Abstract
Numerous studies have stated that comorbidities are risk factors for coronavirus disease 2019 (COVID-19) mortality, but few have considered the severity or stage of these comorbidities. The aim of this study was to determine the association between the severity of comorbidity, age, and number of COVID-19 vaccinations with COVID-19 mortality. This case-control study was conducted from July 2021 until December 2022 at the Dr. Soetomo General Academic Hospital, Surabaya, Indonesia. The patients were divided into non-survived patients (case group) and survived patients (control group). The inclusion criteria for cases were adult patients hospitalized with confirmed COVID-19, based on reverse transcriptase-polymerase chain reaction (RT-PCR) testing of nasopharyngeal swabs. Using total sampling, 1,046 confirmed COVID-19 patients, which consisted of 450 (43%) non-survived patients and 596 (57%) survived patients, were included. The most common comorbidity was diabetes mellitus (DM) (82.7%), chronic kidney disease (CKD) (43%), hypertension (25.7%), and obesity (23.6%). Our multivariate analysis indicated that older age (aOR: 1.03; 95%CI: 1.02–1.04, p<0.001), male sex (aOR: 1.29; 95%CI: 1.11– 2.00, p=0.007), severe COVID-19 at first admission (aOR: 3.13; 95%CI: 2.08–4.73, p<0.001), having pneumonia (aOR: 1.99; 95%CI: 1.21–3.33, p=0.005), poorly controlled DM with HbA1c≥9% (aOR: 2.90; 95%CI: 1.72–4.89, p<0.001), severe obesity with body mass index (BMI)≥30 (OR: 2.90; 95%CI: 1.72–4.89, p<0.001), hypertension stage 2 (aOR: 1.99; 95%CI: 1.12–3.53, p=0.019) or stage 3 (aOR: 6.59; 95%CI: 2.39–18.17, p<0.001), CKD stage 3 (aOR: 2.50; 95%CI: 1.36–4.59, p=0.003), stage 4 (aOR: 5.47; 95%CI: 2.18–13.69, p<0.001) or stage 5 (aOR: 1.71; 95%CI: 1.04–2.81, p=0.036), and having chronic lung disease (aOR: 3.08; 95%CI: 1.22–7.77, p=0.017) significantly increased the risk of COVID-19 mortality. In contrast, COVID-19 vaccination reduced the risk of COVID-19-associated death. This study highlights that more severe comorbidities, advanced age, and incomplete vaccination were associated with COVID-19 mortality.
Keywords: COVID-19, risk factor, mortality, severity, vaccination
Introduction
The coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, is a significant health problem associated with a significant strain on the global economy, civic societies, and healthcare system [1]. While some SARS-CoV-2 infected individuals continue to show no symptoms, others may develop serious respiratory and systemic complications, which can result in multiple organ failure and even death [2]. The clinical features of patients with severe COVID-19 have been documented in a number of studies [3,4]. The number and type of risk factors taken into consideration are different in each study.
Age has been identified as the primary predictor of outcomes in COVID-19 patients since the beginning of the epidemic. According to China’s early statistics data, the case-fatality rate (CRF) rises sharply beyond the age of 60 and reaches 14.8% for those over the age of 80 [5]. This may be due to the physiological aging process as well as the high incidence of frailty and comorbidities leading to lower functional reserve, impaired intrinsic capacity and resilience, and immune senescence [6]. In addition, the other common risk factors identified were diabetes mellitus (DM), hypertension, chronic kidney disease (CKD), obesity, cardiovascular disease, chronic lung disease, malignancy, liver disease, HIV, male gender, older age, and vaccination status [7-14]. Numerous studies have stated that comorbidities are risk factors for COVID-19 mortality [15-18], but few have considered the severity (spectrum) of the comorbidities. A meta-analysis of seven studies indicated that unvaccinated patients were 2.46 times more likely to die from COVID-19 [17]. Another study found that the mortality rate might drop by 7.6% for every 10% increase in vaccination coverage [19]. However, studies in Indonesia remain limited. The aim of this study was to determine the relationship between the severity of comorbidity, age, and the number of vaccinations received and COVID-19 mortality.
Methods
Study design, setting and sampling
A case-control study was conducted at Dr. Soetomo General Academic Hospital, Surabaya, Indonesia, between July 1, 2021, and December 31, 2022, among hospitalized COVID-19 patients. Total sampling was employed in this study. Patients were followed up and further classified into two groups based on mortality status: patients who died and the survivors.
Patients and criteria
This study included hospitalized COVID-19 patients. The diagnosis and clinical severity of COVID-19 at first admission were based on the Guidelines for COVID-19 by the Indonesian Health Ministry [20]. A positive result on the reverse transcriptase-polymerase chain reaction (RT-PCR) assay employing a nasopharyngeal swab was considered to be the COVID-19-confirmed patient [20]. The presence of pneumonia and the severity of COVID were assessed using the diagnosis criteria of the 4th Edition Indonesian Guideline of COVID-19 Management Protocol [21] at hospital admission. Pneumonia was defined as fever, cough, dyspnea/ shortness of breath, tachypnea, or respiratory distress symptoms with radiologic abnormalities on chest x-ray which indicated pneumonia such as infiltrate, reticular opacities, and consolidation on lungs. Mild COVID-19 was considered for asymptomatic patients or patients with mild symptoms such as fever, cough, fatigue, anorexia, anosmia, diarrhea, and vomiting without dehydration. Moderate COVID-19 consists of patients with pneumonia without respiratory distress and a peripheral oxygen saturation of ≥93%. Severe COVID-19 was defined as pneumonia with respiratory distress, decrease of consciousness, convulsion, or the need for an intensive care unit (ICU) for mechanical ventilator or vasopressor support [21]. All severity types of the COVID-19 patients were included in this study. Patients under 18 years old, pregnant women, those who left against medical advice, and those with secondary COVID-19 infections were excluded from the study.
Data collection and study variables
We collected data on age, sex, diagnosis of pneumonia, severity of COVID-19, length of stay, history of COVID-19 vaccinations, presence of comorbidities, and severity of the four most common comorbidities in the study: DM, hypertension, obesity, and CKD. Patients with DM were grouped into three based on their hemoglobin A1c (HbA1c): well-controlled (HbA1c<9%), poorly controlled (HbA1c>9%), and no history of DM [22]. Patients with hypertension were categorized into three categories based on the 2018 European Society of Cardiology (ESC) guidelines [23]: stage 1 hypertension (systolic blood pressure (SBP) of 140–159 mmHg and/or diastolic blood pressure (DBP) of 90–99 mmHg); stage 2 hypertension (SBP of 160–179 mmHg and/or DBP of 100–109 mmHg); and stage 3 hypertension (SBP of 180 mmHg or greater and/or DBP of 110 mmHg or greater). The body mass index (BMI) classification system from the Indonesian Health Ministry [24] was employed to categorize obesity as follows: normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), class 1 obesity (25.0–29.9 kg/m2), class 2 obesity (≥30.0 kg/m2), and underweight (18.4–16.4 kg/m2). Our study defined patients with normal BMI and underweight as “not obese,” overweight and class 1 obesity as “mildly obese,” and class 2 obesity as “severely obese.” CKD patients were grouped based on the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [25], which classify CKD based on glomerular filtration rate (GFR). These classifications included stage 1 CKD (GFR>90 mL/min per 1.73 m2), stage 2 CKD (GFR 60–89 mL/min per 1.73 m2), stage 3 CKD (GFR 30–59 mL/min per 1.73 m2), CKD stage 4 (GFR 15–29 mL/min per 1.73 m2), and stage 5 CKD (GFR<15 mL/min per 1.73 m2 or treatment by dialysis).
COVID-19-associated death was the main outcome determined in this study. The mortality is defined as all-cause mortality of COVID-19 infection.
Statistical analysis
An independent Student’s t-test and the Chi-squared test or Mann-Whitney test were employed to assess the associated determinants with COVID-19 mortality according to categorical variables. Significant variables were then further analyzed using multivariate logistic regression with the backward likelihood ratio selection method. Statistical significance was considered at p<0.05. All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, USA).
Results
Characteristics of the patients
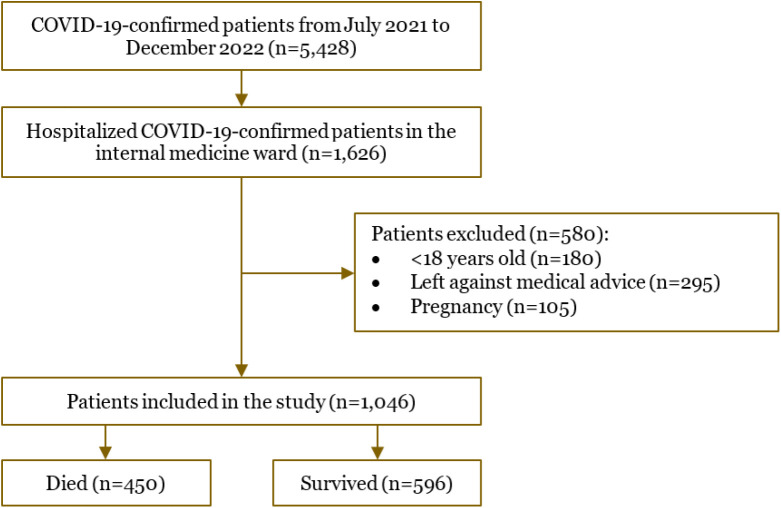
A total of 1,046 COVID-19-confirmed patients were recruited and followed during the study. We recorded 450 (43%) patients who died and 596 (57%) patients who survived. The patient selection flowchart is depicted in Figure 1. The basic characteristics of the COVID-19 patients included in this study are presented in Table 1. The majority of patients presented to the hospital with pneumonia (92.5%) at the time of admission and severe COVID-19 symptoms (81.7%). More than half of the patients were unvaccinated (58.4%). Most patients had multiple comorbidities (71.3%), 23.5% had one comorbidity and only 5.3% had no comorbidity. The most common comorbidities were DM (82.7%), CKD (43.0%), hypertension (25.7%), and obesity (23.6%).
Figure 1.
Flowchart of the coronavirus disease 2019 (COVID-19) patient’s selection in the study.
Table 1.
Characteristics of the COVID-19 patients (n=1,046)
| Characteristics | Frequency (%) |
|---|---|
| Age, mean±SD (years) | 51.22±15.05 |
| Sex, n (%) | |
| Male | 560 (53.5) |
| Female | 486 (46.5) |
| Length of stay, mean±SD (days) | 15.44±19.64 |
| Pneumonia | |
| Present | 968 (92.5) |
| Absent | 78 (7.5) |
| COVID-19 severity | |
| Mild to moderate | 191 (18.3) |
| Severe | 855 (81.7) |
| Number of COVID-19 vaccines | |
| 0 | 611 (58.4) |
| 1 | 104 (9.9) |
| 2 | 251 (24) |
| 3 | 78 (7.5) |
| 4 | 2 (0.2) |
| Number of comorbidities | |
| 0 | 55 (5.3) |
| 1 | 246 (23.5) |
| 2 | 351 (33.6) |
| 3 | 273 (26.1) |
| 4 | 111 (10.6) |
| 5 | 9 (0.9) |
| 6 | 1 (0.1) |
| Comorbidities | |
| Diabetes mellitus (DM) | 865 (82.7) |
| Hypertension | 269 (25.7) |
| Obesity | 247 (23.6) |
| Chronic kidney disease (CKD) | 450 (43.0) |
| Stroke | 45 (4.3) |
| Coronary artery disease | 34 (3.3) |
| Chronic lung disease | 26 (2.5) |
| Chronic liver disease | 42 (4.0) |
| Malignancy | 86 (8.2) |
| Human immunodeficiency virus (HIV) | 46 (4.4) |
| Autoimmune disease | 40 (3.8) |
| Hematologic disease | 25 (2.7) |
| Mortality | |
| Died | 450 (43.0) |
| Survived | 596 (57.0) |
Associations of age, COVID-19 vaccinations, and comorbidities with mortality
Our univariate analysis showed that age (p=0.004), length of stay (p=0.031), the presence of pneumonia (p<0.001), COVID-19 severity (p<0.001), number of COVID-19 vaccinations (p<0.001), DM (p<0.001), hypertension (p<0.001), obesity (p<0.001), CKD (p<0.001), stroke (p=0.046), and chronic lung disease (p=0.026) were associated with mortality (Table 2). The mean age in the deceased group was found to be older compared to the surviving groups. The proportion of unvaccinated patients in the deceased group was found to be higher (66.0%) compared to the surviving group (52.7%). The proportion of multiple comorbidities was also found to be higher in the deceased group (89.3%) than in the surviving group (52.7%) (Table 2).
Table 2.
Factors associated with COVID-19 mortality
| Variables | Survived (n=596) | Deceased (n=450) | value |
|---|---|---|---|
| Age (years), mean±SD | 55-35±11.54 | 57.78±11.54 | 0.004 a* |
| Sex, n (%) | |||
| Male | 310 (52) | 250 (55.6) | 0.255 c |
| Female | 286 (48) | 200 (44.4) | |
| Length of stay (days), mean±SD | 14.21±13.25 | 17.08±25.697 | 0.031 a* |
| Pneumonia, n (%) | |||
| Present | 518 (86.9) | 450 (100.0) | <0.001 c** |
| Absent | 78 (13.1) | 0 (0.0) | |
| COVID-19 severity, n (%) | |||
| Mild to moderate | 178 (29.9) | 13 (6.8) | <0.001 c** |
| Severe | 418 (70.1) | 437 (97.1) | |
| Number of COVID-19 vaccine, n (%) | |||
| 0 | 314 (52.7) | 297 (66.0) | <0.001 b |
| 1 | 60 (10.1) | 44 (9.8) | |
| 2 | 162 (27.2) | 89 (19.8) | |
| 3 | 58 (9.7) | 20 (4.4) | |
| 4 | 2 (0.3) | 0 (0.0) | |
| Number of comorbidities, n (%) | |||
| 0 | 53 (8.9) | 2 (0.4) | <0.001 b** |
| 1 | 200 (33.6) | 46 (10.2) | |
| 2 | 193 (32.4) | 158 (35.1) | |
| 3 | 111 (18.6) | 162 (36) | |
| 4 | 37 (6.2) | 74 (16.4) | |
| 5 | 2 (0.3) | 7 (1.6) | |
| 6 | 0 (0.0) | 0 (0.0) | |
| Comorbidity, n (%) | |||
| Diabetes mellitus (DM) | 443 (74.3) | 422 (93.8) | <0.001 c** |
| Hypertension | 112 (18.8) | 157 (34.9) | <0.001 c** |
| Obesity | 107 (18) | 140 (31.1) | <0.001 c |
| Chronic kidney disease (CKD) | 188 (31.5) | 262 (58.2) | <0.001 c** |
| Stroke | 19 (3.2) | 26 (5.8) | 0.046 c* |
| Coronary artery disease | 14 (2.3) | 20 (4.4) | 0.077 c |
| Chronic lung disease | 9 (1.5) | 17 (3.8) | 0.026 c* |
| Chronic liver disease | 22 (3.7) | 20 (4.4) | 0.634 c |
| Malignancy | 45 (7.6) | 41 (9.1) | 0.366 c |
| Human immunodeficiency virus (HIV) | 29 (4.9) | 17 (3.8) | 0.448 c |
| Autoimmune disease | 27 (4.5) | 13 (2.9) | 0.194 c |
| Hematologic disease | 19 (3.2) | 9 (2.0) | 0.254 c |
Analyzed using independent Student’s t-test
Analyzed using Mann-Whitney test
Analyzed using Chi-squared test
Statistically significant at p=0.05
Statistically significant at p=0.001
Multivariate analysis of factors associated with COVID-19 mortality
All significant variables in univariate analysis (age, length of stay, the presence of pneumonia, severity of COVID-19, number of COVID-19 vaccinations, number of comorbidities, and the presence of comorbidities such as DM, hypertension, obesity, CKD, and chronic lung disease) were included in the multivariate analysis. In this analysis, the comorbidities (DM, obesity, hypertension, and CKD) were classified based on their severity (Table 3). Variables eliminated from logistic regression using backward likelihood ratio were the length of stay, stroke, and the number of comorbidities.
Table 3.
Multivariate logistic regression analysis showing factors associated with mortality
| Variables | Multivariate analysis | |
|---|---|---|
| Odds ratio (95%CI) | p-value | |
| Age | 1.03 (1.02–1.04) | <0.001** |
| Sex | ||
| Male | Reference | |
| Female | 0.67 (0.50-0.90) | 0.007* |
| Severity of COVID-19 infection | ||
| Mild to moderate | Reference | |
| Severe | 3.13 (2.08–4.73) | <0.001** |
| Pneumonia | ||
| Present | Reference | |
| Absent | 0.50 (0.30–0.81) | 0.005* |
| Severity of diabetes mellitus | ||
| Absent | Reference | |
| Well-controlled (HbAic<9%) | 1.36 (0.97–1.90) | 0.072 |
| Poorly controlled (HbA1c ≥9%) | 7.05 (4.64–11.39) | <0.001** |
| Severity of obesity | ||
| Absent | Reference | |
| Mild obesity (BMI 23.0-29.9 kg/m2) | 1.14 (0.84–1.55) | 0.389 |
| Severe obesity (BMI ≥30 kg/m2) | 2.90 (1.72–4.89) | <0.001** |
| Severity of hypertension | ||
| Absent | Reference | |
| Stage 1 | 0.94 (0.62–1.43) | 0.792 |
| Stage 2 | 1.99 (1.12–3.53) | 0.019* |
| Stage 3 | 6.59 (2.39–18.17) | <0.001** |
| Severity of chronic kidney disease | ||
| Absent | Reference | |
| Stage 1 | 1.43 (0.85–2.42) | 0.182 |
| Stage 2 | 1.40 (0.88-2.22) | 0.153 |
| Stage 3 | 2.5 (1.36–4.59) | 0.003* |
| Stage 4 | 5.47 (2.18–13.69) | <0.001** |
| Stage 5 | 1.71 (1.04–2.81) | 0.036* |
| Chronic lung disease | ||
| Absent | Reference | |
| Present | 3.08 (1.22–7.77) | 0.017* |
| Number of vaccinations | ||
| 0 | Reference | |
| 1 | 0.52 (0.32–0.85) | 0.009* |
| 2 | 0.43 (0.31–0.61) | <0.001** |
| 3 | 0.28 (0.15–0.52) | <0.001** |
| 4 | 0.00 | 0.999 |
BMI: body mass index
The analysis showed that the severity of comorbidity was a more significant factor than the number of comorbidities in predicting increased COVID-19 mortality. Older age (aOR: 1.03; 95%CI: 1.02–1.04), poorly controlled DM (aOR: 7.05; 95%CI: 4.63–11.38), stage 2 hypertension (aOR: 1.98; 95%CI: 1.11–3.52), stage 3 hypertension (aOR: 6.59; 95%CI: 2.39–18.17), CKD with GFR≤60 (stage 3, 4, and 5), and chronic lung disease (aOR: 3.08; 95%CI: 1.22–7.77) were associated with COVID-19 mortality (Table 3). The number of vaccinations and being female reduced the risk of COVID-19 mortality (Table 3).
Discussion
This study found that hypertension, DM, CKD, and obesity, increased linearly with the stage of disease severity. Two meta-analyses revealed that patients of all ages had an increased chance of critical COVID-19, ICU needs, and mortality outcomes when they had coexisting conditions of DM and hypertension [10,26]. Diabetic and hypertension patients will have decreased resistance to viral infections. Patients with long-term DM and hypertension are more susceptible to developing critical illness in COVID-19 because these conditions can deteriorate cardiac function and damage vascular structure [27]. A study reported an association between higher blood pressure and reduced antibody response to an inactivated viral vaccine (p=0.01) [28]. Other studies found that obesity (BMI≥30 kg/m2) was linked to a worse prognosis and a more severe illness [29,30]. The biological pathways that could be responsible for severe COVID-19 in obese people include inflammation and widely expressed angiotensin-converting enzyme 2 (ACE2) in adipose tissue [31]. A previous study observed the risk of critical COVID-19 as CKD stages progressed [32]. A study compared COVID-19 inpatients with CKD stages 3 and 4 to those without CKD, it was discovered that 12-week mortality odds increased with CKD stage [33]. However, small cohort sizes led to statistically insignificant findings for stage 5 CKD or those on dialysis [33]. Multifactorial pathways are probably responsible for the increasing likelihood of critical COVID-19 related to declining estimated glomerular filtration rate (eGFR). Uremia has been linked to a reduced number of lymphocytes and decreased neutrophil activity in people with end-stage CKD [34].
This study reported that chronic lung disease is a risk factor for COVID-19 mortality. Another study has suggested that there is a greater hospitalization and mortality rate among COPD patients with COVID-19 [7]. This might result from infections in COPD populations, which worsen multi-organ inflammation and cause a delayed recovery of symptoms [35,36].
Age is thought to have an impact on both the risk of COVID-19 mortality and the severity of the illness. Previous studies have shown that the proportion of infections that eventually result in mortality or severe and critical illness increases with age [3,4,37]. According to this study, COVID-19 mortality increased with age. A meta-analysis involving 423,117 patients showed that older age increased the risk of COVID-19 death (HR 1.31; 95%CI 1.11–1.51) [10]. A different meta-analysis revealed that COVID-19 patients older than 70 years of age seem to be at an increased risk of developing a serious illness, requiring critical care, and passing away [38]. Lower immunity levels and/or age-related chronic medical disorders could be a feasible explanation for this [39]. Furthermore, the functions of B- and T-cells are impacted by aging. A decreased ability to respond effectively to viral infections is linked to a decline in B- and T-cell clonal diversity [40].
According to a study in the United Kingdom on vaccine efficacy, immunization increased protection against serious illness in adults [41]. A prior study found that the death rate might drop by 7.6% for every 10% increase in vaccination coverage [19]. A study carried out after the introduction of the vaccine showed that individuals who were not fully vaccinated had much greater rates of COVID-19 infection, hospitalization, and mortality than those who had received all recommended vaccinations [42]. Similarly, in our study, the mortality risk of COVID-19 was reduced as more vaccinations were received by the patient. According to theory, a successful COVID-19 vaccination can lessen the severity of a SARS-CoV-2 infection-related illness. The growth of immunological memory and the induction of T-cells should also play a role in the protective impact of the vaccination, rather than just the creation of antibodies [43].
There are some limitations of this study. The population only included patients from a single department. A larger population size from other departments for further studies is warranted. Additionally, comorbidities that were classified based on severity only included the four most common comorbidities. Moreover, the CKD population did not differentiate whether patients were on dialysis or not. Furthermore, vaccine types were also not differentiated as homologous or heterologous.
Conclusion
We found that the worsening severity of comorbidities (such as DM, hypertension, CKD, and obesity), older age, chronic lung disease, male, pneumonia occurrence, COVID-19 severity infection at first admission, and incomplete vaccination were increasing the mortality risk of COVID-19 hospitalized patients. Therefore, it is recommended to prioritize these high-risk groups for early intervention and complete vaccination to reduce mortality rates.
Acknowledgments
The author would like to thank all clinical colleagues at the Department of Internal Medicine, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia for their assistance and discussions throughout this work.
Ethics approval
The study was approved by the Dr. Soetomo General Academic Hospital’s Ethical Committee (No. 2074/101/4/III/2023).
Competing interests
All the authors declare that there are no conflicts of interest.
Funding
This study received no external funding.
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Pradhevi L, Soegiarto G, Wulandari L, et al. More severe comorbidities, advanced age, and incomplete vaccination increase the risk of COVID-19 mortality. Narra J 2024; 4 (2): e949 - http://doi.org/10.52225/narra.v4i2.949.
References
- 1.Dong E, Du H, Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20(5):533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother 2020;52(2):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China. Jama 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 5.United Nations International Children’s Emergency Fund. COVID-19 Vaccine Acceptance Survey in Indonesia. Jakarta; UNICEF Indonesia: 2020. [Google Scholar]
- 6.Levin AT, Hanage WP, Owusu-Boaitey N, et al. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur J Epidemiol 2020 3512 2020;35(12):1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS ONE 2020;15(5):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel Coronavirus Disease. Chin Med J (Engl) 2020;133(9):1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. The Lancet 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dessie ZG, Zewotir T.. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 2021;21(1):855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID-19 severity: A systematic review and meta-analysis. F1000Research 2021;9:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu P, Faraone JN, Evans JP, et al. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med 2022;387(14):1329–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurvita R, Nuswantoro D, Prajitno Hendro J. Correlation between physical activity and fasting blood glucose in patient with type 2 diabetes mellitus. Curr Intern Med Res Pract Surabaya J 2022;3(2):40–42. [Google Scholar]
- 14.Harfonso EEB, Mardiana N Atika. Association between albuminuria and serum phosphate levels in non-dialysis stage 3-5 chronic kidney disease patients. Curr Intern Med Res Pract Surabaya J 2023;4(2):2019–2023. [Google Scholar]
- 15.Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy Eur J Allergy Clin Immunol 2020;75(7):1742–1752. [DOI] [PubMed] [Google Scholar]
- 16.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 2020;21(3):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence - 25 U.S. jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep 2022;71(4):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 2020;92(7):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang LL, Kuo HS, Ho HJ, et al. COVID-19 vaccinations are associated with reduced fatality rates: Evidence from cross-county quasi-experiments. J Glob Health 2021;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burhan E, Susanto AS, Isbaniah F, et al. Pedoman tatalaksana COVID-19 Edisi 3 Desember 2020. Jakarta; PDPI, PERKI, PAPDI, PERDATIN, IDAI: 2020. [Google Scholar]
- 21.Burhan E, Susanto AD, Nasution SA, et al. Pedoman tatalaksana COVID-19 Edisi 4 Januari 2022. Jakarta; PDPI, PERKI, PAPDI, PERDATIN, IDAI: 2022. [Google Scholar]
- 22.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021;44 Suppl 1:S15–S33. [DOI] [PubMed] [Google Scholar]
- 23.Stergiou GS, Palatini P, Parati G, et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens 2021;39(7):1293–1302. [DOI] [PubMed] [Google Scholar]
- 24.Kementerian Kesehatan Republik Indonesia. Klasifikasi obesitas setelah pengukuran IMT. Jakarta; P2PTM Kemenkes: 2018. [Google Scholar]
- 25.Milik A, Hrynkiewicz E.. On translation of LD, IL and SFC given according to IEC-61131 for hardware synthesis of reconfigurable logic controller. IFAC Proc Vol IFAC-Pap 2014;19(1):4477–4483. [Google Scholar]
- 26.Bae SA, Kim SR, Kim MN, et al. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2021;107(5):373–380. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020;81(2):e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soegiarto G, Wulandari L, Purnomosari D, et al. Hypertension is associated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine 2022;40(30):4046–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020;43(7):1392–1398. [DOI] [PubMed] [Google Scholar]
- 30.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382(24):2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy Eur J Allergy Clin Immunol 2021;76(2):428–455. [DOI] [PubMed] [Google Scholar]
- 32.Artborg A, Caldinelli A, Wijkström J, et al. Risk factors for COVID-19 hospitalization and mortality in patients with chronic kidney disease : A nationwide cohort study. Clin Kidney J 2023;17(1):sfad283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appelman B, Oppelaar JJ, Broeders L, et al. Mortality and readmission rates among hospitalized COVID-19 patients with varying stages of chronic kidney disease: A multicenter retrospective cohort. Sci Rep 2022;12(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3(5):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams NP, Ostridge K, Devaster JM, et al. Impact of radiologically stratified exacerbations: Insights into pneumonia aetiology in COPD. Respir Res 2018;19(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismayana V, Yanti B, Kurniawan FD, et al. Risk factors of early mortality in COVID-19 patients in Indonesia: A retrospective cohort study in a provincial referral hospital of Aceh. Narra J 2023;3(3):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis 2020;20(6):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K, Zuo P, Liu Y, et al. Clinical and laboratory predictors of in-hospital mortality in patients with Coronavirus Disease-2019: A cohort study in Wuhan, China. Clin Infect Dis 2020;71:2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goronzy JJ, Weyand CM. Successful and maladaptive T cell aging. Immunity 2017;46(3):364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021;373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scobie HM, Johnson AG, Suthar AB, et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status - 13 U.S. jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep 2021;70(37):1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JJ, Dong X, Liu GH, et al. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol 2023;64(1):90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]