Abstract
The hard tick-borne relapsing fever spirochete, Borrelia miyamotoi, has recently gained attention as a cause of human illness, but fundamental aspects of its enzootic maintenance are still poorly understood. Challenges to experimental studies with B. miyamotoi-infected vector ticks include low prevalence of infection in field-collected ticks and seemingly inefficient horizontal transmission from infected immunocompetent rodents to feeding ticks. To reliably produce large numbers of B. miyamotoi-infected ticks in support of experimental studies, we developed an animal model where immunocompromised Mus musculus SCID mice were used as a source of B. miyamotoi-infection for larval and nymphal Ixodes scapularis ticks. Following needle inoculation with 1 × 105 spirochetes, the SCID mice developed a high spirochetemia (greater than 1 × 107 copies of B. miyamotoi purB per mL of blood) that persisted for at least 30 d after inoculation. In comparison, immunocompetent M. musculus CD-1 mice developed transient infections, detectable for only 2–8 d within the first 16 d after needle inoculation, with a brief, lower peak spirochetemia (8.5 × 104 – 5.6 × 105 purB copies per mL of blood). All larval or nymphal ticks fed on infected SCID mice acquired B. miyamotoi, but frequent loss of infection during the molt led to the proportion infected ticks of the resulting nymphal or adult stages declining to 22–29%. The ticks that remained infected after the molt had well-disseminated infections which then persisted through successive life stages, including transmission to larval offspring.
Keywords: Borrelia miyamotoi, Ixodes scapularis, Mouse model, Transmission, Transovarial transmission
1. Introduction
The relapsing fever spirochete, Borrelia miyamotoi, is increasingly recognized as a cause of human illness (named hard tick-borne relapsing fever or Borrelia miyamotoi disease) in North America, Europe, and Asia (Platonov et al., 2011; Krause et al., 2013, 2014; Sato et al., 2014; Molloy et al., 2015; Wagemakers et al., 2015; Fiorito et al., 2017). The primary vector of B. miyamotoi to humans in the eastern United States is the blacklegged tick, Ixodes scapularis, which also transmits the Lyme disease spirochetes, Borrelia burgdorferi sensu stricto (s.s.) and Borrelia mayonii (Krause et al., 2015a; Eisen, 2018; Eisen and Eisen, 2018). In nature, infection of nymphal and adult I. scapularis ticks with B. miyamotoi is relatively infrequent, often with a 10-fold lower infection prevalence compared with B. burgdorferi s.s. (Tsao et al., 2004; Barbour et al., 2009; Krause et al., 2015b; Nelder et al., 2016; Johnson et al., 2018). This discrepancy raises the question of whether natural maintenance relies on the same modes of transmission for both spirochetes. Elucidating how B. miyamotoi is maintained in enzootic transmission cycles also will clarify whether or not control strategies specifically targeting enzootic transmission of B. burgdorferi s.s. among tick vectors and rodent reservoirs can be assumed to also impact B. miyamotoi.
Horizontal transmission, where infected ticks transmit spirochetes to vertebrate hosts, and naïve ticks then acquire spirochetes while feeding on the infectious hosts, is the predominant mode of natural transmission for B. burgdorferi s.s. (Anderson et al., 1985; Donahue et al., 1987; Rollend et al., 2013). Natural maintenance of B. miyamotoi is still poorly understood, but in contrast to B. burgdorferi s.s., it is perpetuated not only via horizontal transmission among tick vectors and vertebrate reservoirs but also by transovarial (vertical) spirochete passage from infected females to their offspring (Scoles et al., 2001; Richter et al., 2012; Rollend et al., 2013; Breuner et al., 2018). If transovarial transmission proves to be of primary importance in maintaining B. miyamotoi in tick populations, control efforts focused on reducing abundance of host-seeking ticks will be more effective than strategies attempting to disrupt horizontal transmission of this spirochete. In addition, if both horizontal and transovarial transmission contribute substantially to the natural maintenance of B. miyamotoi, it will require further investigation as to why this spirochete nevertheless consistently is less prevalent in nymphal and adult I. scapularis ticks compared with B. burgdorferi s.s. (Scoles et al., 2001; Barbour et al., 2009; Hoen et al., 2009; Nelder et al., 2016).
Previous studies assessed the efficiency of transmission of B. miyamotoi from transovarially-infected I. scapularis larvae or nymphs to experimental hosts (Breuner et al., 2017, 2018). However, neither included an evaluation of the efficiency of acquisition of B. miyamotoi by larval ticks from infected hosts and subsequent transstadial passage to the nymphal stage, nor did they assess the efficiency of transmission from horizontally B. miyamotoi-infected I. scapularis ticks to experimental hosts. Though field-collected Ixodes ticks have been used for previous studies of this nature (Scoles et al., 2001; van Duijvendjik et al., 2016), the low prevalence of B. miyamotoi in ticks collected from the wild presents an added challenge for experimental work, and limits the ability to control for factors including pathogen strain and co-infections with other pathogens. Here we report on the use of a mouse model to infect immature I. scapularis ticks with a North American isolate of B. miyamotoi. Experiments were conducted to describe 1) kinetics of spirochetemia in immunocompetent and immunocompromised Mus musculus mice, and 2) infection dynamics in ticks fed on B. miyamotoi-infected immunocompromised mice, including spirochete acquisition by larvae and nymphs, and transstadial passage of infections to successive life stages.
2. Materials and methods
2.1. Borrelia miyamotoi isolate, experimental vertebrate hosts, and I. scapularis ticks
The North American CT13–2396 B. miyamotoi isolate (Kingry et al., 2017) was used to needle-inoculate Mus musculus mice (Sections 2.2 and 2.3). Experimental mouse hosts included 6 wk old female Fox Chase SCID mice (CB17/Icr-Prkdc/scid/IcrlcoCrl) and 8 wk old female CD-1 mice (Charles River Laboratories, Wilmington, MA, USA). Larval and nymphal I. scapularis ticks used in transmission experiments with infected SCID mice (Section 2.3) were sourced from the Oklahoma State University Tick Rearing Facility (Stillwater, OK, USA) and the Medical Entomology Laboratory at the Centers for Disease Control and Prevention (Atlanta, GA, USA) and a subset (a pool of 50 larvae per clutch, or a pool of 10 nymphs for every 100 used) were tested at CDC using the assay described below, and determined to be B. miyamotoi-free (purB negative). Ixodes scapularis nymphs with transovarially acquired infections, descended from field-collected females (see supplemental materials), were included in the study solely for comparison of spirochete load with nymphs harboring laboratory-acquired infections.
2.2. Duration of infection with B. miyamotoi in needle-inoculated mice
Ten CD-1 mice and 10 SCID mice were inoculated via needle with low passage (P3) B. miyamotoi spirochetes grown at 34 °C in modified Barbour-Stoenner-Kelly (BSK) medium (in-house BSK-R medium) without antibiotics. The mice were inoculated intraperitoneally with 100 μl suspended culture at a concentration of approximately 1 × 106 spirochetes/mL (1 × 105 spirochetes inoculated per mouse). Starting at 24 h after spirochete inoculation, a small amount of blood (15 μl) was extracted from each mouse by lateral tail vein nick and collected using MICROSAFE capillary tubes (Medicore, Nashville, TN, USA). To comply with Institutional Animal Care and Use guidelines for weekly limit of blood volume extracted, one of two groups of each mouse strain were sampled on alternating days, so that samples were collected from each individual mouse at 48 h intervals, and five mice of each strain were sampled daily. Blood collection was concluded for CD-1 mice at 15 or 16 days post-inoculation (d.p.i.), when three consecutive samples had yielded negative results for 9 of 10 mice. We collected blood from SCID mice over a period of 30 d.p.i., with frequency of collection reduced from 48 h intervals to biweekly intervals after 16 d.p.i. The methods for detection and quantification of B. miyamotoi spirochetes in mouse blood are described in Section 2.4. All animal procedures were approved by the Centers for Disease Control and Prevention Division of Vector-Borne Diseases Animal Care and Use Committee, in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011).
2.3. Acquisition of B. miyamotoi from infected SCID mice by feeding larval or nymphal ticks and transstadial passage
After first assessing the kinetics of infection in mice, 2 additional sets of 10 SCID mice were inoculated with B. miyamotoi via needle (as described in Section 2.2) and then exposed to feeding ticks. Ticks were introduced following an incubation period of at least 7 d.p.i. and not exceeding 21 d.p.i., a time period during which mouse spirochetemia was expected to be high and stable in these immunodeficient mice based on the first experiment outlined in Section 2.2. Mice were exposed to either naïve larval ticks (50–100 larvae for each of 10 mice) or naïve nymphal ticks (25 nymphs for each of 10 mice). Mouse blood was collected (as described in Section 2.2) on the final day of tick feeding and then tested by qPCR (see Section 2.5) to confirm B. miyamotoi infection. Within 24 h of tick drop off, a subset of freshly fed larval ticks (15 per infected mouse) were set aside for detection and quantification of B. miyamotoi spirochetes, as described in Section 2.4. The remaining fed larvae were placed within glass desiccators (90 to 95% relative humidity) in a growth chamber maintained at 21 to 23 °C with a 16:8 h light:dark cycle. Detection and quantification of B. miyamotoi spirochetes was also done for a subset of larvae harvested 3 wk post-drop off (9 per infected mouse) and for nymphs harvested shortly after the molt (10–15 per infected mouse) and 2 mo post-molt (up to 10 per infected mouse). The remaining nymphs were fed on non-infected CD-1 mice, and approximately 2 mo after adults had molted, females and males from this tick cohort were placed on a New Zealand White rabbit (Charles River Laboratories) and allowed to feed. Males and unfed females were collected and stored in 70% ethanol at 4 °C until qPCR was performed. Engorged females were tested after oviposition was completed or after being removed during unsuccessful feeds. A similar process was followed for ticks first fed on infectious mice as nymphs, with detection and quantification of B. miyamotoi spirochetes performed both on freshly molted adults, as well as on adults following exposure to a rabbit. Engorged females were included in our estimate of proportion infected with B. miyamotoi, but were excluded from median copy number calculation.
2.4. Detection and quantification of B. miyamotoi spirochetes from mouse blood and ticks
Collected mouse blood (15 μl) was immediately transferred into microcentrifuge tubes containing 200 μl lysis buffer (180 μl ATL - Qiagen, Valencia, CA, USA; 20 μl Proteinase K – Qiagen; 1 μl Carrier RNA – Applied Biosystems, ThermoFisher Scientific, Houston, TX, USA; and 1.5 μl DX Reagent - Qiagen) before clotting could occur. Samples were then incubated at 56 °C for 60 min and the lysates were stored at 4 °C until DNA extraction was performed. Ticks were surface cleaned with sodium hypochlorite diluted to 0.01% in water and rinsed in Milli-Q water (Millipore Corporation, Billerica, MA, USA). Dry ticks were stored at −80 °C until DNA extraction was performed. Whole ticks were ground using a Mini-Beadbeater-96 (BioSpec Products, Bartlesville, OK, USA) and 2.0 mm Very High Density Yttria stabilized zirconium oxide beads (GlenMills, Clifton, NJ, USA) immersed in 350 μl tissue lysis buffer (327.25 μl ATL, 20 μl Proteinase K, 1 μl Carrier RNA, and 1.5 μl DX Reagent). DNA was extracted from blood lysates or whole tick triturates using the KingFisher Flex system with MagMAX kit (ThermoFisher Scientific) and eluted into 90 μl elution buffer.
To detect and quantify spirochetes in mouse blood, a duplex assay targeting the B. miyamotoi adenylosuccinate lyase (purB) gene (forward primer - TCC TCA ATG ATG AAA GCT TTA, reverse primer - GGA TCA ACT GTC TCT TTA ATA AAG, probe – CalRd610-TCG ACT TGC AAT GAT GCA AAA CCT-BHQ2) (Graham et al., 2016) and rodent GAPDH (Applied Biosystems® TaqMan® Rodent GAPDH ControlReagents kit; ThermoFisher Scientific) was used. For tick samples, a duplex assay targeting purB and the I. scapularis actin gene (forward primer - GCC CTG GAC TCC GAG CAG, reverse primer - CCG TCG GGA AGC TCG TAG G, probe – Quas705-CCA CCG CCG CCT CCT CTT CTT CC-BHQ3) (Hojgaard et al., 2014) was used. Actin and GAPDH served as controls for DNA purification and qPCR. The Biotechnology Core Facility Branch at the Centers for Disease Control and Prevention (Atlanta, GA, USA) synthesized all oligonucleotides.
A recombinant plasmid containing the purB sequence (Graham et al., 2016) was used to create standard curves for each plate of samples, allowing quantitation cycle (Cq) values to be converted to gene copy numbers. Reactions were performed in 96 well plates using 1X iQ Multiplex Powermix (Bio-Rad Laboratories, Hercules, CA, USA) containing 300 nM of each primer and 200 nM of each probe per 10 μl reaction. Between 4.8 to 5.3% of DNA extracted from a tick was used per reaction as template. Real-time cycling conditions followed a previously described protocol (Graham et al., 2016), and qPCR samples were analyzed using CFX Manager 3.1 software (Bio-Rad) with Cq determination set to regression.
We used a dilution series of the recombinant plasmid, which included six replicates per dilution, to determine the limit of detection for purB copies. Though as few as a single copy per reaction could be detected in some samples, the assay used for blood and ticks was consistently able to detect 6 copies per reaction, resulting in a limit of detection of 8.2 copies per microliter of blood (8.2 × 103 copies per mL of blood) and 113 copies per tick. A single purB copy was considered equivalent to one spirochete, and whole tick spirochete loads were estimated by correcting purB copy number for the fraction of tick DNA included in the qPCR assay.
2.5. Studies on dissemination of B. miyamotoi spirochetes in infected nymphs
Dissemination of B. miyamotoi spirochetes in horizontally infected, unfed nymphs (see Section 2.3) was assessed by qPCR and direct fluorescence assay (DFA). Individual tick tissues, including midguts and salivary glands were dissected out on microscope slides using an Olympus SZX12 stereo microscope (Olympus, Tokyo, Japan). Sterile PBS was used to facilitate tissue extraction. For qPCR, individual tick tissues were then transferred to tubes containing 200 μl lysis buffer. Tick tissue lysates were incubated and stored as described for mouse blood lysates, and DNA extraction and detection and quantification of B. miyamotoi spirochetes was performed as described for whole ticks in Section 2.4. For DFA, tick tissues were smeared on slides, air dried, fixed in acetone, and stored at – 20 °C until further use. Prior to labelling, slides were washed twice in sterile PBS and blocked with SuperBlock Blocking Buffer (Thermo Scientific) for 30 min, followed by three washes with PBS. Tissues were then treated with murine monoclonal anti-flagellin antibody H9724 (Barbour et al., 1986; Gugliotta et al., 2013) (1:200) for 1 h, washed 3 times in PBS, and stained with anti-mouse IgG H + L Alexa Fluor 594 (1:500) (Molecular Probes by Life Technologies, Carlsbad, CA, USA) for 1 h. Finally, slides were washed 3 times with PBS and rinsed with dd H2O, and coverslips were mounted using ProLong DIAMOND anti-fade (Molecular Probes by Life Technologies) and allowed to dry overnight at 4 °C. Samples were viewed using a Zeiss LSM 800 confocal laser scanning microscope (Carl Zeiss, Inc. Oberkochen, Germany) and images produced using the LSM Software Zen 2 (Blue Edition).
2.6. Statistical analysis
Data were analyzed and graphed using JMP 13 software (SAS Institute, Cary, NC, USA). Kruskal-Wallis or Mann-Whitney U tests were used for comparisons of median purB copy numbers among or between groups, respectively, including for mouse strain, ticks originating from different mice, and tick groups tested at different life stages. H and U statistics were approximated as the χ2 values produced using JMP.
3. Results
3.1. Duration and magnitude of spirochetemia in mice infected with B. miyamotoi via needle inoculation
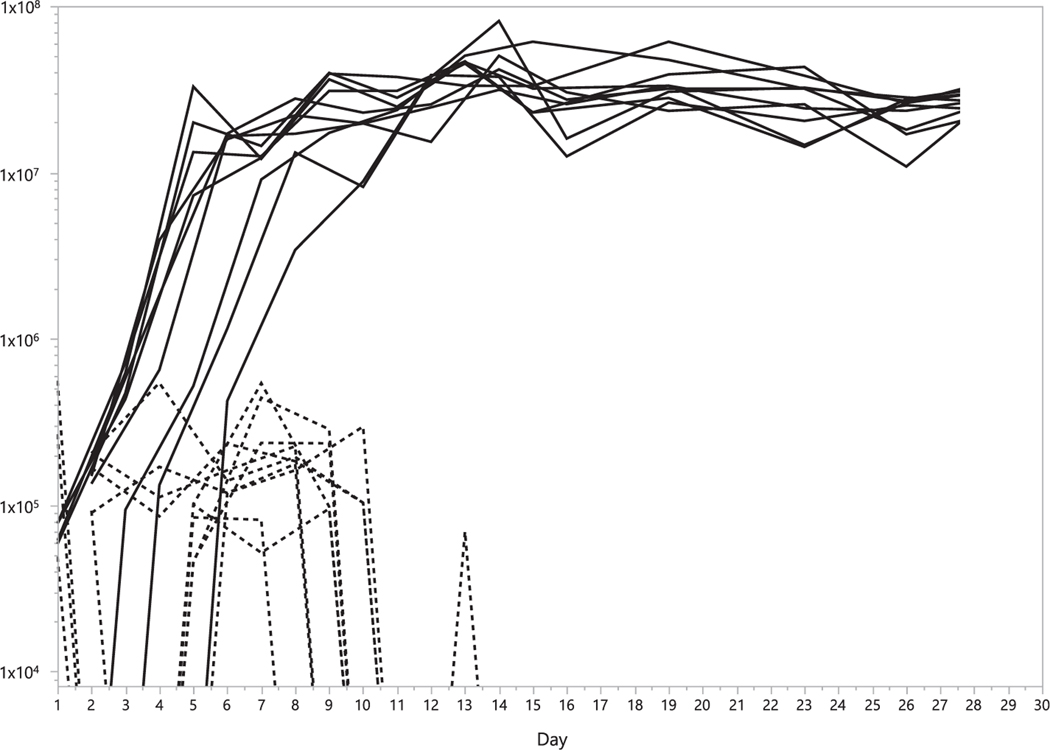
Throughout the sampling period, spirochetemia (estimated as purB copy number per mL blood) was variable among immunocompetent CD-1 mice, fluctuating between peaks as high as 6 × 105 copies/mL and concentrations below the limit of detection of our assay (Fig. 1). All mice had detectable infections on both sampling dates between 5–8 d.p.i., yet, by 16 d.p.i., three consecutive blood samples had tested negative for 9 of 10 mice. In the single exception, only one (13 d.p.i.) of the last three blood samples taken had detectable purB. In each of the immunocompromised SCID mice, purB copy numbers increased rapidly, reaching > 1 × 107 copies/mL of blood by 11 d.p.i. (Fig. 1), after which all 10 mice maintained between 1 × 107 and 7 × 107 copies/mL of blood through the conclusion of the sampling period at 30 d.p.i. The median peak spirochetemia for SCID mice (4.6 × 107 purB copies/mL) was 2 logs higher than for CD-1 mice (2.7 × 105 purB copies/mL) (Table 1; U = 14.3; d.f. = 1; P = 0.0002). The median number of d.p.i. until the first detection of purB did not differ significantly between the mouse strains (2 d in both cases). However, median peak spirochetemia was reached later for SCID mice (14 d; range = 12–30 d) than for CD-1 mice (6.5 d, 1–10 d) (U = 14.4; d.f. = 1; P = 0.0001).
Fig. 1.
Spirochetemia in mice. Changes in blood concentration of purB (copies/mL) over time for individual mice following inoculation of B. miyamotoi CT13–2396 culture. Copy numbers are represented by dashed lines for immunocompetent CD-1 mice and solid lines for immunocompromised SCID mice. Blood collection was discontinued after 16 d for CD-1 mice.
Table 1.
Comparison of infection kinetics by mouse strain. For each of the two mouse strains, time of first and last detection of spirochetes are listed as days post-inoculation, along with peak blood concentration of purB (copies/mL), time of peak blood concentration, and duration of detectable infection in days.
| 1 st detected (dpi) |
peak concentration (copies/mL) |
peak (dpi) |
last detected (dpi) |
duration (d) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| strain | median | range | median | range | median | range | median | range | median | range |
| CD-1 | 2 | (1– 5) | 2.66 × 105 | (8.45 × 104 - 5.60 × 105) | 6.5 | (1– 10) | 9 | (7–13) | 8 | (2–8) |
| SCID | 2 | (1– 6) | 4.64 × 107 | (3.42 × 107 - 8.15 × 107) | 14 | (12–30) | 30 | (30) | NA | NA |
d.p.i. = days post-inoculation.
d = days.
3.2. Acquisition of B. miyamotoi from infected SCID mice by feeding ticks and transstadial passage
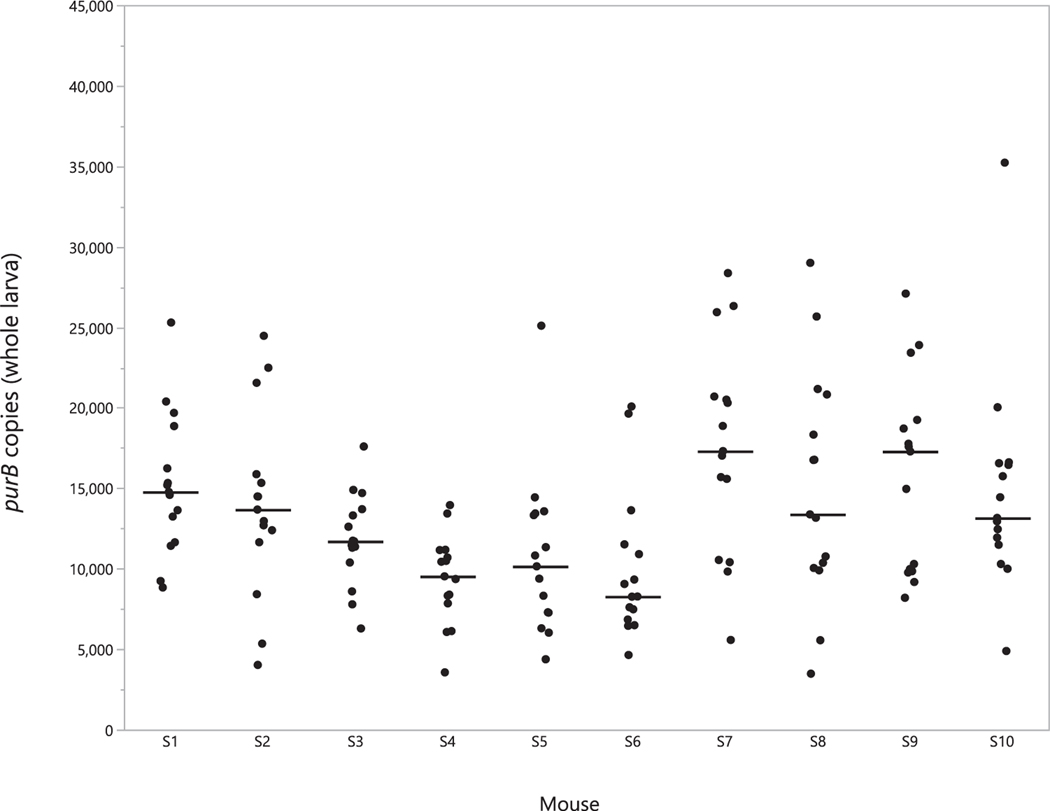
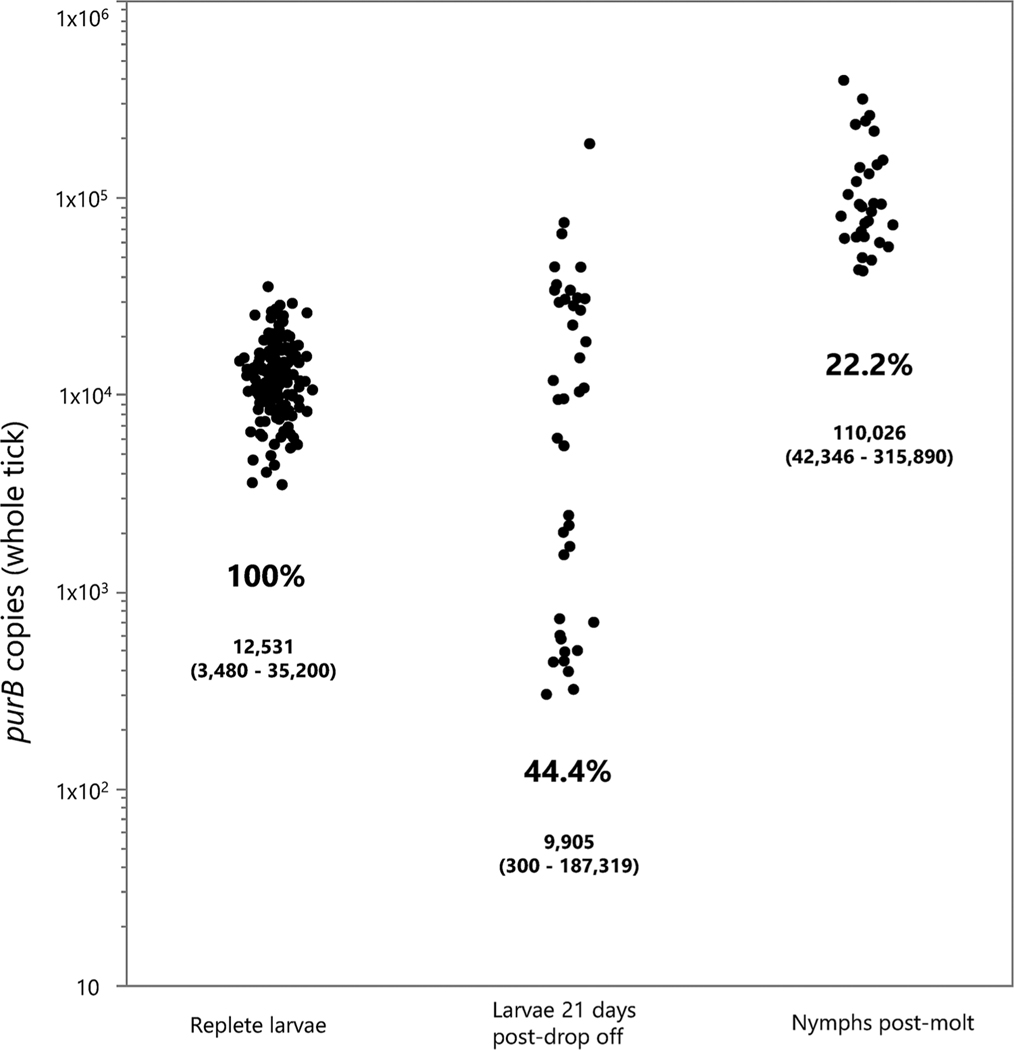
PurB copies were quantified in blood from the SCID mice used to infect larval I. scapularis directly following cessation of tick drop off, confirming that elevated spirochetemia (4.3 × 106–4.1 × 107 copies/mL blood) was attained in all 10 tick-exposed mice. All 150 freshly fed larvae examined contained B. miyamotoi DNA (median: 12,500 copies per tick, range: 3500–35,200) (Fig. 2). The highest median purB copy number for ticks collected from an individual mouse was 17,300 (range of 5600–28,400), whereas the lowest was 8300 (range of 4600–20,100). While statistically significant differences in median values for tick purB copy number were detected among individual mice (χ2 = 33.6; d.f., = 9; P = 0.0001), all ticks tested contained at least 3500 copies and no more than 35,000 copies. At three weeks post-drop off, 9 larvae from each mouse were tested and the percentage positive had declined to 46%, while the median purB copy number for positive ticks had decreased slightly to 9900 copies (Fig. 3). Infection prevalence then declined further to 24.6% in molted, flat nymphs, with a median purB copy number of 89,800 per tick (Fig. 3). Copy numbers for infected molted nymphs (median 89,800; range 42,300–258,000) were significantly higher than for replete larvae both at drop off (12,500; 3500–21,100; χ2 = 74.6; d.f. = 1; P = 0.0001) and at 21 d after drop off (9900; 300–44,400; χ2 = 45.0; d.f. = 1; P = 0.0001). There was no statistically significant difference in median copy number between the two groups of fed larvae. Prevalence of infection between groups of nymphs tested shortly after molting, and at 60 d post-molt was similar (Table 2) and median copy number did not differ significantly (χ2 = 3.12; d.f. = 1; P = 0.077). Molted nymphs retained high purB copy numbers well beyond ecdysis; three individuals among those dissected 6 mo post-molt had combined tissue totals exceeding 130,000 copies each. Finally, we observed high success of transstadial retention of infection between the nymphal and adult stages (Table 2).
Fig. 2.
purB copy number in freshly fed larval ticks by source SCID mouse. Black dots indicate purB copy number for a single engorged larva (whole), grouped according to the mouse the tick was fed upon. The solid bar indicates the median copy number for ticks sourced from that mouse.
Fig. 3.
Transstadial kinetics of infection. Black dots represent purB copy numbers for individual ticks tested at one of three time points: Replete larvae (at drop off), larvae 21 d post-drop off, and nymphs tested shortly after molting. Percent infected is listed for each group, along with median and range of copy number for each time point.
Table 2.
Interstage infection dynamics in ticks fed on SCID mice. Proportion infected (% prevalence) and purB copy numbers are listed (median copies, range) for life stages for I. scapularis exposed to infected SCID mice as larvae (top) or nymphs (bottom).
| life stage | stage infection introduced | timepoint | % prevalence (no. + /no. tested) | median copies (range) |
|---|---|---|---|---|
| larva | larva | drop-off | 100 (150/150) | 12,531 (3480 – 35,220) |
| larva | larva | 21 d.p.d. | 44.4 (40/90) | 9905 (300–187,319) |
| nymph | larva | 0 d.p.m. | 22.2 (30/135) | 110,026 (42,346 – 315,890) |
| nymph | larva | 60 d.p.m. | 24.0 (18/75) | 74,888 (42,913 – 393,235) |
| adult male | larva | p.r.f. | 37.0 (10/27) | NA |
| adult female | larva | p.r.f. | 55.6 (15/27) | NA |
| F1 larva | larva | eclosure | 100 (15/15) | 24,816 (3709– 109,101) |
| nymph | nymph | drop-off | 100 (8/8) | 72,148 (3480– 35,220) |
| adult male | nymph | 2–3 m.p.m. | 25.0 (9/36) | 199,300 (20–3,367,500) |
| adult female | nymph | 2–3 m.p.m. | 30.9 (21/68) | 916,900 (200–5,191,162)a |
d.p.d. = days post-drop off; d.p.m. = days post-molt; p.r.f. = post-rabbit feed; m.p.m. = months post-molt; NA = not available.
5 females that were engorged were excluded from calculations.
3.3. Dissemination of B. miyamotoi spirochetes in infected nymphs
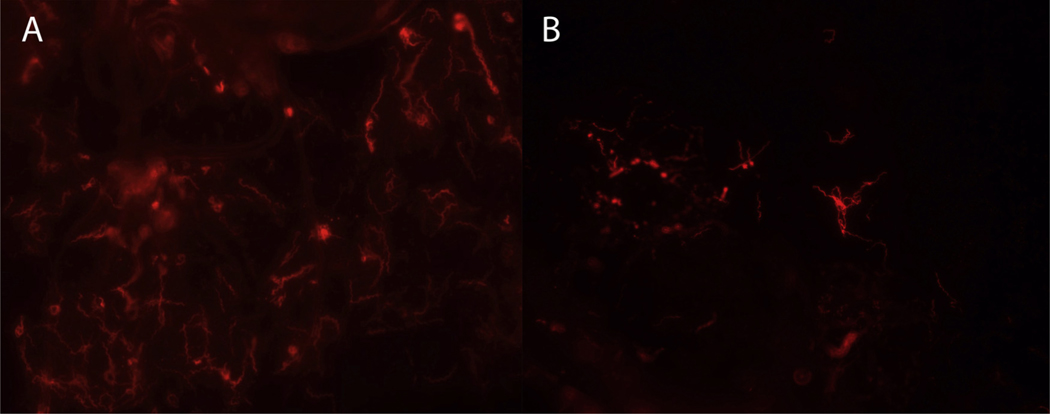
Salivary glands and midguts were extracted from 50 additional nymphs at time points between 1 wk and 6 mo post-molt. Sixteen (32%) of these ticks tested positive for B. miyamotoi and both tissue types were positive in each of the 16 infected ticks. Median purB copy number was estimated at 53,400 (8900–225,900) in midguts and 10,500 (2,000–24,200) in salivary glands. Moreover, midguts and salivary glands from four nymphs testing positive by qPCR were processed for DFA and each of the tissues contained large numbers of distinguishable spirochetes (Fig. 4).
Fig. 4.
IFA of Borrelia miyamotoi in tick tissues. A. Midgut tissue and B. salivary gland tissue from nymphal Ixodes scapularis fed on infected SCID mice as larvae, with spirochetes labelled with anti-flagellin monoclonal antibody (red) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
As expected, the dynamics of B. miyamotoi infection differed markedly between immunocompetent and immunocompromised mouse strains. In immunocompetent CD-1 mice, which had previously been shown susceptible to B. miyamotoi infection via tick bite (Breuner et al., 2017, 2018), spirochetemia fluctuated between peak and nondetectable levels for a period of 10–14 d after needle inoculation, with purB blood concentrations peaking at 2–3 orders of magnitude below those of immunocompromised SCID mice. For the SCID mice, purB copy numbers increased steadily, until peak blood concentration was attained at 10–12 d following inoculation, and remained at, or near peak concentration through the 30-day sampling period in all individuals. Despite differences in strain and inoculums, the kinetics of spirochetemia in our SCID mice were consistent with previous descriptions of infection in immunocompromised mice where the LB-2001 B. miyamotoi isolate was used (Krause et al., 2015b; Wagemakers et al., 2016) and we attribute the delay in peak spirochetemia in SCID mice (relative to CD-1 mice) to the additional period of spirochete replication required to reach higher blood concentrations.
Feeding I. scapularis on infected SCID mice proved a highly effective method of experimentally exposing ticks to B. miyamotoi. All larvae and nymphs contained B. miyamotoi DNA when tested shortly after completion of blood feeding on an infected mouse, and even at the low end of the range, copy numbers were substantial. However, despite universal exposure to spirochetes by feeding larvae, overall transstadial survival was inefficient, as evidenced by the decline in infection prevalence from drop off through ecdysis, at which point only one in four nymphs retained spirochete loads that were detectable by our assay. Interestingly, while a majority of ticks exposed to a large quantity of B. miyamotoi via SCID mouse cleared spirochetes (at least to numbers below detectable levels) during the period between exposure and completion of molt, qPCR results indicate that robust spirochete replication occurred in a subset of these ticks during the molting process. Inclusion of direct culture of tick tissue or a molecular assay targeting spirochete mRNA should be used to confirm these findings in future experimental work. While we were not able to investigate the mechanism(s) leading to clearance of high spirochete loads from our engorged ticks, we speculate that serotype may play a role in the likelihood of successful colonization of ticks. Wagemakers et al. (2016) previously demonstrated that B. miyamotoi, like two closely related relapsing fever spirochetes, Borrelia hermsii and Borrelia turicatae, expresses alternating surface proteins known as variable membrane proteins (VMPs) which contribute to evasion of host immunity. In SCID mice, both B. hermsii and B. turicatae are known to express multiple serotypes simultaneously, with distinctive tissue tropisms (Cadavid et al., 1994, 2001; Mehra et al., 2009). Though the relationship between serotype and arthropod host has not been described for relapsing fever spirochetes, it has been shown that variants within an isogenic population of B. burgdorferi circulating in a host differ in their ability to colonize ticks fed on that host (Rego et al., 2014). We therefore suggest that further consideration should be given to the potential relationship between serotype and vector susceptibility.
We also noted an increase in infection prevalence between nymphs and adults of the same cohort. While it is possible that uninfected ticks acquired infection while co-feeding with infected ticks as nymphs, a previous study suggests that little, if any amplification in infection prevalence of B. miyamotoi occurs during co-feeding of I. ricinus larvae (van Duijvendijk et al., 2016). It is therefore more likely that some nymphs in our experiment were infected at levels below detection, and subsequent spirochete replication that occurred as a result of the nymphal blood meal may have increased copy numbers above the threshold of detection in some adult ticks. The stable infection prevalence of the cohort we exposed to infection as larvae presents in contrast to a similar study of B. burgdorferi s.l., where infection prevalence and spirochete load were observed to decline between the nymphal and adult stages (Jacquet et al., 2017). This difference may reflect two distinctive biological strategies of Borrelia species, where a peak in infection prevalence at the nymphal stage would maximize the likelihood of horizontal transmission of infection, whereas a continued increase in spirochete load and stable infection prevalence in a tick population through the adult stage would be more beneficial to vertical transmission of infection.
Though infection dynamics in SCID mice may offer insights into pathogenesis of tick-borne infections such as B. miyamotoi disease, it is important to acknowledge that they do not reflect natural exposure of a tick to an infected immunocompetent host, and are described here for the purpose of production of infected ticks for use in experimental studies. However, along with the unfed nymphs infected with B. miyamotoi as larvae via SCID mice in the laboratory, we also quantified infection in transovarially-infected unfed nymphs originating from field-collected females collected in Connecticut or Minnesota (Breuner et al., 2017, 2018). In support of the biological relevance of our SCID mouse model, the transovarially-infected nymphs from field-collected females had similarly high to higher spirochete loads (CT nymphs, median purB copy number of 147,700 per tick; MN nymphs, median purB copy number of 301,400 per tick, listed in Supplemental material) compared with the laboratory-infected nymphs (see Table 2; median purB copy number of 110,026 for recently molted ticks). The lone prior study reporting quantitative figures from field-collected I. scapularis nymphs infected with B. miyamotoi listed spirochete loads of 1–2 orders of magnitude lower than those described here (Barbour et al., 2009). Notably, the quantitative assay we used measured genetic copies, which do not equate to single spirochetes. So while we were not able to make a direct comparison between our lab-infected ticks and previously described field-collected specimens, Kitten and Barbour (1992) reported that B. hermsii obtained both from mice and culture contained multiple chromosomal copies per cell, which suggests that purB copy number may overestimate the quantity of B. miyamotoi spirochetes in mouse blood and ticks.
Additionally, we determined that I. scapularis feeding as nymphs were similarly susceptible to infection as feeding larvae. In a natural setting, though phenology and host association for this tick species are likely to limit the significance of infected adults for horizontal transmission relative to infected nymphs, acquisition of infection during the bloodmeal of either immature stage would facilitate vertical transmission of spirochetes. As it concerns the further study of transovarial transmission, experimental introduction of infection at the nymphal stage includes the potential benefits of reduction in tick developmental period and animal resources associated with an additional life stage. Based on our results, infestation of an infected SCID mouse with 100 naïve larvae would result in the production of approximately 20–25 molted infected nymphs, should all larvae feed and molt successfully. In addition, 100% of tested larvae produced by one infected female were purB-positive, indicating that transovarially-infected tick lines can be established using our mouse model.
Examination of individual tick tissues added further insight into the biology of B. miyamotoi infection within the vector. Salivary glands and midguts dissected from flat nymphs contained numerous visible spirochetes, and purB copy numbers were detected at high quantities in these tissues as long as 6 months after the molt. The disseminated infections observed in these nymphs correspond with our previous observation that nymphs can transmit B. miyamotoi to mice within 24 h of attachment (Breuner et al., 2017). Other relapsing fever spirochetes, including B. hermsii and B. turicatae are known to establish persistent infections in salivary glands (Schwan and Hinnebusch, 1998; Takano et al., 2012; Boyle et al., 2014). While this is evidently advantageous for spirochete transmission by rapidly feeding soft ticks, the extent of any benefit conveyed to spirochete transmission by hard ticks, which are attached to their hosts for days, remains to be elucidated.
Further progress toward the prevention of human B. miyamotoi disease cases should include a more robust comparison of the relative contributions of horizontal and vertical transmission in the natural maintenance of this spirochete. For the latter, this should include determination of both the frequency of transovarial transmission among a population of females, as well as quantifying successful acquisition of infection within filial clutch. The SCID mouse model described here allows for reliable production of I. scapularis ticks infected with specific, well-characterized B. miyamotoi isolates, which is a prerequisite for downstream experimental studies focusing on the biology of this emerging human pathogen.
Supplementary Material
Acknowledgements
We thank Alan Barbour of U.C. Irvine for sharing the H9724 antibody; we also thank Karen Boegler, Christine Graham, Christopher Sexton, and Shanna Williams, all of the Centers for Disease Control and Prevention for technical assistance and/or advice with experimental procedures.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ttbdis.2018.11.017.
References
- Anderson JF, Johnson RC, Magnarelli LA, Hyde FW, 1985. Identification of endemic foci of Lyme disease: isolation of Borrelia burgdorferi from feral rodents and ticks (Dermacentor variabilis). J. Clin. Microbiol 22, 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI, 2009. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg 81, 1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF, Heiland RA, Schrumpf ME, Tessier SL, 1986. A Borrelia-specific antibody binds to a flagellar epitope. Infect. Immun 52, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WK, Wilder HK, Lawrence AM, Lopez JE, 2014. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl. Trop. Dis 8, e2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Hojgaard AH, Replogle AJ, Boegler KA, Eisen L, 2018. Transmission of the relapsing fever spirochete, Borrelia miyamotoi, by single transovarially-infected larval Ixodes scapularis ticks. Ticks Tick-Borne Dis. 9, 1464–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Dolan MC, Replogle AJ, Sexton C, Hojgaard A, Boegler KA, Clark RJ, Eisen L, 2017. Transmission of Borrelia miyamotoi sensu lato relapsing fever group spirochetes in relation to duration of attachment by Ixodes scapularis nymphs. Ticks Tick-Borne Dis. 8, 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Pachner AR, Estanislao L, Patalapati R, Barbour AG, 2001. Isogenic serotypes of Borrelia turicatae show different localization in the brain and skin of mice. Infect. Immun 69, 3389–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Thomas DD, Crawley R, Barbour AG, 1994. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J. Exp. Med 179, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JG, Piesman J, Spielman A, 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg 36, 92–96. [DOI] [PubMed] [Google Scholar]
- Eisen L, 2018. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick-Borne Dis. 9, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, 2018. The Blacklegged Tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito TM, Reece R, Flanigan TP, Silverblatt FJ, 2017. Borrelia miyamotoi polymerasechain reactionpositivity on a tick-borne disease panel in an endemic region of Rhode island: a case series. Infect. Dis. Clin. Pract 25, 250–254. [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ, 2016. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: ixodidae). Ticks Tick-Borne Dis. 7, 1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliotta JL, Goethert HK, Berardi VP, Telford III SR, 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med 368, 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen AG, Rollend LG, Papero MA, Carroll JF, Daniels TJ, Mather TN, Schulze TL, Stafford IIIKC, Fish D, 2009. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Dis. 9, 431–438. [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J, 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick Borne-Dis. 5, 349–351. [DOI] [PubMed] [Google Scholar]
- Jacquet M, Genne D, Belli A, Maluenda E, Sarr A, Voordouw MJ, 2017. The abundance of the Lyme disease pathogen Borrelia afzelii declines over time in the tick vector Ixodes ricinus. Parasit. Vectors 10, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, Hojgaard AH, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, Neitzel DF, Schiffman EK, Eisen RJ, 2018. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: ixodidae) nymphs in Minnesota, USA. Ticks Tick-Borne Dis. 10.1016/j.ttbdis.2018.07.009. In press. [DOI] [PMC free article] [PubMed]
- Kingry LC, Replogle A, Batra D, Rowe LA, Sexton C, Dolan M, Connally N, Petersen JM, Schriefer ME, 2017. Toward a complete North American Borrelia miyamotoi genome. Genome Announc. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten T, Barbour AG, 1992. The relapsing fever agent Borrelia hermsii has multiple copies of its chromosome and linear plasmids. Genetics 132, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Hendrickson JE, Steeves TK, Fish D, 2015a. Blood transfusion transmission of the tick-borne relapsing fever spirochete Borrelia miyamotoi in mice. Transfusion 55, 593–597. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, Barbour AG, 2015b. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect 21, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D, 2014. Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg. Infect. Dis 20, 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D, 2013. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med 368, 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R, Londono D, Sondey M, Lawson C, Cadavid D, 2009. Structure-function investigation of vsp serotypes of the spirochete Borrelia hermsii. PLoS One 4, e7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy PJ, Telford IIISR, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP, 2015. Borrelia miyamotoi disease in the Northeastern United States: a case series. Ann. Intern. Med 163, 91–98. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011. Guide for the Care and Use of Laboratory Animals, 8th ed. National Academies Press, Washington, D.C. [Google Scholar]
- Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, Johnson S, Patel SN, Sider D, 2016. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit. Vectors 9, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ, 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis 17, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego RO, Bestor A, Stefka J, Rosa PA, 2014. Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PLoS One 9, e101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Debsk A, Hubalek Z, Matuschka FR, 2012. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 12, 21–27. [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, Childs JE, 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick-Borne Dis. 4, 46–51. [DOI] [PubMed] [Google Scholar]
- Sato K, Takano A, Konnai S, Nakao M, Ito T, Koyama K, Kaneko M, Ohnishi M, Kawabata H, 2014. Human infections with Borrelia miyamotoi, Japan. Emerg. Infect. Dis 20, 1391–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Hinnebusch BJ, 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280, 1938–1940. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D, 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1, 21–34. [DOI] [PubMed] [Google Scholar]
- Takano A, Sugimori C, Fujita H, Kadosaka T, Taylor KR, Tsubota T, Konnai S, Tajima T, Sato K, Watanabe H, Ohnishi M, Kawabata H, 2012. A novel relapsing fever Borrelia sp. Infects the salivary glands of the molted hard tick, Amblyomma geoemydae. Ticks Tick-Borne Dis. 3, 259–261. [DOI] [PubMed] [Google Scholar]
- van Duijvendijk G, Coipan C, Wagemakers A, Fonville M, Ersoz J, Oei A, Foldvari G, Hovius J, Takken W, Sprong H, 2016. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. Miyamotoi to vertebrate hosts. Parasit. Vectors 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG, 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci 101, 18159–18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemakers A, Koetsveld J, Narasimhan S, Wickel M, Deponte K, Bleijlevens B, Jahfari S, Sprong H, Karan LS, Sarksyan DS, van der Poll T, Bockenstedt LK, Bins AD, Platonov AE, Fikrig E, Hovius JW, 2016. Variable major proteins as targets for specific antibodies against Borrelia miyamotoi. J. Immunol 196, 4185–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW, 2015. In vectors and hosts in The Netherlands. Ticks Tick-Borne Dis. 8, 370–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.