Abstract
Vectors derived from the adeno-associated virus (AAV) have been successfully used for the long-term expression of therapeutic genes in animal models and patients. One of the major advantages of these vectors is the absence of deleterious immune responses following gene transfer. However, AAV vectors, when used in vaccination studies, can result in efficient humoral and cellular responses against the transgene product. It is therefore important to understand the factors which influence the establishment of these immune responses in order to design safe and efficient procedures for AAV-based gene therapies. We have compared T-cell activation against a strongly immunogenic protein, the influenza virus hemagglutinin (HA), which is synthesized in skeletal muscle following gene transfer with an adenovirus (Ad) or an AAV vector. In both cases, cellular immune responses resulted in the elimination of transduced muscle fibers within 4 weeks. However, the kinetics of CD4+ T-cell activation were markedly delayed when AAV vectors were used. Upon recombinant Ad (rAd) gene transfer, T cells were activated both by direct transduction of dendritic cells and by cross-presentation of the transgene product, while upon rAAV gene transfer T cells were only activated by the latter mechanism. These results suggested that activation of the immune system by the transgene product following rAAV-mediated gene transfer might be easier to control than that following rAd-mediated gene transfer. Therefore, we tested protocols aimed at interfering with either antigen presentation by blocking the CD40/CD40L pathway or with the T-cell response by inducing transgene-specific tolerance. Long-term expression of the AAV-HA was achieved in both cases, whereas immune responses against Ad-HA could not be prevented. These data clearly underline the importance of understanding the mechanisms by which vector-encoded proteins are recognized by the immune system in order to specifically interfere with them and to achieve safe and stable gene transfer in clinical trials.
In animal models and clinical trials, long-term transgene expression has been described following gene transfer utilizing recombinant adeno-associated virus vectors (rAAV) (11, 14, 15, 20, 30, 35). In these studies no immunological response to the transgene product has been described. This stands in stark contrast to studies using recombinant adenovirus (rAd) vectors, which elicit strong cellular immune responses to the vector as well as to the vector-encoded proteins (16, 33, 40). rAAV vectors do not contain any viral open reading frames, leaving the transgene product and the virus capsid as the only source of non-self antigen. In particular, the observation that the Escherichia coli β-galactosidase (β-Gal) protein, which was immunogenic in the context of an rAd infection, appeared not to cause any immune reaction when introduced via an rAAV vector raised the question as to whether rAAV vectors avoided immunity by delivering the non-self proteins in such a way that they are either ignored or induce tolerance by deletion, by anergy, or by activating suppressive cells (17). In other studies, however, rAAV vectors have been demonstrated to elicit cellular and humoral immune responses to the encoded transgene product and have been used successfully for vaccination purposes (2, 8, 26, 27). In order to use rAAV vectors in a safe manner in gene transfer protocols, one needs to precisely understand the factors influencing the activation of cellular immune responses to proteins encoded by these vectors. This is difficult to study in a normal mouse, where the fraction of cells responsive to one particular protein is very low and therefore difficult to physically track. To circumvent this problem, we have taken advantage of T-cell-receptor (TCR) transgenic animals, which have an increased precursor frequency of antigen-specific T cells that can be followed by a monoclonal antibody (MAb) directed toward the receptor (23). This enabled us to study in detail the activation status of the T cells after they encountered the antigen in the context of different virus vectors. We chose influenza virus hemagglutinin (HA) as our model antigen because its antigenic structure is well defined and in BALB/c mice there is one major class II-restricted epitope (amino acids 111 to 119), presented by major histocompatibility complex class II molecule I-Ed (6, 10).
Using mice transgenic for an HA-specific TCR (21), we report here that rAAV-mediated gene transfer of the HA gene into the muscle triggered the activation of HA-specific CD4+ T cells and target cell destruction. Although immune responses also occurred with E1-deleted Ad vectors, the mechanisms of immune activation differed for the two vector types. rAd vectors were able to directly transduce dendritic cells (DCs) in vivo following intramuscular (i.m.) gene transfer, leading to expression of the transgene within the antigen-presenting cells (APCs). In contrast, APCs in rAAV-injected animals exclusively took up antigenic material released from transduced cells and activated T cells through cross-presentation. In accordance with these results, protocols aimed at interfering with the immune response by blocking antigen presentation or by inducing transgene-specific T-cell tolerance were successful with rAAV but not with rAd vectors.
MATERIALS AND METHODS
Mice.
TCR-HA mice, described previously (21), are on a BALB/c background and express an α/β TCR specific for peptide 111-119 from influenza virus HA, presented by I-Ed. For the experimental protocols, heterozygous TCR transgenic mice at between 6 and 10 weeks of age were used. BALB/c mice were obtained from IFFA CREDO.
Plasmid constructions.
For HAwt-SMD2, a 1.8-kb fragment of HAwt-pCMV (HindIII/BamHI, kindly provided by Drew Pardoll) was cloned into SMD2 (PmlI/BglII) (34). For HAwt-SCII, a 1.8-kb fragment of HAwt-pCMV (KpnI/NotI) was cloned into the KpnI/NotI sites of pSCII (16). For HAwt-pTG6600, the plasmid HAwt-pCMV was cut with HindIII/BamHI and cloned into the EcoRI site of pTG6600 (7) via blunt-end ligation.
Generation of recombinant virus vectors.
rAAV vector was produced by triple transfection into 293 cells as described in Xiao et al. (39). The E1-deleted rAd expressing HA was generated as described by Chartier et al. (7).
i.m. injections.
Mice were anesthetized, and rAAV-HA (1011 particles) or rAd-HA (1011 particles) was injected in a 25-μl volume into the tibialis anterior muscle, after a small incision was made to lay open the muscle. Incisions were closed with a Vicryl suture. The muscles were harvested at various time points after injection and snap frozen.
HA staining.
Frozen muscle sections were fixed in acetone and incubated with biotinylated H37/38 MAb (directed against HA; kindly provided by Walther Gerhard, Wistar Institute, Philadelphia, Pa.) in phosphate-buffered saline–2% goat serum, washed, and incubated in ABC (avidin-biotin-chromagen) solution (Vector Laboratories). The slides were revealed in diaminobenzidine (DAB Fast; Sigma) and counterstained with methyl green or hematoxylin.
X-Gal staining.
Muscle sections were fixed in 0.5% glutaraldehyde and incubated with X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) (as described in reference 17) for 6 h at 37°C. Tissue was counterstained with hematoxylin-eosine.
Antibodies and fluorescence-activated cell sorter (FACS) analysis.
MAb 6.5 was labeled using fluorescein succinyl ester (FLUOS; Boehringer Mannheim), and H37/38 was biotinylated using Biotin-X-NHS (Calbiochem, La Jolla, Calif.). The following antibodies were used: α-CD4-PE (Becton Dickinson, Mountain View, Calif.); biotin-conjugated α-CD25, α-CD45Rb, and α-CD62L (Pharmingen, San Diego, Calif.); and SA-PE (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Flow cytometry was performed on a FACScan, and data were analyzed using the Cellquest software (Becton Dickinson).
B-cell and DC separation.
Draining lymph nodes, lymph nodes, and spleens were digested in Collagenase D (Worthington, Freehold, N.J.). For this, tissue was minced in 100 U of collagenase per ml and further digested in 400 U of collagenase per ml for 30 min at 37°C. For magnetic sorting, cells were incubated with CD11c or CD19 magnetic beads (Miltenyi Biotec, Inc.). For flow cytometry sorting, cells were centrifuged on a bovine serum albumin gradient (Sigma, St. Louis, Mo.), and low-density cells recovered from the interphase were stained with CD11c-fluorescein isothiocyanate (FITC) and CD19-phycoerythrin (PE) antibodies. Sorting of CD11c+ and CD19+ populations was performed on a FACSVantage machine (Becton Dickinson). The purity of the DCs was higher than 75% after magnetic sorting and higher than 95% after fluorescence sorting.
TcH assay.
A total of 105 APCs (B cells or DCs) in 200 μl of Iscove modified Dulbecco medium (IMDM) were incubated for 18 h together with 105 HT-1080 cells transduced with either rAd-HA (multiplicity of infection of 100) or rAAV-HA (105 particles/cell) before the addition of 105 HA-specific T-cell hybridoma (TcH) cells (described by Weber et al. [36]). At 24 h after the addition of the TcH cells, the cells were centrifuged, and the pellets were taken up in 100 μl of IMDM and 100 μl of 2 mM fluorescein di-β-d-galactopyranoside (Molecular Probes) in distilled water and incubated for 1 min at 37°C and for 30 min in ice. The percentage of β-Gal+ TcH cells was determined by cytofluorometry.
CTL assay and generation of recombinant vaccinia virus.
The cytotoxic-T-lymphocyte (CTL) assay and the generation of recombinant vaccinia virus were done as previously described by Jooss et al. (16).
RT-PCR.
Total RNA was isolated from sorted B cells or DCs by using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Reverse transcription was carried out using Superscript II reverse transcriptase (RT; Gibco-BRL, Gaithersburg, Md.). RT-PCR was performed with primers specific for HA, giving rise to a fragment of 440 bp or, for β2-microglobulin, giving rise to a fragment of 230 bp. cDNA isolated from rAd-HA-transduced HT-1080 cells was included in each PCR. H2O samples served as negative controls.
MR1 or YTA3.1.2-IgHA treatment.
A total of 2.5 × 107 lymph node cells were adoptively transferred into BALB/c animals at day −6. Mice were injected (day 0 for the MR1 study and day 8 for YTA3.1.2-IgHA study) into the tibialis anterior muscle with rAAV-HA (1011 particles) or rAd-HA (1011 particles). Some of the animals received, in addition, a neutralizing CD40L antibody (MR1 [28], 100 μg/injection, i.v. [intravenous]) at days −2, 0, 2, 4, and 12 or a semidepleting CD4 antibody (YTA3.1.2 [29], 50 μg/injection, i.v.) at days −2 and 0 in combination with a chimeric immunoglobulin containing the HA peptide, termed IgHA (43) (100 μg/injection, i.v.), at days 0, 7, 10, and 13. The muscles were harvested for immunohistochemistry (day 22 for the MR1 study and day 28 for the YTA3.1.2-IgHA study), and the lymph nodes were harvested for FACS analysis as well as for the proliferation assays.
Proliferation assay.
Lymph node cells were isolated and cultured (2 × 105 cells/well) with irradiated BALB/c splenocytes (5 × 105 cells/well) in the presence of HA peptide (10, 1, and 0.1 μg/ml) or with medium alone. 3H incorporation was measured over the last 18 h of a 90-h culture.
RESULTS
Antigen-specific T-cell activation and target cell destruction following rAAV-mediated gene transfer.
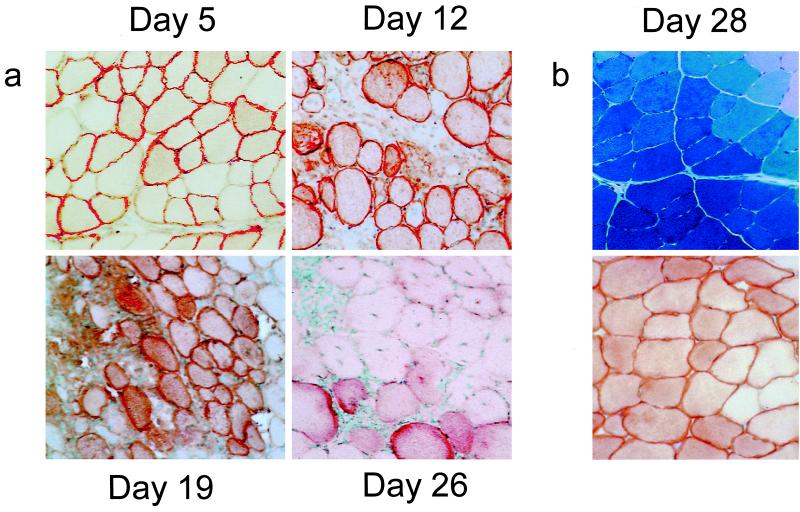
An AAV vector containing the HA gene was constructed (rAAV-HA; see Materials and Methods) and injected into the tibialis anterior muscle of immunocompetent BALB/c mice. The expression of the HA transgene was monitored by immunohistochemistry on muscle sections from injected animals sacrificed at different time points and compared to that of animals injected with an E1-deleted Ad vector carrying HA (rAd-HA). As expected, the rAd-HA control showed a strong infiltration after 5 days, with destruction of the transduced tissue (data not shown). Mice receiving the rAAV-HA vector showed HA expression as early as 5 days after gene transfer (Fig. 1a). Infiltrating cells were recruited into the area of HA-expressing muscle fibers by day 12, and most fibers expressing the transgene had disappeared by day 26 (Fig. 1a). This transient expression profile stands in stark contrast to the stability of transgene expression observed after administration of rAAV vectors expressing various other transgenes (9, 11, 14, 15, 35, 37) and is exemplified in Fig. 1b (upper panel), which shows stable lacZ expression in immunocompetent BALB/c animals 28 days after i.m. injection of an rAAV-LacZ vector. Muscle sections from immunodeficient RAG−/− mice injected with rAAV-HA showed stable HA expression 28 days after transduction (Fig. 1b, lower panel), indicating that the destruction of target cells in immunocompetent BALB/c animals was dependent on an adaptive immune response.
FIG. 1.
rAAV gene transfer to muscle. (a) rAAV-HA (1011 particles) was injected into the tibialis anterior muscle of immunocompetent BALB/c mice. Muscles were isolated and stained for HA at various time points. (b) The same rAAV-HA vector was injected into immunodeficient RAG−/− mice (lower panel) or rAAV-LacZ (1011 particles) was injected into immunocompetent BALB/c animals (upper panel), and the muscles were stained for HA or lacZ expression 28 days later. Magnification, ×150.
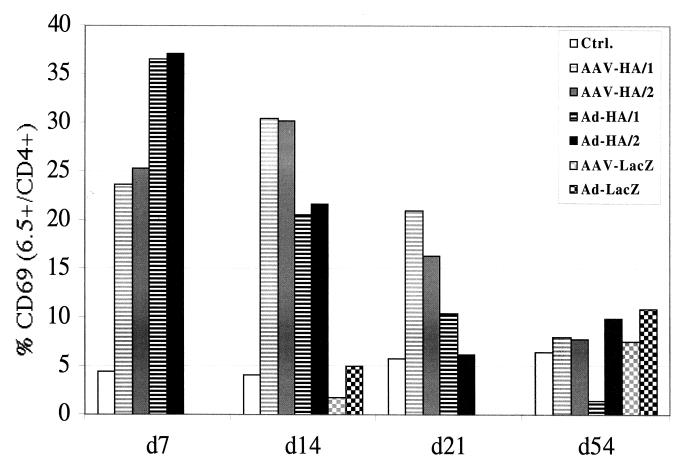
The transgene-specific T-cell response was then monitored in greater detail. For this, we injected rAAV-HA or rAd-HA into the muscle of transgenic mice (TCR-HA) expressing an antigen receptor which recognizes the HA epitope 111-119 on 10 to 15% of their CD4+ T cells. Due to the increased precursor frequency of HA-specific CD4+ T cells in these mice, the kinetics of muscle infiltration after administration of either vector were accelerated (already significantly by day 7) compared to that observed with BALB/c mice (data not shown). The draining (aortic, inguinal, and popliteal) lymph nodes of mice injected with either vector were significantly enlarged compared to nodes draining the noninjected leg, suggesting that the immune response was localized. In accordance with this, HA-specific CD4+ T cells isolated from the draining lymph nodes, but not from the other lymph nodes, had significantly upregulated levels of CD69, an early marker of T-cell activation, 7 days after transduction (Fig. 2). This activation had disappeared by day 54. Interestingly, both vectors resulted in upregulation of the CD69 activation marker but with different kinetics. CD69 expression after rAd gene transfer peaked at day 7 and declined rapidly thereafter, whereas after rAAV-HA injection it reached a peak at 2 weeks and declined slowly thereafter. HA-specific T cells isolated from TCR-HA animals injected with either vector expressing the lacZ transgene did not upregulate CD69 above background levels, confirming the antigen specificity of T-cell activation (Fig. 2, d14 and d54). This was further confirmed in a more physiological setting by performing adoptive transfer of limiting numbers of HA-specific CD4+ T cells (2.5 × 106 cells) from TCR-HA animals into BALB/c mice and injecting the recipients 5 days later with either virus vector. Again, a significant enrichment and activation of TCR-HA-positive CD4+ T cells in the draining lymph nodes but not in the other lymph nodes was found using both vectors (data not shown).
FIG. 2.
Activation of HA-specific T cells following i.m. gene transfer. TCR-HA animals were injected i.m. with 2.5 × 1010 particles of rAd-HA, rAAV-HA, rAd-LacZ, or rAAV-LacZ or were mock injected. Single-cell suspensions from lymph nodes draining the injected muscle were prepared at different time points after gene transfer, and three-color staining was performed using CD4, 6.5 (transgenic TCR), and CD69 antibodies. The histograms show CD69 expression gated for CD4+ and 6.5+ T cells. For animals injected with rAd-HA or rAAV-HA, two mice for each vector are shown.
Different mechanisms of T-cell activation following rAAV- and rAd-mediated gene transfer.
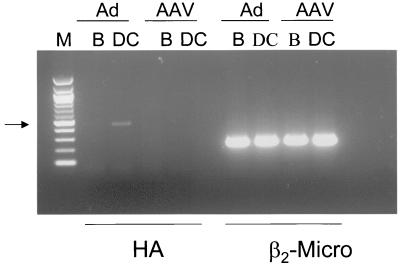
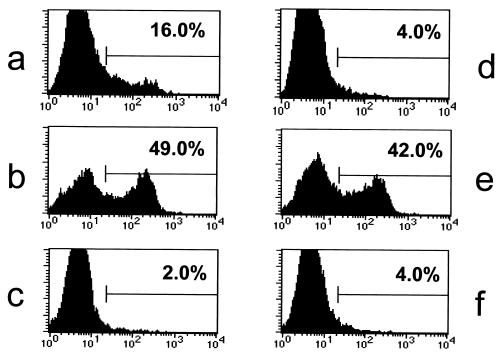
The difference observed in the kinetics of T-cell activation (Fig. 2) could be due to differences in timing of the onset of transgene expression and/or to different mechanisms of T-cell activation. Since muscle cells do not express major histocompatibility complex (MHC) class II molecules, the HA-specific CD4+ T cells must become activated by the presentation of antigen by APCs. After vector administration, APCs can acquire antigen for presentation either by being directly transduced and expressing the antigenic proteins themselves or by taking up antigen from transduced cells in the vicinity, a mechanism termed cross-presentation (1, 4, 13). In order to determine whether direct transduction of DCs was taking place in vivo, we looked at the presence of HA transcript in B cells (CD19+) and DCs (CD11c+) sorted from the draining lymph nodes of TCR-HA animals injected with either vector (Fig. 3). DCs, but not B cells, from rAd-HA-injected animals were found to be positive for the HA transcript by RT-PCR. No HA-specific signal could be detected in either B cells or DCs isolated from rAAV-HA-transduced animals. These data indicate that DCs are directly transduced in vivo after i.m. administration of rAd-HA but not after administration of rAAV-HA. In order to determine how nontransduced APCs can present the HA antigen, we used a TcH which expresses the transgenic, class II-restricted TCR-HA. Although it is well understood that this system has its limitations—TcH cells are more easily activated than are naive T cells—it is nevertheless an elegant tool for studying cross-presentation in vitro. Activation of these T cells can be monitored by measuring the expression of a lacZ gene placed under the control of the interleukin-2 promoter (5). In agreement with our in vivo data, BALB/c splenic DCs transduced with rAd-HA but not those transduced with rAAV-HA were able to activate the TcH cells and induced a fivefold lacZ expression (Fig. 4a and d). We observed a 15- to 20-fold increase in lacZ activity when the TcH cells were incubated with BALB/c DCs that had been in contact with HT-1080 cells transduced with either vector (Fig. 4b and e). As expected, no lacZ activity was detected when the TcH cells were incubated with transduced HT-1080 cells alone (Fig. 4c and f), since they do not express the appropriate MHC. These results indicate that the HA protein, or parts of it, is released from transduced cells, taken up, and efficiently presented by surrounding DCs.
FIG. 3.
RT-PCR of sorted B cells (B) and DCs (DC) following gene transfer. B cells and DCs were sorted from lymph nodes draining the muscle of TCR-HA mice which had been injected 12 days before with rAAV-HA (1011 particles, AAV) or rAd-HA (1011 particles, Ad). RNA was isolated, cDNA was generated, and RT-PCR was performed using primer pairs specific for HA or β2-microglobulin (β2-Micro). A 100-bp marker (M) was used for size estimation.
FIG. 4.
Mechanisms of CD4+ T-cell activation following gene transfer. Activation of the MHC class II-restricted HA-specific TcH cells was measured by determining lacZ expression. The TcH cells were incubated with BALB/c splenic DCs directly transduced with rAd-HA (a), directly transduced with rAAV-HA (d), coincubated with rAd-HA-transduced HT-1080 cells (b), or coincubated with rAAV-HA-transduced HT-1080 cells (e). No activation was observed when the TcH cells were incubated only with the rAd-HA- or rAAV-HA-transduced HT-1080 cells in the absence of DCs (c and f).
Interference with the cellular immune response by blocking the CD40/CD40L pathway or by inducing HA-specific tolerance.
The fact that the viral backbone of rAd, but not of rAAV, is immunogenic (16), together with the observation that rAd, but not rAAV, was capable of directly transducing DCs, suggested that successful interference with cellular immune responses generated toward transgenes encoded by rAAV vectors would be easier to achieve. We tested two approaches that interfered with the immune response at different levels: one approach was to block the T-cell–APC interaction with an anti-CD40L MAb (MR1), which has been successfully used in many studies for transiently preventing transplantation reactions (3, 24), delaying the onset of autoimmune disease (12, 32) and enhancing the persistence of rAd-mediated gene transfer (41). The second approach was to induce transgene-specific T-cell tolerance by using a protocol, previously described, which combines systemic administration of a semidepleting CD4 MAb (YTA3.1.2) and repeated doses of a chimeric immunoglobulin containing the HA peptide 110-119 (IgHA). This regimen can induce long-lasting tolerance in TCR-HA animals (23). In order to be able to determine the effect of these protocols on the activation and proliferative capacity of transgene-specific T cells upon vector administration, we performed adoptive transfer of TCR-HA T cells into BALB/c mice (described above). Recipients were sacrificed at different time points following vector administration, and HA staining on muscle fibers, as well as FACS analysis and proliferation assays of cells recovered from the lymph nodes, was performed.
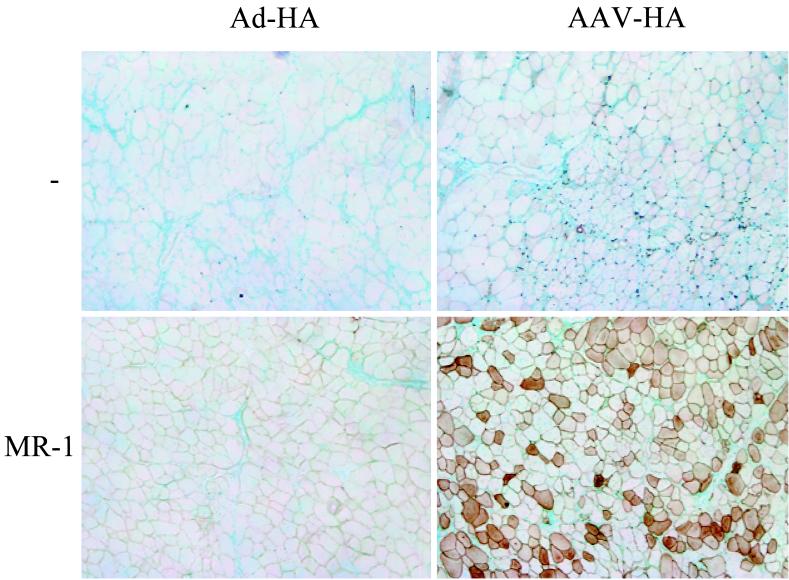
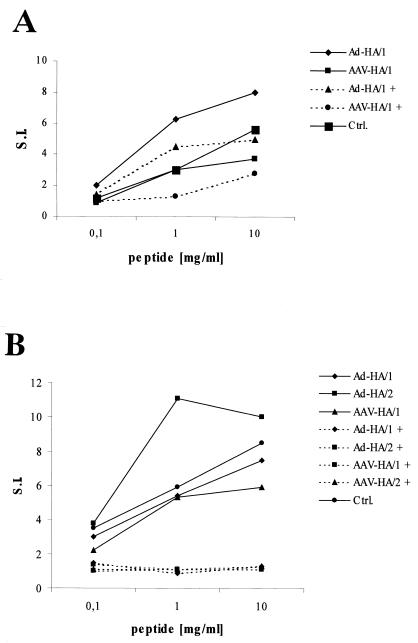
As seen in Fig. 5, MR1-treated animals injected with rAAV-HA showed stable expression of HA and no tissue infiltration 22 days after vector administration. In contrast, mice that had not received MR1 treatment did not exhibit any HA expression at that point in time. MR1 treatment did not significantly protect mice receiving rAd-HA. The protective effect of MR1 in rAAV-HA-transduced mice did not last since, by day 50, the muscles showed signs of infiltration and regenerating fibers, even though HA-positive fibers were still detectable (not shown). In order to determine whether the cells with the transgenic HA-specific TCR in MR1-treated mice had been rendered tolerant, we determined their capacity to proliferate in vitro in response to antigenic stimulation. T cells from MR1-treated mice proliferated less well in response to HA peptide than cells from untreated mice, but they still responded, especially at high concentrations of peptide (see Fig. 7A).
FIG. 5.
Interference with APC–T-cell interaction upon gene transfer. TCR-HA T cells (2.5 × 107 cells) were adoptively transferred into BALB/c animals (day −6). rAAV-HA (1011 particles, right panels) or rAd-HA (1011 particles, left panels) was injected into the tibialis anterior muscles of the recipients on day 0. Some of the animals received α-CD40L MAb (MR-1, 100 μg/injection, i.v.) on days −2, 0, 2, 4, and 12 (lower panels), whereas others remained untreated (upper panels). Muscle tissues were isolated on day 22 following gene transfer, and frozen sections were stained for HA. Original magnification, ×200.
FIG. 7.
Proliferative responses of HA-specific CD4+ T cells after MR1 or YTA3.1.2-IgHA treatment. Lymph node cells were isolated from the animals described in Fig. 5 (A) and Fig. 6 (B) and cultured with irradiated BALB/c splenocytes in the presence of HA peptide (10, 1, and 0.1 μg/ml) or medium alone. 3H incorporation was measured over the last 18 h of a 90-h culture. The counts per minute per 6.5+ CD4+ T cell were determined, and the data are presented as the fold induction over that for the medium control. S.I., stimulation index.
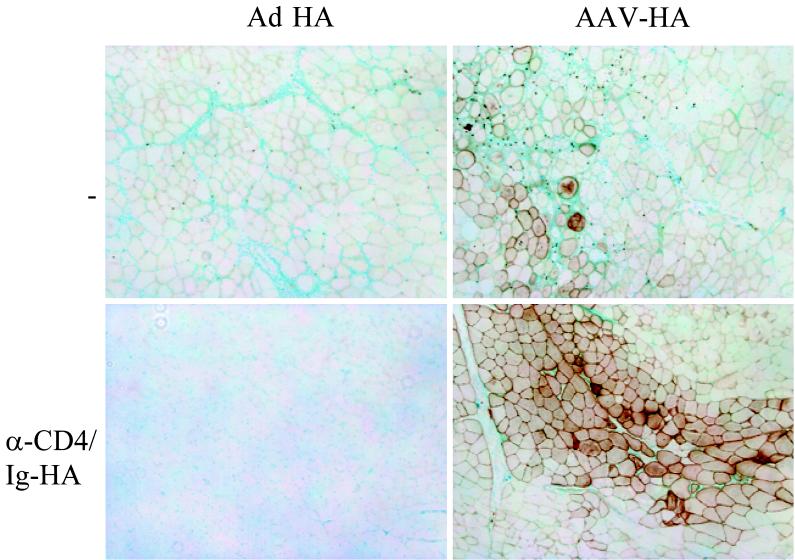
In mice that received the anti-CD4 (YTA3.1.2)-IgHA treatment, rAAV-HA was stably expressed in the muscle and no infiltration was detected 28 days after vector administration. In contrast, muscles from nontreated mice that had received rAAV-HA or from treated mice that had received rAd-HA contained few to no HA-positive fibers and were significantly infiltrated (Fig. 6). Nevertheless, TCR-HA T cells isolated from the lymph nodes of YTA3.1.2-IgHA-treated mice were incapable of responding to HA upon in vitro stimulation with different doses of peptide, indicating that they had been efficiently tolerized (Fig. 7B). Also, gamma interferon secretion by the TCR-HA T cells in response to peptide was significantly reduced in mice that had received YTA3.1.2-IgHA (not shown). By day 50, muscles from YTA3.1.2-IgHA-treated mice transduced with rAAV-HA also showed signs of infiltration, in spite of the fact that the proliferative response of the TCR-HA T cells was still completely suppressed at this point in time (not shown). This suggests that the infiltration at day 50 could be due to recent thymic emigrants from the BALB/c recipient, which were generated after tolerization. This finding needs to be verified by thymectomy. It is interesting that TCR-HA T cells from treated and rAd-HA-transduced mice were also unresponsive at all time points, indicating that the muscle infiltration and target cell destruction observed in these mice was due to an immune response directed against the viral backbone rather than against the HA. A previous study, identifying highly immunogenic proteins within the adenoviral backbone, strongly supports this hypothesis (16). This is also supported by experiments using mice expressing HA under the control of the immunoglobulin κ promoter (18), which drives transgene expression in circulating hemopoietic cells. These mice, which are tolerant for HA, tolerate rAAV-HA but not rAd-HA, indicating that the tissue destruction in the case of rAd-HA is due to an immune response directed against the viral backbone and that rAAV vectors do not break tolerance toward the transgene they encode (data not shown).
FIG. 6.
Induction of transgene-specific CD4+ T-cell tolerance before gene transfer. TCR-HA T cells (2.5 × 107 cells) were adoptively transferred into BALB/c animals (day −6). Some of the animals were treated with a semidepleting CD4 MAb (YTA3.1.2, 50 μg/injection, i.v.) on days −2 and 0, along with repeated doses of a chimeric immunoglobulin containing the HA peptide 110-119 (IgHA, 100 μg/injection) on days 0, 7, 10, and 13 (lower panels). rAAV-HA (1011 particles, right panels) or rAd-HA (1011 particles, left panels) was injected into the tibialis anterior muscles of the BALB/c animals at day 8. Muscles were isolated at day 28, and frozen sections were stained for HA. Original magnification, ×200.
DISCUSSION
It has been demonstrated in many cases that foreign proteins can be produced following gene transfer with rAAV vectors without triggering an obvious immune response (9, 11, 14, 15, 35). However, certain combinations of rAAV-borne transgene products and target tissues do result in the stimulation of immunity, implying that there is no general mechanism by which AAV blunts the immune system (2, 8, 26, 27). Here we have studied the nature of the cellular immune response against the strongly immunogenic HA protein following rAAV-mediated gene transfer and have tested whether such a response could be efficiently prevented. Using a system in which the number and activation status of antigen-specific CD4+ T cells could be precisely monitored, we observed that, in the context of rAAV vectors and, not surprisingly, of rAd vectors, HA elicited an immune response which resulted in destruction of the transduced muscle cells. With both viral vectors, the priming of antigen-specific T cells occurred only in the lymph nodes draining the injected muscle. We show that, in the case of rAd vectors, priming was due to direct transduction of DCs in vivo, as well as to cross-presentation of HA epitopes by DCs in the draining lymph nodes. In contrast, rAAV-mediated gene transfer activated T cells exclusively through cross-presentation. Whether cross-presentation is a phenomena occurring for all transgenes in the context of rAAV vectors is not clear. In some studies using rAAV vectors, the transgene has been reported to lead to the activation of the immune system, suggesting cross-presentation of the transgene product by APCs. For example, in a study by Manning et al., an rAAV vector expressing secreted herpes simplex virus glycoprotein B (gB), led to the activation of gB-specific CTLs, which were most likely activated via cross-presentation of the secreted protein by DCs. In other studies, however, stable expression of transgenes following rAAV-mediated transfer has been observed; for example, the δ-sarcoglycan gene in a hamster model of limb-girdle muscular dystrophy (11, 25, 38). One could imagine that this protein, although localized at the cell membrane, much like the HA in our study, does not lead to the activation of antigen-specific CTLs via cross-presentation because it is part of the dystrophin-associated protein complex, which may reduce its extracellular shedding and hinder efficient cross-presentation. Ongoing studies, aimed at the identification of the nature of the DC cross-presented material following rAAV- or rAd-mediated gene transfer (e.g., apoptotic bodies, exosomes, etc.), should address this point. Interestingly, we could demonstrate, by retargeting lacZ from the cytoplasm to the cell surface, that the cellular localization of the transgene product following rAAV-mediated gene transfer has an impact on subsequent immune responses. LacZ protein expressed on the cell surface—but not in the cytoplasm—activated antigen-specific CTLs, leading to efficient target cell destruction, most likely by being more accessible to APCs (manuscript in preparation).
The amount of transgene product synthesized by the transduced cells will likely influence the priming of the immune system. The expression of antigens in peripheral tissues must be relatively high to facilitate priming of naive CD8+ T cells by cross-presentation (22). Thus, the strength of promoters used in rAAV vectors will have an impact on T-cell activation, and low activity and regulatable promoters may facilitate escape from immune recognition. However, it has been shown that antigens which are weakly expressed in peripheral tissues do activate naive CD8+ T cells via cross-presentation if the target tissue is destroyed (22). This should be kept in mind, especially in cases where the tissue targeted by gene transfer is inflamed as, for instance, in muscular dystrophies, where even intracellular or nuclear transgene products may become cross-presented due to excessive cell death (31).
In the present study we demonstrate that, even if cross-presentation of the transgene in the context of gene therapy is unavoidable, one might still be able to avoid harmful cellular immune responses following gene transfer. The fact that rAd vectors transduced DCs whereas rAAV vectors did not, together with the fact that rAd vectors contain an immunogenic backbone, suggested that controlling immune responses might be easier to achieve in the context of rAAV vectors. In effect, here we show that stable gene transfer in the context of rAAV, but not rAd, vectors was facilitated by two different approaches: blocking the T-cell–APC interaction by using an MAb directed against the CD40L and inducing transgene-specific T-cell tolerance by using a combination of semidepleting CD4 MAb and a chimeric immunoglobulin containing the immunodominant HA epitope. While both prevented target cell destruction upon rAAV- but not rAd-mediated gene transfer, the latter protocol but not the former abrogated transgene-specific T-cell responses in an efficient and long-lasting manner even though it appeared to fail silencing newly produced antigen-specific T cells that, because of their low frequency, could not be detected in the proliferative assay but nevertheless caused infiltration.
In our study, MR1 did not significantly enhance rAd-HA stability, although target cell destruction was slightly delayed. While anti-CD40L has been reported in other studies to delay immune responses upon rAd-mediated gene transfer, it did not completely block them since vector-specific CTLs could still be detected (41, 42). Furthermore, even in CD40L−/− mice where transgene expression was reported to be stable 28 days after vector administration, LacZ-specific CTLs and tissue destruction were evident at later time points (K.J., unpublished results). In another study, MR1 significantly enhanced persistence of rAd-mediated gene transfer only when combined with CTLA4-Ig (19). The fact that MR1 neither induced a long-lasting effect nor induced a profound unresponsiveness of HA-specific T cells suggests that the MAb is interfering with the T-cell priming rather than changing T-cell function. Since, in our study, the immunogenic transgene product is being continuously produced, activation of T cells by APCs cross-presenting the transgene product is possible as soon as the antibody effect is gone, thus resulting in tissue destruction by day 50. This seems to be supported by our observation that two doses of MR1 were less efficient than the five-dose treatment in delaying the immune response. Thus, continuous administration of the MR1 antibody would appear necessary to achieve long-lasting rAAV-mediated gene transfer.
On the other hand, the fact that transgene-specific T cells were still unresponsive at day 50 upon α-CD4–IgHA treatment suggests effective tolerization of cells that exist at the time of treatment. The tissue infiltration observed at day 50 could be due to BALB/c thymic emigrants that were generated after the treatment. To overcome this obstacle, maintaining CD4 antibody administration would likely be sufficient since the antigen is continuously expressed by the vector.
Overall, these studies underline the importance of carefully monitoring T-cell responses in the patients enrolled in currently ongoing clinical trials and provide important information for designing new protocols aimed at interfering with the immune response, either by targeting T-cell activation or by inducing T-cell tolerance, in order to achieve long-term expression of therapeutic genes.
ACKNOWLEDGMENTS
We thank Drew Pardoll and Adam Adler for initial discussions and the HA cDNA, Walther Gerhard for the HA-specific antibody, Monika Lusky for the pTG3652 and pTG6600 plasmids, Cecile Fiamma and Otto Merten for the production of IgHA, Corinne Garcia for the cell sorting, Carole Zober for the mouse typing, the Laboratoire de Therapie Genique in Nantes for support in rAAV production, and Estelle Morillon for technical assistance.
A.S. is supported by the Juvenile Diabetes Foundation, and H.V.B. is supported by the Korber Foundation and the Juvenile Diabetes Foundation. This study was supported by the Association Française contre les Myopathies.
REFERENCES
- 1.Bevan M. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brockstedt D G, Podsakoff G M, Fong L, Kurtzman G, Mueller-Ruchholtz W, Engelman E G. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- 3.Buhlmann J, Foy T, Aruffo A, Crassi K, Ledbetter J, Green W, Xu J, Shultz L, Roopesian D, Flavell R, et al. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995;2:645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 4.Carbone F R, Kurts C, Bennet S R M, Miller J F A P, Heath W R. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 5.Casares S, Inaba K, Brumeanu T-D, Steinman R M, Bona C A. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caton A, Brownlee G. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 7.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.During M J, Symes C W, Lawlor P A, Lin J, Dunning J, Fitzsimons H L, Poulsen D, Leone P, Xu R, Dicker B L, Lipski J, Young D. An oral vaccine against NMDAR1 with efficacy in experimental stroke and epilepsy. Science. 2000;287:1453–1460. doi: 10.1126/science.287.5457.1453. [DOI] [PubMed] [Google Scholar]
- 9.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 10.Gerhard W, Haberman A, Scherle P, Taylor A, Palladino G, Caton A. Identification of eight determinants in the hemagglutinin molecule of influenza virus A/PR/8/34 (H1N1) which are recognized by class II-restricted T cells from BALB/c mice. J Virol. 1991;65:364–372. doi: 10.1128/jvi.65.1.364-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greelish J P, Su L T, Lankford I M, Burkman J M, Chen H, Konig S K, Mercier I M, Desjardins P R, Mitchell M A, Zheng X G, Leferovich J, Gao G P, Balice-Gordon R J, Wilson J M, Stedman H H. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- 12.Griggs N, Agersborg S, Noelle R, Ledbetter J, Linsley P, Tung K. The relative contribution of the CD28 and gp39 costimulatory pathways in the clonal expansion and pathogenic acquisition of self-reactive T cells. J Exp Med. 1996;183:801–810. doi: 10.1084/jem.183.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath W R, Kurts C, Miller J F A P, Carbone F R. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J Exp Med. 1998;187:1549–1553. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog R, Hagstrom J, Kung S, Tai S, Wilson J, Fisher K, High K. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzog R W, Yang E Y, Couto L B, Hagstrom J N, Elwell D, Fields P A, Burton M, Bellinger D A, Read M S, Brinkhous K M, Podsakoff G M, Nichols T C, Kurtzman G J, High K A. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 16.Jooss K, Ertl H C J, Wilson J M. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jooss K, Yang Y, Fisher K, Wilson J. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fiber. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalberer C, Reininger L, Melchers F, Rolink A. Priming of helper T cell-dependent antibody responses by hemagglutinin-transgenic B cells. Eur J Immunol. 1997;27:2400–2407. doi: 10.1002/eji.1830270939. [DOI] [PubMed] [Google Scholar]
- 19.Kay M, Meuse L, Gown A, Linsley P, Hollenbaugh D, Aruffo A, Ochs H, Wilson C. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay M A, Manno C S, Ragni M V, Larson P J, Couto L B, McClelland A, Glader B, Chew A J, Tai S J, Herzog R W, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake A W, High K A. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 21.Kirberg J, Baron A, Jakob S, Rolink A, Karjalaien K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurts C, Miller J F A P, Subramaniam R M, Carbone F R, Heath W R. Major histocompatibility complex class I-restricted cross-presentation is biased toward high-dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanoue A, Bona C, von Boehmer H, Sarukhan A. Conditions that induce tolerance in mature CD4+ T cells. J Exp Med. 1997;185:405–414. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen C, Elwood E, Alexander D, Ritchie S, Hendrix R, Tucker-Burden C, Cho H, Aruffo A, Hollenbaugh D, Linsley P, Winn K, Pearson T. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Dressman D, Tsao Y, Sakamoto A, Hoffman E, Xiao X. rAAV vector-mediated sarcoglycan gene transfer in a hamster model for limb girdle muscular dystrophy. Gene Ther. 1999;6:74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- 26.Liu D-W, Tsao Y-P, Kung J T, Ding Y-A, Sytwu H-K, Xiao X, Chen S-L. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000;74:2888–2894. doi: 10.1128/jvi.74.6.2888-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning W C, Paliard X, Zhou S, Bland M P, Lee A Y, Hong K, Walker C M, Escobedo J A, Dwarki V. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noelle R, Roy M, Sheperd D, Stamenkovic I, Ledbetter J, Aruffo A. A 39-kDa protein on activated helper T cells bind CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin S, Cobbold S, Tighe H, Benjamin R, Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987;17:1159–1165. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- 30.Snyder R. Adeno-associated virus-mediated gene delivery. J Gene Med. 1999;1:166–175. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<166::AID-JGM34>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Snyder R, Spratt S, Lagarde C, Bohl D, Kaspar B, Sloan B, Cohen L, Danos O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- 32.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathy S, Black H, Goldwasser E, Leiden J. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-deficient adenovirus vector. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 34.Vincent-Lacaze N, Snyder R, Gluzman R, Bohl D, Lagarde C, Danos O. Structure of adeno-associated virus vector DNA following transduction of the skeletal muscle. J Virol. 1999;73:1949–1955. doi: 10.1128/jvi.73.3.1949-1955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Takabe K, Bidlingmaier S M, Ill C R, Verma I M. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber S, Trannecker A, Olivery F, Gerhard W V, Karjalainen K. Specific low-affinity recognition of MHC complex plus peptide by soluble T-cell receptor. Nature. 1992;356:793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- 37.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao X, Li J, Tsao Y, Dressman D, Hoffman E, Watchko J. Full functional rescue of a complete muscle (TA) in dystrophic hamsters by adeno-associated virus vector-directed gene therapy. J Virol. 2000;74:1436–1442. doi: 10.1128/jvi.74.3.1436-1442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao X, Samulski R. Production of high-titer recombinant adeno-associated virus vector in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Jooss K U, Su Q, Ertl H C J, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 41.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Wilson J. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 43.Zaghouani H, Steinman R, Nonacs R, Shah H, Gerhard W, Bona C. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science. 1993;259:224–227. doi: 10.1126/science.7678469. [DOI] [PubMed] [Google Scholar]