Abstract
Background:
Traumatic brain injury (TBI) is one of the diseases with high disability and mortality worldwide. Recent studies have shown that TBI-related factors may change the complex balance between bleeding and thrombosis, leading to coagulation disorders. The aim of this retrospective study was to investigate the prediction of coagulopathy and subdural hematoma thickness at admission using the Glasgow Outcome Scale (GOS) in patients with severe TBI at 6 months after discharge.
Methods:
In this retrospective cohort study, a total of 1006 patients with severe TBI in large medical centers in three different provinces of China from June 2015 to June 2021 were enrolled after the exclusion criteria, and 800 patients who met the enrollment criteria were included. A receiver operating characteristic (ROC) curve was used to determine the best cut-off values of platelet (PLT), international normalized ratio (INR), activated partial thromboplastin time (APTT), and subdural hematoma (SDH) thickness. The ROC curve, nomogram, calibration curve, and the decision curve were used to evaluate the predictive effect of the coagulopathy and Coagulopathy-SDH(X1) models on the prognoses of patients with severe TBI, and the importance of predictive indicators was ranked by machine learning.
Results:
Among the patients with severe TBI on admission, 576/800 (72%) had coagulopathy, 494/800 (61%) had SDH thickness ≥14.05 mm, and 385/800 (48%) had coagulopathy combined with SDH thickness ≥14.05 mm. Multivariate logistic regression analyses showed that age, pupil, brain herniation, WBC, CRP, SDH, coagulopathy, and X1 were independent prognostic factors for GOS after severe TBI. Compared with other single indicators, X1 as a predictor of the prognosis of severe TBI was more accurate. The GOS of patients with coagulopathy and thick SDH (X1, 1 point) at 6 months after discharge was significantly worse than that of patients with coagulopathy and thin SDH (X1, 2 points), patients without coagulopathy and thick SDH (X1, 3 point), and patients without coagulopathy and thin SDH (X1, 4 points). In the training group, the C-index based on the coagulopathy nomogram was 0.900. The C-index of the X1-based nomogram was 0.912. In the validation group, the C-index based on the coagulopathy nomogram was 0.858. The C-index of the X1-based nomogram was 0.877. Decision curve analysis also confirmed that the X1-based model had a higher clinical net benefit of GOS at 6 months after discharge than the coagulopathy-based model in most cases, both in the training and validation groups. In addition, compared with the calibration curve based on the coagulopathy model, the prediction of the X1 model-based calibration curve for the probability of GOS at 6 months after discharge showed better agreement with actual observations. Machine learning compared the importance of each independent influencing factor in the evaluation of GOS prediction after TBI, with results showing that the importance of X1 was better than that of coagulopathy alone.
Conclusion:
Coagulopathy combined with SDH thickness could be used as a new, accurate, and objective clinical predictor, and X1, based on combining coagulopathy with SDH thickness could be used to improve the accuracy of GOS prediction in patients with TBI, 6 months after discharge.
Keywords: coagulopathy, Glasgow outcome scale, nomogram, prognosis, subdural hematoma, traumatic brain injury
Introduction
Highlights
To establish and validate a novel nomogram model for evaluating the 6-month prognosis of severe traumatic brain injury (TBI) patients with coagulopathy combined with subdural hematoma (SDH) thickness.
Coagulopathy combined with SDH thickness as a new indicator had better predictive performance than coagulopathy alone.
Coagulopathy combined with SDH thickness could be used as a new, accurate, and objective clinical predictor for clinical workers to evaluate prognoses.
The core of the establishment of the X1 model was to combine coagulopathy with SDH thickness and combine other indicators, which could be used to improve the accuracy of Glasgow Outcome Scale (GOS) prediction in severe TBI patients at 6 months after discharge.
Traumatic brain injury (TBI) is one of the leading causes of death and disability in the world, with more than 50 million new cases every year. More than half of the world’s population will experience TBI in their lifetime, and the world spends more than 40 billion US dollars on TBI every year1–3. At present, the main treatment methods are craniectomy, decompression surgery, and basic vital signs support4–6. However, the differences in patients’ brain injuries may lead to uncertain prognoses7, which indirectly lead to a failure to fully and accurately determine the prognoses of patients and provide appropriate diagnoses and treatment plans during the early stages of injury. It is therefore necessary to develop a model for early prediction of patient outcomes to help physicians and families make optimal decisions.
Coagulopathy is a common clinical symptom affecting the course of TBI in patients. More than 60% of patients with severe TBI will have abnormalities in routine coagulation tests on admission to the emergency department, which is more common compared with the incidence of mild TBI (<1%)8–10. The incidence of coagulopathy after TBI also increases with the severity of injury11–13. The coexistence of TBI patients with varying degrees of coagulopathy at admission is often associated with poor outcomes, with reported mortalities ranging from 17 to 86% in TBI patients with coagulopathy14. This may be related to a variety of mechanisms associated with coagulopathy after TBI, including disturbances in PLT number and function, changes in endogenous procoagulant and anticoagulant factors, endothelial cell activation, hypoperfusion, and release of inflammatory factors15. The effects of these mechanistic changes on survival and functional outcomes, and whether they might be targeted for intervention to improve prognostic outcomes, remain to be determined, suggesting that coagulopathy may be a key indicator for predicting the prognoses of patients with severe TBI.
Coagulopathy has been shown to be associated with poor outcomes in patients with severe TBI16–20. However, few studies have evaluated the role of coagulopathy in predicting outcomes at 6 months after severe TBI. In addition, clinical studies have shown that space occupying position caused by SDH after TBI was a common cause of clinical death, and the mortality of patients with TBI complicated with SDH was significantly higher than those of patients without hematomas21,22. TBI patients with intracranial hemorrhage and coagulopathy have a faster progression of intracranial hemorrhage, which is also one of the causes of death23. However, previous prediction models have not considered the importance of coagulopathy combined with SDH in predicting the prognostic outcome of severe TBI24–26. This may be one of the reasons why previous single factor prediction models have not been accurate.
In recent years, nomograms have been widely used in the prognoses of cancer patients and the prognostic models of common diseases in clinical medicine27. Nomograms are able to generate individual probabilities of clinical events by integrating different prognostic and decisive variables, thus fulfilling the need for integrated biological and clinical models and our need for personalized medicine28. The present study therefore used coagulopathy combined with SDH thickness in patients with severe TBI, to establish and validate a novel nomogram model for evaluating the 6-month prognoses of patients with severe TBI, and to compare the combined model with coagulopathy alone. Finally, this study established the importance of ranking of machine learning for the indicators of coagulopathy and X1.
Materials and methods
Patient section
This was a retrospective cohort study that collected data from a total of 1006 patients with severe TBI in large medical centers in three different provinces of China from June 2015 to June 2021. All the selected patients had not received any treatment for coagulopathy at the time of admission, and the patients who were transferred to our hospital after coagulopathy intervention from other hospitals were excluded from this study, and all the operations were performed by the trauma treatment group doctors. Inclusion criteria were the following (1): Glasgow Coma Scale (GCS) ≤8 at admission (2), isolated TBI patients, and (3) blood tests performed within 12 h after injury, in the emergency department. Exclusion criteria were the following (1): death on admission (2); patients with bilateral dilated pupil sizes, no spontaneous breathing, and no indication for surgery (3); patients with a history of cancer, consumptive disease, acute infection, atrial fibrillation, cerebral infarction, venous thrombosis, liver disease and/or hematological diseases (4); a history of taking anticoagulant drugs; and (5) among the patients transferred to our hospital after surgical treatment in other hospitals. A total of 1006 patients with severe TBI collected in this study were enrolled after the above exclusion criteria, and 800 patients who met the enrollment criteria were included. In this study, 619 patients from the 900th Hospital were selected as the training group, and 181 patients from the Affiliated Hospital of Guilin Medical University and Ganzhou People’s Hospital of Jiangxi Province were selected as the external validation group.
This study was approved by the Ethics Committee of the 900th Hospital and was conducted in accordance with the Declaration of Helsinki. Because it was a retrospective study, the ethics committee approved it without the need for patients to sign informed consent forms in accordance with national laws and institutional consent. Furthermore, the patient’s personal identifiable information was anonymized and replaced with a coding system.
Data collection and definition
Clinical characteristics of patients included the following: sex, age, blood pressure, pupil size, brain herniation, subarachnoid hemorrhage (SAH), Glasgow Coma Scale (GCS), cerebral ischemia (CI), white blood cell (WBC), neutrophil (NEU), lymphocyte (LYM), monocyte (MNC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), creatinine (CR), admission blood glucose (Glu), total protein (TP), albumin (ALB), globulin (GLB), creatine kinase (CK), creatine kinase isoenzyme (CK-MB), potassium (K), sodium (Na), calcium (Ca), fibrin degradation product (FDP), plasma D D-Dimer, plasma thrombin time (TT), fibrinogen (FIB), plasma C-reactive protein (CRP).
In the absence of a generally accepted definition of coagulopathy after TBI, coagulopathy was defined as any of the following conditions based on previous studies9,13,14,29, and the definitions and reference values proposed by local institutions and laboratories: INR >1.2, APTT >35 s, or PLT <100 000/μl. All CT and laboratory data were obtained on admission.
X1 was defined as a new combined index of coagulopathy with subdural hematoma thickness (Coagulopathy-SDH). The ROC curve showed that the thickness of the best SDH cut-off value was 14.05 mm, the thick group of SDH was ≥14.05 mm (n=494), and the thin group of SDH was <14.05 mm (n=306). Patients with coagulopathy and a thick SDH thickness were assigned X1 (1 point), those with coagulopathy and a thin SDH thickness were assigned X1 (2 points), those without coagulopathy and a thick SDH thickness were assigned X1 (3 points), and those without coagulopathy and a thin SDH thickness were assigned X1 (4 points).
Application of machine learning in clinical prediction models
The significance and role of machine learning in clinical prediction models are self-evident. First, machine learning is able to learn underlying patterns and regularities from large amounts of clinical data, thereby helping physicians to more accurately predict a patient’s disease risk, diagnosis, or treatment effect. Secondly, machine learning can automatically process complex data, including different types of clinical indicators, biomarkers, imaging data, etc., so as to provide doctors with more comprehensive and multiangle information. In addition, machine learning is capable of continuously optimizing and improving predictive models to greater accuracy and reliability. In the process of building clinical prediction models, the common machine learning steps include data preprocessing, feature selection, model selection, and evaluation. First, raw data needs to be cleaned, processed, and transformed to ensure data quality and consistency. Then, through feature selection, the feature variables that have important influence on the prediction target are screened out. Next, according to the different prediction targets, the appropriate machine learning algorithm is selected for model training, and the performance of the model is evaluated by cross-validation and other methods. Finally, the model was optimized according to its performance metrics and validated on independent datasets to verify the generalization ability and stability of the model.
In conclusion, machine learning plays an important role in clinical prediction models, which can help doctors predict and diagnose diseases more accurately and improve the treatment effect and survival rate of patients.
Follow-up
All patients were followed up regularly by the professional staff, and the prognostic function score was followed up 6 months after discharge using outpatient examinations, SMS, and telephone interviews. The GOS was used to evaluate the results. Under this scoring system, a score of 1 indicated death, 2 indicated a persistent vegetative state, 3 indicated severe disability (conscious but disabled), 4 indicated a moderate disability, and 5 indicated good recovery and return to baseline functional status. According to the GOS score at 6 months after discharge, the patients were divided into good prognosis (GOS >3) and poor prognosis groups (GOS ≤3). The work has been reported in line with the strengthening the reporting of cohort, cross-sectional, and case–control studies in surgery (STROCSS) criteria30.
Statistical analysis
Data analysis was performed using SPSS27.0 (SPSS). R Studio (version 4.2.2) (R Foundation for Statistical Computing) was used for nomogram drawing, C-index calculations, calibration plots, and DCA drawing. The XGBoost package was used for machine learning. Prism 9.5.0 (Graphpad) was used to draw bar charts and ROC curves. The Kolmogorov–Smirnow test was used to determine the normality of the data. Data consistent with a normal distribution were expressed as the mean±SD and compared between groups using the t-test. Measurement data not consistent with a normal distribution were expressed as the median and quartile, and compared between groups using the rank-sum test. Count data were expressed as a percentage n (%), and the χ 2 test or Fisher’s test was used for comparison between groups. The ROC curve was used to calculate the optimal cut-off values of the INR, APTT, PLT, and SDH thickness, and the endpoint was based on the patient’s GOS score at 6 months after discharge. Binary logistic analysis was performed on factors that showed significance using univariate analysis, to evaluate independent predictors of poor outcomes in patients with severe TBI. All tests were bidirectional, and P<0.05 was considered significant. Factors with P<0.05 using univariate analysis were included in multivariate analysis. All statistical analyses were performed using a two-sided test, and P<0.05 was considered statistically significant. A small number of missing data were deleted.
Results
Relationship between clinical characteristics and outcomes in patients with severe TBI
A total of 1006 patients with severe TBI in three large hospitals from June 2015 to June 2021 were collected in this study, of which 206 patients did not meet the inclusion criteria and were excluded from the study, and 800 patients were included for analysis. The flow chart of patient selection is shown in Figure 1. There were 169 females (21.1%) and 631 males (78.9%), and the age [interquartile range (IQR)] of the cohort was 50 years (35–63 years). The follow-up time was 6 months after discharge. The median GOS IQR was 3 (1–5), 344 patients (41.8%) died, and 456 patients (58.2%) survived. The relationship between clinical characteristics and outcomes in patients with severe TBI is shown in Table 1.
Figure 1.
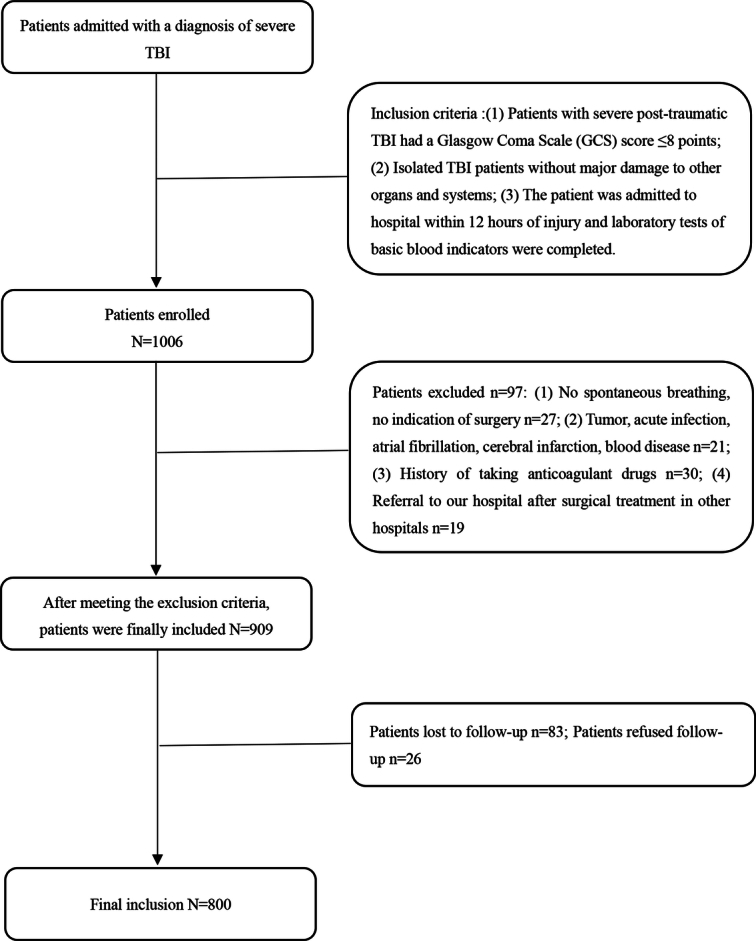
Flowchart for patient selection.
Table 1.
The relationship between clinical characteristics and prognosis in patients with severe traumatic brain injury.
| Variables | Classification | GOS>3 | GOS≤3 |
|---|---|---|---|
| Sex | Male | 292 (80.2%) | 339 (77.8%) |
| Female | 72 (19.8%) | 97 (22.2%) | |
| Miss | 0 | 0 | |
| Age | 44.78±20.006 | 51.98±18.286 | |
| Miss | 0 | 0 | |
| Hypertension | Y | 67 (18.4%) | 136 (31.2%) |
| N | 297 (81.6%) | 300 (68.8%) | |
| Miss | 0 | 0 | |
| Pupil | Unequal | 64 (17.6%) | 230 (52.8%) |
| Equal | 300 (82.4%) | 206 (47.2%) | |
| Miss | 0 | 0 | |
| SAH | Y | 210 (57.7%) | 299 (68.6%) |
| N | 154 (42.3%) | 136 (31.2%) | |
| Miss | 0 | 1 | |
| Brain herniation | Y | 30 (8.2%) | 280 (64.2%) |
| N | 334 (91.8%) | 156 (35.8%) | |
| Miss | 0 | 0 | |
| CI | Y | 9 (2.5%) | 31 (7.1%) |
| N | 354 (97.3%) | 405 (92.9%) | |
| Miss | 1 | 0 | |
| WBC | 13.25 (10.6075–17.62) | 18.185 (14.395–22.975) | |
| Miss | 0 | 6 | |
| NEU | 11.22 (8.7425–14.9725) | 15.805 (12.242–20.4125) | |
| Miss | 0 | 6 | |
| LYM | 1.17 (0.85–1.71) | 1.21 (0.8375–1.8825) | |
| Miss | 0 | 6 | |
| MON | 0.57 (0.34–0.78) | 0.71 (0.41–1.03) | |
| Miss | 0 | 6 | |
| MCV | 89 (85.525–92.2) | 89.7 (86.9–92.5) | |
| Miss | 0 | 6 | |
| MCH | 30.5 (29.1–31.7) | 30.3 (29.3–31.4) | |
| Miss | 0 | 6 | |
| CR | 68 (56.1–78) | 79 (64–98.15) | |
| Miss | 0 | 7 | |
| GLU | 7.78 (6.2075–9.3475) | 10.2 (7.975–13.75) | |
| Miss | 0 | 7 | |
| TP | 68 (63–73.475) | 65.2 (56.05–72) | |
| Miss | 0 | 11 | |
| ALB | 41 (37.525–44) | 39 (33–43) | |
| Miss | 0 | 11 | |
| GLB | 24.95 (21.7–29.3) | 24 (19.4–28.7) | |
| Miss | 142 | 190 | |
| CK | 311 (140.1–605) | 410 (207.5–892.75) | |
| Miss | 13 | 28 | |
| CK-MB | 21 (13.5–36) | 37 (20–71.75) | |
| Miss | 13 | 28 | |
| K | 3.9 (3.6–4.2) | 3.72 (3.325–4.195) | |
| Miss | 0 | 7 | |
| Na | 138.6 (136–141) | 139 (136–142.2) | |
| Miss | 0 | 7 | |
| Ca | 2.16 (2.07–2.26) | 2.1 (1.96–2.22) | |
| Miss | 0 | 7 | |
| FDP | 40.4 (16.2–120) | 120 (62.4–211.535) | |
| Miss | 43 | 71 | |
| D-Dimer | 12.805 (4.3325–35.2) | 35.2 (12.735–35.2) | |
| Miss | 10 | 27 | |
| PT | 11.9 (11.3–12.5) | 12.5 (11.5–13.9) | |
| Miss | 5 | 27 | |
| TT | 17.5 (16.4–18.9) | 18.7 (16.6–21.2) | |
| Miss | 5 | 27 | |
| FIB | 2.2 (1.67–3.15) | 1.88 (1.2–2.955) | |
| Miss | 5 | 27 | |
| CRP | 7.2 (7.2–30.95) | 26.5 (7.2–73.6) | |
| Miss | 36 | 42 | |
| SDH | High (≥14.05) | 139 (38.2%) | 355 (81.4%) |
| Low (<14.05) | 225 (61.8%) | 81 (18.6%) | |
| Miss | 0 | 0 | |
| Coagulopathy | Yes | 218 (59.9%) | 358 (82.1%) |
| No | 144 (39.6%) | 68 (15.6%) | |
| Miss | 2 | 10 | |
| X1 | 1 point | 88 (24.2%) | 297 (68.1%) |
| 2 points | 50 (13.7%) | 50 (11.5%) | |
| 3 points | 130 (35.7%) | 61 (14%) | |
| 4 points | 94 (25.8%) | 18 (4.1%) | |
| Miss | 2 | 10 |
ALB, albumin; APTT, activated partial thromboplastin time; CI, cerebral ischemic; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; Cr, creatinine; CRP, C-reactive protein; FDP, fibrin degradation products; FIB, Fibrinogen; GLB, globulin; Glu, glucose; INR, International Normalized Ratio; LYM, lymphocyte; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MON, monocyte; NEU, neutrophile; PT, Prothrombin time; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; TP, total protein; TT, thrombin time; WBC, white blood cell; X1 (1 point), patients with coagulopathy and thick SDH thickness; X1 (2 points), patients with coagulopathy and thin SDH thickness; X1 (3 points), patients without coagulopathy and with thick SDH thickness; X1 (4 points), patients without coagulopathy and with thin SDH thickness; X1, coagulopathy combined with subdural hematoma thickness.
All data were analyzed using the chi-square test, rank-sum test, and Fisher’s precision probability test, and P<0.05 was considered to indicate statistically significant differences.
Relationships among coagulopathy, SDH thickness, X1, and GOS scores at 6 months after discharge in patients with severe TBI
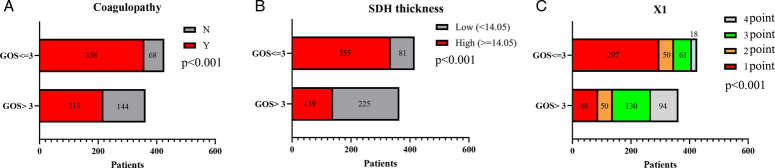
The GOS significantly differed from coagulopathy, SDH thickness and X1 in patients with severe TBI (P<0.001). Patients with GOS ≤3 tended to have coagulopathy (83%), high SDH thickness (81%), and X1 (1 point, 88%). Patients with GOS >3 showed opposite trends (Fig. 2 and Table 2). CT images of SDH thickness with coagulopathy after TBI in four patients were selected as representative. Figure 3A, B are typical CT images of patients with GOS >3, and Fig. 3C, D are typical CT images of patients with GOS ≤3.
Figure 2.
Relationships among coagulopathy, SDH thickness, X1 and GOS scores at 6 months after discharge in patients with severe TBI. (A) Patients with coagulopathy had poorer GOS scores than patients without coagulopathy (P<0.001). (B) Patients with high SDH thickness ≥14.05 mm had poorer GOS scores than patients with low SDH thickness <14.05 mm (P<0.001). (C) The proportion of patients with GOS score >3 was analyzed. X1 (4 points, 84%) > X1 (3 points, 68%) > X1 (2 points, 50%) > X1 (1 point, 23%) (P<0.001). X1 (1 point), patients with coagulopathy and thick SDH thickness; X1 (2 points), patients with coagulopathy and thin SDH thickness; X1 (3 points), patients without coagulopathy and with thick SDH thickness; and X1 (4 points), patients without coagulopathy but with a thin SDH thickness.
Table 2.
The relationship between the Coagulopathy, SDH thickness and X1, and GOS score in severe TBI patients.
| Coagulopathy | SDH thickness | X1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | High (≥14.05) | Low (<14.05) | 1 point | 2 points | 3 points | 4 points | ||||
| Variables | n=576 | n=212 | P | n=494 | n=306 | P | n=485 | n=100 | n=191 | n=112 | P |
| GOS | <0.001 | <0.001 | <0.001 | ||||||||
| >3 | 218 | 144 | 139 | 225 | 88 | 50 | 130 | 94 | |||
| ≤3 | 358 | 68 | 355 | 81 | 297 | 50 | 61 | 18 | |||
X1 (1 point), patients with coagulopathy and thick SDH thickness; X1 (2 points), patients with coagulopathy and thin SDH thickness; X1 (3 points), patients without coagulopathy and with thick SDH thickness; X1 (4 points), patients without coagulopathy and with thin SDH thickness; X1, coagulopathy combined with subdural hematoma thickness.
All data were analyzed using the χ 2 test, and P<0.05 was considered to indicate statistically significant differences.
Figure 3.
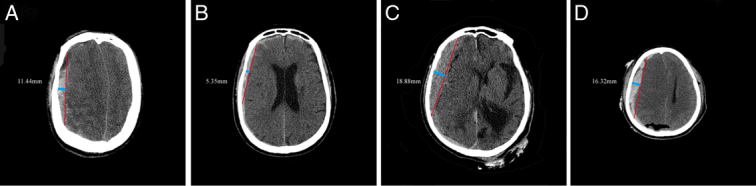
Typical CT images of coagulopathy with subdural hematoma after traumatic brain injury. (A, B) Prognosis of the patient was Glasgow Outcome Scale score >3, and the widths of the final hematoma were 11.44 mm and 5.35 mm. (C, D) Prognosis of the patient was GOS score ≤3, and the widths of the final hematoma was 18.88 mm and 16.32 mm.
Univariate and multivariate analysis of factors influencing GOS scores in patients with severe TBI
Univariate logistic regression analysis showed that age, hypertension, pupil size, SAH, brain herniation, CI, WBC, NEU, LYM, CR, Glu, TP, ALB, CK-MB, Na, Ca, FDP, D-Dimer, TT, FIB, CRP, SDH, coagulopathy, X1 (1 point), X1 (2 points), and X1 (3 points) were important prognostic factors affecting GOS scores in patients with severe TBI (Table 3).
Table 3.
Univariate logistic regression models used to analyze the factors influencing GOS in patients with severe traumatic brain injury.
| Variable | B | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Sex | −0.149 | 0.395 | 0.862 | 0.612 | 1.214 |
| Age | 0.020 | <0.001 | 1.020 | 1.012 | 1.027 |
| Hypertension | 0.698 | <0.001 | 2.010 | 1.439 | 2.805 |
| Pupil | 1.655 | <0.001 | 5.234 | 3.767 | 7.272 |
| SAH | 0.478 | 0.001 | 1.612 | 1.206 | 2.156 |
| Brain herniation | 2.995 | <0.001 | 19.983 | 13.106 | 30.467 |
| CI | 1.102 | 0.004 | 3.011 | 1.414 | 6.410 |
| WBC | 0.113 | <0.001 | 1.120 | 1.091 | 1.149 |
| NEU | 0.088 | <0.001 | 1.092 | 1.064 | 1.120 |
| LYM | 0.166 | 0.038 | 1.181 | 1.010 | 1.381 |
| MON | −0.005 | 0.843 | 0.995 | 0.952 | 1.041 |
| MCV | 0.016 | 0.073 | 1.016 | 0.998 | 1.035 |
| MCH | −0.008 | 0.282 | 0.992 | 0.977 | 1.007 |
| CR | 0.017 | <0.001 | 1.017 | 1.011 | 1.023 |
| GLU | 0.272 | <0.001 | 1.313 | 1.245 | 1.386 |
| TP | −0.036 | <0.001 | 0.965 | 0.952 | 0.978 |
| ALB | −0.070 | <0.001 | 0.932 | 0.911 | 0.954 |
| GLB | −0.029 | 0.052 | 0.972 | 0.944 | 1.000 |
| CK | 0.000 | 0.058 | 1.000 | 1.000 | 1.000 |
| CK-MB | 0.016 | <0.001 | 1.016 | 1.011 | 1.021 |
| K | −0.143 | 0.194 | 0.867 | 0.699 | 1.075 |
| Na | 0.044 | 0.002 | 1.045 | 1.016 | 1.074 |
| Ca | −2.530 | <0.001 | 0.080 | 0.034 | 0.185 |
| FDP | 0.006 | <0.001 | 1.006 | 1.004 | 1.007 |
| D-Dimer | 0.052 | <0.001 | 1.053 | 1.041 | 1.065 |
| PT | 0.051 | 0.111 | 1.052 | 0.988 | 1.120 |
| TT | 0.102 | <0.001 | 1.108 | 1.058 | 1.159 |
| FIB | −0.211 | <0.001 | 0.810 | 0.725 | 0.905 |
| CRP | 0.011 | <0.001 | 1.011 | 1.007 | 1.015 |
| SDH | 1.959 | <0.001 | 7.094 | 5.147 | 9.778 |
| Coagulopathy | 1.246 | <0.001 | 3.478 | 2.490 | 4.856 |
| X1 (1point) | 2.869 | <0.001 | 17.625 | 10.092 | 30.780 |
| X1 (2point) | 1.653 | <0.001 | 5.222 | 2.757 | 9.891 |
| X1 (3point) | 0.896 | 0.003 | 2.450 | 1.360 | 4.416 |
ALB, albumin; APTT, activated partial thromboplastin time; CI, cerebral ischemic; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; Cr, creatinine; CRP, C-reactive protein; FDP, fibrin degradation products; FIB, Fibrinogen; GLB, globulin; Glu, glucose; INR, international normalized ratio; LYM, lymphocyte; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MON, monocyte; NEU, neutrophile; PT, Prothrombin time; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; TP, total protein; TT, thrombin time; WBC, white blood cell; X1, coagulopathy combined with subdural hematoma thickness.
P<0.05 was considered to indicate statistically significant differences.
Before the X1 index was established, multivariate logistic regression analysis showed that age [odds ratio (OR) = 1.038, 95% CI: 1.020−1.056, P<0.001), pupil size (OR: 2.122, 95% CI: 1.194−3.770, P=0.010), brain herniation (OR: 8.035, 95% CI: 4.183−15.506, P<0.001), WBC (OR: 1.012, 95% CI: 1.026−1.185, P=0.008), CRP (OR: 1.009, 95% CI: 1.003−1.014, P=0.003), SDH (OR: 4.151, 95% CI: 2.465−6.991, P<0.001), and coagulopathy (OR: 2.647, 95% CI: 1.488−4.709, P=0.001) were independent prognostic factors for severe TBI (Table 4).
Table 4.
Multivariate logistic regression models used to analyze the factors influencing GOS in patients with severe traumatic brain injury.
| Variable | B | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Age | 0.037 | <0.001 | 1.038 | 1.020 | 1.056 |
| Pupil | 0.752 | 0.010 | 2.122 | 1.194 | 3.770 |
| Brain herniation | 2.086 | <0.001 | 8.053 | 4.183 | 15.506 |
| WBC | 0.097 | 0.008 | 1.102 | 1.026 | 1.185 |
| CRP | 0.009 | 0.003 | 1.009 | 1.003 | 1.014 |
| SDH | 1.423 | <0.001 | 4.151 | 2.465 | 6.991 |
| Coagulopathy | 0.973 | 0.001 | 2.647 | 1.488 | 4.709 |
CRP, C-reactive protein; WBC, white blood cell.
P<0.05 was considered to indicate statistically significant differences.
After the X1 index was established, factors significantly related to the prognoses of severe TBI patients were included in the multiple regression analyses. The results showed that age (OR: 1.039, 95% CI: 1.021−1.057, P<0.001), pupil size (OR: 2.071, 95% CI: 1.156−3.710, P=0.014), brain herniation (OR: 7.905, 95% CI: 4.084−15.300, P<0.001), WBC (OR: 1.099, 95% CI: 1.024−1.180, P=0.009), CRP (OR: 1.009, 95% CI: 1.003−1.015, P=0.002), X1 (1 point) (OR: 8.807, 95% CI: 3.989−19.444, P<0.001) were independent prognostic factors in patients with severe TBI (Table 5). We found that X1: coagulopathy combined with subdural hematoma thickness was an independent prognostic factor affecting GOS in patients with severe TBI.
Table 5.
Multivariate logistic regression models used to analyze the factors influencing GOS in patients with severe traumatic brain injury.
| Variable | B | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Age | 0.038 | <0.001 | 1.039 | 1.021 | 1.057 |
| Pupil | 0.728 | 0.014 | 2.071 | 1.156 | 3.710 |
| Brain herniation | 2.067 | <0.001 | 7.905 | 4.084 | 15.300 |
| WBC | 0.094 | 0.009 | 1.099 | 1.024 | 1.180 |
| CRP | 0.009 | 0.002 | 1.009 | 1.003 | 1.015 |
| X1(1point) | 2.176 | <0.001 | 8.807 | 3.989 | 19.444 |
CRP, C-reactive protein; WBC, white blood cell; X1 (1 point), patients with coagulopathy and thick SDH; X1, coagulopathy combined with subdural hematoma thickness.
P<0.05 was considered to indicate statistically significant differences.
The predictive value of X1 in patients with severe TBI, and determination of the best cut-off value
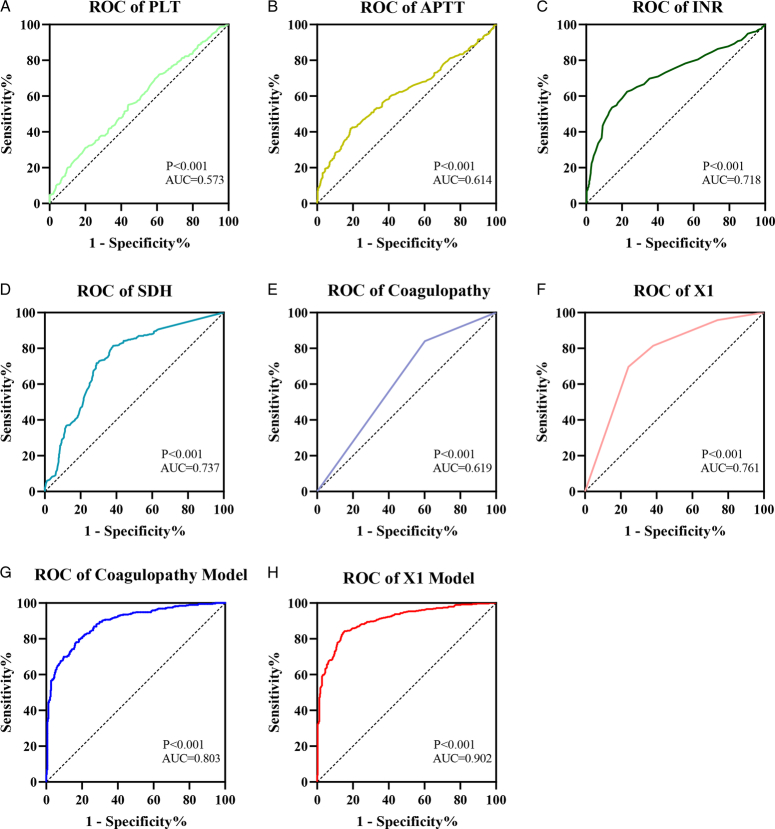
The ROC curve was constructed with the PLT, INR, APTT, and SDH thickness, with coagulopathy and X1 as the independent variables and GOS score at 6 months after discharge as the dependent variable. The results showed that the optimal cut-off values of PLT, INR, APTT, and SDH thickness were 182.5, 1.105, 30.05, and 14.05, respectively. In addition, the area under the curve (AUC) of PLT was 0.573 (95% CI: 0.534−0.613), APTT 0.614 (95% CI: 0.575−0.654), INR 0.718 (95% CI: 0.682−0.755), SDH 0.737 (95% CI: 0.682−0.755), 0.702−0.773), coagulopathy 0.619 (95% CI: 0.579−0.659), and X1 0.761 (95% CI: 0.727−0.796). The results showed that X1 had the highest AUC value, indicating that X1 was more accurate than the other independent indicators, which was beneficial to the prognostic evaluation of severe TBI. The AUC of the coagulopathy model was 0.803, and that of the X1 model 0.902. The coagulopathy model included age, pupil size, cerebral hernia, WBC, CRP, and coagulopathy. The X1 model included age, pupil size, cerebral hernia, WBC, CRP, and X1, which indicated that in the prediction model, the joint prediction model of X1 constructed by our group was better than the traditional coagulopathy model (Fig. 4).
Figure 4.
Area under the curve (AUC) of the PLT, INR, APTT, SDH thickness, coagulopathy, and X1. (A) The AUC of the PLT was 0.573. (B) The AUC of APTT was 0.614. (C) The AUC of INR was 0.718. (D) The AUC of SDH was 0.737. (E) The AUC of coagulopathy was 0.619. (F) The AUC of X1 was 0.761. (G) The AUC of the coagulopathy model was 0.803. (H) The AUC the curve of the X1 Model was 0.902.
In the prediction of GOS prognoses in patients with severe TBI, the prediction accuracy and predictive value of the X1 model were higher than those of the traditional coagulopathy model.
The basic clinical characteristics of the training and validation groups were compared
The three hospitals had long discussed the treatment and care of patients, and formulated consistent nursing principles. A total of 619 patients from our hospital (the 900th Hospital) who met the criteria were used as the training group (n=619), and 181 patients from two centers of Guilin Medical University Affiliated Hospital and Ganzhou People’s Hospital of Jiangxi Province who met the criteria, were used as the external validation group (n=181). The baseline characteristic data of the patients in the training group were basically the same as those in the validation group, and there was no significant difference between the two groups (P>0.05), which indirectly proved that the clinical characteristics data of this study were not affected by different central sources (Table 6).
Table 6.
The baseline characteristics of the patients in the training and validation groups were compared.
| Variable | Classification | Training | Validation | P |
|---|---|---|---|---|
| Sex | Male | 497 (80.3%) | 134 (74%) | 0.07 |
| Female | 122 (19.7%) | 47 (26%) | ||
| Miss | 0 | 0 | ||
| Age | 48.47±19.676 | 49.51±18.5 | 0.527 | |
| Miss | 0 | 0 | ||
| Hypertension | Y | 156 (25.2%) | 47 (26%) | 0.835 |
| N | 463 (74.8%) | 134 (74%) | ||
| Miss | 0 | 0 | ||
| Pupil | Unequal | 221 (35.7%) | 73 (40.3%) | 0.256 |
| Equal | 398 (64.3%) | 108 (59.7%) | ||
| Miss | 0 | 0 | ||
| SAH | Y | 392 (63.3%) | 117 (64.6%) | 0.766 |
| N | 226 (36.5%) | 64 (35.4%) | ||
| Miss | 1 | 0 | ||
| Brain herniation | Y | 239 (38.6%) | 71 (39.2%) | 0.881 |
| N | 380 (61.4%) | 110 (60.8%) | ||
| Miss | 0 | 0 | ||
| CI | Y | 34 (5.5%) | 6 (3.3%) | 0.235 |
| N | 584 (94.3%) | 175 (96.7%) | ||
| Miss | 1 | 0 | ||
| WBC | 16.135 (12.015–20.5275) | 16.205 (11.99–20.945) | 0.974 | |
| Miss | 5 | 1 | ||
| NEU | 13.715 (9.8175–18.145) | 13.66 (10.155–18.1025) | 0.93 | |
| Miss | 5 | 1 | ||
| LYM | 1.21 (0.85–1.84) | 1.14 (0.82–1.7975) | 0.451 | |
| Miss | 5 | 1 | ||
| MON | 0.63 (0.38–0.92) | 0.63 (0.4–0.9475) | 0.593 | |
| Miss | 5 | 1 | ||
| MCV | 89.4 (86.4–92.5) | 89.3 (86.6–92.05) | 0.697 | |
| Miss | 5 | 1 | ||
| MCH | 30.3 (29.2–31.6) | 30.4 (29.4–31.375) | 0.741 | |
| Miss | 5 | 1 | ||
| CR | 72 (59.5–90) | 74 (61.25–87) | 0.779 | |
| Miss | 6 | 1 | ||
| GLU | 8.8 (6.98–11.45) | 9.17 (7.125–12.1075) | 0.219 | |
| Miss | 6 | 1 | ||
| TP | 67 (59.9–73) | 66.15 (58.525–73) | 0.494 | |
| Miss | 8 | 3 | ||
| ALB | 40 (36–43.6) | 39.9 (34.825–44) | 0.352 | |
| Miss | 8 | 3 | ||
| GLB | 24.5 (20.8–29.075) | 24.1 (20.375–28.825) | 0.387 | |
| Miss | 255 | 77 | ||
| CK | 367 (174–756) | 348.5 (191.5–803) | 0.682 | |
| Miss | 30 | 11 | ||
| CK-MB | 27 (17–51) | 33.4 (17–59.25) | 0.082 | |
| Miss | 30 | 11 | ||
| K | 3.81 (3.5–4.2) | 3.8 (3.343–4.1) | 0.127 | |
| Miss | 6 | 1 | ||
| Na | 138.8 (136–142) | 139 (136.525–141.1) | 0.697 | |
| Miss | 6 | 1 | ||
| Ca | 2.13 (2.02–2.24) | 2.14 (2.01–2.2375) | 0.916 | |
| Miss | 6 | 1 | ||
| FDP | 86.7 (27.25–152.0425) | 119.8 (26.225–154.425) | 0.596 | |
| Miss | 87 | 27 | ||
| D-Dimer | 24.14 (7.48–35.2) | 25.855 (6.67–35.2) | 0.728 | |
| Miss | 28 | 9 | ||
| PT | 12.1 (11.4–13.2) | 12 (11.4–13.1) | 0.799 | |
| Miss | 25 | 7 | ||
| TT | 18 (16.5–19.9) | 18.15 (16.4–20.125) | 0.584 | |
| Miss | 25 | 7 | ||
| FIB | 2.04 (1.46–2.995) | 1.995 (1.5075–3.095) | 0.944 | |
| Miss | 25 | 7 | ||
| CRP | 12.85 (7.2–47.325) | 18.75 (7.2–62.775) | 0.094 | |
| Miss | 65 | 13 | ||
| Miss | 25 | 7 | ||
| SDH | High (≥14.05) | 387 (62.5%) | 107 (59.1%) | 0.407 |
| Low (<14.05) | 226 (36.5%) | 74 (40.9%) | ||
| Miss | 0 | 0 | ||
| Coagulopathy | Y | 438 (70.8%) | 138 (76.2%) | 0.17 |
| N | 171 (27.6%) | 41 (22.7%) | ||
| Miss | 10 | 2 | ||
| X1 | 1point | 298 (48.1%) | 87 (48.1%) | 0.347 |
| 2 points | 82 (13.2%) | 18 (9.9%) | ||
| 3 points | 140 (22.6%) | 51 (28.2%) | ||
| 4 points | 89 (14.4%) | 23 (12.7%) | ||
| Miss | 10 | 2 |
ALB, albumin; APTT, activated partial thromboplastin time; CI, cerebral ischemic; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; Cr, creatinine; CRP, C-reactive protein; FDP, fibrin degradation products; FIB, Fibrinogen; GLB, globulin; Glu, glucose; INR, International Normalized Ratio; LYM, lymphocyte; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MON, monocyte; NEU, neutrophile; PT, Prothrombin time; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; TP, total protein; TT, thrombin time; WBC, white blood cell; X1 (1 point), patients with coagulopathy and thick SDH thickness; X1 (2 points), patients with coagulopathy and thin SDH thickness; X1 (3 points), patients without coagulopathy and with thick SDH thickness; X1 (4 points), patients without coagulopathy and with thin SDH thickness; X1, coagulopathy combined with subdural hematoma thickness.
All data were analyzed using the chi-square test, rank-sum test, and Fisher’s precision probability test, and P<0.05 was considered to indicate statistically significant differences.
Comparison of the predictive performance of the nomogram based on the coagulopathy model and the nomogram based on the coagulopathy-hematoma thickness (X1) model
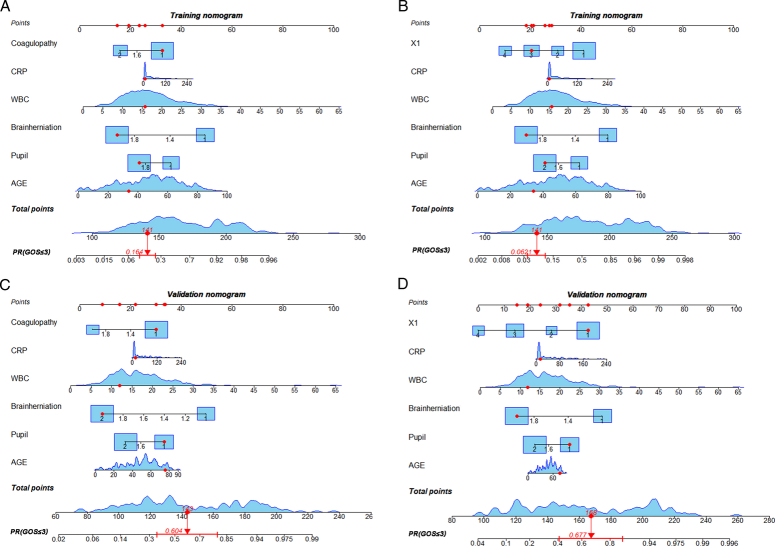
To predict the GOS score of severe TBI patients at 6 months after discharge, based on the results of the multivariate analysis regression model, the coagulopathy, age, pupil size abnormality, cerebral hernia, WBC, and CRP were used to construct the nomogram in Figure 5A, B for the training group and Figure 5C, D for the validation group. In the training group, the C-index of the nomogram based on the coagulopathy model (including age, pupil size, brain herniation, WBC, CRP, and coagulopathy) was 0.900. The C-index of the nomogram based on the X1 model (including age, pupil size, brain herniation, WBC, CRP, and X1) was 0.912 (Fig. 6A). In the validation group, the C-index of the nomogram based on the coagulopathy model (including age, pupil size, brain herniation, WBC, CRP, and coagulopathy) was 0.858. The C-index of the nomogram based on the X1 model (including age, pupil size, brain herniation, WBC, CRP, and X1) was 0.877 (Fig. 6B).
Figure 5.
Nomograms based on the coagulopathy and coagulopathy-hematoma thickness (X1) models were compared in the training and validation groups. (A) Coagulopathy-based nomograms in the training group (B) X1-based nomograms in the training group. (C) Coagulopathy-based nomograms in the validation group. (D) X1-based nomograms in the validation group.
Figure 6.
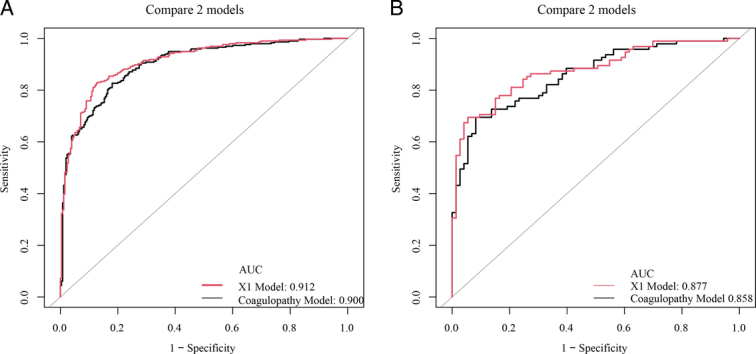
The C-index based on the coagulopathy and coagulopathy-hematoma thickness (X1) models were compared in the training and validation groups. (A) The C-index of coagulopathy was compared with the C-index of X1 in the training group. (B) The C-index of coagulopathy was compared with the C-index of X1 in the validation group.
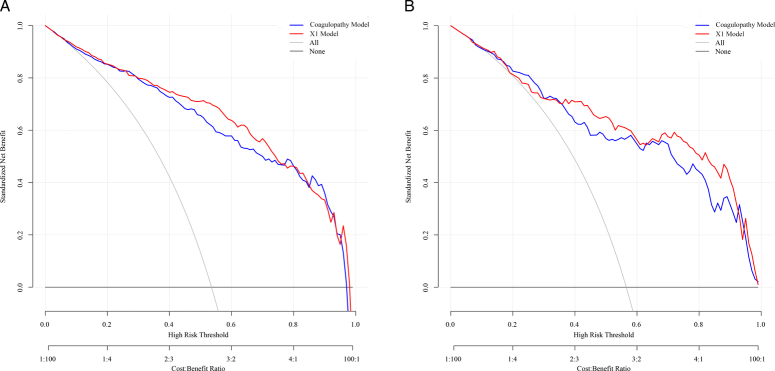
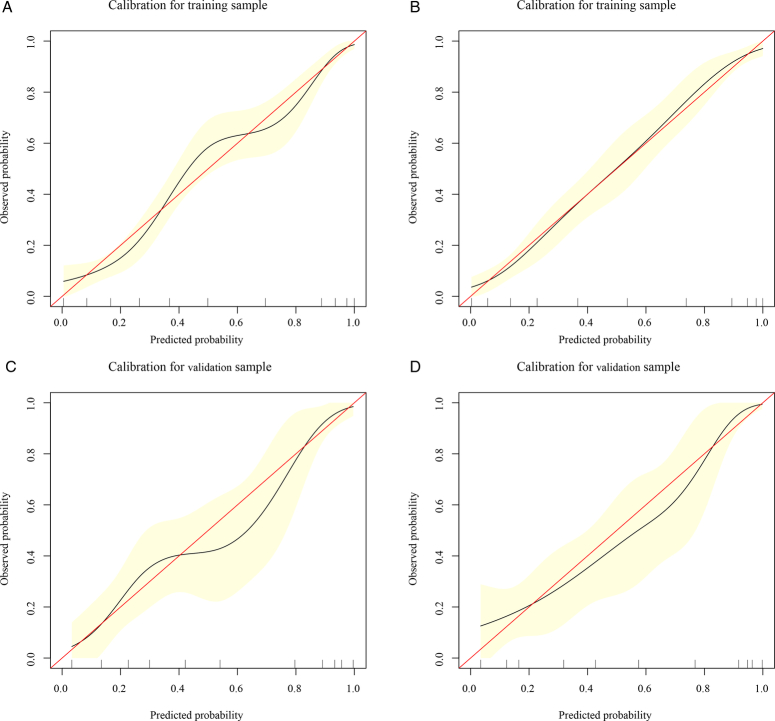
DCA showed that both in the training and validation groups, the clinical net benefit of GOS at 6 months after discharge of the nomogram based on the X1 model was better than that based on the coagulopathy model in most cases (Fig. 7). In addition, compared with the calibration curve of the nomogram based on the coagulopathy model, prediction of the nomogram calibration curve based on the X1 model for the probability of GOS at 6 months after discharge showed better consistency with the actual observation results (Fig. 8). Based on the above results, the C-index, DCA, and calibration curve of the nomogram based on the X1 model were more accurate than the nomogram based on the coagulopathy model in both the training and validation groups, which indicated that the X1 model constructed by our group was a reliable indicator for predicting the GOS at 6 months after discharge, and could be used as a clinical tool to predict the outcome of severe TBI.
Figure 7.
The decision curve analysis (DCA) based on the coagulopathy model and coagulopathy-hematoma thickness (X1) models were compared in the training and validation groups. (A) DCA of the X1 model was compared with DCA of the coagulopathy model in the training group. (B) DCA of the X1 model was compared with DCA of the coagulopathy model in the validation group.
Figure 8.
The decision curve analysis based on the coagulopathy and coagulopathy-hematoma thickness (X1) models were compared in the training and validation groups. (A) Calibration curve analysis of the coagulopathy model in the training group. (B) Calibration curve analysis of the X1 model in the training group. (C) Calibration curve analysis of the coagulopathy model in the validation group. (D) Calibration curve analysis of the X1 model in the validation group.
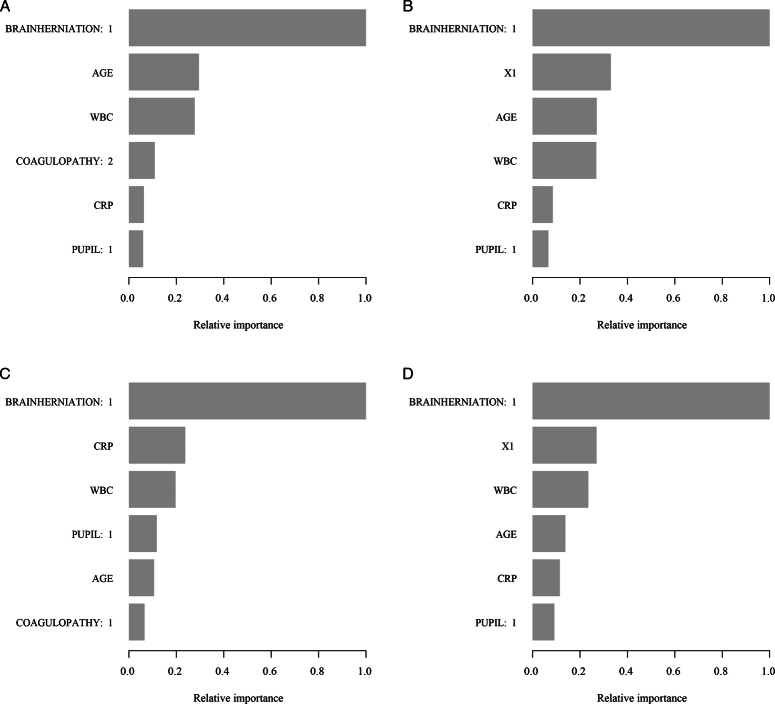
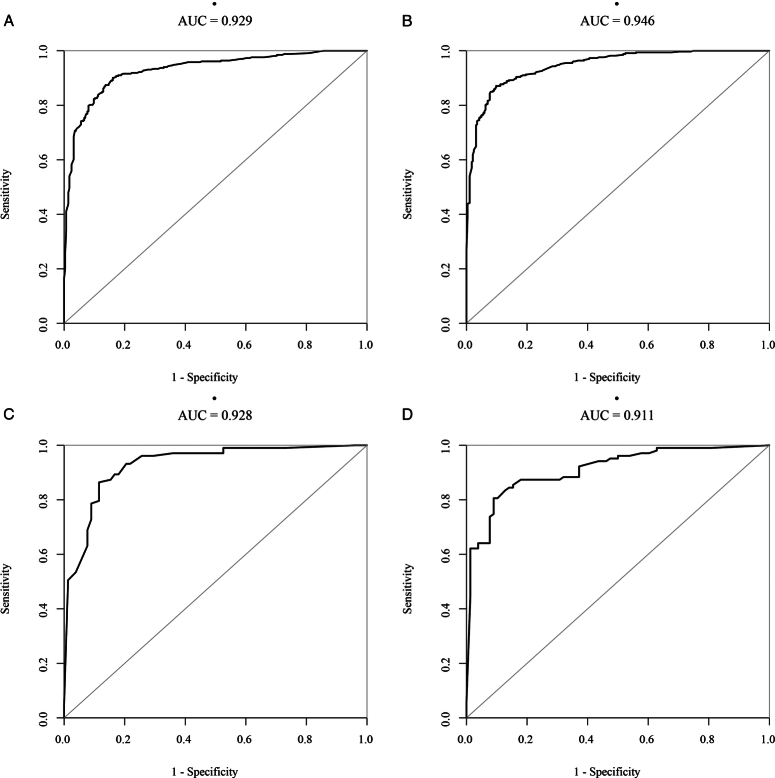
Machine learning was used to compare the importance of each independent factor in predicting the prognoses of TBI patients.
The independent prognostic factors (age, pupil size, brain herniation, WBC, CRP, coagulopathy, and X1), which were screened based on the multivariate risk regression model, were ranked by machine learning using R language in both the training or validation groups. The importance of ranking the coagulopathy and X1 models was determined. In the coagulopathy model, coagulopathy occupied the fourth level, while in the X1 model, X1 occupied the second level. The importance of the X1 model was significantly higher than that of the coagulopathy prediction model alone (Fig. 9). In the training groups, the AUCs of the coagulopathy prediction and X1 prediction models were 0.929 and 0.946, respectively (Fig. 10A, B), and the AUCs of the validation groups was 0.928 and 0.911, respectively (Fig. 10C, D).
Figure 9.
Machine learning was used to compare the importance ranking of each independent influencing factor in predicting the Glasgow Outcome Scale in traumatic brain injury patients. (A) The importance ranking based on the coagulopathy model was obtained by machine learning in the training group. (B) The importance ranking based on the X1 model was obtained by machine learning in the training group. (C) The importance ranking based on the coagulopathy model was obtained by machine learning in the validation group. (D) The importance ranking based on the X1 model was obtained by machine learning in the validation group.
Figure 10.
Machine learning was used to evaluate the accuracy of the coagulopathy model and X1 models in the training and validation groups. (A) The receiver operating characteristic (ROC) curve based on the coagulopathy model in the training group. (B) The ROC curve based on the X1 model in the training group. (C) The ROC curve based on the coagulopathy model in the validation group. (D) The ROC curve based on the X1 model in the validation group.
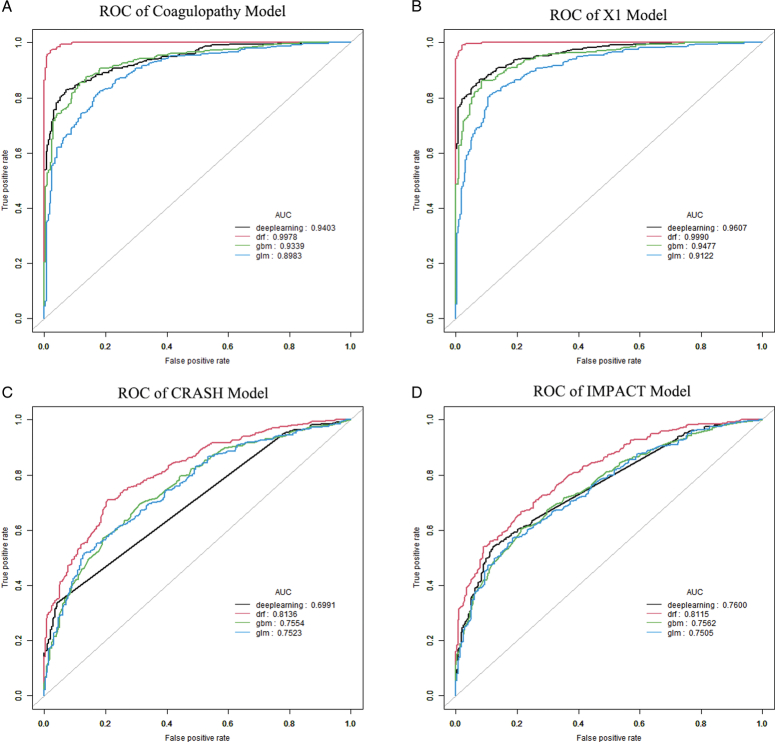
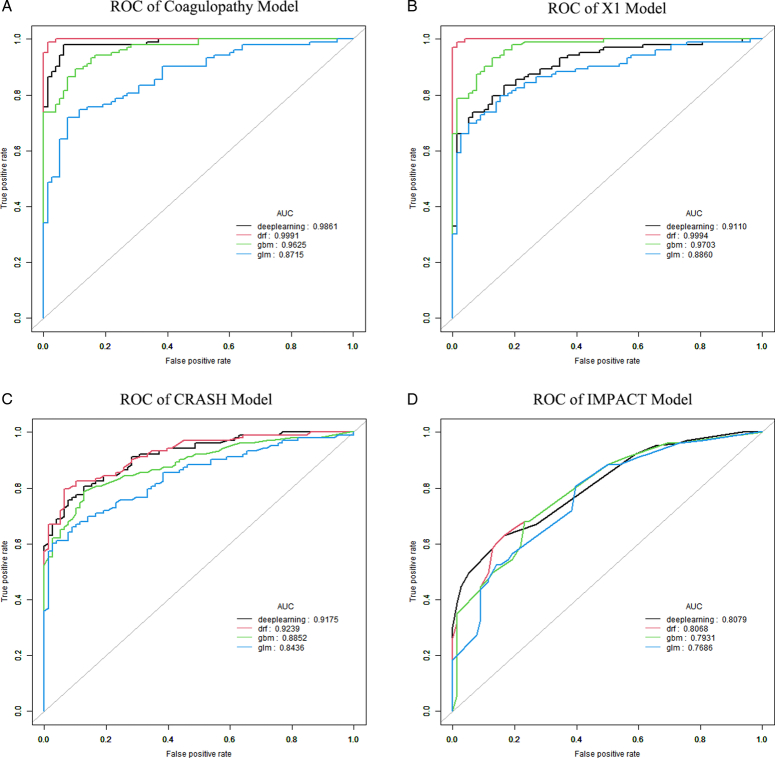
Comparison of the performances of the X1 model with the classical CRASH and IMPACT models using machine learning
The CRASH31 model and IMPACT7 models were selected for comparisons with the coagulopathy model and X1 model in this study. The role of machine learning in clinical research is increasingly widespread, especially in building new classifiers and clinical predictors32–35. Different machine learning algorithms were selected as deep learning (fully connected deep neural networks), DRF (distributed random forest), GBM (gradient boosting machine), and GLM (generalized linear model) algorithms, and were used to evaluate the predictive performances of the four models in the training group (Fig. 11) and the validation group (Fig. 12). The results showed that the AUC values of the X1 model-based on four different machine learning algorithms were better than those of the classic CRASH and IMPACT models in both the training group and the validation group. Based on the data of this study, the prediction performance of the X1 model established by our research group was better than that of the CRASH and IMPACT models. Therefore, in the process of treating patients with severe TBI, the X1 model can be used as a more reliable model to predict the prognoses of patients. Clinicians can intervene and correct the prognoses of patients at an earlier time, according to the prediction results of the X1 model, to obtain a better quality of life.
Figure 11.
ROC curves derived from different machine learning algorithms for the four models in the training group. (A) The area under the curve (AUC) values of different algorithms were obtained based on the coagulopathy model. (B) The AUC values of different algorithms were obtained based on the X1 model. (C) The AUC values of different algorithms were obtained based on the CRASH model. (D) The AUC values of different algorithms were obtained based on the IMPACT model.
Figure 12.
Receiver operating characteristic (ROC) curves derived from different machine learning algorithms for the four models in the validation group. (A) The area under the curve (AUC) values of different algorithms were obtained based on the coagulopathy model. (B) The AUC values of different algorithms were obtained based on the X1 model. (C) The AUC values of different algorithms were obtained based on the CRASH model. (D) The AUC values of different algorithms were obtained based on the IMPACT model.
Discussion
In the present study, we determined the prognostic values of coagulopathy indicators and subdural hematoma thickness in 800 patients with severe TBI. The prediction performance was evaluated by the corresponding ROC, nomogram, DCA, C-index, and machine learning importance ranking. Patients with severe TBI with coagulopathy and subdural hematoma thickness more than 14.05 mm at admission had a worse prognostic GOS score. In addition, X1 and coagulopathy were shown to be independent prognostic factors for GOS at 6 months in patients with severe TBI.
Coagulopathy induced by TBI is one of the common risk factors for poor clinical outcomes, but its pathogenesis remains unknown, and treatment options are limited and ineffective36. Coagulopathy has been reported to occur in up to two-thirds of patients after severe TBI, and TBI-related factors may alter the complex balance between bleeding and thrombosis, resulting in coagulopathy and associated with increased mortality14,18,37–40. The clinical course of coagulopathy and bleeding progression after TBI typically reflects a rapid progression from a hypercoagulable to a hypocoagulable state, whereby coagulopathy develops due to the early release of procoagulant tissue factor from the injured brain, which is then continuously consumed, leading to coagulopathy15,41. In addition, soon after TBI, due to the formation of multiple clots, systemic depletion of coagulation factors and platelets leads to a decrease in fibrinogen concentrations and platelet counts, which is also an important factor leading to increased bleeding42. In patients with severe TBI, the imbalance between coagulation and anticoagulation systems caused by various factors often leads to coagulopathy, and further aggravates bleeding, including the occurrence and progressive expansion of dural or intracranial hematomas.
The presence of coagulopathy in TBI patients at presentation is an independent risk factor for poor clinical outcomes, several times increasing the risk of adverse outcomes and death9,43. Hypocoagulable coagulopathy has been reported to appear early after severe TBI, occurring in up to 67% of patients, which is close to the 72% incidence of coagulopathy in patients with severe TBI in our study, and is associated with increased mortality20. Approximately 50% of TBI patients with coagulopathy experience hemorrhagic progression of initial brain contusion or intracerebral hematoma within hours of TBI44,45. Juratli et al.44, in a prospective study of patients with TBI, reported a 47.1% incidence of coagulopathy, with 43.5% of these patients showing early hemorrhagic progression of brain contuses within the first 6 h. Previous studies have also confirmed that coagulopathy, epidural hematoma, subdural hematoma, and intracranial hemorrhage enlargement were closely related to the prognosis of TBI12,43,46. Subdural hematoma is a major determinant of short-term outcomes in TBI patients. TBI patients with SDH have a lower discharge mortality but a higher in-hospital mortality21. Local consumptive coagulopathy and excessive fibrinolysis after TBI can lead to recurrent hemorrhage and progressive expansion of subdural hematomas. The mass effects of hematomas can lead to a series of fatal complications such as increased intracranial pressure, brain herniation, and respiratory depression. Coagulopathy is a poor prognostic factor for patients with TBI, but also aggravates the progression and mass effect of subdural hematomas. The effects of coagulopathy after TBI on the prognoses of patients with TBI may therefore be multidirectional. Traditional studies generally use coagulopathy alone to predict the prognosis of TBI patients, but do not pay attention to the further pathological phenomenon caused by coagulopathy. Our research group believes that severe coagulopathy can partly promote the formation of SDH, and the severity of SDH directly affects the prognosis of patients with GOS score, coagulopathy can affect the prognosis of TBI patients and the formation of SDH. We determined the best cut-off value of SDH thickness (14.05 mm) in patients with severe TBI and classified it into high and low levels, and found that the prognosis of patients with coagulopathy combined with SDH thickness ≥14.05 mm was worse. For the first time, we combined coagulopathy with SDH thickness and developed a new prediction model, which was validated by the latest machine learning technology. The data of this study were applied to the IMPACT7 and CRASH31 prediction models, which are widely used in the world, and compared with the performance of our prediction model. It was found that the AUC values of the X1 model established by us in four different machine learning algorithms were higher than those of the IMPACT and CRASH models. This further illustrates the importance of coagulopathy combined with SDH thickness. Of course, IMPACT and CRASH are international standard prediction models, while our data is based on national research, which may be affected by differences in patients from different countries and regions.
The prevalence of coagulopathy in TBI varies considerably among studies and proportions between 10 and 90% have been reported. This variation may be ascribed to various reasons, including inconsistency in the definition of coagulopathy, diversity in the level of injury severity among studies, and the mixture of early and delayed hemostatic disorders to calculate the prevalence of TBI-associated coagulopathy. Most studies rely on classical laboratory parameters like the activated partial thromboplastin time (aPTT), the prothrombin time (PT), the international normalized ratio (INR) in the PT, fibrinogen levels and platelet count. Olson et al.47 earlier reported that mild alterations in the aPTT (>34 s) and platelet count (<150×109/μl) may already be indicative for early coagulopathy in isolated head injury. Others reported aPTT and PT values ranging from 34 to 60 and 13 to 18 s, respectively, and an INR of >1.1 to 1.5 and platelet counts of 100 to 150×109/μl as definition for coagulopathy associated with TBI47–49. The definition of coagulopathy used in this study is consistent with the above range. However, because the prediction of outcomes associated with coagulopathy in TBI depends on the definition of coagulopathy, there is an international need to develop a standard evidence-based guideline.
In conclusion, determining the occurrence of coagulopathy and the progression of subdural hematoma after severe TBI was valuable for the prognoses of patients and the planning of treatments. Clinicians can use the thickness of coagulopathy and subdural hematoma on admission to calculate the nomogram score and evaluate the short-term poor prognosis in advance.
Despite demonstrating the predictive prognostic value of coagulopathy and SDH thickness in patients with severe TBI, this study had some limitations. First, this was a retrospective analysis, and some potential factors may have influenced the findings. Second, although the data source of this study was multicenter, the number of patients was not large enough, and this study only calculated coagulation indexes and SDH thickness at admission, without dynamic monitoring. Dynamic monitoring of changes in coagulation indexes and hematoma thickness may provide more reliable results. Finally, the conclusions of this paper still need to be verified by prospective studies.
Conclusions
In conclusion, our study showed that coagulopathy combined with SDH thickness (X1) had better predictive performance than coagulopathy alone. The X1 model constructed by combining coagulopathy with SDH thickness could therefore be used to improve the accuracy of GOS score predictions in severe TBI patients at 6 months after discharge, to carry out early planned interventions for patients with a poor prognosis.
Ethical approval
The study was approved by the Fuzong Clinical Medical College of Fujian Medical University (No: 2023-022). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Consent
We have obtained ethics committee approval and fully informed written consent, which should be recorded in the paper.
Sources of funding
This work was supported by Fujian Provincial Science and Technology Innovation Joint Fund Project (2019Y9045), Joint Logistics Medical Key Specialty Program (LQZD-SW), Huai’an Health Research Project (HAWJ202111) and The Open Project of Jiangsu Key Laboratory of Xuzhou Medical University (XZSYSKF2021036).
Author contribution
L.C.: conceptualization; L.C., Y.L., and S.X.: data curation; Y.Y., L.X., and X.Z.: formal analysis; Y.Z. and S.W.: funding acquisition; L.C., Y.Z., and S.W.: methodology; X.X. and S.W.: project administration; L.C. and S.X.: resources; L.C. L.X. and X.Q: writing – original draft; X.X., T.F., and S.W.: writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Research registration unique identifying number (UIN)
Name of the registry: Research Registry Shousen Wang.
Unique identifying number or registration ID: researchregistry9642.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-theregistry#home/registrationdetails/653a374ea00c0d0025ffd6b2/
Guarantor
Li Chen and Shousen Wang are guarantors.
Data availability statement
The data is available by contacting the corresponding authors.
Provenance and peer review
Not applicable.
Acknowledgements
Assistance with the study: The authors thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Footnotes
Li Chen, Shaohuai Xia, and Yinghong Lin have contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 15 May 2024
Contributor Information
Li Chen, Email: 431062918@qq.com.
Shaohuai Xia, Email: zx5182388@163.com.
Yinghong Lin, Email: LYH2020FZ@163.com.
Yuhui Chen, Email: 764689594@qq.com.
Liang Xian, Email: 627461879@qq.com.
Yang Yang, Email: yang15142022@163.com.
Xianshen Qiu, Email: Dr_qxs@163.com.
Limei Xu, Email: xulimeiqz@163.com.
Zhu Xingshu, Email: 862379991@qq.com.
Dujuan Chen, Email: 2418751098@qq.com.
Xuewei Xia, Email: xxw7456@163.com.
Yi Zuo, Email: zuoyyy2020@163.com.
Shousen Wang, Email: wshsen1965@126.com.
References
- 1. Jamjoom AAB, Rhodes J, Andrews PJD, et al. The synapse in traumatic brain injury. Brain 2021;144:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rui T, Wang H, Li Q, et al. Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. J Pineal Res 2021;70:e12704. [DOI] [PubMed] [Google Scholar]
- 3. Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048. [DOI] [PubMed] [Google Scholar]
- 4. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 2013;9:231–236. [DOI] [PubMed] [Google Scholar]
- 5. Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol 2017;16:452–464. [DOI] [PubMed] [Google Scholar]
- 6. Blennow K, Brody DL, Kochanek PM, et al. Traumatic brain injuries. Nat Rev Dis Primers 2016;2:16084. [DOI] [PubMed] [Google Scholar]
- 7. Roozenbeek B, Lingsma HF, Lecky FE, et al. Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit Care Med 2012;40:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoyt DB. A clinical review of bleeding dilemmas in trauma. Semin Hematol 2004;41(1 Suppl 1):40–43. [DOI] [PubMed] [Google Scholar]
- 9. Harhangi BS, Kompanje EJ, Leebeek FW, et al. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 2008;150:165–175; discussion 75. [DOI] [PubMed] [Google Scholar]
- 10. Gomez PA, Lobato RD, Ortega JM, et al. Mild head injury: differences in prognosis among patients with a Glasgow Coma Scale score of 13 to 15 and analysis of factors associated with abnormal CT findings. Br J Neurosurg 1996;10:453–460. [DOI] [PubMed] [Google Scholar]
- 11. Lustenberger T, Talving P, Kobayashi L, et al. Early coagulopathy after isolated severe traumatic brain injury: relationship with hypoperfusion challenged. J Trauma 2010;69:1410–1414. [DOI] [PubMed] [Google Scholar]
- 12. Wafaisade A, Lefering R, Tjardes T, et al. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit Care 2010;12:211–219. [DOI] [PubMed] [Google Scholar]
- 13. Alexiou GA, Lianos G, Fotakopoulos G, et al. Admission glucose and coagulopathy occurrence in patients with traumatic brain injury. Brain Inj 2014;28:438–441. [DOI] [PubMed] [Google Scholar]
- 14. Maegele M, Schochl H, Menovsky T, et al. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol 2017;16:630–647. [DOI] [PubMed] [Google Scholar]
- 15. Laroche M, Kutcher ME, Huang MC, et al. Coagulopathy after traumatic brain injury. Neurosurgery 2012;70:1334–1345. [DOI] [PubMed] [Google Scholar]
- 16. Greuters S, van den Berg A, Franschman G, et al. Acute and delayed mild coagulopathy are related to outcome in patients with isolated traumatic brain injury. Crit Care 2011;15:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cucher D, Harmon L, Myer B, et al. Critical traumatic brain injury is associated with worse coagulopathy. J Trauma Acute Care Surg 2021;91:331–335. [DOI] [PubMed] [Google Scholar]
- 18. Talving P, Benfield R, Hadjizacharia P, et al. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma 2009;66:55–61; discussion -2. [DOI] [PubMed] [Google Scholar]
- 19. Talving P, Lustenberger T, Lam L, et al. Coagulopathy after isolated severe traumatic brain injury in children. J Trauma 2011;71:1205–1210. [DOI] [PubMed] [Google Scholar]
- 20. Mansour A, Loggini A, Goldenberg FD, et al. Coagulopathy as a surrogate of severity of injury in penetrating brain injury. J Neurotrauma 2021;38:1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee JJ, Segar DJ, Morrison JF, et al. Subdural hematoma as a major determinant of short-term outcomes in traumatic brain injury. J Neurosurg 2018;128:236–249. [DOI] [PubMed] [Google Scholar]
- 22. Aromatario M, Torsello A, D’Errico S, et al. Traumatic epidural and subdural hematoma: epidemiology, outcome, and dating. Medicina (Kaunas) 2021;57:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinnunen J, Satopaa J, Niemela M, et al. Coagulopathy and its effect on treatment and mortality in patients with traumatic intracranial hemorrhage. Acta Neurochir (Wien) 2021;163:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folkerson LE, Sloan D, Davis E, et al. Coagulopathy as a predictor of mortality after penetrating traumatic brain injury. Am J Emerg Med 2018;36:38–42. [DOI] [PubMed] [Google Scholar]
- 25. Han JX, See AAQ, Gandhi M, et al. Models of mortality and morbidity in severe traumatic brain injury: an analysis of a Singapore neurotrauma database. World Neurosurg 2017;108:885–93 e1. [DOI] [PubMed] [Google Scholar]
- 26. Xu L, An T, Li C, et al. Development and verification of prognostic prediction models for patients with brain trauma based on coagulation function indexes. J Immunol Res 2022;2022:3876805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364–1370. [DOI] [PubMed] [Google Scholar]
- 28. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Gent JAN, van Essen TA, Bos MHA, et al. Coagulopathy after hemorrhagic traumatic brain injury, an observational study of the incidence and prognosis. Acta Neurochir (Wien) 2020;162:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 31. Collaborators MCT, Perel P, Arango M, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 2008;336:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oh S, Ryu J, Shin HJ, et al. Deep learning using computed tomography to identify high-risk patients for acute small bowel obstruction: development and validation of a prediction model: a retrospective cohort study. Int J Surg 2023;109:4091–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zou Y, Xie J, Zheng S, et al. Leveraging diverse cell-death patterns to predict the prognosis and drug sensitivity of triple-negative breast cancer patients after surgery. Int J Surg 2022;107:106936. [DOI] [PubMed] [Google Scholar]
- 34. Liu P, Zhu H, Zhu H, et al. Predicting survival for hepatic arterial infusion chemotherapy of unresectable colorectal liver metastases: radiomics analysis of pretreatment computed tomography. J Transl Int Med 2022;10:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Chen L, Yao C, et al. Early plasma proteomic biomarkers and prediction model of acute respiratory distress syndrome after cardiopulmonary bypass: a prospective nested cohort study. Int J Surg 2023;109:2561–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Zhang F, Dong JF. Coagulopathy induced by traumatic brain injury: systemic manifestation of a localized injury. Blood 2018;131:2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit Care 2004;1:479–488. [DOI] [PubMed] [Google Scholar]
- 38. Hulka F, Mullins RJ, Frank EH. Blunt brain injury activates the coagulation process. Arch Surg 1996;131:923–927; discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 39. Hay JR, Johnson VE, Young AM, et al. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol 2015;74:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang F, Peng C, Peng L, et al. A Machine learning approach for the prediction of traumatic brain injury induced coagulopathy. Front Med (Lausanne) 2021;8:792689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar MA. Coagulopathy associated with traumatic brain injury. Curr Neurol Neurosci Rep 2013;13:391. [DOI] [PubMed] [Google Scholar]
- 42. Tong WS, Zheng P, Zeng JS, et al. Prognosis analysis and risk factors related to progressive intracranial haemorrhage in patients with acute traumatic brain injury. Brain Inj 2012;26:1136–1142. [DOI] [PubMed] [Google Scholar]
- 43. Epstein DS, Mitra B, O’Reilly G, et al. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: a systematic review and meta-analysis. Injury 2014;45:819–824. [DOI] [PubMed] [Google Scholar]
- 44. Juratli TA, Zang B, Litz RJ, et al. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. J Neurotrauma 2014;31:1521–1527. [DOI] [PubMed] [Google Scholar]
- 45. Tian HL, Chen H, Wu BS, et al. D-dimer as a predictor of progressive hemorrhagic injury in patients with traumatic brain injury: analysis of 194 cases. Neurosurg Rev 2010;33:359–365; discussion 65-6. [DOI] [PubMed] [Google Scholar]
- 46. Yuan Q, Sun YR, Wu X, et al. Coagulopathy in traumatic brain injury and its correlation with progressive hemorrhagic injury: a systematic review and meta-analysis. J Neurotrauma 2016;33:1279–1291. [DOI] [PubMed] [Google Scholar]
- 47. Olson JD, Kaufman HH, Moake J, et al. The incidence and significance of hemostatic abnormalities in patients with head injuries. Neurosurgery 1989;24:825–832. [DOI] [PubMed] [Google Scholar]
- 48. Carrick MM, Tyroch AH, Youens CA, et al. Subsequent development of thrombocytopenia and coagulopathy in moderate and severe head injury: support for serial laboratory examination. J Trauma 2005;58:725–729; discussion 9-30. [DOI] [PubMed] [Google Scholar]
- 49. Zehtabchi S, Soghoian S, Liu Y, et al. The association of coagulopathy and traumatic brain injury in patients with isolated head injury. Resuscitation 2008;76:52–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available by contacting the corresponding authors.