Abstract
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic pain condition creating a wide range of urologic and pain symptoms. There is currently limited evidence to understand the mechanisms of IC/BPS. There have been recent studies suggesting that altered function in brain motor areas, particularly the supplementary motor cortex (SMA), relates to altered bladder sensorimotor control and may play an important role in IC/BPS. This study aims to provide evidence that non-invasive stimulation targeting the motor cortex may help reduce IC/BPS pain, as well as better understand the neural mechanism by which this stimulation targets neuromuscular dysfunction.
This study is a two-group quadruple-blinded randomized controlled trial (RCT) of active vs. sham repetitive transmagnetic stimulation (rTMS). In addition, our study will also include functional magnetic resonance imaging (fMRI), pelvic floor electromyography (EMG), pelvic exam, and outcome measures and questionnaires to further study outcomes.
Ethics and dissemination
All aspects of the study were approved by the Institutional Review Board of the University of Southern California (protocol HS-20–01021). All participants provided informed consent by the research coordinator/assistants. The results will be submitted for publication in peer-reviewed journals and disseminated at scientific conferences.
Trial registration
ClinicalTrials.gov NCT04734847. Registered on February 1, 2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08450-w.
Keywords: Chronic pain, Transcranial magnetic stimulation, Pelvic floor muscles, Bladder pain syndrome, BPS, Interstitial cystitis, IC, Pelvic pain, Chronic overlapping pain condition, Motor cortical neuromodulation, Functional magnetic resonance imaging, Supplementary motor area
Strengths and limitations of this RCT design
Randomized controlled trial (RCT) examining the changes in functional magnetic resonance imaging (fMRI) and brain or pelvic floor muscle electromyographic (EMG) activity to determine the mechanism of rTMS for IC/BPS.
Study design quadruple-blinded RCT with an interventional model of parallel assignment and multiple levels of blinding (participant, care provider, investigator, outcome assessor).
High-frequency repetitive transcranial magnetic stimulation (rTMS) to the pelvic region of supplementary motor area, which we refer to as pelvic-SMA, that regulates pelvic floor muscle activity. Pelvic-SMA was previously identified to be a region of neural dysfunction in IC/BPS.
Functional magnetic resonance imaging (fMRI) to measure changes in pelvic-SMA target previously described, performed 1 h before and after rTMS treatment to show the comparative change in brain activity post-rTMS treatment.
Electromyography (EMG) via internal pelvic floor sensor will be examined during the first treatment (day 1) and during the last treatment (day 5) to assess rTMS-related changes in pelvic floor muscle (PFM) activity.
Study design reduces risk of treatment variables impacting the effect of rTMS on outcomes, as participants are asked to discontinue other IC/BPS treatments during the 6-week participation in the study, if possible. If this is not medically advised by the study physician, participants will be asked to report other concurrent treatments.
Limitations include not varying the stimulation intensity or number of sessions per group.
Background
New potential mechanisms of interstitial cystitis/bladder pain syndrome (IC/BPS) in women have recently been proposed [1]. One newly proposed mechanism identified by functional magnetic resonance imaging (fMRI) is altered activity in sensorimotor cortex, particularly supplementary motor area (SMA), that may lead to dysregulated pelvic floor activity [2, 3]. This mechanism is of particular interest because there is strong evidence showing the benefits of motor cortical rTMS, aligned with the cortical representation of the painful body region, in treating neuropathic pain [4]. Previous studies of rTMS for pain do not typically target a particular marker of altered brain function. Therefore, we designed a study to evaluate whether active rTMS can improve IC/BPS symptoms and whether these changes are related to fMRI-identified changes in brain function would be valuable.
Objectives
(1) Primary objective: to reduce pain in IC/BPS, (2) to improve brain and muscle activity in IC/BPS, (3) tertiary objective: to explore if reduced pain is mediated by brain and muscle activity marker improvements.
Study design and methods
Study design
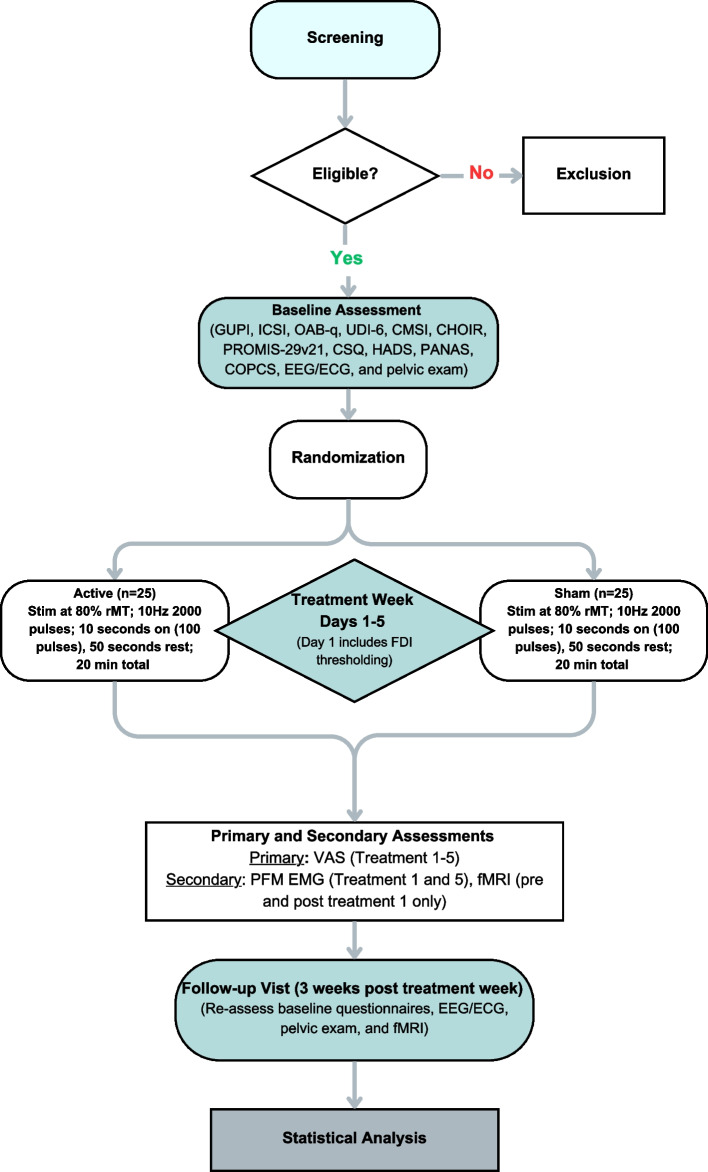
Two-group randomized controlled trial (RCT) of active versus sham rTMS. rTMS uses pulses of magnetic energy to non-invasively stimulate the brain. The rTMS device to be used in this study is the Magstim Rapid2. The rTMS device can either connect to a “active” coil (used in the active group) which rests against the scalp and delivers the magnetic energy to the brain or can be connected to an identically appearing “sham” coil (used in the sham group) that also rests against the scalp but does not deliver any magnetic energy to the brain. The rTMS protocol is specified by the parameters of pulse frequency, duration of pulse delivery (“train duration”), intensity, and stimulation location.
Our study proposes to have the active rTMS group receive a previously described rTMS protocol for treatment of patients with chronic pain in research studies (a “high-frequency protocol”) [4]: a pulse frequency of 10 pulses per second, with 10-s pulse trains, at an intensity of 80% of resting motor threshold, delivered over the pelvic-SMA region, defined in Montreal Neurological Institute (MNI) coordinates to be X = − 2, Y = − 16, and Z = 68 mm. A “session” of rTMS in our study will consist of 20 pulse trains, each separated by 50 s. Should protocol changes be needed, the proposed changes are submitted to the DSMB first, then the home institute’s internal review board (IRB) to be reviewed and approved. Protocol changes may not be implemented until approved. Any protocol deviations are reported to the IRB to be documented and acknowledged.
Study population
Our study population is actively being recruited through various avenues, including the University of Southern California’s broad medical system, focusing on, but not limited to those in the greater Los Angeles area. Additionally, recruitment has been mindful of inclusivity and equity within our system, recruiting from various settings, including but not limited to our USC Keck hospitals and LA County General Hospital, and outpatient PT clinics in the greater Los Angeles area, in hopes to improve diversity in our participants.
Study setting
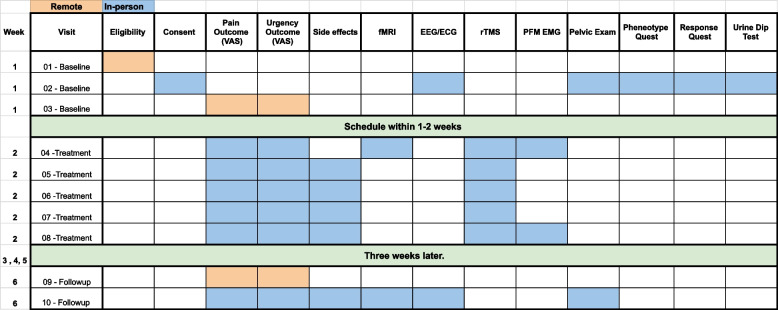
Participants will be treated at the University of Southern California Division of Biokinesiology and Physical Therapy Applied Movement and Pain Laboratory (AMPL) with MRI being performed onsite at the USC Stevens Institute for Neuroimaging and Neuroinformatics. Study workflow is summarized in Fig. 1.
Fig. 1.
Study workflow
Inclusion criteria
Female at least 18 years old.
Have a diagnosis of IC/BPS by a physician.
Urologic symptoms present a majority of the time during the most recent 3 months.
Report 4/10 pain or greater pain in response to “At its worst, on scale 0–10 how bad did your pelvic pain get in the past 3 months?” asked at screening.
Exclusion criteria
This study uses several measures to exclude individuals who have a known physical cause of their symptoms such as an active infection, urethral strictures, active cancer treatments/pelvic malignancies, and neurological conditions affecting the bladder. In addition, rTMS and fMRI pose higher than average health consequences to individuals with personal factors such as pregnancy, seizure disorders, and chronic headaches. As such, participants with these factors were designated ineligible for this study.
Participants will be excluded from the study if they report:
Symptomatic urethral stricture
On-going neurological conditions affecting the bladder or bowel
Active auto-immune or infectious disorders
History of cystitis caused by tuberculosis or radiation or chemotherapies
History of non-dermatologic cancer
Current major psychiatric disorders
Severe cardiac, pulmonary, renal, or hepatic disease
Conditions or the use of medical devices that are contraindications for either fMRI or rTMS procedures, including pregnancy, seizure disorders, or chronic headaches
Physicians with expertise in clinical TMS or IC/BPS are consulted if there are any questions about eligibility for the study, and their expert opinion documented.
Deferral criteria and management
Participants are deferred if:
They test positive on the McKesson urinalysis test during the baseline visit.
They report having started any new treatments or medications for IC/BPS in the past 3 months during screening.
For either of the following, interested participants will be re-evaluated in no sooner than 6 weeks.
Recruitment
Recruitment for this study was conducted through the Applied Movement and Pain Laboratory (AMPL) in the Department of Biokinesiology and Physical Therapy at the University of Southern California (USC). This study worked with providers in the USC Medical System and outpatient Pelvic Health Physical Therapy centers in the greater Los Angeles area to identify potentially appropriate participants. Additionally, research coordinators used digital platforms such as YouTube, social media, and email to inform the broader population of this study as well as subscribers and members of the national support groups. Interested participants were then screened for appropriateness and safety for the trial.
Randomization, allocation concealment, and blinding
We will randomize treatment assignments using a randomized block sequence as implemented in the blockrand function in the R statistical package [5]. To ensure approximately equal allocation to the two arms throughout the study, randomization is conducted in blocks, of sizes 2, 4 and 6. The randomization order will be administered by a statistician independent of the participant recruitment and evaluation. A list of 50 record IDs was generated paired with a 4-letter code for the active and sham coil. As participants are enrolled in the study, they are assigned a record ID and matched to the subsequent coil/treatment group. Study staff delivering the intervention know the coil only by code and are specifically instructed that they must remain blinded to which coil is active. This is a double quadruple-blinded study where both the interventionists and participants are blinded to which treatment group they have been assigned (sham vs. active), as are the investigators and outcome assessors.
Intervention
The active and sham groups will undergo identical protocol, with the only difference being that the active vs. sham coil used per designated group for the 5-day treatment week. During the participant’s 6-week participation and treatment period, they will be asked to discontinue any other IC/BPS treatments and therapies, to reduce any confounding treatment variables. If this is not medically advised by the study physician, participants will be asked to report other concurrent treatments.
Outcome assessment
Outcomes will be assessed at the initial baseline visit, throughout the treatment week, and at a follow-up visit 3 weeks later. At baseline, participants will complete a urine dip test, subjective questionnaires, and a pelvic floor muscle exam. Day 1 of treatment will involve outcome assessment via subjective questionnaires, and fMRI pre- and post-rTMS, and pelvic floor muscle assessment during rTMS via a surface electromyographic (EMG) sensor that rests just inside the rectum. Treatments 2 through 4 involve outcome assessment via questionnaires and rTMS treatments. Treatment 5 involve outcome assessment via questionnaires (listed below) and rTMS treatment with pelvic floor muscle assessment. A follow-up visit 3 weeks later will include outcome assessment via questionnaires, fMRI, and pelvic exam.
Outcome measures assessed include demographic information, Genitourinary Pain Index (GUPI) [6], Interstitial Cystitis Symptom Index (ICSI) [7], Overactive Bladder Questionnaire (OAB-q) [8], Urinary Distress Inventory (UDI-6) [9], Complex Multisystem Inventory (CMSI) [10], Collaborative Health Outcomes Registry Body Map (CHOIR) [11], Patient-Reported Outcomes Measurement Information System (PROMIS) [12], Coping Strategies Questionnaire (CSQ) [13], Hospital Anxiety and Depression Scale (HADS) [14], and The Positive and Negative Affect Schedule (PANAS) [15].
Primary outcomes
Subjective ratings of pain symptoms are the primary outcome measures of the study. Pain will be assessed using a visual analog scale (VAS): the scale ranges from 0 (no pain) to 10 (worst pain imaginable). Pain will also be assessed using a global response assessment (GRA) asking “As compared to when you started the study treatment, how would you rate your interstitial cystitis/bladder pain syndrome (IC/BPS) symptoms now?.” The 3 pain primary outcome measures are:
Longer-term VAS change: before first treatment to 3 weeks after last treatment
Shorter-term VAS change: before first treatment to 1 day later just before second treatment
GRA: 3 weeks after last treatment
Secondary outcomes
Secondary outcomes from fMRI and pelvic EMG will be computed exactly as described previously [3]. In summary:
Change in fractional amplitude of low frequency fluctuations (fALFF) in pelvic-SMA determined from fMRI. This change will be assessed between 1 h before and one hour after first treatment.
Change in pelvic floor muscle activity from pelvic EMG. This change will be assessed between just before to between 5 and 10 min after the start of first treatment.
Safety and participant compensation
Transient headache is one of the known side effects of rTMS treatment. During and following every TMS session, we will ask the participant about any incidence of headache and neck pain. If the participant complains of any discomfort or pain during the TMS session, the session will be terminated immediately. The participant’s symptoms and side effects will be monitored both during treatment and longer-term side effects will be assessed at the follow-up visit (Fig. 2). There is no anticipated harm and compensation for trial participation and there are no plans to offer any type of payment for injury. If the participant requires treatment because they were injured, from participating in the study, treatment will be provided and the participants’ health insurance plan will be billed for treatment. The study sponsor will not pay for this treatment. The rTMS is being performed at a low intensity (80% of the FDI-thresholding) and the treatment duration is brief; no dosage changes are proposed. If a participant were to miss a treatment session, they can continue with the remaining scheduled visits and no treatment sessions will be rescheduled. Any moderate or severe side effect will be reported to the IRB as an adverse event within 24 h.
Fig. 2.
Study schedule of enrolment, event, and assessments
The participant can withdraw from the study at any time for any reason. Treatment will be discontinued and all side effects will be documented. The study personnel will notify the PI as soon as any possible adverse event occurs and the PI will submit the adverse event reports. IRB will be notified immediately of all adverse and serious adverse events and the DSMB will be notified, as well as receive an annual summary of all adverse events.
Oversight and monitoring
A 3-member Data and Safety Monitoring Board (DSMB) has been established for this study in accordance with NIH guidelines. The study’s full case report forms and standard operating procedures (CRF + SOP) stored in secure files. All data collected will be stored in a secure REDCap database without any participant identifying information. Identifying information will be kept securely by the principal investigator (PI). A single password-protected spreadsheet will be maintained by the PI with the participant’s name and contact information as a key to codes and this spreadsheet will be destroyed at study completion to ensure all data analyses are de-identified. We will do an annual progress report, continued review and DSMB meeting to go over the status of the study and compliance. Should any other concern for patient safety occur throughout the study, the DSMB is consulted immediately.
Sample size
We designed our study to have N = 50 participants, with N = 25 for each of the two groups (N = 25 sham, N = 25 high-frequency rTMS), to provide 84% power for the primary hypothesis. The primary hypothesis will be tested with a two-sample t-test for a difference in mean pain outcome measure in the high-frequency rTMS group versus the sham group. We performed this power calculation using G*Power version 3.1 under the assumptions of a two-sided hypothesis test, a type I error rate of 0.05, and the following effect sizes from the literature. In a prior study with N = 13 [16], pain (measured on a 100-point scale) was reduced an average of 15 points in the high-frequency rTMS group (SD = 20) and 3 points in the sham group (SD = 22) at 3 weeks after the last rTMS session, compared to baseline.
Statistical analyses
Data will be analyzed using R [17] by a blinded statistician not involved with participants or data collection. Initiative descriptive analyses will include evaluation of the distributions of all variables to evaluate modeling assumptions (e.g., Shapiro–Wilk test for normality) and identify potential extreme values. All data will be included in the primary analysis, but any extreme values will be discussed with the study team and potentially excluded in a sensitivity analysis should the extreme value be suspected to be erroneous. Hypothesis tests will be two-tailed, using a significance level of α = 0.05. The primary hypotheses will be evaluated using a two-sample t-test for a difference in mean pain outcome between the two groups, assuming normality of the outcome (or its natural log transformation) in each group. If normality assumptions are not satisfied, we will instead use the nonparametric alternative to the two-sample t-test, the Wilcoxon rank sum test to test for a difference in medians between the two groups. The number of adverse events will be tabulated and compared across the two groups using a chi-square test or Fisher’s exact test. Final primary hypothesis results and R code for producing these results will be presented to the Independent Monitoring Committee by the blinded statistician for certification. The independent DSMB statistician will unblind the results and assign the true group labels. Secondary and tertiary analyses will be performed unblinded, using two-sample t-tests (or Wilcoxon rank sum tests) and standard mediation analysis methods [18].
Collection and management of data
Participants will receive seven in-person visits at the University of Southern California Health Science Campus Applied Movement and Pain Laboratory (AMPL) over the course of approximately 1 month. On the first (baseline) in-person visit, the participant will be tested to rule out any urinary tract infection (UTI) and pending the test is negative, they will proceed with the process of collecting EMG data and a pelvic examination. Additionally, the participant will complete a series of online questionnaires. Treatment week, which consists of 5 in-person (Monday–Friday) sessions, is then scheduled no greater than 2 weeks post-baseline visit. In the 3 days preceding this treatment week, the participants will fill out several online questionnaires pertaining to their pain level each morning, as well as the same questionnaires prior to each rTMS treatment. During the treatment week, the participant will receive rTMS treatment, based on their randomization group (treatment vs. sham). On the first of the 5-day treatments, brain imaging fMRI will be taken before and after brain stimulation (rTMS). Additionally, the use of a rectal EMG sensor will be used Monday and Friday during rTMS treatment.
The final portion of data collection occurs 3 weeks post Friday of the 5-day treatment week, preceded by 3 days of the same questionnaires to understand any changes in their pelvic pain or urinary urgency. During this final in-person visit, the participant completed the same set of questionnaires administered at the baseline visit, followed by fMRI, an EEG, and pelvic examination.
We will summarize the availability of key data needed for the primary analysis. All treatment assignments will be available and due to randomization of treatment assignment no covariates will be included in the primary intent-to-treat statistical analysis. The primary outcome variable is the change in pain scale from before first treatment (baseline) to 3 weeks after the last treatment, and the goal is to compare the mean change in pain between treatment groups. Should there be missing values in the primary outcome measurements of pain, we will modify the initial statistical analysis plan of a two-sample t-test for differences in the pain change score to an analogous linear mixed model, which provides estimates that are valid under a missing at random assumption.
Bias
Several measures to reduce bias have been implemented, including that all questionnaires are self-reported and have the option of being provided in English or Spanish, with the option for the participant given the option of “I prefer not to answer” for any questions, due to the sensitive and intimate nature of some questionnaires. Standardized screening processes are utilized, which can be provided either in English or Spanish, depending on the potential participants’ preference to improve communication, understanding, and inclusivity.
While we are recruiting from various settings, one bias noted is that the majority of our participants captured from advertisements/flyers (ICD-10 code based) are those with healthcare insurance vs. those without health care insurance, which can be linked to differences in socioeconomic factors. Additionally, our study is recruiting females only, assuming they not only identify as female but have female anatomy; however, we realize this is a limit in our inclusion criteria description, as we are basing our recruitment on female anatomy and not gender.
Discussion
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a debilitating condition occurring in millions of women in the USA [19, 20]. The underlying mechanism of IC/BPS is not well understood; clinical trials of agents directed at potential targets in the bladder have not identified any generally effective treatments [21]. Recent studies have suggested that altered function in brain motor areas, not directly related to bladder sensation, may play a critical role in IC/BPS [2, 22–24].
Emerging evidence suggests that altered control of pelvic floor muscles may contribute to IC/BPS. Patients with IC/BPS have increased pelvic floor muscle activity [25], potentially caused by changes in supplementary motor area (SMA) [2]. Although pelvic floor physical therapy may be beneficial [26], its results may be suboptimal since it may not directly target dysfunction in SMA. Our laboratory has demonstrated unique expertise in understanding the neural control of pelvic floor muscles [27–29]. We have found that women with IC/BPS have altered activity compared to healthy women in a subregion of SMA that controls pelvic floor muscles (that we refer to as pelvic-SMA) [2]. Our findings fit with an expanding literature suggesting that chronic pain is caused by motor cortical changes that, while initially beneficial to generate protective muscle activity, are ultimately maladaptive and perpetuate pain through involuntary and uncomfortable muscle activity [30–32].
Because pelvic-SMA is a superficial cortical region, it may be possible to use repetitive transcranial magnetic stimulation (rTMS) to treat IC/BPS. Our published resting-state functional magnetic resonance imaging (rs-fMRI) findings hint at reduced activity in pelvic-SMA in women with IC/BPS [2], which would be consistent with the large body of evidence suggesting high-frequency excitatory rTMS over motor cortex is the best stimulation protocol to reduce pain [4]. Increased resting pelvic floor muscle activity in IC/BPS could result, since pelvic-SMA controls pelvic floor muscles with both excitation and inhibition [33–35]. Using rs-fMRI, we will determine if a high-frequency rTMS protocol improves pelvic-SMA activity in women with IC/BPS by moving it in the direction of healthy women.
Our published work has identified that pelvic-SMA is an important brain region-of-interest in IC/BPS. Our current trial will test whether modulation of pelvic-SMA function provides a mechanism by which IC/BPS symptoms can be reduced. We will then be able to conduct future studies to determine if neuromodulation of pelvic-SMA, by itself or in conjunction with other therapies, may be able to generate long-term reduction or even remission of IC/BPS symptoms.
Conclusions
This RCT will provide further insight into the mechanisms of IC/BPS by applying rTMS to the pelvic-SMA and examining its impact on pelvic pain outcomes, brain activity, and pelvic floor muscle activity.
Supplementary Information
Acknowledgements
We would like to thank the participants for their time and willingness to help.
Trial status
NCT04734847, https://clinicaltrials.gov/study/NCT04734847. Recruitment start date 2021–06-01 and approximate end date 2025–08-15.
Authors’ contributions
JK and LR serve as principal investigators for this study. The authors confirm contribution to the paper as follows: study conception and design: JK and LR; statistical analysis of data: SE; data collection: MY, EJ, GG; analysis and interpretation of results: JK, SE, LR; draft manuscript preparation: MY, EJ, MB, SE, GG, JJ, LR. All authors reviewed the results and approved the final version of the manuscript.
Funding
This study is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) (R01 DK121724). The NIH funding plays no role in the study design of the study and collection, analysis, and interpretation of data and in writing the manuscript. ClinicalTrials.gov NCT04734847 dates 08/2020–04/2025.
Availability of data and materials
The data from this study will not be shared publicly.
Ethics approval and consent to participate
All aspects of the study were approved by the Institutional Review Board of the University of Southern California (protocol HS-20–01021). All participants provided informed consent by the research coordinator/assistants. The results will be submitted for publication in peer-reviewed journals and disseminated at scientific conferences.
Consent for publication
Post-completion of the study, we will publish our results in peer-reviewed journals. These publications will be publicly accessible through the National Library of Medicine (NLM). Links to these publications will be shared with the participants. Not applicable—no identifiable personal or clinical details or identifying images of images are presented here or will be presented in the reports of the trial results.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, et al. Urologic chronic pelvic pain syndrome: insights from the MAPP research network. Nat Rev Urol. 2019;16:187–200. 10.1038/s41585-018-0135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilpatrick LA, Tillisch K, Naliboff B, Labus J, Jiang Z, Farmer M, et al. Alterations in resting state oscillations and connectivity within sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192:947. 10.1016/j.juro.2014.03.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yani MS, Fenske SJ, Rodriguez LV, Kutch JJ. Motor cortical neuromodulation of pelvic floor muscle tone: potential implications for the treatment of urologic conditions. Neurourol Urodyn. 2019;38:1517–23. 10.1002/nau.24014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefaucheur J-P, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131:474–528. 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002;359. Available from: https://pubmed.ncbi.nlm.nih.gov/11918933/. Cited 16 Oct 2023. [DOI] [PubMed]
- 6.Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urol. 2009;74:983–7 quiz 987.e1–3. 10.1016/j.urology.2009.06.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temml C, Wehrberger C, Riedl C, Ponholzer A, Marszalek M, Madersbacher S. Prevalence and correlates for interstitial cystitis symptoms in women participating in a health screening project. Eur Urol. 2007;51:803–8 discussion 809. 10.1016/j.eururo.2006.08.028 [DOI] [PubMed] [Google Scholar]
- 8.Coyne KS, Thompson CL, Lai J-S, Sexton CC. An overactive bladder symptom and health-related quality of life short-form: validation of the OAB-q SF. Neurourol Urodyn. 2015;34:255–63. 10.1002/nau.22559 [DOI] [PubMed] [Google Scholar]
- 9.Skorupska K, Grzybowska ME, Kubik-Komar A, Rechberger T, Miotla P. Identification of the Urogenital Distress Inventory-6 and the Incontinence Impact Questionnaire-7 cutoff scores in urinary incontinent women. Health Qual Life Outcomes. 2021;19:87. 10.1186/s12955-021-01721-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabia M, Sehdev J, Bentley W. Urogenital pain: a clinicians guide to diagnosis and interventional treatments. Springer; 2017.
- 11.Sturgeon JA, Darnall BD, Kao M-CJ, Mackey SC. Physical and psychological correlates of fatigue and physical function: a Collaborative Health Outcomes Information Registry (CHOIR) study. J Pain. 2015;16:291–8.e1. [DOI] [PMC free article] [PubMed]
- 12.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verra ML, Angst F, Lehmann S, Aeschlimann A. Translation, cross-cultural adaptation, reliability, and validity of the German version of the Coping Strategies Questionnaire (CSQ-D). J Pain. 2006;7:327–36. 10.1016/j.jpain.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 15.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–65. 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- 16.Cervigni M, Onesti E, Ceccanti M, Gori MC, Tartaglia G, Campagna G, et al. Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2018;37. Available from: https://pubmed.ncbi.nlm.nih.gov/29797500/ Cited 16 Oct 2023. [DOI] [PubMed]
- 17.Aslam M, Ullah MI. Practicing R for statistical computing. Springer Nature; 2023.
- 18.Structural equations with latent variables. Available from: https://onlinelibrary.wiley.com/doi/book/ 10.1002/9781118619179. Cited 29 Oct 2023.
- 19.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–4. 10.1016/j.juro.2011.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemens JQ, Link CL, Eggers PW, Kusek JW, Nyberg LM Jr, McKinlay JB, et al. Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol. 2007;177:1390–4. 10.1016/j.juro.2006.11.084 [DOI] [PubMed] [Google Scholar]
- 21.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodríguez LV, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. 10.1186/1471-2490-14-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Kutch JJ, Ellingson BM, Martucci KT, Harris RE, Clauw DJ, et al. Brain white matter changes associated with urological chronic pelvic pain syndrome: multisite neuroimaging from a MAPP case-control study. Pain. 2016;157:2782–91. 10.1097/j.pain.0000000000000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2015;193:131–7. 10.1016/j.juro.2014.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, et al. Unique microstructural changes in the brain associated with urological chronic pelvic pain syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: a MAPP network neuroimaging study. PLoS ONE. 2015;10: e0140250. 10.1371/journal.pone.0140250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman AL, Lee UJ, Jellison FC, Tan N, Patel M, Raman SS, et al. MRI suggests increased tonicity of the levator ani in women with interstitial cystitis/bladder pain syndrome. Int Urogynecol J. 2016;27:77–83. 10.1007/s00192-015-2794-6 [DOI] [PubMed] [Google Scholar]
- 26.FitzGerald MP, Payne CK, Lukacz ES, Yang CC, Peters KM, Chai TC, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012;187:2113–8. 10.1016/j.juro.2012.01.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yani MS, Wondolowski JH, Eckel SP, Kulig K, Fisher BE, Gordon JE, et al. Distributed representation of pelvic floor muscles in human motor cortex. Sci Rep. 2018;8:1–16. 10.1038/s41598-018-25705-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rana M, Yani MS, Asavasopon S, Fisher BE, Kutch JJ. Brain connectivity associated with muscle synergies in humans. J Neurosci. 2015;35:14708–16. 10.1523/JNEUROSCI.1971-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asavasopon S, Rana M, Kirages DJ, Yani MS, Fisher BE, Hwang DH, et al. Cortical activation associated with muscle synergies of the human male pelvic floor. J Neurosci. 2014;34:13811–8. 10.1523/JNEUROSCI.2073-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, et al. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS ONE. 2017;12: e0182976. 10.1371/journal.pone.0182976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: a MAPP: research network neuroimaging study. Neuroimage Clin. 2015;8:493–502. 10.1016/j.nicl.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152:S90–8. 10.1016/j.pain.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 33.Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol. 1980;44:773–91. 10.1152/jn.1980.44.4.773 [DOI] [PubMed] [Google Scholar]
- 34.Cheney PD, Fetz EE, Palmer SS. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. J Neurophysiol. 1985;53:805–20. 10.1152/jn.1985.53.3.805 [DOI] [PubMed] [Google Scholar]
- 35.Sherrington CS. The integrative action of the nervous system. In Scientific and Medical Knowledge Production. Chicago: Routledge; 2023. p. 1796–918 (pp. 217–53).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study will not be shared publicly.