Abstract
Background:
With a rising emphasis on public reporting, we hypothesized that select hospitals are becoming increasingly risk-averse by avoiding high-risk operations. Further, we evaluated the association between risk-averse practices, outcomes and publicly reported quality measures.
Methods:
Clinical data from 78,417 patients undergoing cardiac surgery (2002–2016) from a regional consortium was paired with publicly available reimbursement and quality data. High-risk surgery was defined as predicted risk of mortality ≥5%. Hospital risk aversion was defined as a significant decrease in both high-risk volume and proportion, with cases stratified by hospital risk aversion status for univariate analysis.
Results:
The rate of high-risk cases decreased from 17.9% in 2002 to 12.6% in 2016. Significant risk aversion was seen in 39% of hospitals, which had a 59% decrease in high-risk volume versus a 16% decrease at non-risk-averse hospitals. In the last five years, declining high-risk cases at risk-averse hospitals were driven by fewer cases from transfers (19.2% vs 28.1%, p<0.0001) and the emergency department (17.6% vs 19.2%, p=0.001). Only non-risk-averse hospitals had mortality rates lower than expected (risk-averse: 0.97 [0.91–1.03], p=0.30; non-risk-averse: 0.88 [0.83–0.94], p<0.0001). There were no differences by risk aversion status in reported ratings or financial incentives (all p>0.05).
Conclusions:
Over 60% of hospitals continue to operate on high-risk patients, with concentration of care driven by transfer patterns. These non-risk-averse hospitals are high-performing with better than expected outcomes, particularly in high risk cases. Transparency and objectivity in reporting are essential to ensure continued access for these high-risk patients.
Keywords: Health Policy, Cardiac Surgery, Risk Modeling
Cardiac surgery public reporting expanded in 2010 with the launch of the Society of Thoracic Surgeons (STS) public reporting initiative.[1, 2] It is currently voluntary, utilizes STS outcomes and National Quality Forum quality metrics, and covers three separate operations, coronary artery bypass grafting (CABG), aortic valve replacement (AVR), and CABG+AVR, and will soon expand to five.[2] Also in 2010, the passage of the Patient Protection and Affordable Care Act required the Centers for Medicare and Medicaid Services to report quality and patient experience measures through the physician and hospital compare websites.[3–5] These practice-based ratings were rolled out in 2013 and are available for all hospitals performing cardiac surgery. Numerous other public ratings programs now exist, ranging from Angie’s List™ to US News and World Report™, with limited transparency, differing standards, and in some cases, questionable validity.[6, 7]
Between the Patient Protection and Affordable Care Act and the Medicare Access and Children’s Health Insurance Program Reauthorization Act there is a hard shift to value-based reimbursement models.[5, 8] Hospital based programs currently include the hospital-acquired condition reduction program (HACRP), readmission reduction program (HRRP), and value based purchasing program (HVBP).[4] Attempts at further expanding this with a bundled payment program have been retracted, at least for the time being.[9] This combination of increasing public scrutiny, access to outcomes data, and value based financial incentives is raising concerns.[3, 10–16] Sometimes reporting is risk-adjusted, high-quality data, while in other cases the methods are cloaked in secrecy, sometimes overly subjective or utilize charge data rather than medically-vetted patient data.[6, 7, 17–20] With serious potential ramifications, it is entirely reasonable to expect hospitals and cardiac surgical practices to try and improve their statistics, including by selecting only optimal surgical candidates or optimizing coding to their advantage. Therefore, we hypothesized that select hospitals are becoming increasingly risk-averse by avoiding high-risk operations. Further, we sought to determine the association between risk-averse practices, outcomes, and publicly reported quality measures.
PATIENTS AND METHODS
Patient Data
The Virginia Cardiac Services Quality Initiative (VCSQI) is comprised of 18 hospitals and cardiac surgical practices in the Commonwealth of Virginia. The VCSQI database is comprised of Society of Thoracic Surgery (STS) data paired with cost information, with data acquisition and matching described previously.[9, 21] In brief, each participating institution submits clinical and administrative data using the Society of Thoracic Surgeons (STS) clinical data entry form and matched to cost data derived from Uniform Billing-04/92 files. Cost-to-charge ratios submitted to Centers for Medicare and Medicaid Services (CMS) by each institution are used to estimate costs. Cost data was adjusted to 2016 dollars using the market basket for the CMS inpatient prospective payment system to account for medical-specific inflation.
Additionally, hospital level data was obtained from publicly available quality and value-based repayment models. STS quality star ratings were obtained through their public reporting interface.[1] Additional publicly reported quality ratings were obtained from the CMS hospital quality initiative.[4] These rating were paired with data on value-based purchasing, readmission reduction program, and hospital-acquired condition reduction programs. These three programs assess separate quality measures and are included in the CMS prospective payment system as adjustment factors.
Records for coronary artery bypass grafting, mitral and aortic valve operations (n=78,417) from 2002–2016 were extracted from the VCSQI database. Patients were required to have a STS risk score available with no exclusions applied. High risk was defined as a STS predicted risk of mortality (PROM) ≥5%, very high risk as PROM ≥10% and extreme risk as PROM ≥20%. Risk aversion was defined as a decrease in both volume of high-risk cases and proportion of cases being high risk. The decline had to be statistically significant on linear regression, thus hospitals with either a non-statistically significant decline or an increase were defined as non-risk-averse. Standard STS definitions were utilized including operative mortality (in-hospital or 30-day mortality) and major morbidity (permanent stroke, prolonged ventilation, reoperation, renal failure and deep sternal wound infection). This investigation was a secondary analysis of the VCSQI data registry without Health Insurance Portability and Accountability Act patient identifiers where quality data was paired and de-identified prior to data extraction, thus was exempt from institutional review board review.
Statistical Analysis
Categorical data was summarized as count (percent) and analyzed by Chi-Square test. Continuous data is presented as median [interquartile range] or mean ± standard deviation based on skewedness, except for cost data which is presented as mean ± standard deviation to better represent complete costs incurred. Linear regression was utilized to assess trends over time in high risk case volume and proportion for each individual hospital. A hospital was classified as risk-averse if the time trend demonstrated a significant decrease in both high-risk case volume and proportion. Patients were stratified by hospital risk aversion for univariate analysis. Trends over time in clinical and operative factors were analyzed by linear regression stratified by risk aversion status. Observed to expected ratios were calculated as observed count of the event divided by the sum of the appropriate risk score, with statistical difference assessed by Chi-Square test. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The significance level of all tests was set at alpha=0.05.
RESULTS
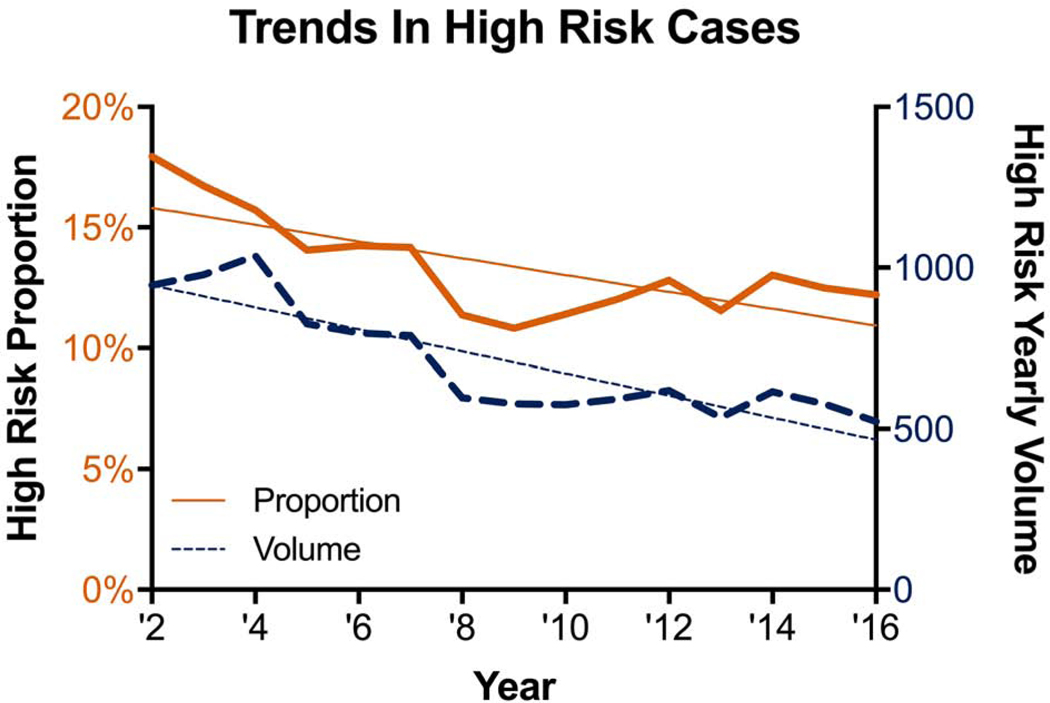
A total of 10,589 (13.5%) patients were classified as high risk with PROM ≥5%. Over time, both the volume and proportion of high-risk cases declined in a linear fashion (Figure 1). From 2002–2016, the proportion of high-risk cases declined by 0.35% per year (R2=0.553, p=0.002) while the volume declined by 34 cases per year (R2=0.774, p<0.0001). The proportion of very high-risk cases (PROM ≥10%) declined by 0.13% per year (R2=0.483, p=0.004), while the number of very high-risk cases declined by 12 per year (R2=0.728, p<0.0001). The proportion of extreme risk (PROM ≥20%) declined by 0.03% per year (R2=0.241, p=0.063) and the number of cases by 3 per year (R2=0.521, p=0.002).
Figure 1:
Volume and proportion of high-risk cases over time.
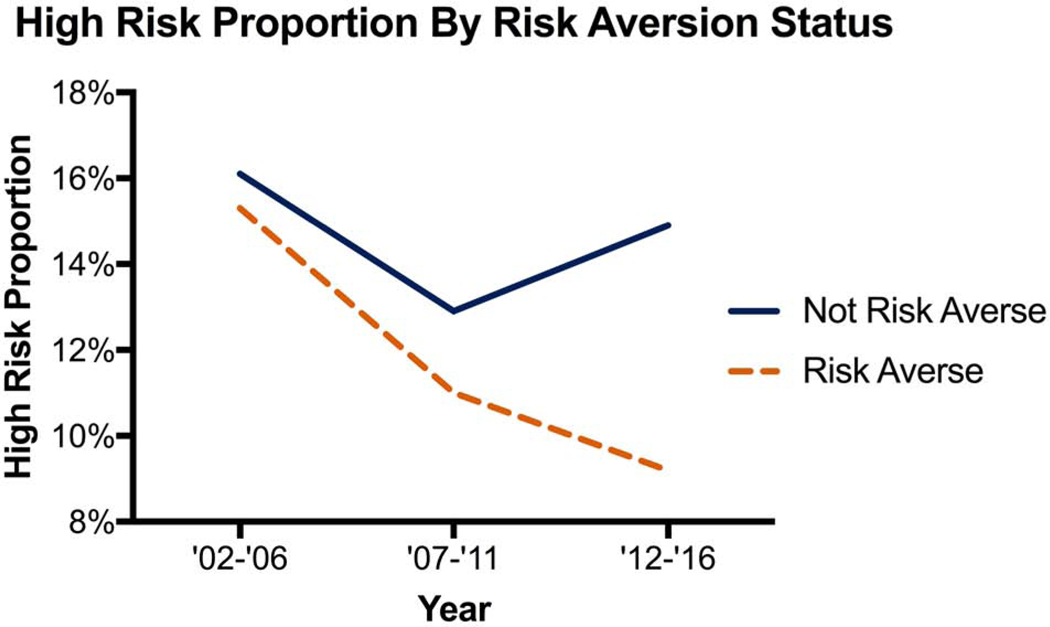
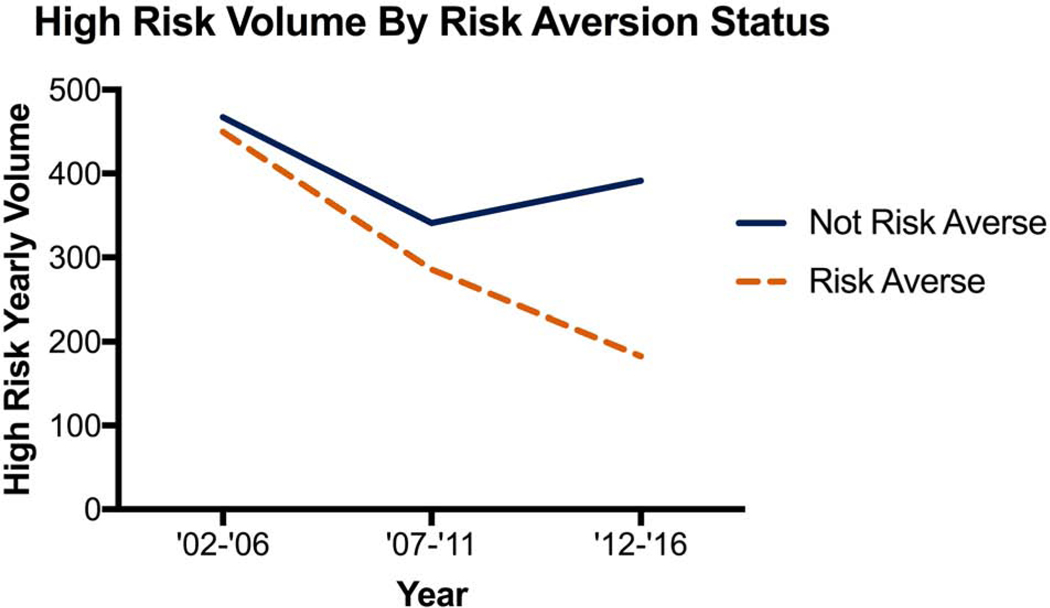
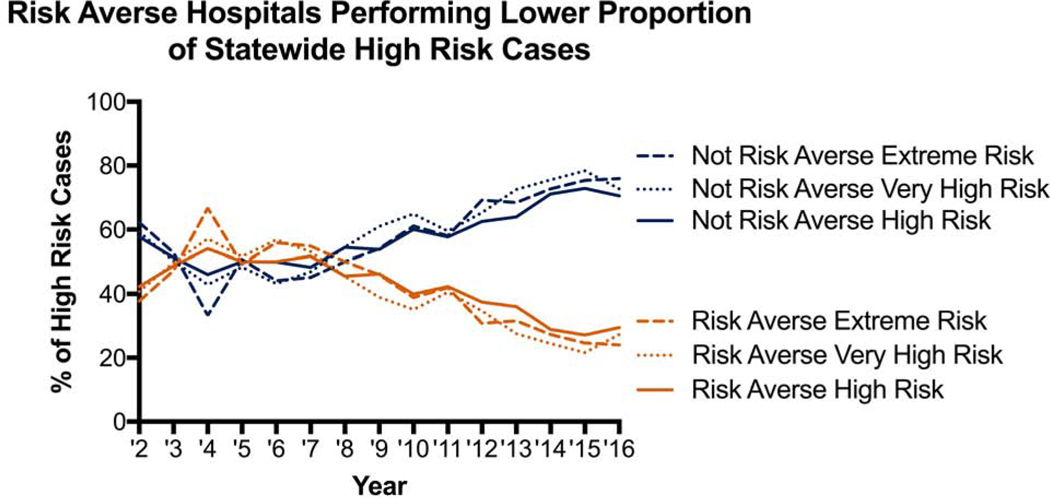
A total of 11 (61%) hospitals had a significant decline in the proportion of high-risk cases and 10 (56%) hospitals with a significant decline in volume of high-risk cases. Only 7 (39%) hospitals met both proportion and volume criteria to be labeled as risk averse. Stratification by risk-aversion status demonstrates the divergent trend in hospitals proportion and volume of high-risk cases (Figure 2). This divergent trend is further shown in Figure 3 where non-risk-averse hospitals see an increase in high-risk, very high-risk and extreme risk cases. There was no significant difference in the average yearly total volume with the median and interquartile range [IQR] for risk-averse hospitals being 316 [IQR 219–438] with a range of 153–767, while for non-risk-averse hospitals it was 198 [IQR 142–281] with a range of 89–860, p=0.151. Risk-averse and non-risk-averse hospitals saw a similar decline in elective case volume (Supplemental Figure 1) while risk-averse hospitals saw a decline in urgent and emergent case volume (Supplemental Figures 2 and 3).
Figure 2:
(A) Proportion of and (B) Case volume of high-risk cases by hospital risk-aversion status over time.
Figure 3.
Proportion of high-risk, very high risk and extreme risk cases performed each year by risk-aversion status.
As expected, risk-averse hospitals operated on lower risk patients, with significantly fewer high-risk cases (Table 1). Although many risk factors were statistically different between groups, the clinical difference was often small. This is most pronounced in the last 5 years, where risk-averse hospitals have operated on proportionally fewer high-risk patients (9.1% vs 14.9%), very-high-risk patients (2.5% vs 5.2%) and extreme risk patients (0.8% vs 1.4%). Risk-averse hospitals performed surgery on patients with lower median PROM (1.3% vs 1.5%, p<0.0001), yet a greater frequency of urgent/emergent cases (56% vs 48%, p<0.0001). Additionally, risk-averse hospitals performed fewer mitral, aortic and combination valve/coronary artery bypass grafting (CABG) cases (Table 1). Data regarding sources of admissions are available since 2011. Patients who underwent surgery at risk-averse hospitals included 1,893 (17.6%) admitted from the emergency department, 2,114 (19.2%) transferred from an outside hospital and 6,776 (62.8%) were elective admissions. This is significantly different from non-risk-averse hospitals with 2,686 (19.2%) of patients admitted through the emergency department, 4,032 (28.1%) transferred from an outside hospital and only 7,287 (52.0%) elective admissions (all p<0.001). For patients with admission data available, 7,444 (53.2%) at risk-averse hospitals were deemed urgent or emergent cases, while only 5,486 (50.9%) were classified as urgent/emergent at non-risk-averse hospitals. Of transfer patients, only 92 patients (4.4%) at risk-averse hospitals came from other cardiac surgical centers compared to 342 (8.5%) at non-risk-averse-hospitals (p<0.0001).
TABLE 1.
Baseline and operative characteristics by risk aversion status
| Baseline Characteristics | Risk Averse (n=37,574) | Not Risk Averse (n=40,843) | p-value |
|---|---|---|---|
|
| |||
| Age (years) | 65 [57–73] | 66 [57–73] | 0.274 |
| Female | 10481 (27.9%) | 12274 (30.1%) | <0.0001 |
| Prior stroke | 2179 (5.9%) | 2912 (7.3%) | <0.0001 |
| Hypertension | 29578 (78.7%) | 33513 (82.1%) | <0.0001 |
| Prior myocardial infarction | 14654 (39.3%) | 16362 (40.5%) | 0.002 |
| Heart failure symptoms | 10829 (18.2%) | 5686 (30.1%) | <0.0001 |
| Ejection fraction (%) | 55 [45–60] | 55 [45–60] | 0.680 |
| Diabetes | 14015 (37.3%) | 16368 (40.1%) | <0.0001 |
| Dialysis | 809 (2.2%) | 1183 (2.9%) | <0.0001 |
| Last creatinine level (mg/Dl) | 1.0 [0.8–1.2] | 1.0 [0.8–1.2] | 0.001 |
| Moderate/severe chronic lung disease | 3148 (8.5%) | 3656 (9.2%) | 0.0006 |
| Peripheral arterial disease | 7660 (12.9%) | 2940 (15.5%) | <0.0001 |
| Endocarditis | 728 (1.2%) | 396 (2.1%) | <0.0001 |
| Urgent or emergent status | 21143 (56.3%) | 19529 (47.9%) | <0.0001 |
| Predicted risk of mortality | 1.3 [0.7–2.9] | 1.5 [0.7–3.2] | <0.0001 |
| High risk | 4590 (12.2%) | 5999 (14.7%) | <0.0001 |
| Very high risk | 1499 (4.0%) | 2030 (5.0%) | <0.0001 |
| Extreme risk | 410 (1.1%) | 522 (1.3%) | 0.016 |
| Operative Characteristics | p-value | ||
|
| |||
| Prior cardiac surgery | 1501 (4.7%) | 2201 (6.4%) | <0.0001 |
| CABG | 32669 (87.0%) | 34982 (85.7%) | <0.0001 |
| Mitral Valve Surgery | 2442 (6.5%) | 2970 (7.3%) | <0.0001 |
| Aortic Valve Surgery | 5925 (15.8%) | 6869 (16.8%) | <0.0001 |
| Combination Valve/CABG | 3,463 (9.2%) | 3,980 (9.7%) | <0.0001 |
| Cross Clamp Time (min) | 69 [53–88] | 71 [54–94] | <0.0001 |
| Cardiopulmonary bypass Time (min) | 98 [77–124] | 97 [76–125] | 0.004 |
CABG = coronary artery bypass grafting
Risk-averse hospitals had an equivalent unadjusted mortality rate as non-risk-averse hospitals (2.6% vs 2.6%, p=0.51). When compared to the predicted mortality rate, risk-averse hospitals performed as expected with an observed-to-expected ratio (O:E) of 0.97, while non-risk-averse hospitals were better than expected, O:E 0.88 (Table 2). The major morbidity rate was lower at risk-averse hospitals (14.3% vs 15.4%, p<0.0001) with risk-averse hospitals performing as expected (O:E 1.01) while non-risk-averse hospitals performed better than expected (O:E 0.97; Table 2). These differences are even more pronounced when isolated to high-risk only cases, with risk-averse hospitals having expected rates of mortality (O:E 1.01, p= 0.848) and morbidity and mortality (O:E 0.98, p= 0.254). Alternatively, non-risk-averse hospitals had better than expected mortality (O:E 0.87, p=0.0005) and morbidity and mortality (O:E 0.89, p<0.0001) for high risk cases. Other unadjusted results are shown in Table 2, with risk-averse hospitals demonstrating shorter length of stay and lower cost but equivalent readmission rates.
TABLE 2.
Operative outcomes by risk aversion status
| Short-Term Outcomes | Risk Averse (n=37,574) | Not Risk Averse (n=40,843) | p-value |
|---|---|---|---|
| Operative mortality | 962 (2.6%) | 1076 (2.6%) | 0.514 |
| Operative mortality (O:E [95% CI]) | 0.97 [0.91–1.03] | 0.88 [0.83–0.94]* | |
| Major morbidity | 5367 (14.3%) | 6274 (15.4%) | <0.0001 |
| Morbidity or mortality | 5644 (15.0%) | 6579 (16.2%) | <0.0001 |
| Morbidity or mortality (O:E [95% CI]) | 1.01 [0.99–1.04] | 0.97 [0.95–0.99]* | |
| Postoperative length of stay (days) | 5 [4–7] | 5 [4–8] | <0.0001 |
| Discharge to a facility | 5246 (14.6%) | 6615 (17.2%) | <0.0001 |
| Readmission | 3216 (8.9%) | 3430 (8.7%) | 0.281 |
| Hospital Cost (mean ± SD) | 39,664 ± 33,930 | 46,501 ± 37,795 | <0.0001 |
statistically lower than expected
CI = confidence interval; O:E = observed to expected ratio; SD = standard deviation
As seen in Table 3, there were no differences in CMS or STS hospital ratings between risk-averse and and non-risk-averse hospitals. STS rating participation rates were similar between risk-averse and non-risk-averse hospitals (57% vs 55%, p=0.99). The most recent CMS quality based financial incentives from 2017 demonstrated no statistical differences between groups (Table 3). There were also no differences between groups in either trends or changes in metrics over time.
TABLE 3.
Volume, reimbursement and quality metrics by hospital
| Publicly Available Quality Ratings | Risk Averse (n=7) | Not Risk Averse (n=11) | p-value |
|---|---|---|---|
|
| |||
| Medicare Hospital Rating | 0.688 | ||
| 1 star | 0 (0%) | 0 (0%) | |
| 2 star | 2 (29%) | 2 (18%) | |
| 3 star | 3 (43%) | 7 (64%) | |
| 4 star | 2 (29%) | 2 (18%) | |
| 5 star | 0 (0%) | 0 (0%) | |
| STS CABG Rating | 0.858 | ||
| 1 star | 0 (0%) | 0 (0%) | |
| 2 stars | 3 (75%) | 4 (80%) | |
| 3 stars | 1 (25%) | 1 (20%) | |
| STS AVR Rating | 0.257 | ||
| 1 star | 0 (0%) | 0 (0%) | |
| 2 stars | 3 (100%) | 4 (67%) | |
| 3 stars | 0 (0%) | 2 (33%) | |
| STS CABG/AVR Rating | 0.145 | ||
| 1 star | 0 (0%) | 0 (0%) | |
| 2 stars | 3 (100%) | 2 (50%) | |
| 3 stars | 0 (0%) | 2 (50%) | |
| Medicare Penalties | |||
|
| |||
| 2017 HACRP Scores | 6.23 ± 1.07 | 6.39 ± 0.95 | 0.754 |
| Change in HACRP score | 0.86 ± 2.19 | −0.11 ± 1.61 | 0.330 |
| HACRP penalty | 3 (42.9%) | 5 (45.5%) | 0.914 |
| 2017 HVBP adjustment | 0.999 ± 0.004 | 1.000 ± 0.006 | 0.654 |
| 2017 HVBP penalty | 3 (42.9%) | 5 (45.5%) | 0.914 |
| HVBP adjustment trend | −0.0025 ± 0.0035 | −0.0005 ± 0.0052 | 0.384 |
| 2017 HRRP adjustment | 0.991 ± 0.009 | 0.993 ± 0.007 | 0.640 |
| 2017 HRRP penalty | 6 (85.7%) | 11 (100%) | 0.197 |
| HRRP adjustment trend | −0.0084 ± 0.0089 | −0.0024 ± 0.0088 | 0.177 |
Hospital-acquired condition reduction program = HACRP; Hospital value-based purchasing program = HVBP; Hospital readmission reduction program = HRRP
COMMENT
Within a regional collaborative, 39% of hospitals demonstrated increasingly risk-averse behavior over the past 15 years. The statewide volume of high-risk cases is declining with a notable divergence as high-risk cases shift to non-risk-averse hospitals. Interestingly, risk-averse hospitals recorded a higher rate of urgent/emergent cases despite lower risk patients. Specifically, since 2011 risk-averse hospitals had 63% elective admissions and yet 53% were coded as urgent/emergent cases. While risk-averse hospitals performed as expected, non-risk-averse hospitals had lower than expected rates of morbidity and mortality. Despite the difference in risk-adjusted outcomes, there were no differences in CMS or STS pubic ratings. Further, there were no differences in three financial incentive programs run by CMS.
In the second half of the study period there was a clear divergence where risk-averse hospitals continue to see a decline in high-risk cases while non-risk-averse hospitals actually see an increase in high-risk case volume. The change in distribution of high-risk cases throughout the state is rather abrupt and roughly corresponds with the time public reporting was starting to become more prominent. A similar trend of high-risk cases shifting to high-performing hospitals was seen after the release of CABG records in California.[22] An alternative explanation is that changing referral patterns such as consolidation and closure of practices has shifted patients regardless of risk-aversion preference. However, the changes are limited to urgent and emergent cases as seen in the supplemental figures, limiting this alternative possibility. The changing risk-distribution could also be explained by advanced centers introducing new technology. However, this explanation is intricately linked to risk-aversion. Advanced technologies such as transcatheter valves and ventricular assist devices carry financial risk but also inherently attract high-risk patients. High-risk patient care is higher cost, and not in a predictable way that can be compensated for with an alternative payment model.[23] Surgical and medical advances explain some of the overall decline in high risk volumes throughout the study period and is reflected in changes to the STS risk score, but this does not relate to the divergence across hospitals in the second half.[24]
Despite non-risk-averse hospitals taking on the financial risk of higher risk patients with better than expected results, this was not reflected in the public reporting we analyzed. This is not entirely unexpected given the very low number of hospitals that are given 3-star ratings. However, it is notable given the publicity that hospitals give to the ratings. Additionally, there were no differences noted in the financial incentives analyzed. Consideration should be given to improving the recognition of these hospitals in order to reverse the perverse incentives currently derived from lower performing risk-averse hospitals having similar ratings and reimbursement despite lower costs, either truly due to or simply perceived to be because of offloading difficult cases.
Shahian and colleagues have posited that risk aversion may lead to denial of interventions to high-risk patients who would benefit, avoidance of futile operations and a shift of high-risk cases to the most capable providers.[13] While it is impossible to analyze denials from this dataset and therefore answer the question of whether the first two options are changing over time, we can see the third option of shifting risk is on the rise. There are also indications that the shifting of high-risk cases to high performing providers is in part due to transfer patterns. Risk-averse hospitals now operate on few transfer patients (19%) compared to non-risk-averse hospitals where more than a quarter of their cases come from transfers. Moreover, these non-risk-averse hospitals accept approximately double the number of transfers from hospitals that also perform cardiac surgery. Ultimately, higher risk patients are now more likely to have their operation at a high performing center. In an era of rapidly changing organizational paradigms such as Accountable Care Organizations, these patterns could be codified to help improve quality and payment stability.[9] While traditionally applied to insurers, alternative payment models expand financial risks to hospitals. Therefore, “risk corridors” could help hospitals ensure high-risk patients are offered appropriate operations and that they are performed in the most capable hands.
Another concern among providers is ensuring a level playing field. The variety of rating systems, number of data sources and lack of standards makes this difficult. The STS database is the gold standard in data quality and risk-adjustment-modeling making their public score cards particularly important. However, the objectivity of the variables ranges from lab values to vague definitions such as urgent status: “Procedure required during same hospitalization in order to minimize chance of further clinical deterioration.”[25] It is notable that since 2011, risk-averse hospitals had 63% elective admissions, yet 53% of cases were coded as urgent or emergent. This compares with non-risk-averse hospitals that had 52% elective admissions and coded a total of 50% of patients as urgent/emergent. If even within STS data there is the flexibility to have such a discrepancy, the myriad other hospital ratings organizations likely fare much worse. Numerous strategies have been proposed to minimize risk-aversion and they all focus on improving risk-adjustment while ensuring fair and transparent reporting.[18, 19, 26–28] Oversight of the ratings agencies is needed in order to increase both accuracy and transparency.[20] Improving the risk-model specifically for public reporting could also help, such as by excluding or separating out extreme risk patients and removing subjective assessments.[29]
Inherent to all retrospective analyses, this study cannot determine causation and is susceptible to selection bias. While the VCSQI database utilizes high quality, audited, clinical and financial data, only variables captured and patients included can be analyzed. Hospital characteristics such as teaching status, size, population density and socioeconomic status of patients are unknown due to the blinded nature of the data. A complete picture of risk aversion would include patients denied surgery or transferred because of their risk profile, which must be estimated for this analysis. While the decline in high-risk operations over time is linear and data was normally distributed, utilization of linear regression to define risk-aversion may underestimate the practice in low volume centers with high variability in their high-risk case volume. In addition, this definition may miscategorize hospitals that have always been risk averse, although this would be not be due to factors that have changed over time such as public reporting, reimbursement, penalties, etc.
Almost 40% of hospitals demonstrate risk aversion with significant declines in high-risk volume. The result is concentration of high-risk cases at select high performing hospitals. The non-risk-averse hospitals absorbing these patients maintain better than expected outcomes, particularly in high-risk cases. Novel statewide high-risk care agreements could help ensure access to care for those who might otherwise not be offered surgery at a risk-averse hospital. These partnerships could also address the financial risk these hospitals accrue due to the variable high costs of these patients. Publicly reported scorecards and financial incentives were no better for non-risk-averse hospitals, a finding that may be warranted but should be closely scrutinized. The methods and standards for the large number of publicly reported ratings could benefit from oversight, transparency and increased objectivity in order to accurately account for risk and incentivize appropriate utilization of potentially lifesaving surgery.
Supplementary Material
Abbreviations
- AVR
Aortic valve replacement
- CMS
Centers for Medicare and Medicaid Services
- CI
Confidence Interval
- CABG
Coronary Artery Bypass Grafting
- O:E
Observed-to-expected ratio
- PROM
Predicted Risk of Mortality
- STS
Society of Thoracic Surgeons
- SD
Standard deviation
- VCSQI
Virginia Cardiac Services Quality Initiative
- HVBP
Hospital value-based purchasing program
- HRRP
Hospital readmission reduction program
- HACRP
Hospital-acquired condition reduction program
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.2017 Sts public reporting online. Available at https://publicreporting.sts.org/. September 30, 2017.
- 2.Shahian DM, Grover FL, Prager RL et al. The society of thoracic surgeons voluntary public reporting initiative: The first 4 years. Ann Surg 2015;262:526–535; discussion 533–525. [DOI] [PubMed] [Google Scholar]
- 3.Dehmer GJ, Drozda JP Jr, Brindis RG et al. Public reporting of clinical quality data: An update for cardiovascular specialists. J Am Coll Cardiol 2014;63:1239–1245. [DOI] [PubMed] [Google Scholar]
- 4.2017 Cms hospital quality initiative. Available at https://publicreporting.sts.org/. September 30, 2017.
- 5.Blumenthal D, Abrams M, Nuzum R. The affordable care act at 5 years. N Engl J Med 2015;372:2451–2458. [DOI] [PubMed] [Google Scholar]
- 6.Shahian DM, Mort EA, Pronovost PJ. The quality measurement crisis: An urgent need for methodological standards and transparency. Jt Comm J Qual Patient Saf 2016;42:435–438. [DOI] [PubMed] [Google Scholar]
- 7.Hota B, Webb TA, Stein BD, Gupta R, Ansell D, Lateef O. Consumer rankings and health care: Toward validation and transparency. Jt Comm J Qual Patient Saf 2016;42:439–446. [DOI] [PubMed] [Google Scholar]
- 8.Rich J, Fonner CE, Eller J, Berkel I, Nadzam D. Maximize reimbursement and minimize risk under the medicare access and children’s health insurance program reauthorization act (macra) and the quality payment program (qpp). Ann Thorac Surg 2018. [DOI] [PubMed]
- 9.Hawkins RB, Mehaffey JH, Yount KW et al. Coronary artery bypass grafting bundled payment proposal will have significant financial impact on hospitals. J Thorac Cardiovasc Surg 2018;155:182–188. [DOI] [PubMed] [Google Scholar]
- 10.Chassin MR, Hannan EL, DeBuono BA. Benefits and hazards of reporting medical outcomes publicly. N Engl J Med 1996;334:394–398. [DOI] [PubMed] [Google Scholar]
- 11.Hannan EL. The public reporting risk of performing high-risk procedures: Perception or reality? JACC Cardiovasc Interv 2015;8:17–19. [DOI] [PubMed] [Google Scholar]
- 12.Shahian DM. Public reporting of cardiac surgery performance: Introduction. Ann Thorac Surg 2011;92:S1. [DOI] [PubMed] [Google Scholar]
- 13.Shahian DM, Jacobs JP, Badhwar V, D’Agostino RS, Bavaria JE, Prager RL. Risk aversion and public reporting. Part 1: Observations from cardiac surgery and interventional cardiology. Ann Thorac Surg 2017;104:2093–2101. [DOI] [PubMed] [Google Scholar]
- 14.Turi ZG. The big chill: The deleterious effects of public reporting on access to health care for the sickest patients. J Am Coll Cardiol 2005;45:1766–1768. [DOI] [PubMed] [Google Scholar]
- 15.Werner RM, Asch DA. The unintended consequences of publicly reporting quality information. JAMA 2005;293:1239–1244. [DOI] [PubMed] [Google Scholar]
- 16.Westaby S. Publishing individual surgeons’ death rates prompts risk averse behaviour. BMJ 2014;349:g5026. [DOI] [PubMed] [Google Scholar]
- 17.Dimick JB, Birkmeyer JD. Ranking hospitals on surgical quality: Does risk-adjustment always matter? J Am Coll Surg 2008;207:347–351. [DOI] [PubMed] [Google Scholar]
- 18.Girling AJ, Hofer TP, Wu J et al. Case-mix adjusted hospital mortality is a poor proxy for preventable mortality: A modelling study. BMJ Qual Saf 2012;21:1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahian DM, Edwards FH, Jacobs JP et al. Public reporting of cardiac surgery performance: Part 2-- implementation. Ann Thorac Surg 2011;92:S12–23. [DOI] [PubMed] [Google Scholar]
- 20.Shahian DM, Normand SL, Friedberg MW, Hutter MM, Pronovost PJ. Rating the raters: The inconsistent quality of health care performance measurement. Ann Surg 2016;264:36–38. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins RB, Downs EA, Johnston LE et al. Impact of transcatheter technology on surgical aortic valve replacement volume, outcomes, and cost. Ann Thorac Surg 2017;103:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano PS, Marcin JP, Dai JJ et al. Impact of public reporting of coronary artery bypass graft surgery performance data on market share, mortality, and patient selection. Med Care 2011;49:1118–1125. [DOI] [PubMed] [Google Scholar]
- 23.Yount KW, Isbell JM, Lichtendahl C et al. Bundled payments in cardiac surgery: Is risk adjustment sufficient to make it feasible? Ann Thorac Surg 2015;100:1646–1652; discussion 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin R, Furnary AP, Fine SC, Blackstone EH, Grunkemeier GL. Using society of thoracic surgeons risk models for risk-adjusting cardiac surgery results. Ann Thorac Surg 2010;89:677–682. [DOI] [PubMed] [Google Scholar]
- 25.2017 Adult cardiac surgery data collection. Available at http://www.sts.org/sts-national-database/database-managers/adult-cardiac-surgery-database/data-collection#data. April 3, 2017.
- 26.Shahian DM, Jacobs JP, Badhwar V, D’Agostino RS, Bavaria JE, Prager RL. Risk aversion and public reporting. Part 2: Mitigation strategies. Ann Thorac Surg 2017;104:2102–2110. [DOI] [PubMed] [Google Scholar]
- 27.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: Structure, process, or outcomes? J Am Coll Surg 2004;198:626–632. [DOI] [PubMed] [Google Scholar]
- 28.Elbardissi AW, Duclos A, Rawn JD, Orgill DP, Carty MJ. Cumulative team experience matters more than individual surgeon experience in cardiac surgery. J Thorac Cardiovasc Surg 2013;145:328–333. [DOI] [PubMed] [Google Scholar]
- 29.Chancellor WZ, Mehaffey JH, Beller JP et al. Current quality reporting methods are not adequate for salvage cardiac operations. J Thorac Cardiovasc Surg 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.