about M2-exo on improvement of periodontitis are insufficient, the underlying mechanism through which M2-exo treats periodontitis remains to be elucidated.
CXCL12 is considered to be a homeostatic chemokine, which is involved in embryogenesis, neurogenesis, hematopoietic, angiogenesis and other physiological processes by inducing migration and activation of hematopoietic stem cells, endothelial cells and white blood cells [16]. CXCL12 has been observed to be upregulated in multiple inflammatory diseases [17]. In periodontal disease, CXCL12 was found to be upregulated in a previous study, which may be involved in the immune defense pathway activated during periodontal disease [18]. Moreover, Hosokawa et al. [19]. proposed that CXCL12 may be associated with cell infiltration and angiogenesis in normal periodontal tissue and periodontal lesion tissue. Notably, several recent studies demonstrated that CXCL12 promotes proliferation, migration and osteogenic differentiation and enhances the potential of angiogenesis of hPDLSCs [20, 21]. Therefore, we speculate that CXCL12 may play a key role in the pathogenesis of periodontitis. However, whether M2-exo mediates CXCL12 in hPDLSCs remains unclear.
In this study, hPDLSCs were treated with lipopolysaccharide (LPS) to simulate periodontitis conditions, and we aimed to investigate the effect and mechanism of exosomes isolated from M2 macrophages on osteogenic differentiation and inflammatory in LPS-induced hPDLSCs to provide a theoretical guide for the treatment of periodontitis. This study will provide a new theoretical basis for the treatment of periodontitis.
Method
Cell culture and treatment
RAW264.7 macrophages and hPDLSCs were applied in this study. RAW264.7 macrophages were provided from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin and incubated at 37℃ and 5% CO2. To induce RAW264.7 macrophages M2 polarization, the cells were treated with 20 ng/ml IL-4 for 24 h. hPDLSCs were isolated according to previous protocols [15]. The experiments were approved by the Clinical Ethics Committee of The First Affiliated Hospital of Bengbu Medical University. Informed consent was obtained from all individual participants or their legal guardians included in the study. hPDLSCs were isolated from periodontally healthy third molars and premolars of systemically healthy patients aged between 12 and 25 years at the hospital. PDL tissues were washed with PBS (Gibco) containing 5% penicillin/streptomycin, cut into small pieces and cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37℃ and 5% CO2. To simulate the periodontitis environment, hPDLSCs were treated with 20 µg/ml LPS. To induce hPDLSCs osteogenic differentiation, cells were treated with 50 µg/mL β-ascorbic acid, 20 nM dexamethasone, and 8 mM β‐glycerol phosphate for subsequent experiments.To induce hPDLSCs adipogenic differentiation, cells were treated with 0.5 mmol/L dexamethasone, 10 mmol/L insulin and 0.5 mmol/L 3-isobutyl−1-methylxanthine.
Flow cytometry
Evaluation of M2 polarization of RAW264.7 macrophages and identification of hPDLSCs were performed by flow cytometry. M2 macrophages were incubated with anti-CD163 and anti-CD206, and hPDLSCs were incubated with CD11b, CD45 and CD90 for 30 min protected from light and detected by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Isolation of exosomes
M2-exo was isolated from M2 macrophages by ultracentrifugation according to previous experiments [22]. Supernatant of medium was centrifuged at 300×g for 15 min, 3000×g for 15 min and 20,000×g for 70 min, respectively. Finally, the exosomes were purified and the supernatant was centrifuged at 120,000×g for 70 min, and the exosomes was suspended in PBS and stored at−80℃ for use.
Identification of M2-exo
M2-exos were identified by transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA). For TEM, exosomes were loaded into a copper mesh for 3–5 min, stained with 2% (w/v) phosphotungstic acid for 2–3 min and then identified by (TEM JEOL, Tokyo, Japan). NTA was performed using a NanoSight NS300 instrument (Malvern, Worcestershire, UK) to measure the size of exosomes.
PKH67 staining
Hieff® exosome tracker kit (Yeasen, Shanghai, China) was applied to stain exosomes. Briefly, PKH6 storage buffer was diluted 10 times with dilution buffer and the working solution was configured at a concentration of 100 µM. The working solution was added to exosomes at the recommended dose according to the manufacturer’s protocol. The mixture was well-mixed and incubated for 10 min. Next, 10 ml PBS was added to mixture, and the excess dye was removed and the PKH67-labelled exosomes were incubated with hPDLSCs for 1 h. Cells were incubated with 4’,6-diamidino−2-phenylindole (DAPI; Beyotime, Shanghai, China) to stain nucleus. The images were captured using a fluorescence microscope.
Cell transfection
Short hairpin RNA targeting CXCL12 (shCXCL12) and its negative control (shNC) were purchased from GenePharma (Shanghai, China). hPDLSCs were seeded in 24-well plate (4 × 104 cells/well) and transfected with these shCXCL12 or shNC using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The cells were harvested after 48 h of transfection.
Western blot
Total protein from hPDLSCs was isolated using RIPA lysis buffer (Beyotime). M2-exo protein was isolated using an exosome protein extraction kit (Proteintech Group, Chicago, IL, USA). Protein samples were loaded on 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% milk for 1 h and incubated with primary antibodies overnight at 4℃. Next, samples were incubated with secondary antibodies (1/10000, ab6721, Abcam, Cambridge, UK) for 2 h at room temperature. The membranes were wash with 1×TBST for three times and visualized using the ECL reagent (Thermo Scientific, Waltham, MA, USA). The primary antibodies used in this study were as follows: anti-TSG101 (1/1000, ab125011, Abcam), anti-CD9 (1/1000, ab236630, Abcam), anti-CD36 (1/1000, ab133625, Abcam), anti-Arg-1 (1/1000, ab133543, Abcam), anti-CD206 (1/1000, ab64693, Abcam), anti-ALP (1/1000, ab229126, Abcam), anti-OCN (1/1000, ab133612, Abcam), anti-Runx2 (1/1000, ab236639, Abcam) and anti-β-actin (1/2500, ab8227, Abcam).
Quantitative real-time PCR (qPCR)
TRIzol reagent (Invitrogen) was performed to extract total RNA from RAW264.7 macrophages and hPDLSCs. RNA isolation of M2-exo was conducted using an exosome RNA isolation kit (Thermo Scientific). Reverse transcription was performed using a PrimeScript RT reagent kit (Takara, Tokyo, Japan). qPCR was conducted on ABI 7500 system using SYBR green (Invitrogen). Relative mRNA expression was calculated using the 2−ΔΔCt method as normalized to β-actin. The primers for qPCR were as follows: ALP, 5’-ACCACCACGAGAGTGAACCA-3’ (forward primer) and 5’-CGTTGTCTGAGTACCAGTCCC-3’ (reverse primer); OCN, 5’-CACTCCTCGCCCTATTGGC-3’ (forward primer) and 5’-CCCTCCTGCTTGGACACAAAG-3’ (reverse primer); Runx2, 5’-TGGTTACTGTCATGGCGGGTA-3’ (forward primer) and 5’-TCTCAGATCGTTGAACCTTGCTA-3’ (reverse primer); CXCL12, 5’-ATTCTCAACACTCCAAACTGTGC-3’ (forward primer) and 5’-ACTTTAGCTTCGGGTCAATGC-3’ (reverse primer).
Alkaline phosphatase (ALP) staining and ALP activity
ALP staining was conducted after 7 d of osteogenic differentiation induction using an ALP staining kit (Beyotime). hPDLSCs were stained with staining solution for 30 min protected from light at room temperature. ALP activity was measured using an ALP assay kit (Beyotime). The experiments were carried out following the manufacturer’s protocol, and the absorbance was measured at 405 nm.
Alizarin Red S (ARS) staining
ARS staining was conducted using an ARS staining kit (Beyotime) after 14 d of osteogenic differentiation induction to measure osteogenic mineralized nodules. hPDLSCs were washed with PBS and fixed for 20 min. Then, cells were stained with staining solution for 30 min at room temperature and observed under the microscope. ARS staining was extracted by cetylpyridinium chloride and the absorbance was measured at 562 nm.
Oil Red O staining
Oil Red O staining was performed using an Oil Red O staining kit (Beyotime) 14 d after induction of adipogenic differentiation. Cells were stained with Oil red O staining solution for 20 min and observed using a microscope.
ELISA assay
The levels of IL-1β, IL-6 and TNF-α in hPDLSCs were measured using ELISA kits (Beyotime). The experiments were conducted according to the manufacturer’s protocol, and the absorbance was measured at 450 nm.
Statistical analysis
All statistical analysis was performed using SPSS 22.0. Results were expressed as mean ± standard deviation of at least three replicates. The comparison between two or more groups was performed by student’s t-test or one-way analysis of variance (ANOVA). P < 0.05 was recognized as statistically significant.
Results
Identification of M2 macrophages, M2-exo and hPDLSCs
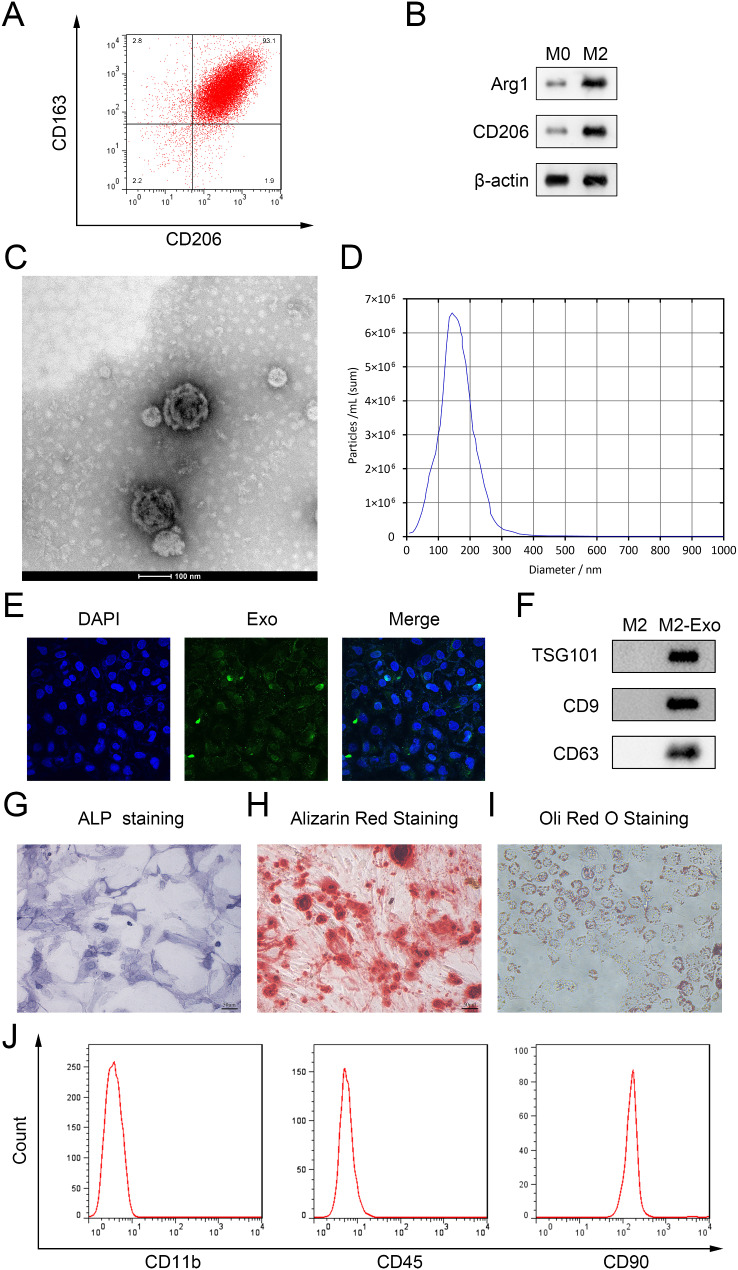
To identify M2 polarization of RAW264.7 cells, we detected several markers of M2 macrophages. Results showed that expression of CD163 and CD206 and the upregulation of Arg1 and CD206 in M2 macrophages (Fig. 1A and B). M2-exos were isolated from M2 macrophages through ultracentrifugation and was identified by TEM. As shown in Fig. 1C, TEM suggested that M2-exos are spherical vesicles with a double membrane. NTA analysis revealed that the diameter of M2-exos mainly concentrated at 100–200 nm (Fig. 1D). PKH67 staining suggested that M2-exos were taken up by hPDLSCs (Fig. 1E). Moreover, we measured the levels of exosome labeled protein through western blot. Results suggested that the levels of TSG101, CD9 and CD63 in M2-exos were increased (Fig. 1F). These results demonstrated that the particles isolated from M2 macrophages were exosomes. Subsequently, we identified the isolated cells from PDL tissues. ALP, ARS and Oil red O staining suggested the multidirectional differentiation potential of the cells (Fig. 1G-H). Moreover, flow cytometry showed that CD11b and CD45 were negatively expressed, while CD90 was positive expressed (Fig. 1J), indicating the isolated cells from PDL tissues were PDLCs.
Fig. 1.
Identification of M2 macrophages, M2-exo and hPDLSCs (A) Markers of M2 macrophage CD163 and CD206 was identified by flow cytometry. (B) The protein levels of Arg1 and CD206 were detected by western blot. (C) The characteristic of M2-exos was identified by TEM. (D) The size of M2-exos was measured by NTA. (E) The PKH67 labeled ADSC-exos taken up by the hPDLSCs was identified by PKH67 staining. (F) Western blot was performed to detect the exosome labeled protein of M2-exos. (G-H) ALP staining, ARS staining and Oil red O staining were performed to identify hPDLSCs
M2-exo restores osteogenic differentiation in hPDLSCs inhibited by LPS
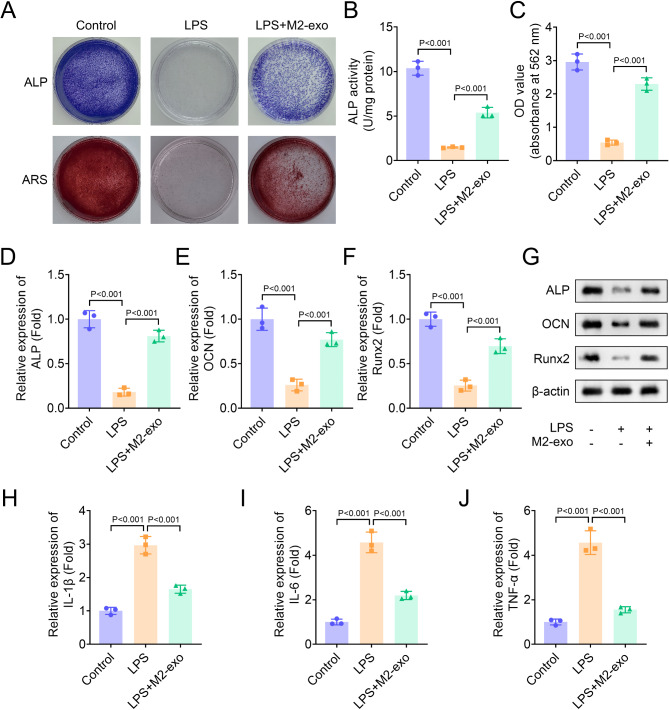
Next, we assessed the effect of M2-exo on osteogenic differentiation in hPDLSCs. Results suggested that LPS inhibited the ALP staining density and osteogenic mineralization nodules in hPDLSCs, which was partially restored by M2-exo, indicating that osteogenic differentiation inhibited by LPS was reversed by M2-exo (Fig. 2A-C). Then, we measured the expression of several osteogenic differentiation-related genes, ALP, OCN and Runx2 in hPDLSCs. Results showed that LPS inhibited the expression of ALP, OCN and Runx2, while this effect was partially reversed by M2-exo (Fig. 2D-F). Moreover, The protein levels of ALP, OCN and Runx2 downregulated by LPS was reversed by M2-exo (Fig. 2G). Additionally, LPS increased the levels of inflammatory factors IL-1β, IL-6 and TNF-α, which was significantly inhibited by M2-exo (Fig. 2H-J). Moreover, we identified the effect of M2-exo derived from THP-1 on hPDLSCs and found that its effect was similar to those of M2-exo from RAW264.7 (Figure S1A-J). In conclusion, these results revealed that M2-exo partially restored osteogenic differentiation in hPDLSCs inhibited by LPS.
Fig. 2.
M2-exo restored osteogenic differentiation in hPDLSCs inhibited by LPS. (A) Osteogenic differentiation was assessed by ALP and ARS staining. (B) ALP activity in hPDLSCs was measured using an ALP activity kit. (C) The absorbance of ARS staining was measured at 562 nm. (D-F) The expression of osteogenic differentiation-related genes ALP, OCN and Runx2 was measured by qPCR. (G) The protein levels of ALP, OCN and Runx2 were identified by western blot. (H-J) The levels of inflammatory factors IL-1β, IL-6 and TNF-α were measured by ELISA assay
Osteogenic differentiation restored by M2-exo in LPS-induced hPDLSCs is suppressed by CXCL12 knockdown
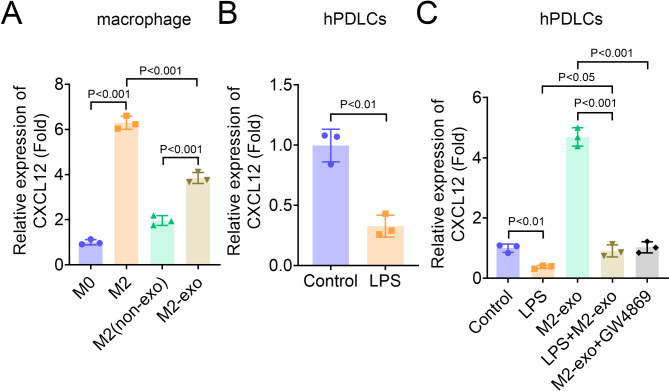
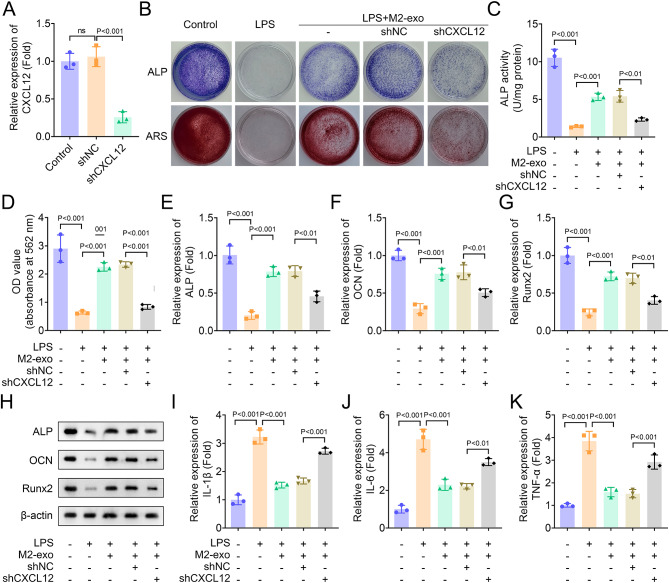
A previous study revealed that CXCL12 promotes proliferation, migration and osteogenic differentiation of hPDLSCs in vitro [20]. To identify whether M2-exo mediates CXCL12 in hPDLSCs, we first measured the expression of CXCL12 in M2 macrophage and hPDLSCs. Results showed that CXCL12 expression was significantly increased in M2 macrophages, and CXC12 expression in M2-exo was higher than that in M2 macrophages without exosomes (Fig. 3A). On the contrary, CXCL12 was decreased in LPS-induced hPDLSCs (Fig. 3B). Furthermore, we detected the expression of CXCL12 in hPDLSCs after co-culture of M2-exo with hPDLSCs induced by LPS or not. Results showed that M2-exo increased CXCL12 expression in hPDLSCs without LPS treatment, while the expression was significantly inhibited by LPS and exosome inhibitor and exosome inhibitor GW4896 (Fig. 3C), indicating that increased CXCL12 expression in hPDLSCs mainly from M2-exo. Then, the role of CXCL12 was identified through rescue experiments. CXCL12 expression was decreased in hPDLSCs by CXCL12 knockdown (Fig. 4A). ALP and ARS staining suggested that CXCL12 knockdown inhibited the ALP staining density and activity and osteogenic mineralization nodules in LPS-induced hPDLSCs restored by M2-exo (Fig. 4B-D). To assess the osteogenic differentiation potential of hPDLSCs, we measured the expression and protein levels of ALP, OCN and Runx2 through qPCR and western blot. Results suggested that the expression of ALP, OCN and Runx2 in LPS-induced hPDLSCs increased by M2-exo was reversed by CXCL12 knockdown (Figure E-H). Moreover, the levels of inflammatory factors IL-1β, IL-6 and TNF-α inhibited by M2-exo in LPS-treated hPDLSCs was partially restored by CXCL12 knockdown, indicating that CXCL12 knockdown promoted inflammation in hPDLSCs (Figure 4I-K).
Fig. 3.
Expression of CXCL12 in macrophages and hPDLSCs (A-C) qPCR was performed to measure the expression of CXCL12 in macrophages and hPDLSCs
Fig. 4.
M2-exo restored osteogenic differentiation in LPS-induced hPDLSCs is suppressed by CXCL12 knockdown (A) The expression of CXCL12 was measured by qPCR. (B) Osteogenic differentiation was assessed by ALP and ARS staining. (C) ALP activity in hPDLSCs was measured using an ALP activity kit. (D) The absorbance of ARS staining was measured at 562 nm. (E-G) The expression of osteogenic differentiation-related genes ALP, OCN and Runx2 was measured by qPCR. (H) The protein levels of ALP, OCN and Runx2 were identified by western blot. (I-K) The levels of inflammatory factors IL-1β, IL-6 and TNF-α were measured by ELISA assay
Discussion
Periodontitis not only damages the integrity of the periodontal tissues, but also increases the risk of diseases such as cancer and autoimmune diseases [23]. Periodontitis is primarily triggered by dysbiosis of the periodontal flora leading to host inflammation, the mechanism of which has not yet been fully elucidated [24]. Notably, elevated levels of inflammation exacerbate osteoclastic bone resorption while inhibiting osteoblastic bone formation, resulting in net bone loss [25]. Therefore, the promotion of periodontal tissue regeneration through the improvement of osteogenic differentiation has become a new direction in the treatment of periodontitis. It is reported that modulation of macrophage polarization adjusts the level of inflammation in periodontal tissues and enhances osteogenic differentiation of hPDLSCs, suggesting the potential of hPDLSCs on periodontal repair [10, 26]. M2-exo exhibits anti-inflammatory effects in several diseases induced by obesity [27]. Moreover, M2-exo plays an important role in bone formation. Zhou et al. [28]. demonstrated that the pre-treatment of M2-exo with hydrogen sulfide significantly enhances its ability to promote bone regeneration in cranial bone defects and facilitates osteogenic differentiation of mesenchymal stem cells. Hou et al. [29]. revealed that M2-exos target OLFML1 through miR-365-2-5p to facilitate osteogenesis. Recently, several studies reported that M2-exo improves the progression of periodontitis by regulating bone formation. Several studies revealed that M2-exo promotes osteogenic differentiation of human periodontal ligament stem cells (hPDLSCs) and enhances mineralization of cementoblast [30, 31]. Moreover, Chen et al. [14]. reported that M2-exo facilitates osteogenic differentiation of bone marrow stromal cells while inhibiting the formation of bone marrow-derived macrophage osteoclast, and prevent pathological alveolar bone resorption through intercellular communication via exosomes. In this study, we isolated exosomes from IL-4-induced RAW264.7 cells, and assessed the effect of M2-exo on hPDLSCs. Similarly to previous studies, our results demonstrated that M2-exo promoted osteogenic differentiation and suppressed levels of inflammatory factors in LPS-induced hPDLSCs, indicating that M2-exo may be a potential treatment for periodontitis.
CXCL12 is a key chemokine in many homeostatic processes including embryonic development and angiogenesis, and has been shown to play a role in the regulation of inflammatory responses. Moreover, CXCL12 has also been reported to be involved in the regulation of bone homeostasis. The mechanism of CXCL12 regulating bone homeostasis is complex. Ponte et al. [32]. demonstrated that CXCL12 restrains bone turnover by suppressing osteoblastogenesis and the osteoclastogenesis support provided by cells of the osteoblast lineage rather than altering the balance between resorption and formation. Tzeng et al. [33]. revealed that deletion of CXCL12 in mesenchymal stem/progenitor cells (MSPCs) reduces trabecular bone content and promotes MSPCs differentiation into adipocytes. Due to the potential of CXCL12 in regulating osteogenic differentiation, the mechanism of CXCL12 mediating periodontitis by regulating bone homeostasis has also been explored. For instance, Liang et al. [20]. confirmed that SDF-1/EX-4 cotherapy synergistically regulates PDLSCs activities, promotes periodontal bone formation, which may be a new strategy for periodontal bone regeneration. However, the mechanism of CXCL12 mediating the development of periodontitis is not been fully explored, and whether CXCL12 is regulated by M2-exo in hPDLSCs remains unclear. In our current study, we demonstrated that CXCL12 was highly expressed in M2 macrophages, and LPS inhibited CXCL12 expression in hPDLSCs. Additionally, promoted osteogenic differentiation and suppressed inflammation levels in LPS-induced hPDLSCs were reversed by CXCL12 knockdown. These results suggested that CXCL12 may promote osteogenic differentiation in hPDLSCs. Zhang et al. [21]. demonstrated that CXCL12 overexpression promotes the angiogenesis of PDLSCs. Moreover, Du et al. [34]. confirmed that PTH and CXCL12 cotherapy facilitates proliferation, migration and osteogenic differentiation of PDLSCs. These results indicated that CXCL12 may be a promoter of periodontal tissue regeneration. Collectively, this study demonstrated that M2-exo may promote osteogenic differentiation and suppress inflammation in LPS-induced hPDLSCs by enhancing CXCL12 expression.
However, limitations still exist in this study. Although we found that THP-1-derived M2-exo and RAW264.7 cell-derived M2-exo presented close inhibitory effects at osteogenic differentiation and inflammation level, whether it also exert these effects by targeting CXCL12 remains unclear, which may be investigated in the future work. Moreover, animal study will be conducted in the future work to provide the theoretical and experimental foundation for the clinical treatment of periodontitis.
In conclusion, we confirmed that M2-exo promoted osteogenic differentiation and suppressed inflammation in LPS-induced hPDLSCs through promoting CXCL12 expression. This study may provide a new theory for the treatment of periodontitis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. J G drafted the work and revised it critically for important intellectual content and was responsible for the acquisition, analysis and interpretation of data for the work; Z W made substantial contributions to the conception or design of the work. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experiments were approved by the Clinical Ethics Committee of The First Affiliated Hospital of Bengbu Medical University. Informed consent was obtained from all individual participants included in the study. This study was performed in line with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang B, Pang X, Li Z, Chen Z, Wang Y. Immunomodulation in the treatment of Periodontitis: Progress and perspectives. FRONT IMMUNOL. 2021;12:781378. 10.3389/fimmu.2021.781378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Zhou H, Kong N, et al. l-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration. BIOACT MATER. 2021;6:3288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wangzhou K, Lai Z, Lu Z, et al. MiR-143-3p inhibits osteogenic differentiation of Human Periodontal Ligament cells by targeting KLF5 and inactivating the Wnt/beta-Catenin pathway. FRONT PHYSIOL. 2020;11:606967. 10.3389/fphys.2020.606967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Wang Z, Huang M, Zhang Y, Xu L. Circ_0099630 participates in SPRY1-Mediated repression in Periodontitis. INT DENT J. 2023;73:136–43. 10.1016/j.identj.2022.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin SS, He DQ, Wang Y, et al. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. CELL PROLIFERAT. 2020;53:e12912. 10.1111/cpr.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (American Association for the Advancement of Science; 2020. p. 367. [DOI] [PMC free article] [PubMed]
- 7.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. IMMUNOL LETT. 2006;107:102–8. 10.1016/j.imlet.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a Distance. DEV CELL. 2019;49:347–60. 10.1016/j.devcel.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Lin H, Chen H, Zhao X, et al. Advances of exosomes in periodontitis treatment. J TRANSL MED. 2022;20:279. 10.1186/s12967-022-03487-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao Y, Fukuda T, Zhang Q, et al. Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. ACTA BIOMATER. 2021;122:306–24. 10.1016/j.actbio.2020.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao X, Tang J, Dou L, et al. Dental Pulp Stem Cell-Derived exosomes regulate anti-inflammatory and Osteogenesis in Periodontal Ligament Stem cells and promote the repair of experimental periodontitis in rats. INT J NANOMED. 2023;18:4683–703. 10.2147/IJN.S420967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. TRENDS IMMUNOL. 2002;23:549–55. 10.1016/S1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 13.Ruytinx P, Proost P, Van Damme J, Struyf S. Chemokine-Induced Macrophage polarization in inflammatory conditions. FRONT IMMUNOL. 2018;9:1930. 10.3389/fimmu.2018.01930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Wan Z, Yang L, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J NANOBIOTECHNOL. 2022;20:110. 10.1186/s12951-022-01314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Hong S, Xia Y, et al. Melatonin Engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and Immune Reprogramming for Periodontitis Therapy. ADV SCI. 2023;10:e2302029. 10.1002/advs.202302029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cambier S, Gouwy M, Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. CELL MOL IMMUNOL. 2023;20:217–51. 10.1038/s41423-023-00974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner L, Guzner-Gur H, Dotan I. Involvement of CXCR4/CXCR7/CXCL12 interactions in inflammatory bowel disease. THERANOSTICS. 2013;3:40–6. 10.7150/thno.5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havens AM, Chiu E, Taba M, et al. Stromal-derived factor-1alpha (CXCL12) levels increase in periodontal disease. J PERIODONTOL. 2008;79:845–53. 10.1902/jop.2008.070514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosokawa Y, Hosokawa I, Ozaki K, et al. CXCL12 and CXCR4 expression by human gingival fibroblasts in periodontal disease. CLIN EXP IMMUNOL. 2005;141:467–74. 10.1111/j.1365-2249.2005.02852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Q, Du L, Zhang R, Kang W, Ge S. Stromal cell-derived factor-1/Exendin-4 cotherapy facilitates the proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells in vitro and promotes periodontal bone regeneration in vivo. CELL PROLIFERAT. 2021;54:e12997. 10.1111/cpr.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Zhou Y, Sun X, Zhou J, Yang P. CXCL12 overexpression promotes the angiogenesis potential of periodontal ligament stem cells. SCI REP-UK. 2017;7:10286. 10.1038/s41598-017-10971-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. 10.1016/j.biomaterials.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. NAT REV IMMUNOL. 2021;21:426–40. 10.1038/s41577-020-00488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. NAT REV IMMUNOL. 2015;15:30–44. 10.1038/nri3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Wang CY. Osteoporosis and periodontal diseases - an update on their association and mechanistic links. PERIODONTOL 2000. 2022;89:99–113. 10.1111/prd.12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Liu Q, Liu L, et al. Lipopolysaccharide-preconditioned Dental follicle stem cells derived small extracellular vesicles treating Periodontitis via reactive oxygen Species/Mitogen-Activated protein kinase signaling-mediated antioxidant effect. INT J NANOMED. 2022;17:799–819. 10.2147/IJN.S350869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phu TA, Ng M, Vu NK, Bouchareychas L, Raffai RL. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. MOL THER. 2022;30:2274–97. 10.1016/j.ymthe.2022.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou YK, Han CS, Zhu ZL, et al. M2 exosomes modified by hydrogen sulfide promoted bone regeneration by moesin mediated endocytosis. BIOACT MATER. 2024;31:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou C, Zhang Y, Lv Z, et al. Macrophage exosomes modified by mir-365-2-5p promoted osteoblast osteogenic differentiation by targeting OLFML1. REGEN BIOMATER. 2024;11:e18. 10.1093/rb/rbae018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao XM, Guan Z, Yang ZJ, et al. Comprehensive analysis of M2 macrophage-derived exosomes facilitating osteogenic differentiation of human periodontal ligament stem cells. BMC Oral Health. 2022;22:647. 10.1186/s12903-022-02682-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Huang Y, Liu H, et al. Macrophages with different polarization phenotypes influence cementoblast mineralization through Exosomes. STEM CELLS INT. 2022;2022:4185972. 10.1155/2022/4185972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponte F, Kim HN, Iyer S, et al. Cxcl12 deletion in mesenchymal cells increases bone turnover and attenuates the loss of cortical bone caused by Estrogen Deficiency in mice. J BONE Min RES. 2020;35:1441–51. 10.1002/jbmr.4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzeng YS, Chung NC, Chen YR, et al. Imbalanced Osteogenesis and adipogenesis in mice deficient in the chemokine Cxcl12/Sdf1 in the bone mesenchymal Stem/Progenitor cells. J BONE Min RES. 2018;33:679–90. 10.1002/jbmr.3340 [DOI] [PubMed] [Google Scholar]
- 34.Du L, Feng R, Ge S. PTH/SDF-1alpha cotherapy promotes proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells. CELL PROLIFERAT. 2016;49:599–608. 10.1111/cpr.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.