Abstract
Background
Hydroxychloroquine (HCQ) has proved ineffective in treating patients hospitalised with Coronavirus Disease 2019 (COVID-19), but uncertainty remains over its safety and efficacy in chemoprevention. Previous chemoprevention randomised controlled trials (RCTs) did not individually show benefit of HCQ against COVID-19 and, although meta-analysis did suggest clinical benefit, guidelines recommend against its use.
Methods and findings
Healthy adult participants from the healthcare setting, and later from the community, were enrolled in 26 centres in 11 countries to a double-blind, placebo-controlled, randomised trial of COVID-19 chemoprevention. HCQ was evaluated in Europe and Africa, and chloroquine (CQ) was evaluated in Asia, (both base equivalent of 155 mg once daily). The primary endpoint was symptomatic COVID-19, confirmed by PCR or seroconversion during the 3-month follow-up period. The secondary and tertiary endpoints were: asymptomatic laboratory-confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection; severity of COVID-19 symptoms; all-cause PCR-confirmed symptomatic acute respiratory illness (including SARS-CoV-2 infection); participant reported number of workdays lost; genetic and baseline biochemical markers associated with symptomatic COVID-19, respiratory illness and disease severity (not reported here); and health economic analyses of HCQ and CQ prophylaxis on costs and quality of life measures (not reported here).
The primary and safety analyses were conducted in the intention-to-treat (ITT) population. Recruitment of 40,000 (20,000 HCQ arm, 20,000 CQ arm) participants was planned but was not possible because of protracted delays resulting from controversies over efficacy and adverse events with HCQ use, vaccine rollout in some countries, and other factors. Between 29 April 2020 and 10 March 2022, 4,652 participants (46% females) were enrolled (HCQ/CQ n = 2,320; placebo n = 2,332). The median (IQR) age was 29 (23 to 39) years. SARS-CoV-2 infections (symptomatic and asymptomatic) occurred in 1,071 (23%) participants. For the primary endpoint the incidence of symptomatic COVID-19 was 240/2,320 in the HCQ/CQ versus 284/2,332 in the placebo arms (risk ratio (RR) 0.85 [95% confidence interval, 0.72 to 1.00; p = 0.05]).
For the secondary and tertiary outcomes asymptomatic SARS-CoV-2 infections occurred in 11.5% of HCQ/CQ recipients and 12.0% of placebo recipients: RR: 0.96 (95% CI, 0.82 to 1.12; p = 0.6). There were no differences in the severity of symptoms between the groups and no severe illnesses. HCQ/CQ chemoprevention was associated with fewer PCR-confirmed all-cause respiratory infections (predominantly SARS-CoV-2): RR 0.61 (95% CI, 0.42 to 0.88; p = 0.009) and fewer days lost to work because of illness: 104 days per 1,000 participants over 90 days (95% CI, 12 to 199 days; p < 0.001). The prespecified meta-analysis of all published pre-exposure RCTs indicates that HCQ/CQ prophylaxis provided a moderate protective benefit against symptomatic COVID-19: RR 0.80 (95% CI, 0.71 to 0.91). Both drugs were well tolerated with no drug-related serious adverse events (SAEs). Study limitations include the smaller than planned study size, the relatively low number of PCR-confirmed infections, and the lower comparative accuracy of serology endpoints (in particular, the adapted dried blood spot method) compared to the PCR endpoint. The COPCOV trial was registered with ClinicalTrials.gov; number NCT04303507.
Interpretation
In this large placebo-controlled, double-blind randomised trial, HCQ and CQ were safe and well tolerated in COVID-19 chemoprevention, and there was evidence of moderate protective benefit in a meta-analysis including this trial and similar RCTs.
Trial registration
ClinicalTrials.gov NCT04303507; ISRCTN Registry ISRCTN10207947.
In a randomised controlled trial, William Schilling and co-workers investigate potential prevention of COVID-19 by chloroquine or hydroxychloroquine.
Author summary
Why was this study done?
At the beginning of the Coronavirus Disease 2019 (COVID-19) pandemic, there was an urgent need to find ways to prevent COVID-19.
Laboratory studies showed that the related 4-aminoquinolines, chloroquine (CQ), and hydroxychloroquine (HCQ), which had been used widely for over 50 years, had antiviral activity against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
HCQ proved ineffective in the treatment of hospitalised patients, and individual RCTs testing COVID-19 prophylaxis did not show benefit of HCQ. However, a meta-analysis of trial data did suggest some efficacy in preventing COVID-19.
Current evidence-based guidelines using data from the same studies recommend strongly against the use of HCQ for prophylaxis.
This study aimed to provide a definitive answer whether or not pre-exposure use of these drugs could prevent COVID-19.
What did the researchers do and find?
The COPCOV study was a double-blind placebo-controlled evaluation of CQ and HCQ COVID-19 chemoprevention. It was the largest pre-exposure prophylaxis study in COVID-19.
We found that CQ and HCQ were well tolerated and safe in prophylaxis. There was some evidence for protection against symptomatic COVID-19, and a reduction in workdays lost to illness.
Our updated meta-analysis of all chemoprevention studies in COVID-19 confirms that chemoprophylaxis with CQ or HCQ is well tolerated, safe, and provides a moderate beneficial effect in preventing COVID-19.
What do these findings mean?
Although CQ or HCQ are unlikely to be used in COVID-19 prevention at this stage, they could have been deployed with benefit earlier, and they might have value in future pandemics.
Randomised controlled trial (RCT) evidence is essential in evaluating therapeutics in the context of a pandemic.
Trials should be facilitated and protected so that evidence is generated rapidly and evidence-based policies can be implemented without delay to allow timely interventions.
Introduction
In the 4 years since the start of the Coronavirus Disease 2019 (COVID-19) pandemic, the majority of the world’s population has been infected. It is estimated conservatively that over 6.9 million people have died from COVID-19 [1]. At the beginning of 2020, there were no vaccines and no specific treatments, and there was substantial global concern about the projected consequences of the developing pandemic. Many existing medicines were proposed as potential therapeutics (“repurposing”). Prominent among these were the 4-aminoquinolines, chloroquine (CQ), and hydroxychloroquine (HCQ), as they had been used widely for decades in the prevention and treatment of malaria and rheumatological conditions, and they had in vitro activity against both SARS-CoV-1 and SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) [2,3]. After initial claims of benefit, their use rapidly became politicised and controversial. This unhelpful milieu was worsened in May 2020 by a prominent false claim of lethal cardiovascular toxicity [4]. The clinical trials evaluating the preventive and curative efficacy of the 4-aminoquinolines were caught, and in many cases damaged, by the controversies and regulatory decisions. Soon afterwards, large randomised controlled trials (RCTs) in patients hospitalised with COVID-19 showed definitively that HCQ treatment did not reduce mortality [5,6]. Although it has become clear that antivirals are most effective early in COVID-19, when viral burdens are highest, whereas anti-inflammatory drugs are beneficial in late disease (hospitalised patients), the negative results from the large RCTs in severe COVID-19 were extrapolated to indicate a lack of efficacy for HCQ in all stages of COVID-19 infection [7,8]. Nevertheless, HCQ was recommended widely as COVID-19 chemoprevention [9]. For the chemoprevention clinical trials attempting to provide definitive evidence the widely publicised controversies and negative regulatory responses adversely affected recruitment and study conduct. Despite this, some investigators did successfully complete their RCTs [10–23]. Taken together, these studies point towards moderate preventive efficacy even though individually most were underpowered to demonstrate benefit [24], but the evidence is far from conclusive. In contrast, most authorities recommend against HCQ [7,25]. As a result, there still remains substantial uncertainty regarding the true efficacy of HCQ in COVID-19 prophylaxis. This study’s aim was to characterise the efficacy, tolerability, and safety of HCQ/CQ pre-exposure prophylaxis in the prevention of COVID-19.
Methods
Study design
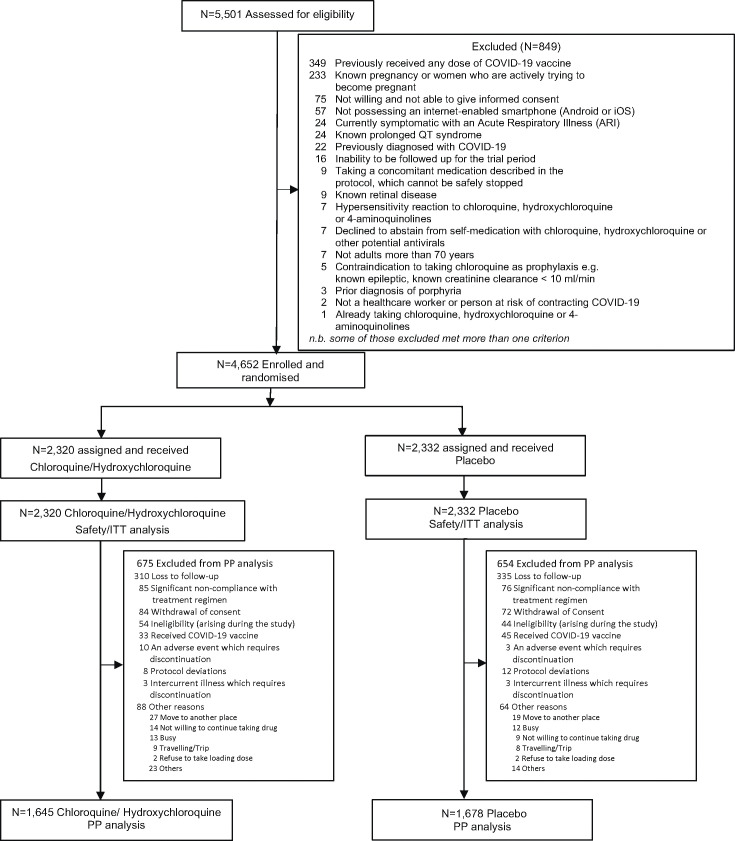
COPCOV was a multinational double-blind, randomised, placebo-controlled trial of COVID-19 chemoprevention (Fig 1). Potential investigators in 76 countries across the world were contacted to seek their interest and ability to conduct the study and follow the protocol (Fig A1 in S1 Appendix). Hydroxychloroquine was evaluated in Africa and the United Kingdom (UK), and chloroquine was evaluated in Asia. As the drugs have comparable in vitro activities, mode of action, and pharmacokinetic properties, they were considered equivalent [26,27].
Fig 1. COPCOV study participant flowchart.
Participants
Initially, in the rapidly spreading pandemic, the focus was on protecting healthcare workers but, as the study progressed, the inclusion criteria were widened (S1 Appendix). The study recruited unvaccinated healthy, nonpregnant, adults aged between 17 and 70 years who were at risk of COVID-19, agreed to the study procedures, could be followed reliably for up to 5 months, and had ready access to an internet-enabled smartphone. Participants with any underlying disease or contraindication to taking 4-aminoquinolines were excluded.
Randomisation and masking
The trial statistician (MM) generated 2 separate permuted-block randomisation sequences in blocks of 10 stratified in sets of 400 drug kits (each kit comprised 10 blister packs of 10 tablets containing either drug or identical placebo) packed into boxes of 50 (hydroxychloroquine and placebo: Accord Healthcare, London, UK) and 200 (chloroquine and placebo: Utopian Pharmaceutical Co., Bangkok, Thailand) kits by Piramal Healthcare (UK) and Utopian, respectively, according to company regulations. The assignments were concealed from investigators, research staff, and participants. Kits were in sequence of randomisation and allocation was by opening in sequential order. The Statistical Analysis Plan (SAP) was completed and signed before the database lock and subsequent unblinding (S1 Statistical Analysis Plan).
After education about the study and provision of voluntary written informed consent, participants were randomised to receive either CQ or HCQ (depending on study site) or identical matched placebos (1:1 randomisation). The tablets were film-coated to conceal the taste and prevent unblinding. A loading dose of 10 mg base/kg (four 155 mg tablets for a 60 kg subject) was followed by 155 mg daily (equivalent to 250 mg chloroquine phosphate or 200 mg hydroxychloroquine sulphate) for 3 months.
Procedures
At the initial visit, participants were examined and baseline screening blood samples were taken. Participants were instructed how to use the mobile ‘phone application (ePRO, Axiom Real-Time Metrics, ON, Canada) and were asked to record their temperature twice daily (an oral electronic thermometer was provided) and symptoms at least once daily. Thereafter, each participant was reviewed in person each month. Serology serum samples were taken on days 0 and 90, or at the last visit if the participant left the study earlier. Contingency dried blood spot samples (DBS) were taken for drug measurement on days 0, 30, 60, and 90 (the analysis of drug concentrations will be reported separately). If symptoms consistent with COVID-19 occurred, the participant was asked to alert the study team by ‘phone, so that nose and throat swabs could be taken. These were stored at −80°C. The methods for SARS-CoV-2 PCR diagnosis and serological quantification of SARS-CoV-2 spike protein IgG antibodies are described in the S1 Appendix. Vaccines were deployed increasingly during the trial. Vaccinated participants were reviewed and an “end of study” serum sample was taken before, or within 3 days, of their vaccination, and were censored from the trial on receiving the first dose of vaccine.
Procedures for case identification, management, and subsequent isolation followed local and national guidelines. Continuation of the blinded study medication in confirmed COVID-19 cases was at the discretion of the attending health care professional. Overall trial monitoring was conducted by the Mahidol Oxford Tropical Medicine Research Unit Clinical Trials Support Group.
Outcomes
The primary outcome was symptomatic laboratory-confirmed COVID-19 defined as symptoms consistent with COVID-19 and laboratory evidence of SARS-CoV-2 infection (S1 Appendix). Laboratory evidence was defined by a prespecified hierarchy: first by a nose and/or throat swab PCR positive for SARS-CoV-2; second, if the PCR was negative, failed, or not done, by seroconversion (S-protein IgG antibody) based on baseline and end of study paired sera [28]; and third, only if paired sera were unavailable or uninterpretable, by seroconversion from the contingency DBS in an adapted assay. The prespecified primary endpoint determination algorithm code is provided in the GitHub repository.
A Serology Endpoint Assessment Committee (SEAC) comprising 2 external experts with extensive SARS-CoV-2 serology experience was convened to adjudicate on equivocal serology endpoints (SAP: S1 Appendix). Their judgements before trial unblinding were included in the database and regarded as final.
Secondary outcomes
There were 3 prespecified secondary outcomes: asymptomatic laboratory-confirmed SARS-CoV-2 infection; severity of COVID-19 symptoms; and all-cause PCR-confirmed symptomatic acute respiratory illness (including SARS-CoV-2 infection).
Tertiary outcomes
There were 3 generic prespecified trial tertiary outcomes: the participant reported number of workdays lost; genetic and baseline biochemical markers associated with symptomatic COVID-19, respiratory illness, and disease severity (not reported here); and health economic analyses of HCQ and CQ prophylaxis on costs and quality of life measures (not reported here).
In addition, we prespecified that a meta-analysis of previously published randomised hydroxychloroquine COVID-19 pre-exposure chemoprevention studies and the current study should be conducted (S1 Appendix and Methods A2 in S1 Appendix).
Statistical analysis
At the beginning of the pandemic, we did not know what would be the subsequent incidence of COVID-19, and estimated conservatively a 90-day incidence of 3%. We therefore planned to enrol 20,000 participants in Asia (chloroquine-based randomisation) and 20,000 in Europe/Africa (hydroxychloroquine-based randomisation). This sample size allowed for approximately 20% loss to follow-up, withdrawals, protocol deviations and non-adherence, and provided 80% power to detect a 23% reduction in symptomatic COVID-19 incidence for each drug individually with 95% confidence. Unfortunately, there were protracted delays and difficulties with recruitment as the study was starting in 2020. These were related to adverse publicity and withdrawal of regulatory approvals resulting from falsified claims of frequent serious cardiotoxicity [4,29]. The Data Safety and Monitoring Board (DSMB) then acknowledged that the original sample size would no longer be achievable, but recommended study continuation, with pooling of the HCQ and CQ results. The primary outcome was subsequently changed to include seroconversion. This resulted in a 4-fold higher event rate than initially forecasted (approximately 12%). Assuming a continued 12% event rate in the control arm a total sample size of 4,600 had >80% power to detect the previously targeted treatment effect (i.e., 23% reduction).
The primary outcome included all participants and was analysed using intention-to-treat (ITT) with a two-sided p-value <0.05 considered significant. A secondary per protocol (PP) analysis excluded participants as described in the CONSORT diagram (Fig 1) and in the SAP (S1 Appendix). Secondary and tertiary endpoints were analysed using the ITT population. Fisher’s exact test was used to compare treatment effects between groups. Risk ratios were obtained from a log-binomial model. Kaplan–Meier survival curves were estimated for the time to PCR-confirmed symptomatic COVID-19 and all-cause respiratory infection and were tested using the log-rank test. The number of workdays lost was analysed under a zero-inflated Poisson regression model. For convergence reasons, we fitted this model using a Bayesian framework in the brms R package (which uses stan to estimate posterior distributions under weakly informative priors). Data analysis was performed using Stata 17.0, StataCorp, College Station, Texas, United States of America and R version 4.2.2. All code and data required to reproduce the primary analysis are provided in https://github.com/jwatowatson/COPCOV. The study is reported according to the CONSORT guidelines for reporting of a randomised trial (S1 CONSORT Checklist).
Trial support and role of the funding source
The COPCOV trial was approved by all local ethics committees and the Oxford Tropical Research Ethics Committee (OxTREC: 25–20) and was sponsored by the University of Oxford. The DSMB met before and during the trial and reviewed all serious adverse events (SAEs). The study sponsor, funder, and the drug manufacturers had no input into the study design, conduct, oversight, analysis, or reporting. The authors vouch for the completeness and accuracy of the data. After critical review all authors agreed to submit the manuscript for publication.
Results
The COPCOV study was conducted in 26 sites in 11 countries (Benin, Côte d’Ivoire, Indonesia, Kenya, Mali, Nepal, Niger, Pakistan, Thailand, UK, and Zambia) (Table A1 and Fig A2 In S1 Appendix) and ran between 29 April 2020 and 10 March 2022 (Fig A3 in S1 Appendix). A total of 4,652 adult participants (Table 1) were randomised to hydroxychloroquine or chloroquine (HCQ: 1,299, CQ: 1,021: total: 2,320) or corresponding matched placebos (N = 2,332) (Fig 1). The median age of the participants was 29 years (interquartile range, 23 to 39). The population was generally healthy; 4.8% (225/4,652) reported having a chronic disease. There were no significant differences in tolerability, safety, or efficacy between chloroquine and hydroxychloroquine.
Table 1. Demographic details of the COPCOV study participants.
| Chloroquine/hydroxychloroquine | Placebo | Total | |
|---|---|---|---|
| N = 2,320 | N = 2,332 | N = 4,652 | |
| Age (years), median (IQR) | 29 (23–39) | 29 (24–39) | 29 (23–39) |
| Sex, n (%) | |||
| Male | 1,252 (54.0) | 1,267 (54.3) | 2,519 (54.1) |
| Female | 1,067 (46.0) | 1,064 (45.6) | 2,131 (45.8) |
| Not specified | 1 (0) | 1 (0) | 2 (0) |
| Temperature (°C), mean (SD) | 36.4 (0.4) | 36.4 (0.5) | 36.4 (0.5) |
| Weight (kg), mean (SD) | 64.8 (14.2) | 65.3 (14.7) | 65.1 (14.4) |
| Height (cm), mean (SD) | 164 (9) | 164 (10) | 164 (9) |
| BMI (kg/m2), median (IQR) | 23.1 (20.3–26.9) | 23.3 (20.5–26.9) | 23.2 (20.4–26.9) |
| Smoking, n (%) | |||
| Yes | 374 (16.12) | 395 (16.94) | 769 (16.53) |
| Never smoked | 1,789 (77.11) | 1,772 (75.99) | 3,561 (76.55) |
| Former smoker | 157 (6.77) | 165 (7.08) | 322 (6.92) |
| COVID-19 in household, n/N (%) | 263 (11.3) | 273 (11.7) | 536 (11.5) |
| Existing comorbidities | |||
| Chronic pulmonary disease (not asthma), n/N (%) | 5 (0.2) | 0 (0) | 5 (0.1) |
| Asthma (physician diagnosed), n/N (%) | 22 (0.9) | 18 (0.8) | 40 (0.9) |
| Chronic kidney disease, n/N (%) | 0 (0) | 0 (0) | 0 (0) |
| Liver disease, n/N (%) | 1 (0.04) | 1 (0.04) | 2 (0.04) |
| AIDS/HIV, n/N (%) | 37 (1.6) | 34 (1.5) | 71 (1.5) |
| Diabetes, n/N (%) | 21 (0.9) | 15 (0.6) | 36 (0.8) |
| Hypertension, n/N (%) | 43 (1.9) | 41 (1.8) | 84 (1.8) |
| Cancer, n/N (%) | 1 (0.04) | 1 (0.04) | 2 (0.04) |
| Condition requiring immunosuppressive drugs, n/N (%) | 1 (0.04) | 0 (0) | 1 (0.02) |
| Ischaemic heart disease, n/N (%) | 2 (0.09) | 0 (0) | 2 (0.04) |
| High cholesterol, n/N (%) | 5 (0.2) | 7 (0.3) | 12 (0.3) |
| Any chronic condition, n (%) | 118 (5.1) | 107 (4.6) | 225 (4.8) |
| Baseline symptoms | |||
| Fever, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Cough, n (%) | 5 (0.2) | 3 (0.1) | 8 (0.2) |
| Sore throat, n (%) | 0 (0) | 1 (0.04) | 1 (0.02) |
| Runny nose (Rhinorrhoea), n (%) | 0 (0) | 1 (0.04) | 1 (0.02) |
| Wheezing, n (%) | 1 (0.04) | 0 (0) | 1 (0.02) |
| Anosmia, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Chest pain, n (%) | 0 (0) | 1 (0.04) | 1 (0.02) |
| Muscle pain (myalgia), n (%) | 2 (0.09) | 5 (0.2) | 7 (0.2) |
| Joint pain (Arthralgia), n (%) | 4 (0.2) | 4 (0.2) | 8 (0.2) |
| Shortness of breath on exertion, n (%) | 0 (0) | 3 (0.13) | 3 (0.06) |
| Shortness of breath at rest, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue/malaise, n (%) | 0 (0) | 3 (0.13) | 3 (0.06) |
| Itching, n (%) | 0 (0) | 1 (0.04) | 1 (0.02) |
| Headache, n (%) | 1 (0.04) | 5 (0.2) | 6 (0.1) |
| Dizziness, n (%) | 2 (0.09) | 3 (0.1) | 5 (0.1) |
| Visual disturbance, n (%) | 0 (0) | 1 (0.04) | 1 (0.02) |
| Abdominal pain, n (%) | 0 (0) | 2 (0.09) | 2 (0.04) |
| Poor appetite, n (%) | 1 (0.04) | 1 (0.04) | 2 (0.04) |
| Nausea, n (%) | 0 (0) | 2 (0.09) | 2 (0.04) |
| Vomiting, n (%) | 0 (0) | 1 (0.04) | 1 (0.02) |
| Diarrhoea, n (%) | 1 (0.04) | 0 (0) | 1 (0.02) |
| Skin rash, n (%) | 0 (0) | 2 (0.09) | 2 (0.04) |
Primary outcome
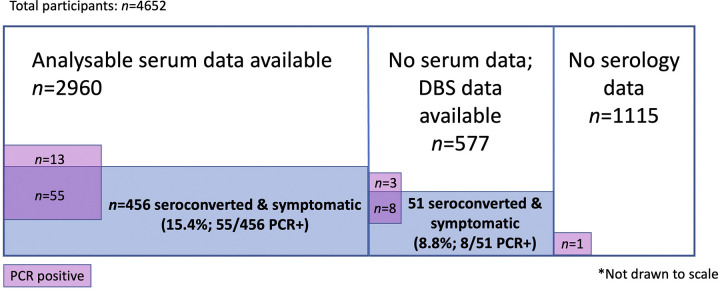
Symptomatic, laboratory-confirmed COVID-19 during the 3-month follow-up period occurred in 524 participants (11.3%); 240/2,320 (10.3% [95% CI, 9.2 to 11.7]) received HCQ/CQ and 284/2,332 (12.3% [95% CI, 10.9 to 13.6]) received placebo (risk ratio (RR) 0.85 [95% CI, 0.72 to 1.00; p = 0.05]) (Table 2). Symptomatic COVID-19 was PCR-confirmed in 24/2,320 HCQ/CQ recipients (1.0% [95%CI, 0.7 to 1.5]) and 56/2,332 (2.4% [95% CI, 1.8 to 3.1]) placebo recipients (RR 0.43 [95% CI, 0.27 to 0.69; p < 0.001]) (Fig 3). Among those with paired sera, symptomatic COVID-19 was diagnosed by seroconversion in 211/1,462 (14.4% [95% CI, 12.7 to 16.3]) HCQ/CQ recipients and 245/1,498 (16.4% [95%CI, 14.5 to 18.3]) placebo recipients (RR 0.88 [95% CI, 0.74 to 1.05], p = 0.2) (Table 2). In the remaining cases analysed by DBS (i.e., without paired sera), symptomatic COVID-19 was diagnosed in 25/280 (8.9% [95% CI, 5.9 to 12.9]) HCQ/CQ recipients and 26/297 (8.8 [95% CI, 5.8 to 12.6]) placebo recipients (RR 1.02 [95% CI, 0.60 to 1.72; p = 0.9]). Primary outcome data were missing for 1,114 participants (24%) for the serology component, mainly because of loss to follow-up (Fig 2). Asymptomatic infection was also common, occurring in 524 participants, thus the overall proportion of SARS-CoV-2 infection within the 3-month study was 23% (1,071 of 4,652).
Table 2. Prespecified endpoints of the COPCOV trial in the intention to treat population.
| Outcome | Chloroquine/hydroxychloroquine (N = 2,320) |
Placebo (N = 2,332) |
Risk ratio (95% CI) |
Fisher’s exact P-value |
|---|---|---|---|---|
| Total participant days | 181,263 | 184,688 | ||
| Primary endpoint: Symptomatic laboratory-confirmed COVID-19. n (%); 95% CI | 240/2,320 10.3 (9.1 to 11.7) |
284/2,332 12.2 (10.9 to 13.6) |
0.85 (0.72 to 1.00) |
0.051 |
| PCR-confirmed diagnosis. n/N (%); 95% CI | 24/2,320 1.0 (0.7 to 1.5) |
56/2,332 2.4 (1.8 to 3.1) |
0.43 (0.27 to 0.69) |
<0.001 |
| Serology confirmed diagnosis (serum). n (%); 95% CI |
211/1,462 14.4 (12.7 to 16.3) |
245/1,498 16.4 (14.5 to 18.3) |
0.88 (0.74 to 1.05) |
0.154 |
| Serology confirmed diagnosis (DBS). n (%); 95% CI |
25/280 8.9 (5.9–12.9) |
26/297 8.8 (5.8 to 12.6) |
1.02 (0.60 to 1.72) |
1.000 |
| Secondary endpoints: | ||||
| Asymptomatic SARS-CoV-2 infection. n (%); 95% CI |
267/2,320 11.5 (10.2 to 12.9) |
280/2,332 12.0 (10.7 to 13.4) |
0.96 (0.82 to 1.12) |
0.617 |
| All SARS-CoV-2 infection n (%); 95% CI | 507/2,320 21.9 (20.2 to 23.6) |
564/2,332 24.2 (22.5 to 26.0) |
0.90 (0.81 to 1.00) |
0.060 |
| All-cause respiratory illness*. n (%); 95% CI | 44/2,320 1.9 (1.4 to 2.5) |
73/2,332 3.1 (2.5 to 3.9) |
0.61 (0.42 to 0.88) |
0.009 |
| Severity score. Median (IQR) | 20.0 (5–85) |
21.5 (5–89) |
NA | 1.000 |
|
Tertiary endpoint: Participant reported workdays lost |
700/181,263 | 932/184,688 | NA | 0.0002** |
*PCR-confirmed respiratory infection including COVID-19.
**Assessed by a zero-inflated Poisson regression model.
Missing outcomes in the primary endpoint ITT analysis were treated as not having had COVID-19 during the study period.
See Fig 2 for Venn diagram depicting breakdown of numbers analysed.
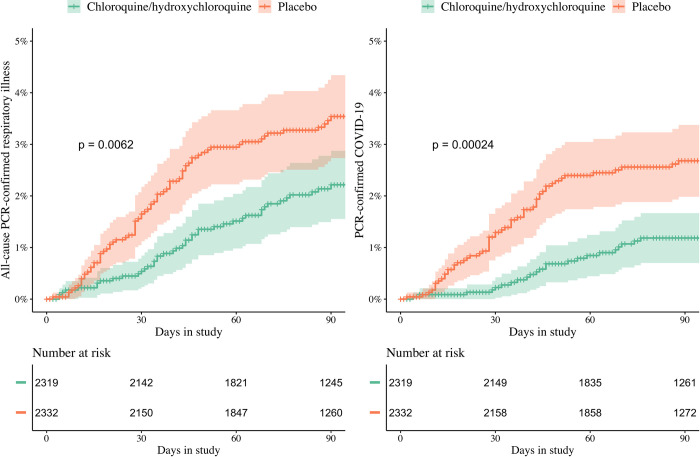
Fig 3.
All-cause PCR-confirmed respiratory infections (left) and PCR-confirmed COVID-19 (right) over time in the HCQ/CQ recipients (green) and placebo recipients (pink). All-cause respiratory infection was a secondary endpoint. The majority (68%, 80/117) of infections were SARS-CoV-2. Log-rank test p-values are shown. Patients are right censored at the date of last visit. COVID-19, Coronavirus Disease 2019; CQ, chloroquine; HCQ, hydroxychloroquine; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Fig 2. Venn diagram depicting the breakdown of numbers analysed.
Those with no serology data were either those who dropped out of the study before day 30, and had no end of study samples, or had missing end of study samples, or those judged unreliable by the serology endpoint assessment committee. This group differed from the PP population, with some overlap.
Prespecified secondary outcomes
Asymptomatic COVID-19 occurred in 267/2,320 (11.5% [95% CI, 10.2 to 12.9]), HCQ/CQ recipients and 280/2,332 (12.0% [95% CI, 10.7 to 13.4]) placebo recipients (RR: 0.96 [95% CI, 0.82 to 1.12; p = 0.6]). In symptomatic patients, there were no significant differences in symptom severity scores (20.0 (5–85) versus 21.5 (5–89)). All-cause respiratory illness (mainly COVID-19) occurred in 44/2,320 (1.9% [95% CI, 1.4 to 2.5]) HCQ/CQ recipients and 73/2,332 (3.1% [95% CI, 2.5 to 3.9]) placebo recipients (RR: 0.61 [95% CI, 0.42 to 0.88; p = 0.009]) (Fig 3 and Table A8 in S1 Appendix).
Prespecified tertiary outcomes
Under a zero-inflated Poisson regression model, the mean number of workdays lost over 90 days of chemoprophylaxis was 337 (95% CI, 279 to 398) per 1,000 participants in HCQ/CQ recipients and 441 (95% CI, 370 to 515) per 1,000 in placebo recipients; a mean difference of 104 days (95% CI, 12 to 199). Similar results were observed in the ITT and per protocol analyses for all comparisons (Tables A2–A3 in S1 Appendix). Tests for genetic and biochemical markers for symptomatic COVID-19, respiratory illness or disease severity, were not conducted, as suitable specific tests were not available. A health economic analysis will be reported separately.
Safety and tolerability
The study medications were well tolerated. One HCQ recipient was hospitalised with confirmed COVID-19. There were 10 SAEs in 9 HCQ/CQ recipients and 8 SAEs in 8 placebo recipients but none were considered drug-related (Table 3 and Table A4 in S1 Appendix) and no one died. Overall, 218/2,320 (9.4%) HCQ/CQ recipients had at least 1 adverse event (AE) compared to 242/2,332 (10.4%) placebo recipients (p = 0.3). Fewer HCQ/CQ recipients had severe AEs (31/2,320; 1.3%) than placebo recipients (58/2,332; 2.5%; p = 0.005). Twelve HCQ/CQ recipients (0.52%) discontinued chemoprophylaxis either because of side-effects (N = 10) or inability to comply with study procedures (N = 2), versus 4 (0.17%) in the placebo group (p = 0.05).
Table 3. Safety and tolerability of the COPCOV study medications.
| Adverse events | Chloroquine/hydroxychloroquine | Placebo | Fisher’s exact P-value | ||
|---|---|---|---|---|---|
| Number of subjects | N = 2,320 | N = 2,332 | |||
| Total participant days | 181,263 | 184,688 | |||
| Number of subjects with at least 1 AE, n (%) Total adverse events, nE (%) |
218 (9.4) 578 (24.9) |
242 (10.4) 656 (28.1) |
0.260 | ||
| Participants with at least 1 SAE, n (%): Total events, nE (%): |
9 (0.4) 10 (0.4) |
8(0.3) 8 (0.3) |
|||
| Deaths, n/N, (%) | 0 (0) | 0 (0) | |||
| Possible, probable, or definite drug related SAEs, n/N, (%) | 0 (0) | 0 (0) | |||
| Grading of adverse events, n E /N, (%) |
Moderate
(grade 2) N = 2,320 |
Severe
(grade 3) N = 2,320 |
Moderate
(grade 2) N = 2,332 |
Severe
(grade 3) N = 2,332 |
P-value for total severe AEs between groups |
| Symptoms | |||||
| Number of adverse events** | 547 (23.6) | 31 (1.3) | 598 (25.6) | 58 (2.5) | 0.005 |
| Itching | 7 (0.3) | 0 (0) | 11 (0.5) | 1 (0) | |
| Headache | 85 (3.7) | 5 (0.2) | 85 (3.6) | 11 (0.5) | |
| Dizziness | 30 (1.3) | 1 (0) | 20 (0.9) | 0 (0) | |
| Visual disturbance | 3 (0.1) | 0 (0) | 7 (0.3) | 0 (0) | |
| Abdominal pain | 22 (0.9) | 5 (0.2) | 19 (0.8) | 6 (0.3) | |
| Poor appetite | 3 (0.1) | 0 (0) | 5 (0.2) | 0 (0) | |
| Nausea | 19 (0.8) | 0 (0) | 19 (0.8) | 2 (0.1) | |
| Vomiting | 9 (0.4) | 1 (0) | 8 (0.3) | 1 (0) | |
| Diarrhoea | 22 (0.9) | 0 (0) | 17 (0.7) | 2 (0.1) | |
| Skin rash | 6 (0.3) | 0 (0) | 3 (0.1) | 1 (0) | |
| Other | 341 (14.7) | 19 (0.8) | 404 (17.3) | 34 (1.5) | |
*There were no participants with grade 4 AEs.
**SAEs are reported separately in the same table.
SAE, serious adverse event.
Prespecified meta-analysis
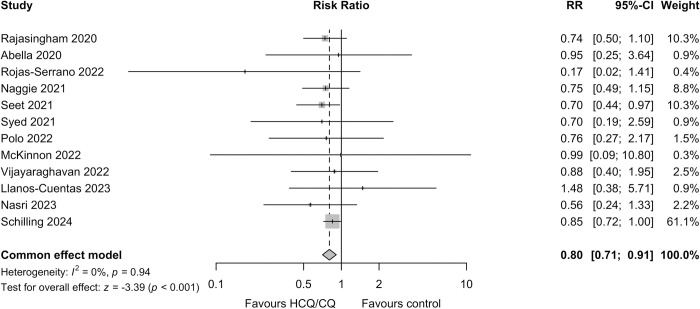
Including this study and the 11 previously published randomised hydroxychloroquine COVID-19 pre-exposure chemoprevention studies (Fig 3), the prespecified meta-analysis showed an overall protective efficacy against symptomatic COVID-19 (RR 0.80 [95% CI, 0.71 to 0.91; p = < 0.001]) [11,12,15–23]. Although the studies reported slightly different outcomes, used different doses and for different durations, there was no clear between-study heterogeneity or evidence of publication bias (Fig A4 in S1 Appendix).
Discussion
This large multinational randomised double-blind COVID-19 chemoprevention trial provides strong evidence of the safety of hydroxychloroquine and chloroquine given daily for 3 months (average 2.4 mg base/kg/day). The trial ended below its original recruitment objective but, because of the higher than anticipated incidence of COVID-19, it was able to provide evidence relevant to protective efficacy. For the trial’s primary endpoint (incidence of symptomatic laboratory-confirmed COVID-19), the apparent benefit observed for HCQ/CQ treatment is consistent with the aggregated results of previous smaller studies [11,12,15–23], all but one of which had a lower incidence of symptomatic COVID-19 in the HCQ treatment arm (Fig 4). Incorporating the COPCOV trial data in a prespecified meta-analysis of pre-exposure RCTs, in which there was little heterogeneity across the studies and no evidence of publication bias, suggests a moderate protective benefit (RR 0.80 [95% CI 0.71 to 0.91]). In other prespecified comparisons in the COPCOV study, HCQ/CQ chemoprevention was associated with fewer adverse events, fewer all-cause PCR-confirmed respiratory infections (mostly COVID-19), and a reduction in workdays lost, but it did not affect the incidence of asymptomatic infections or the severity of respiratory illness (although no patients required hospitalisation for hypoxia).
Fig 4. Prespecified meta-analysis of 4-aminoquinoline COVID-19 pre-exposure chemoprevention RCTs based on individual study primary endpoints according to the method of Garcia-Albeniz and colleagues [24].
Schilling 2024 refers to the current study. The size of the grey squares centred at the treatment effect estimates are proportional to the study weight. Risk ratios were determined for all studies based on the reported data, apart from Seet and colleagues, which was cluster randomised and a recalculated adjusted RR was used. See Methods A2 in S1 Appendix for further details. CQ, chloroquine; HCQ, hydroxychloroquine; RCT, randomised controlled trial; RR, risk ratio.
Despite this study’s size, and the combined evidence, there is still substantial uncertainty as to the true prophylactic benefit of these 4-aminoquinolines in COVID-19. The large difference between the groups in rates of PCR-confirmed diagnosis was not mirrored by a similar difference in seroconversion-based diagnosis. This could be a chance finding from PCR (as numbers were relatively small) or, more likely, results from imprecision of serodiagnosis diluting the power of the study. The DBS samples, used to determine seroconversion when paired sera were unavailable, may have provided unreliable results. The extraction of serum from DBS is variable and inefficient and, unlike the serum-based assays [28], the seroconversion thresholds have not been fully validated, although they have been used in other COVID-19 studies [30]. The absence of a significant protective effect against asymptomatic infection may indicate that protective benefit is proportional to the viral burden and thus disease severity, but it could also reflect the imprecision of the seroconversion-based endpoint.
The tolerability and safety of these well-established 4-aminoquinoline medicines is further reinforced in this double-blind comparison [31]. This was a point of controversy early in the pandemic. Initial reports described a range of toxicities that created a climate of concern and adverse opinion. This trial provides no support for these claims, and it supports the generally good short-term safety profile of these drugs [31]. It challenges the World Health Organization “living” guidelines on COVID-19 chemoprevention which emphasised toxicity in recommending against hydroxychloroquine. The same guideline also stated that prophylactic use “probably has a small or no effect on laboratory-confirmed COVID-19” with “moderate certainty,” and has no effect on mortality with “high certainty” [27]. Yet, the meta-analysis reported here suggests significant benefit. No deaths have been reported in any of the pre-exposure prophylaxis studies, and the drugs have been well tolerated with very low rates of treatment discontinuation (Figs A5–A6 in S1 Appendix) [32].
Few drugs have excited such controversy as hydroxychloroquine in COVID-19. Bureaucracy, politicisation, and polarised debate obstructed the undertaking of clinical trials needed to provide objective evidence early in the pandemic. Now, with increasing vaccine coverage, declining viral virulence and availability of effective medicines, there is little reason to recommend the 4-aminoquinolines for COVID-19 chemoprevention. But earlier in the pandemic mortality was much higher, there were no vaccines or drugs, and healthcare systems were under intense strain as their workforces were depleted by illness. If these results had been available then, a case could have been made to deploy these moderately effective, inexpensive, available, and safe 4-aminoquinolines more widely. To put the moderate prophylactic efficacy of HCQ/CQ in perspective, the WHO stated initially that COVID-19 vaccines with a protective efficacy of at least 30% would be considered [33]. In those early days, the rapid spread and the initial high mortality of COVID-19 in the absence of proven effective therapies or vaccines created global concern but, despite the seriousness of the pandemic, there were major obstacles to the conduct of urgently needed clinical research, particularly in low resource settings. In addition, the endorsement of unproven medicines and remedies (including hydroxychloroquine), and later warnings and revocations by regulatory authorities [29], the associated politicisation, and the intense media scrutiny excited controversy and polarised views. All of these factors compromised objective scientific evaluation.
There are significant limitations to this study. The final sample size was substantially smaller than intended which limits confidence in the preventive effect estimates. At the beginning of the pandemic we did not know what the incidence of COVID-19 would be, nor what magnitude of preventive benefit would be considered enough to warrant use of these medicines in prophylaxis. The trial was conducted in many resource-limited settings where collection of respiratory swab specimens and PCR diagnosis was difficult, so only a small proportion of cases could be confirmed by PCR. Seroconversion over 3 months is a less precise measure of symptomatic infection. In particular, the DBS method of assessment, although employed in other studies [30], was not validated and did not perform well in this study. A significant proportion of participants were lost to follow-up (Fig 1) although these were balanced across arms (Fig A7 in S1 Appendix) and the PP analysis corresponded well with that of the ITT population (Tables A2–A3 in S1 Appendix). In addition, some participant outcomes were not evaluable because the end-of-study results were either unavailable (early drop-out), or were considered to be unreliable by the independent Serology Endpoint Assessment Committee (Table 2 and Table A5 in S1 Appendix). Although the inclusion of the DBS assessment was prespecified in the composite endpoint, this likely reduced the precision of the endpoint ascertainment and thus the estimate of preventive efficacy (Table 2). The study primary endpoints evaluated in the meta-analysis were slightly different, but meta-analysis of the PCR-confirmed infections indicates consistent evidence of moderate protective benefit (Fig A6 in S1 Appendix).
In summary, this large double-blind, placebo-controlled, trial showed that hydroxychloroquine and chloroquine prophylaxis was safe and well tolerated, and combined with data from other similar trials provided some evidence that laboratory-confirmed symptomatic COVID-19 might be reduced. The totality of evidence from this and other RCTs suggests a moderate preventive benefit. This knowledge could have proved beneficial earlier in the COVID-19 pandemic when vaccines and treatments were lacking, and mortality and morbidity were high. RCTs should be supported during pandemics.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(PDF)
(XLSX)
List of COPCOV study sites. Table A2. Baseline characteristics in the COPCOV trial (Per Protocol Analysis). Table A3. Outcomes of Chloroquine/Hydroxychloroquine and Placebo Pre-exposure Prophylaxis against COVID-19 in the COPCOV study (Per Protocol Analysis). Table A4. Summary of Serious Adverse Events in the COPCOV study. Table A5. Primary and secondary outcomes of Chloroquine/Hydroxychloroquine Therapy for Pre-exposure Prophylaxis against COVID-19 (missing outcomes treated as not having had COVID-19 during the study period) ITT–Results presented as “Risk differences.” Table A6. Outcomes of Chloroquine/Hydroxychloroquine and Placebo Pre-exposure Prophylaxis against COVID-19 in the COPCOV study (removing cases for which the SEAC judged that a study endpoint could not be determined). Table A7. Summary characteristics of previously published pre-exposure prophylaxis studies considered for meta-analysis. Table A8. Listing of causes of PCR-confirmed respiratory illness. Fig A1. Atlas showing those countries in which investigators were contacted to enquire whether they would be interested in, and able to join the COPCOV study. Fig A2. Atlas showing the location of the COPCOV trial sites which recruited participants, the 4-aminoquinoline tested, and the approximate numbers recruited. Fig A3. Graph showing cumulative enrollment over time (per week) by country. Fig A4. Funnel plot showing the 4-aminoquinoline COVID-19 pre-exposure chemoprevention RCTs included in the prespecified meta-analysis, and the relationship between point estimate risk ratios for the primary outcome and the corresponding standard errors. Fig A5. Meta-analysis of 4-aminoquinoline COVID-19 pre-exposure chemoprevention RCTs based on individual study primary endpoints using Risk Of Bias tool (RoB 2). Fig A6. Meta-analysis of the safety and tolerability outcomes in COVID-19 chemoprevention RCTs using the same methodology as reported in the WHO living guideline [24]. Fig A7. Meta-analysis of adverse events leading to treatment discontinuation reported in double-blind, placebo-controlled, 4-aminoquinoline COVID-19 pre-exposure chemoprevention RCTs. Fig A8. Graph showing cumulative loss to follow-up (LTFU) for Hydroxychloroquine/ Chloroquine arms and Placebo arm.
(DOCX)
Acknowledgments
We are very grateful to all the participants of this study and the many nurses, technicians, laboratory scientists, doctors, and support staff who helped with the study.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- CQ
chloroquine
- DBS
dried blood spot
- DSMB
Data Safety and Monitoring Board
- HCQ
hydroxychloroquine
- ITT
intention-to-treat
- PP
per protocol
- RCT
randomised controlled trial
- RR
risk ratio
- SAE
serious adverse event
- SAP
Statistical Analysis Plan
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SEAC
Serology Endpoint Assessment Committee
Data Availability
The data underlying the results presented in the study are available from https://github.com/jwatowatson/COPCOV.
Funding Statement
This research was funded by the Wellcome Trust [Grant number 221307/Z/20/Z] through the COVID-19 Therapeutics Accelerator.
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Last accessed 2023 Oct 25.
- 2.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledford H, Van Noorden R. High-profile coronavirus retractions raise concerns about data oversight. Nature. 2020;582:160. doi: 10.1038/d41586-020-01695-w Last accessed 2024 Feb 19. [DOI] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schilling WH, White NJ. Does hydroxychloroquine still have any role in the COVID-19 pandemic? Expert Opin Pharmacother. 2021;22:1257–1266. doi: 10.1080/14656566.2021.1898589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Therapeutics and COVID-19. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2020.1. Last accessed 2023 Oct 25. [Google Scholar]
- 8.White NJ, Strub-Wourgaft N, Faiz A, Guerin PJ. Guidelines should not pool evidence from uncomplicated and severe COVID-19. Lancet. 2021;397(10281):1262–1263. doi: 10.1016/S0140-6736(21)00469-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulla P. India expands use of controversial drug for coronavirus despite safety concerns. Nature. 2020. doi: 10.1038/d41586-020-01619-8 Last accessed 2024. Feb 19. [DOI] [PubMed] [Google Scholar]
- 10.Boulware DR, Pullen MF, Bangdiwala AS, Stankiewicz Karita HC, Johnston C, Thorpe LE, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed F, Hassan M, Arif MA, Batool S, Niazi R, Laila UE, et al. Pre-exposure Prophylaxis With Various Doses of Hydroxychloroquine Among Healthcare Personnel With High-Risk Exposure to COVID-19: A Randomized Controlled Trial. Cureus. 2021;13:e20572. doi: 10.7759/cureus.20572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abella BS, Jolkovsky EL, Biney BT, Uspal JE, Hyman MC, Frank I, et al. Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial. JAMA Intern Med. 2021;181:195–202. doi: 10.1001/jamainternmed.2020.6319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitjà O, Corbacho-Monné M, Ubals M, Alemany A, Suñer C, Tebé C, et al. A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19. N Engl J Med. 2021;384(5):417–427. doi: 10.1056/NEJMoa2021801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grau-Pujol B, Camprubi-Ferrer D, Marti-Soler H, Fernández-Pardos M, Carreras-Abad C, Andrés MV, et al. Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: a double-blind, placebo-controlled randomized clinical trial. Trials. 2021;22:808. doi: 10.1186/s13063-021-05758-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajasingham R, Bangdiwala AS, Nicol MR, Skipper CP, Pastick KA, Axelrod ML, et al. Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial. Clin Infect Dis. 2021;72:e835–e843. doi: 10.1093/cid/ciaa1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seet RCS, Quek AML, Ooi DSQ, Sengupta S, Lakshminarasappa SR, Koo CY, et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int J Infect Dis. 2021;106:314–322. doi: 10.1016/j.ijid.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinnon JE, Wang DD, Zervos M, Saval M, Marshall-Nightengale L, Kilgore P, et al. Safety and tolerability of hydroxychloroquine in health care workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study. Int J Infect Dis. 2022;116:167–173. doi: 10.1016/j.ijid.2021.12.343 ; PMCID: PMC8695310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas-Serrano J, Portillo-Vasquez AM, Thirion-Romero I, Vázquez-Pérez J, Mejía-Nepomuceno F, Ramírez-Venegas A, et al. Hydroxychloroquine for prophylaxis of COVID-19 in health workers: A randomized clinical trial. PLoS ONE. 2022;17:e0261980. doi: 10.1371/journal.pone.0261980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naggie S, Milstone A, Castro M, Collins SP, Lakshmi S, Anderson DJ, et al. Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine (HERO-HCQ). Int J Infect Dis. 2023. Apr;129:40–48. doi: 10.1016/j.ijid.2023.01.019 Epub 2023 Jan 20. ; PMCID: PMC9851717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polo R, Garcia-Albeniz X, Teran C, Morales M, Rial-Crestelo D, Garcinuño MA, et al. Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo-controlled randomized trial in healthcare workers. Clin Microbiol Infect. 2023 Jan;29(1):85–93. doi: 10.1016/j.cmi.2022.07.006 Epub 2022. Aug 5. ; PMCID: PMC9352647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirupakuzhi Vijayaraghavan BK, Jha V, Rajbhandari D, Myatra SN, Ghosh A, Bhattacharya A, et al. Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: a multicentre, parallel-group randomised controlled trial from India. BMJ Open. 2022. Jun 1;12(6):e059540. doi: 10.1136/bmjopen-2021-059540 ; PMCID: PMC9160584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llanos-Cuentas A, Schwalb A, Quintana JL, Delfin B, Alvarez F, Ugarte-Gil C, et al. Hydroxychloroquine to prevent SARS-CoV-2 infection among healthcare workers: early termination of a phase 3, randomised, open-label, controlled clinical trial. BMC Res Notes. 2023;16(1):22. doi: 10.1186/s13104-023-06281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasri E, Fakhim H, Salahi M, Ghafel S, Pourajam S, Darakhshandeh A, et al. Efficacy of Hydroxychloroquine in Pre-exposure Severe Acute Respiratory Syndrome Coronavirus 2 Prophylaxis among High-Risk HealthCare Workers: A Multicenter Study. Adv Biomed Res. 2023;12:3. doi: 10.4103/abr.abr_104_21 ; PMCID: PMC10012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Albeniz X, Del Amo J, Polo R, Morales-Asencio JM, Hernán MA. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. Eur J Epidemiol. 2022;37:789–796. doi: 10.1007/s10654-022-00891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO Living guideline: Drugs to prevent COVID-19. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-prophylaxes-2021-1. Last accessed 2023. Oct 25. [Google Scholar]
- 26.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macedo ACL, Prestes GDS, Colonetti T, Candido ACR, Uggioni MLR, Gomes AC, et al. A systematic review and meta-analysis of the accuracy of SARS-COV-2 IGM and IGG tests in individuals with COVID-19. J Clin Virol. 2022;148:105121. doi: 10.1016/j.jcv.2022.105121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US FDA. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. 2020. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Last accessed 2023. Oct 25. [Google Scholar]
- 30.Lofgren SM, Okafor EC, Colette AA, Pastick KA, Skipper CP, Pullen MF, et al. Feasibility of SARS-CoV-2 Antibody Testing in Remote Outpatient Trials. Open Forum Infect Dis. 2021. Oct 6;8(11):ofab506. doi: 10.1093/ofid/ofab506 ; PMCID: PMC8522439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lofgren SM, Nicol MR, Bangdiwala AS, Pastick KA, Okafor EC, Skipper CP, et al. Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19. Open Forum Infect Dis. 2020. Oct 19;7(11):ofaa500. doi: 10.1093/ofid/ofaa500 ; PMCID: PMC7654376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilling WH, Callery JJ, Chandna A, Hamers RL, Watson JA, White NJ. The WHO guideline on drugs to prevent COVID-19: small numbers- big conclusions. Wellcome Open Res. 2021;6:e71. doi: 10.12688/wellcomeopenres.16741.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause P, Fleming TR, Longini I, Henao-Restrepo AM, Peto R. World Health Organization Solidarity Vaccines Trial Expert Group. COVID-19 vaccine trials should seek worthwhile efficacy. Lancet. 2020;396:741–743. doi: 10.1016/S0140-6736(20)31821-3 ; PMCID: PMC7832749. [DOI] [PMC free article] [PubMed] [Google Scholar]