Abstract
Purpose:
To assess the possible association between MIR200B variations and sight-threatening diabetic retinopathy (STDR).
Methods:
A total number of 141 diabetes mellitus patients were enrolled in the study and divided into two groups including 76 patients diagnosed with STDR assigned to the case group, and 65 subjects without STDR considered in the control group. Peripheral blood specimens were used to extract the DNA content, and the primary MIR200B encoding sequence was amplified using a polymerase chain reaction. Then, the amplified DNA was sequenced by the Sanger method. The sequences were compared to the MIR200B reference sequence to find sequence variations. RNAfold, miRVaS, and Mfold bioinformatics web servers were employed to predict the potential effects of the identified variations on RNA structure.
Results:
Two MIR200B gene variants were identified. Although both variations were found more frequent in cases than controls, statistical analysis of allelic and genotypic features did not reach statistical significance.
Conclusions:
In silico analysis showed mild changes in MIR200B secondary structure and increased free energy in the presence of one of the identified variants (g.1167183G>A; rs72563729). Increasing the sample size in future studies may help a more accurate interpretation of the allelic association of MIR200B variations with STDR.
Keywords: MicroRNA, MIR200B, Polymorphisms, Sight-threatening diabetic retinopathy, Vascular endothelial growth factor
INTRODUCTION
Diabetes mellitus (DM) is a common health problem, that has reached a global alarming level. Almost 463 million adults suffered from DM worldwide in 2019.1 Diabetic retinopathy (DR) and diabetic macular edema (DME), known as the major issues reported for DM patients, are among the main causes of preventable blindness and vision impairment around the world.2 It has been estimated that 3.8 million individuals suffered blindness or moderate-to-severe vision impairment due to DR globally in 2020.3 Early diagnosis and timely therapeutic strategies can be largely effective in preventing DM-induced blindness.4 Prolonged duration of DM, obesity, hypertension (HTN), dyslipidemia, and pregnancy are the major known predisposing factors for developing DR. These items; however, cannot anticipate the definite progression of DR.5,6 In other words, patients with similar metabolic risk factors can exhibit different grades of progression of DR.7 Epigenetic regulations also play a role in the pathophysiology of DR.8
Clinical trials have revealed a genetic predisposition for sight-threatening DR (STDR).9,10,11 However, epidemiological studies have shown differences in DR genetic association among different ethnic groups.12 A handful of studies have been published to determine the genotype–phenotype association of candidate genes with DR development.12 Vascular endothelial growth factor (VEGF), aldose reductase, receptor for advanced glycation end products (RAGE), and endothelial nitric oxide synthase (eNOS) are the most investigated genes in association with DR.13,14 VEGF is one of the most studied genes, for which a number of polymorphisms have been detected in DR-affected patients among various populations.15 However, these reports have failed to reach a consensus among different ethnicities.16 VEGF protein expression is increased in response to hypoxia and inflammatory conditions17,18 and plays a role in the pathogenesis of proliferative DR (PDR) and DME through causing impairment in retinal capillary permeability.19
Recent studies have unveiled unexpected roles of microRNAs (miRNAs) in various types of human diseases suggesting their potential applications in clinical diagnosis, prognosis prediction in the affected patients, and also as therapeutic targets.20,21 MiRNAs are a class of noncoding RNAs characterized by a short length of 19–22 nucleotides, which are known to be involved in the regulation of critical cell functions through affecting the expression of a particular target protein at the posttranscriptional level.22,23 By binding to target messenger RNAs (mRNAs), miRNAs usually “sponge” and inhibit their expression, thus exert their regulatory functions.24 Dysregulation of miRNAs has been reported to be correlated with a variety of human disorders.25,26 Alterations in the sequence of miRNA coding genes can affect their function at different stages, including transcription, maturation, secondary structures, and their ability to bind to target.26 Such changes eventually can facilitate pathologic mechanisms leading to disease development or progression.26
Sequence variations in miRNAs targeting VEGF mRNA could plausibly be associated with microvascular complications of DR.27 By exploring a miRNA-specific search engine, FirePlex Discovery Engine (available online at: https://www.fireflybio.com), we found 89 miRNAs in 73 publications in association with DR due to their effects on the VEGF expression. This tool extracts and releases a list composed of the most important miRNAs and associated genes from the scientific publications for any keyword or topic. Most of the miRNA reports were focused on MIR200B. Statistically significant differences in the MIR200B expression in DR patients compared to control groups have been shown in previous studies.28,29
As far as we found from the literature, just a small number of experiments have explored the association of polymorphisms in miRNA coding genes and DR pathogenesis. For instance, the association of two polymorphisms in MIR-126 and MIR-146A, two other commonly reported miRNAs affecting VEGF expression based on the Fireplex discovery engine, have been investigated in DR patients.30,31 The current study investigated whether MIR200B sequence variations are associated with STDR in a case–control study of Iranian DM patients.
METHODS
Participants were recruited from patients with type 1 or type 2 DM referred to Labbafinejad Medical Center, Shahid Beheshti University of Medical Sciences, and Islamshahr Branch of the Iranian Diabetes Society, between November 2018 and November 2020. The protocol of the study was approved by the Ethics Committee of the Ophthalmic Research Center affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethical approval code number: IR.SBMU.ORC.REC.1397.028). Furthermore, the study protocol conformed to the Declaration of Helsinki32 and written informed consent was obtained from all participants.
The demographic information, history of HTN, hyperlipidemia, and ocular condition were obtained from the patients. Patients with a history of myocardial infarction, cerebrovascular accident, renal insufficiency (serum creatinine levels of ≥1.2 and ≥1.4 mg/dL for females and males, respectively), and the pregnant or breastfeeding subjects were excluded from the study. The eligible subjects underwent comprehensive ophthalmologic evaluations including assessment of visual acuity and intraocular pressure. The fundus examination was performed by the retina specialist using a slit-lamp biomicroscope and + 78 diopter (D)/+90 D lenses. To measure the hemoglobin A1c (HbA1c) levels, serum creatinine, and also to extract DNA, blood specimens were obtained from patients.
The severity of DR and DME were determined based on international classification.33 Assigning the participants to the case and control groups was conducted according to the worse eye severity. Patients with severe nonproliferative DR (NPDR), PDR, and/or DME were categorized as the case group (STDR), and patients without DR or mild and moderate NPDR were categorized as the control group (non-STDR).
DNA extraction was done using the standard salting-out protocol.34 Then, polymerase chain reaction was conducted on the extracted DNA using the Taq DNA Polymerase 2x Master Mix RED (Ampliqon, Cat. No. A180301) to amplify the entire primary miRNA (pri-miRNA) encoding sequence of MIR200B (miRBase Accession Number: MI0000342; http://www.mirbase.org/) and its flanking regions under the condition summarized in Table 1 by MJ Mini Gradient Thermal Cycler (Bio-Rad, Hercules, California, United States). Primers were designed using GeneRunner version 3.05 software (http://www.generunner.net/) and checked for the specificity to the target sequence with NCBI Primer-Blast tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The sequences of the primer pair used for the amplification reaction are also shown in Table 1. Sanger method was employed to sequence the amplicons using ABI BigDye Terminator chemistry with an ABI 3730XL genetic analyzer instrument (Applied Biosystems, Foster City, CA, USA), and the retrieved data were aligned to the reference sequence Ensembl genome browser, ENSG00000207730; https://www.ensembl.org) via utilizing Sequencher 5.0 software (Gene Codes Corporation, Ann Arbor, MI, USA).
Table 1.
Primer sequences and conditions of the amplification
| Primer | Sequence |
|---|---|
| Forward strand | 5’ AGCGAGTCCCATGCAACC 3’ |
| Reverse primer | 5’ CATTCCGGGGTCTCTGAG 3’ |
|
| |
| PCR condition | Temperature and time |
|
| |
| Primary denaturation | 95°C, 10 min |
| 35 repeated cycles including 3 steps of | |
| Denaturation | 95°C, 1 min |
| Annealing | 57°C, 1 min |
| Extension | 72°C, 40 s |
| Final extension | 72°C, 5 min |
PCR: Polymerase chain reaction
To computationally predict the secondary structure and stability of MIR200B in the presence of identified genetic variation positioned in pri-MIR200B encoding sequence, three bioinformatics tools including Mfold (http://mfold.rna.albany.edu/?q=mfold/DNA-Folding-Form),35 miRVaS (http://mirvas.bioinf.be/),36 and RNAfold (http://rna.tbi.univie.ac.at/cgibin/RNAWebSuite/RNAfold.cgi)37 on web servers were employed. First, Gibbs free energy was calculated by the tools to estimate the stability of the predicted structures. Subsequently, structures possessing the lowest levels of free energy were retrieved from the corresponding tools.
Statistical analysis
The demographic features were analyzed with the Student’s t-test in the Statistical Package for the Social Sciences SPSS software (version 25, IBM Corporation, Armonk, NY, USA). The frequencies of alleles and genotypes were first evaluated using a Chi–square test and generalized estimating equations. Then, we constructed the multivariate logistic regression models for the i, and ii models, which then were adjusted using age, and sex for the i model in addition to DM duration, HbA1c levels, HTN, and hyperlipidemia adjusted for the ii model. In data analysis, P < 0.05 were considered significant.
We performed the power analysis based on the odd ratio of g.1167183G>A and considered the sample size of 141 using the G * Power version 3.1.9.6 software (Heinrich Heine University Düsseldorf, Düsseldorf, Germany). We obtained the power of 86%.
RESULTS
A total of 141 patients, comprising 76 cases and 65 controls, were recruited to the study. Table 2 shows the patients’ characteristics. Variations of statistical significance were seen between the groups in several parameters such as age (P < 0.001), type of DM (P < 0.001), duration of DM (P = 0.001), self-reporting HTN (P < 0.001), and HbA1c (P < 0.001).
Table 2.
Demographic features and clinical characteristics of participants
| Group | P | ||
|---|---|---|---|
|
| |||
| Control (n=65) | Case (n=76) | ||
| Sex | |||
| Male, n (%) | 32 (49.2) | 35 (46.1) | 0.706 |
| Female | 33 (50.8) | 41 (53.9) | |
| Age (years) | |||
| Mean±SD | 46.58±18.5 | 58.04±10.87 | <0.001 |
| Median (range) | 52 (18–79) | 59.5 (23–80) | |
| Type of DM, n (%) | |||
| Type 1 | 23 (35.4) | 7 (9.2) | <0.001 |
| Type 2 | 42 (64.6) | 69 (90.8) | |
| Duration of DM (years) | |||
| Mean±SD | 11.52±6.45 | 15.94±7.91 | 0.001 |
| Median (range) | 10 (1–28) | 15 (3–45) | |
| Self-reporting HTN, n (%) | |||
| Yes | 17 (26.6) | 43 (56.6) | <0.001 |
| No | 47 (73.4) | 33 (43.4) | |
| Self-reporting hyperlipidemia, n (%) | |||
| Yes | 23 (36.5) | 38 (50.0) | 0.111 |
| No | 40 (63.5) | 38 (50.0) | |
| HbA1c (mmol/mol) | |||
| Mean±SD | 7.85±1.79 | 9.29±2.05 | <0.001 |
| Median (range) | 7.64 (5–12.1) | 9.05 (5.8–15.6) | |
| Serum ceratinin (mg/dL) | |||
| Mean±SD | 0.96±0.11 | 0.97±0.08 | 0.786 |
| Median (range) | 0.97 (0.7–1.16) | 0.95 (0.86–1.07) | |
n (%). DM: Diabetes mellitus, HbA1c: Hemoglobin A1c, HTN: Hypertension, SD: Standard deviation
A 323 base pairs-length DNA fragment including the entire pri-miRNA encoding sequence of MIR200B (Chromosome 1: 1,167,104–1,167,198) was amplified and sequenced for all samples. The results showed two different variations in MIR200B sequence (NC_000001.11; GRCH38.p12) including g.1167183G>A (rs72563729) and g.1167277C>T [Figure 1]. The g.1167183G>A (rs72563729) variation was found in 3 individuals among the STDR patients and 1 in the control group, all in a heterozygous state [Figure 1]. This polymorphism has been reported at a low frequency of 0.01 in the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) and the combined annotation-dependent depletion score of 13.86 has been calculated for this variant. The corresponding frequency in the healthy Iranian population has been found higher (0.025) according to the Iranome genome database (http://www.iranome.ir/), which presents all discovered genetic variations in 800 healthy Iranian individuals of various ethnic groups.38 The g.1167183G>A (rs72563729) variation is positioned within the genome sequence of pri-MIR200B (NR_029639.1: N.80G>A). The g.1167277C>T variation, which has not been previously reported, was found in four cases (three heterozygous and one homozygous) and one heterozygous control [Figure 1]. It is positioned 79 nucleotides downstream of the pri-miRNA sequence. One of the STDR cases was identified as heterozygous for both of the variations.
Figure 1.
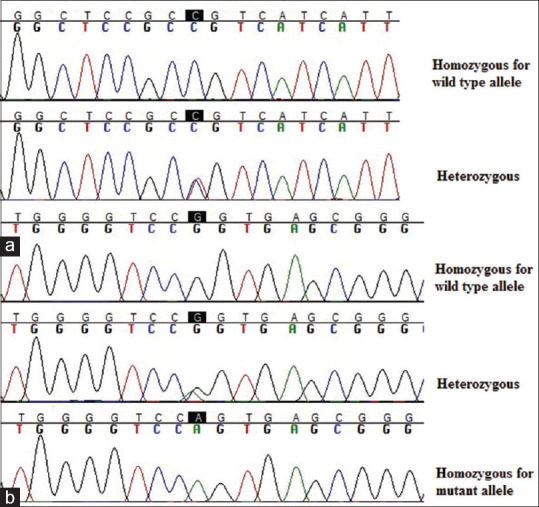
DNA sequence chromatograms of the detected variations in all identified genotypic features. (a) g.1167183G>A (rs72563729) variation; (b) g.1167277C>T variation. The sequences have been read by reverse primer and the site of the variations has been highlighted
Table 3 shows the allelic frequency and association of the identified MIR200B variants in two investigated groups. As most of the clinical features with potential impact on DR were significantly different between the case and control participants, these parameters were imported to two logistic regression models for assessment of the possible correlation between the variations and DR. Statistical analysis showed no significant difference for risk of STDR in association with the MIR200B variants between the studied groups after multivariable adjustment. The genotypic analysis did not demonstrate any significant association of the variants with STDR under either recessive or dominant model [Table 4].
Table 3.
Allelic associations of the g.1167183G>A and g.1167277C>T variations with sight-threatening diabetic retinopathy
| Subjects | Allele | Frequency case (alleles), n=152 (76×2), n (%) | Frequency control (alleles), n=130 (65×2), n (%) | Unadjusted | Adjusteda | Adjustedb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| P | OR | 95% CI | χ 2 | P | OR | 95% CI | t-statistic | P | OR | 95% CI | t-statistic | ||||
| g.1167183G>A | |||||||||||||||
| T1DM | G | 14/14 (100) | 45/46 (97.8) | 0.766 | 0 | - | 0.309 | 0 | - | 0 | 0 | 0 | - | 0 | 0 |
| A | 0/14 (0) | 1/46 (2.2) | |||||||||||||
| T2DM | G | 135/138 (97.8) | 84/84 (100) | 0.238 | 0 | - | 1.851 | 0 | - | 0 | 0 | 0 | - | 0 | 0 |
| A | 3/138 (2.2) | 0/84 (0) | |||||||||||||
| Total | G | 149/152 (98) | 129/130 (99.2) | 0.454 | 2.63 | 0.267–25.919 | 0.727 | 0.322 | 3.459 | 0.296–40.409 | 1.241 | 0.253 | 5.07 | 0.314–81.844 | 1.623 |
| A | 3/152 (2) | 1/130 (0.8) | |||||||||||||
| g.1167277C>T | |||||||||||||||
| T1DM | C | 14/14 (100) | 45/46 (97.8) | 0.766 | 0 | - | 0.309 | 0 | - | 0 | 0 | 0 | - | 0 | 0 |
| T | 0/14 (0) | 1/46 (2.2) | |||||||||||||
| T2DM | C | 133/138 (96.4) | 84/84 (100) | 0.09 | 0.281 | 0.031–2.582 | 3.114 | 0 | - | 0 | 0 | 0 | - | 0 | 0 |
| T | 5/138 (3.6) | 0/84 (0) | |||||||||||||
| Total | C | 147/152 (96.7) | 129/130 (99.2) | 0.171 | 3.56 | 0.387–32.64 | 2.137 | 0.27 | 4.084 | 0.335–49.77 | 1.407 | 0.553 | 2.134 | 0.175–26.065 | 0.758 |
| T | 5/152 (3.3) | 1/130 (0.8) | |||||||||||||
aAdjusted probabilities after controlling for age and sex, bAdjusted probabilities after controlling for age, sex, duration of DM, HbA1c and self-reported HTN and hyperlipidemia. DM: Diabetes mellitus, T1DM: Type 1 DM, T2DM: Type 2 DM, OR: Odds ratio, CI: Confidence interval, χ2: Chi-square statistic, HbA1c: Hemoglobin A1c, HTN: Hypertension
Table 4.
Genotypic associations of the g.1167183G>A and g.1167277C>T variations with sight-threatening diabetic retinopathy
| Subjects | Additive model (AA vs. GG allele)b | Dominant model (AA + AG vs. GG)b | Recessive model (AA vs. AG + GG)b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | ||
| g.1167183G>A | ||||||||||
| T1DM | - | - | - | - | - | - | - | - | - | |
| T2DM | - | - | - | - | - | - | - | - | - | |
| Total | - | - | - | 0.407 | 2.63 | 0.267–25.919 | - | - | - | |
|
| ||||||||||
| Additive model (TT vs. CC allele)b | Dominant model (TT + TC vs. CC)b | Recessive model (TT vs. TC + CC)b | ||||||||
|
| ||||||||||
| g.1167277C>T | ||||||||||
| T1DM | - | - | - | 0.766 | 0.283 | 0.011–2.322 | >0.999 | - | - | |
| T2DM | - | - | - | 0.144 | 0.228 | 0.026–1.976 | 0.621 | 0.227 | 0.03–1.99 | |
| Total | >0.999 | 0.227 | 0.026–1.976 | 0.262 | 3.556 | 0.387–32.64 | >0.999 | 0.281 | 0.031–2.582 | |
bAdjusted probabilities after controlling for age, sex, duration of DM, HbA1c, and self-reported HTN and hyperlipidemia. Additive model: The model usually encodes “AA”, “Aa” and “aa” (“a” represents the minor allele) as three different numbers, implying the contribution of genotype “Aa” to the phenotype is different from “AA” and “aa”. In additive model, a linear increase is assumed based on the number of each copy of the minor or risk allele. DM: Diabetes mellitus, T1DM: Type 1 diabetes mellitus, T2DM: Type 2 diabetes mellitus, OR: Odds ratio, CI: Confidence interval, HbA1c: Hemoglobin A1c, HTN: Hypertension
The Gibbs free energy values (ΔG) for the formation of spontaneous pri-MIR200B structure for both G and A alleles in the 80th nucleotide of pri-MIR200B have been estimated using in silico tools. The conformation with the lowest estimated energy was considered the most stable. The results revealed that g.1167183G>A (rs72563729) variation cause increased ΔG by 1-2 Kcal/mol. The predicted structure also showed a larger loop formation in the presence of the variation [Figure 2].
Figure 2.
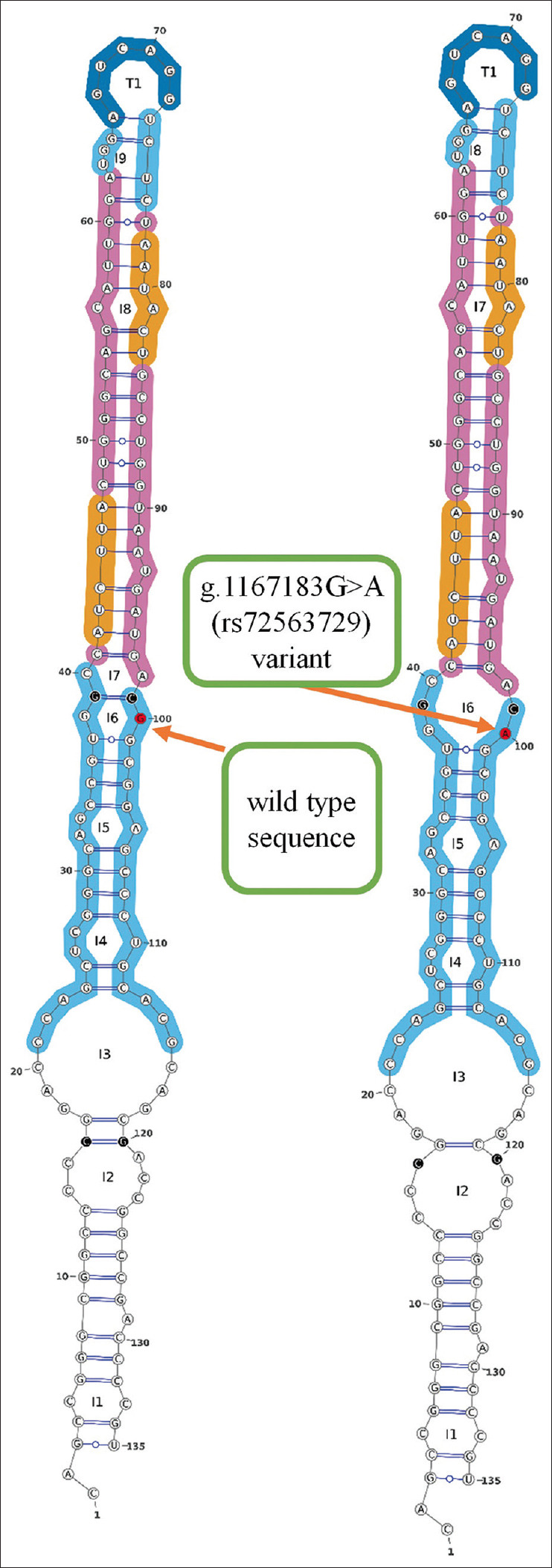
Predicted structure for MIR200B in the presence of g.1167183G>A (rs72563729) variant compared to the wild type sequence. Images retrieved from the miRVaS web server
DISCUSSION
Prompted by lack of studies covering the association of miRNAs variations with STDR and clarified diagnostic and therapeutic importance of miRNAs in various human diseases, in this study, we performed complete sequencing of MIR200B encoding gene, as the most considerable VEGF affecting miRNA in 141 Iranian patients with DM. Furthermore, the frequency of the detected alleles for the MIR200B gene was also calculated among the STDR-affected patients compared to the control group, and possible impacts of variants on the corresponded miRNA structure were predicted using in silico tools.
In this study, we detected two variants in MIR200B, including g.1167183G>A (rs72563729) and g.1167277C>T. The first variation was positioned in the nucleotide 80 of the pri-MIR200B sequence and the second change was found in the 79th nucleotide downstream of the pri-miRNA sequence. Despite the higher prevalence of both detected variants among STDR patients compared with the control group, statistical analysis did not show any significant difference. Retrieved data from RNAfold, miRVaS, and Mfold tools revealed mild changes in the MIR200B secondary structure and stability in the presence of g.1167183G>A (rs72563729) polymorphism compared to the wild-type sequence. A larger loop formation and increased ΔG are predicted in the presence of g.1167183G>A (rs72563729) variation. The predicted structural change is slight and functional analysis is required to assess the putative influence of the variation on MIR200B function.
Not significant, although a higher abundance of both variations among STDR patients, along with the relatively moderate frequency of g.1167183G>A (rs72563729) polymorphism among the healthy Iranian population (allele frequency: 0.02562),38 suggested a low probability for correlation between MIR200B variations and the risk of STDR. So far, MIR200B polymorphisms had not been studied for association with STDR and in this study, we screened, for the first time, the entire coding sequence of MIR200B to investigate this association. In two similar studies, the association of two polymorphisms in MIR-126 and MIR-146A, other VEGF affecting miRNAs, have been investigated in DR patients.30,31 The results showed that the rs4636297 of miR-126 is associated with STDR and the rs2910164 polymorphism in MIR-146A is also significantly associated with microvascular complications diabetic nephropathy in patients with type 1 DM and DME in patients with type 2 DM.30,31
From two detected variations in this study, g.1167277C>T variant is novel and there is no report for this polymorphism. The g.1167183G>A (rs72563729) variation has been proposed in two other studies for association to bone density and risk of fractures and susceptibility to lung cancer. MIR200B variants were considered for association to bone density because one of its target genes is TGFβ2 which has a well-known function in bone regulation and it was considered for association to lung cancer because of its supposed effect on E-cadherin expression and epithelial-to-mesenchymal transition program in lung cancer. No association was identified for the g.1167183G>A (rs72563729) variation in both of the studies.39,40
In conclusion, the small sample size used in this study was the major limitation of the experiment; and so further investigations with larger cohorts are suggested to determine the accurate association between MIR200B polymorphisms and STDR. On the other hand, screening other miRNAs targeting STDR-associated genes such as VEGF, RAGE, AR, and eNOS and assessing their correlation with STDR are recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would appreciate Dr. Davood Abbasi and the staff of the Islamshahr Branch of the Iranian Diabetes Society for their help on this project.
REFERENCES
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Burton MJ, Ramke J, Marques AP, Bourne RR, Congdon N, Jones I, et al. The lancet global health commission on global eye health: Vision beyond 2020. Lancet Glob Health. 2021;9:e489–551. doi: 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The right to sight: An analysis for the global burden of disease study. Lancet Glob Health. 2021;9:e144–60. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill L, Makaroff LE. Early detection and timely treatment can prevent or delay diabetic retinopathy. Diabetes Res Clin Pract. 2016;120:241–3. doi: 10.1016/j.diabres.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S, Chen H, Tinkham NH, Zhang K. Genetic susceptibility of diabetic retinopathy. Curr Diab Rep. 2008;8:257–62. doi: 10.1007/s11892-008-0046-6. [DOI] [PubMed] [Google Scholar]
- 7.Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–70. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852:2474–83. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:1829–39. [PubMed] [Google Scholar]
- 10.Arar NH, Freedman BI, Adler SG, Iyengar SK, Chew EY, Davis MD, et al. Heritability of the severity of diabetic retinopathy: The FIND-Eye study. Invest Ophthalmol Vis Sci. 2008;49:3839–45. doi: 10.1167/iovs.07-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hietala K, Forsblom C, Summanen P, Groop PH FinnDiane Study Group. Heritability of proliferative diabetic retinopathy. Diabetes. 2008;57:2176–80. doi: 10.2337/db07-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlmann K, Kovacs P, Boettcher Y, Hammes HP, Paschke R. Genetics of diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2006;114:275–94. doi: 10.1055/s-2006-924260. [DOI] [PubMed] [Google Scholar]
- 13.Jehanzeb M, Khan NU, Hussain M, Subrina J, Ayub S, Mustafa A. Association of candidate genes (ALR2, RAGE, and VEGF) polymorphisms with diabetic retinopathy in type 2 diabetic patients of Khyber Pakhtunkhwa, Pakistan. Mol Biol Rep. 2023;50:227–34. doi: 10.1007/s11033-022-08057-x. [DOI] [PubMed] [Google Scholar]
- 14.Suganthalakshmi B, Anand R, Kim R, Mahalakshmi R, Karthikprakash S, Namperumalsamy P, et al. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis. 2006;12:336–41. [PubMed] [Google Scholar]
- 15.Gong JY, Sun YH. Association of VEGF gene polymorphisms with diabetic retinopathy: A meta-analysis. PLoS One. 2013;8:e84069. doi: 10.1371/journal.pone.0084069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampton BM, Schwartz SG, Brantley MA, Jr, Flynn HW., Jr Update on genetics and diabetic retinopathy. Clin Ophthalmol. 2015;9:2175–93. doi: 10.2147/OPTH.S94508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: Pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–94. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–9. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–6. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- 20.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat Rev Drug Discov. 2012;11:860–72. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–22. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 22.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Najafi S, Tan SC, Raee P, Rahmati Y, Asemani Y, Lee EH, et al. Gene regulation by antisense transcription: A focus on neurological and cancer diseases. Biomed Pharmacother. 2022;145:112265. doi: 10.1016/j.biopha.2021.112265. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Sall A, Yang D. MicroRNA: An emerging therapeutic target and intervention tool. Int J Mol Sci. 2008;9:978–99. doi: 10.3390/ijms9060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, et al. Interplay between miRNAs and human diseases. J Cell Physiol. 2018;233:2007–18. doi: 10.1002/jcp.25854. [DOI] [PubMed] [Google Scholar]
- 26.Cammaerts S, Strazisar M, De Rijk P, Del Favero J. Genetic variants in microRNA genes: Impact on microRNA expression, function, and disease. Front Genet. 2015;6:186. doi: 10.3389/fgene.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massignam ET, Dieter C, Pellenz FM, Assmann TS, Crispim D. Involvement of miR-126 rs4636297 and miR-146a rs2910164 polymorphisms in the susceptibility for diabetic retinopathy: A case-control study in a type 1 diabetes population. Acta Ophthalmol. 2021;99:e461–9. doi: 10.1111/aos.14638. [DOI] [PubMed] [Google Scholar]
- 28.Li EH, Huang QZ, Li GC, Xiang ZY, Zhang X. Effects of miRNA-200b on the development of diabetic retinopathy by targeting VEGFA gene. Biosci Rep. 2017;37:BSR20160572. doi: 10.1042/BSR20160572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomaa AR, Elsayed ET, Moftah RF. MicroRNA-200b expression in the vitreous humor of patients with proliferative diabetic retinopathy. Ophthalmic Res. 2017;58:168–75. doi: 10.1159/000475671. [DOI] [PubMed] [Google Scholar]
- 30.Kaidonis G, Gillies MC, Abhary S, Liu E, Essex RW, Chang JH, et al. Asingle-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasian patients. Acta Diabetol. 2016;53:643–50. doi: 10.1007/s00592-016-0850-4. [DOI] [PubMed] [Google Scholar]
- 31.McAuley AK, Dirani M, Wang JJ, Connell PP, Lamoureux EL, Hewitt AW. A genetic variant regulating miR-126 is associated with sight threatening diabetic retinopathy. Diab Vasc Dis Res. 2015;12:133–8. doi: 10.1177/1479164114560160. [DOI] [PubMed] [Google Scholar]
- 32.World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 34.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–15. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cammaerts S, Strazisar M, Dierckx J, Del Favero J, De Rijk P. miRVaS: A tool to predict the impact of genetic variants on miRNAs. Nucleic Acids Res. 2016;44:e23. doi: 10.1093/nar/gkv921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. The vienna RNA websuite. Nucleic Acids Res. 2008;36:W70–4. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattahi Z, Beheshtian M, Mohseni M, Poustchi H, Sellars E, Nezhadi SH, et al. Iranome: A catalog of genomic variations in the Iranian population. Hum Mutat. 2019;40:1968–84. doi: 10.1002/humu.23880. [DOI] [PubMed] [Google Scholar]
- 39.De-Ugarte L, Caro-Molina E, Rodríguez-Sanz M, García-Pérez MA, Olmos JM, Sosa-Henríquez M, et al. SNPs in bone-related miRNAs are associated with the osteoporotic phenotype. Sci Rep. 2017;7:516. doi: 10.1038/s41598-017-00641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leng S, Bernauer AM, Zhai R, Tellez CS, Su L, Burki EA, et al. Discovery of common SNPs in the miR-205/200 family-regulated epithelial to mesenchymal transition pathway and their association with risk for non-small cell lung cancer. Int J Mol Epidemiol Genet. 2011;2:145–55. [PMC free article] [PubMed] [Google Scholar]


