Abstract
The products of the A33R and A36R genes of vaccinia virus are incorporated into the membranes of intracellular enveloped virions (IEV). When extracts of cells that had been infected with vaccinia virus and labeled with H332PO4 were immunoprecipitated with antibodies against the A33R protein, two prominent bands were resolved. The moderately and more intensely labeled bands were identified as phosphorylated A33R and A36R proteins, respectively. The immunoprecipitated complex contained disulfide-bonded dimers of A33R protein that were noncovalently linked to A36R protein. Biochemical analysis indicated that the two proteins were phosphorylated predominantly on serine residues, with lesser amounts on threonines. The A36R protein was also phosphorylated on tyrosine, as determined by specific binding to an anti-phosphotyrosine antibody. Serine phosphorylation and A33R-A36R protein complex formation occurred even when virus assembly was blocked at an early stage with the drug rifampin. Tyrosine phosphorylation was selectively reduced in cells infected with F13L or A34R gene deletion mutants that were impaired in the membrane-wrapping step of IEV formation. In addition, tyrosine phosphorylation was specifically inhibited in cells infected with an A33R deletion mutant that still formed IEV. Immunofluorescence and immunoelectron microscopy indicated that in the absence of the A33R protein, the A36R protein was localized in Golgi membranes but not in IEV. In the absence of the A36R protein, however, the A33R protein still localized to IEV membranes. These studies together with others suggest that the A33R protein guides the A36R protein to the IEV membrane, where it subsequently becomes tyrosine phosphorylated as a signal for actin tail formation.
Vaccinia virus, the prototypic member of the poxvirus family, is a complex enveloped DNA virus with antigenically distinct intracellular and extracellular infectious forms (1, 5). Although the majority of intracellular mature virions (IMV) reside in the cytoplasm until cell lysis, some are wrapped by additional membranes and transported to the cell surface. Two related types of extracellular virions have been identified: adherent cell-associated enveloped virions (CEV), which are needed for efficient cell-to-cell spread (4), and released extracellular enveloped virions (EEV), which promote long range dissemination (19).
Although the mechanism of formation of IMV is still poorly understood, considerable progress has been made in identifying the cellular membranes and viral proteins required for extracellular virion formation. The double membranes that wrap IMV to form intracellular enveloped virions (IEV) are derived from late Golgi or endosomal cisternae (15, 25). IEV are transported to the cell periphery, where they acquire actin tails, similar to those made by other intracellular pathogens such as Listeria and Shigella spp., and form the tips of protruding microvilli (6, 14, 16, 27). Virions are externalized at the plasma membrane, apparently losing the outermost of the two IEV-specific membranes in the process.
Six proteins, encoded by the F13L, B5R, A33R, A34R, A36R, and A56R open reading frames (ORFs), have been identified as constituents of the IEV or EEV membrane (8, 10, 17, 18, 20, 22, 29). Deletion of any of these ORFs, except for A56R, which encodes the viral hemagglutinin, yields a mutant with a small-plaque phenotype. Mutants with deleted F13L or B5R (3, 11, 30) ORFs have defects in membrane wrapping that lead to reduced production of CEV and EEV. The small-plaque phenotypes of A33R, A34R, or A36R deletion mutants, however, correlated with defects in actin tail formation rather than with reduced production of EEV (21, 24, 31, 33). The latter results indicated that actin tails were important for virus spread rather than for the egress of vaccinia virus.
Based on the above observations, we proposed that the A33R, A34R, and A36R proteins interact to form a platform for nucleation of actin tails (33). While we were carrying out experiments to further investigate this hypothesis, evidence for physical associations among EEV proteins and between tyrosine-phosphorylated A36R protein and the cellular adapter protein Nck, leading to the recruitment of N-WASP to the site of actin assembly, was reported (12, 23). Here, we confirm interactions between the A33R and A36R proteins and demonstrate that both are phosphorylated predominantly on serine residues and that viral membrane localization and tyrosine phosphorylation of the A36R protein are dependent on expression of the A33R protein.
MATERIALS AND METHODS
Cells and viruses.
Cells and virus were propagated as previously described (9). Unless otherwise specified, HeLa cells were infected with trypsin-treated vaccinia virus stocks at a multiplicity of 10 PFU in medium containing 2.5% fetal bovine serum.
Antibodies.
Rabbit antiserum α-A36RC was raised to a C-terminal peptide of A36R (EHDDIESSVVSLV) coupled to keyhole limpet hemocyanin. Monoclonal antibody (MAb) 4 against the A33R protein (α-A33R) was a kind gift of L. Payne, MAb 19C2 (25) against the B5R protein and polyclonal antiserum against the F13L and B5R proteins were generous gifts from G. Hiller, and MAb PY99 against phosphotyrosine was purchased from Santa Cruz Laboratories.
Immunoprecipitations.
Cells were labeled with 50 to 100 μCi of [35S]methionine (Amersham) per ml of methionine-free medium or with 100 to 200 μCi of H332PO4 (ICN Biochemicals) per ml of phosphate-free medium for the indicated times. The cells were then incubated for 20 min in half-strength phosphate-buffered saline (PBS) containing 1% NP-40 supplemented with protease (complete protease inhibitor tablets; Roche Molecular Biochemicals) and phosphatase (sodium fluoride and sodium vanadate; Sigma-Aldrich) inhibitors. The lysates were cleared by centrifugation at 30,000 rpm for 60 min in a Beckman 42.2 Ti rotor and were incubated first with preimmune serum and then with protein A-Sepharose. Proteins were incubated with either α-A36RC or PY99. The antigen-antibody complexes were bound to protein A-Sepharose beads, washed three times with half-strength PBS containing 1% NP-40 and protease inhibitors, resuspended in Laemmli sample buffer in the presence of sodium dodecyl sulfate (SDS) and dithiothreitol (DTT), and boiled for 3 min before application to an SDS-polyacrylamide gel. After electrophoresis, the gels were dried and subjected to autoradiography.
Phosphoamino acid analysis.
BS-C-1 cells were infected with vaccinia virus and metabolically labeled with H332PO4 as described above. After immunoprecipitation with α-A33R or α-A36RC, the phosphoproteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene difluoride membrane (Millipore). The band of interest was excised, and the protein was hydrolyzed in 6 M HCl for 60 min at 110°C. The hydrolysate was dried under a vacuum, resuspended, and analyzed by two-dimensional thin-layer electrophoresis (26).
Immunofluorescence.
At 8 h after infection, HeLa cells were fixed in 3% paraformaldehyde and stained with α-A36RC followed by rhodamine-conjugated anti-rabbit immunoglobulin G (IgG) (Dako Corp.). Cells were incubated with rat MAb 19C2 against the B5R protein followed by fluorescein isothiocyanate (FITC)-conjugated anti-rat IgG (Dako Corp.). Finally, cells were stained with 5 μg of Hoechst 33258 (Pierce)/ml, washed, and mounted in Fluoromount G (Southern Biotechnology Associates) before observation by confocal microscopy. Images were collected on a Leica model TCS NT laser scanning confocal microscope with an attached UV laser; each channel was collected separately and then merged.
Electron microscopy.
Infected BS-C-1 cells, grown in 60-mm dishes, were fixed with paraformaldehyde and cryosectioned as previously described (32). Thawed cryosections were incubated with either α-A36RC, α-A33R, or an antibody to the F13L protein. Sections were washed and incubated with protein A conjugated to 10-nm colloidal gold (Department of Cell Biology, Utrecht University School of Medicine, Utrecht, The Netherlands). Immunostained sections were viewed using a Philips CM100 transmission electron microscope.
RESULTS
Coimmunoprecipitation and phosphorylation of the A33R and A36R proteins.
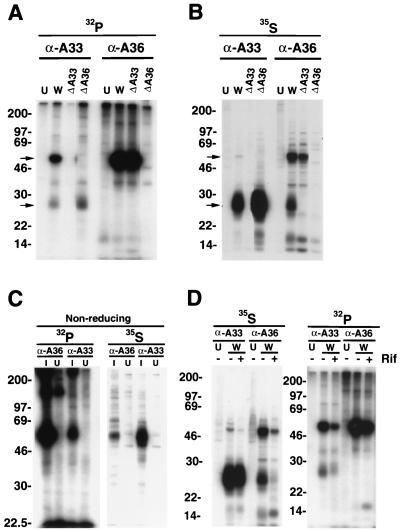
Recent studies indicating that some IMV membrane proteins were phosphorylated on serine, threonine, and tyrosine residues (2, 7, 28) prompted us to determine whether EEV proteins are also phosphorylated. Initially, we focused on the A33R protein because of its relatively long cytoplasmic tail (22). Cells were infected with wild-type (WR) or mutant vaccinia viruses and labeled from 8 to 24 h with H332PO4. NP-40 detergent lysates of these cells were incubated with a mouse MAb (α-A33R) to the A33R protein followed by protein A-Sepharose. The bound proteins were analyzed by SDS-PAGE and subjected to autoradiography. From 32P-labeled extracts of cells infected with WR, α-A33R immunoprecipitated a species of 28 to 30 kDa (referred to below as a 28-kDa band) with the expected molecular mass of the A33R protein (Fig. 1A). Unexpectedly, a more highly 32P-labeled protein of approximately 50 kDa coprecipitated with the A33R protein. Neither protein was immunoprecipitated from uninfected cells or from cells infected with an A33R deletion mutant (vΔA33R; Fig. 1A). We considered that the 50-kDa protein might be a dimer of the A33R protein (22) that was unusually resistant to reducing agents and SDS or another cellular or viral protein that interacted with the A33R protein. With regard to the latter possibility, the A36R protein seemed a likely candidate because of its size. However, the A36R protein had been classified as a type 2 membrane protein with an N-terminal hydrophobic domain (18) and therefore had no predicted cytoplasmic sites of phosphorylation. Nevertheless, the 32P-labeled 50-kDa protein was not precipitated from lysates of cells infected with the A36R deletion mutant vΔA36R (Fig. 1A).
FIG. 1.
SDS-PAGE analysis of metabolically labeled proteins from vaccinia virus-infected cells. BS-C-1 cells were uninfected (U) or were infected with vaccinia virus WR (W) or recombinant vaccinia viruses from which the A33R ORF (ΔA33) or the A36R ORF (ΔA36) had been deleted. After 8 h, infected and uninfected cells were labeled with H332PO4 (A, C, and D) or [35S]methionine (B, C, and D) for 18 h. Lysates were immunoprecipitated with either α-A33R (α-A33) or α-A36RC (α-A36). In panel D, cells were infected in the absence (−) or presence (+) of rifampin (Rif). Immunoprecipitated proteins were run on SDS-PAGE gels under reducing conditions (A, B, and D) or nonreducing conditions (C) and visualized by autoradiography. Masses of marker proteins (in kilodaltons) are given on the left. Arrows point to the 28-kDa A33R or the 50-kDa A36R protein.
To more directly identify the 50-kDa protein, rabbit polyclonal antiserum α-A36RC directed against a C-terminal peptide of the A36R protein was used. This antibody brought down a 32P-labeled 50-kDa band from extracts of cells infected with WR or vΔA33R but not from cells infected with vΔA36R or from uninfected cells, confirming its identity as the product of the A36R gene (Fig. 1A). Although immunoprecipitation of the 50-kDa band by α-A36RC seemed efficient, coprecipitation of the 32P-labeled 28-kDa band was not detected (Fig. 1A). The latter result could be due to excess uncomplexed 50-kDa protein and the much lower 32P labeling of A33R protein compared to A36R protein. As will be shown below, α-A36RC did coprecipitate [35S]methionine-labeled A36R and A33R proteins. Thus, these data indicated that 32P-labeled 28- and 50-kDa bands were derived from the A33R and A36R ORFs, respectively, and that the A36R and A33R proteins coimmunoprecipitated when a MAb to the latter was used. The phosphorylation of the A36R protein meant that it was unlikely to have a type 2 membrane topology. Evidence that the A36R protein is, in fact, a type 1b membrane protein with a long cytoplasmic tail was recently reported (23).
Parallel experiments, in which the infected cells were incubated with [35S]methionine, were also carried out. Because of the inhibition of host protein synthesis, only viral proteins should be labeled during an 8- to 24-h period. A predominant 28-kDa band was seen when extracts of [35S]methionine-labeled cells that had been infected with WR were immunoprecipitated with α-A33R (Fig. 1B). The labeled 50-kDa species was faint compared to the 28-kDa band. However, neither the 28- nor the 50-kDa 35S-labeled band was immunoprecipitated with α-A33R when the extracts were from uninfected cells or cells infected with vΔA33R, and only the 28-kDa band was observed when the extracts were from cells infected with vΔA36R (Fig. 1B). The difference between the 32P and 35S labeling of the A33R and A36R bands could not be explained by the relative numbers of methionines in the two proteins, because the A33R ORF contains six residues compared to five for the A36R ORF. Therefore, the difference in labeling signified that the A36R protein is more highly phosphorylated than the A33R protein.
Immunoprecipitation of extracts of the [35S]methionine-labeled cells was also carried out with α-A36RC. Apparently due to the greater labeling of the A33R protein with [35S]methionine compared to H332PO4, coprecipitation of the A33R and A36R proteins from extracts of cells infected with WR was clearly seen (Fig. 1B). As expected, the 28-kDa band was not immunoprecipitated from uninfected cells or from cells infected with vΔA33R, and neither the 50- nor the 28-kDa band was immunoprecipitated from cells infected with vΔA36R (Fig. 1B). In some individual experiments, however, we did not achieve efficient coprecipitation of the A33R protein with the A36R antibody.
The 32P- and 35S-labeled proteins from cells infected with WR were also analyzed by SDS-PAGE under nonreducing conditions. The major labeled protein that was immunoprecipitated with either α-A33R or α-A36RC migrated as a diffuse band of approximately 50 kDa (Fig. 1C), consistent with dimeric A33R (22) and monomeric A36R.
To determine whether IEV membrane formation was required for the interaction and phosphorylation of A33R and A36R, we immunoprecipitated extracts of cells that had been infected with WR and labeled with H332PO4 or [35S]methionine in the presence of rifampin, an inhibitor of virus assembly (13). Although rifampin caused an overall decrease in 32P and 35S labeling, α-A33R precipitated phosphorylated proteins corresponding in size to A33R and A36R even in the presence of the drug (Fig. 1D), indicating that some complex formation and phosphorylation could occur in the absence of assembly.
Taken together, our data demonstrated that the A33R and A36R proteins formed a complex and were both phosphorylated. In addition, phosphorylation of each occurred independently of the other, as indicated by labeling of the proteins in cells infected by deletion mutants, and did not require virus assembly.
Phosphoamino acid analysis of the A33R and A36R proteins.
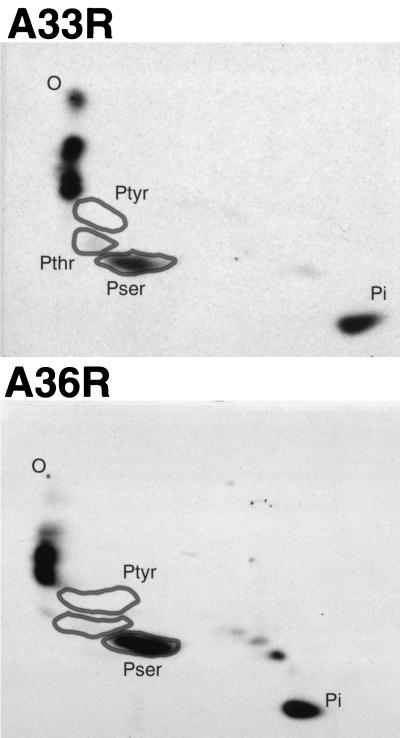
The 32P-labeled A33R and A36R proteins were purified by immunoprecipitation with specific antibodies, followed by SDS-PAGE. The proteins were transferred to a membrane, and the labeled bands were excised and acid hydrolyzed. The phosphoamino acids were resolved by two-dimensional thin-layer electrophoresis and detected by autoradiography. Major and minor spots from the A33R protein comigrated with phosphoserine and phosphothreonine standards, respectively (Fig. 2). Additional radioactive spots represented incompletely hydrolyzed peptides and inorganic phosphate. Of the radioactivity in these phosphoamino acids, 93% was phosphoserine and 7% phosphothreonine. Phosphoserine was also the major hydrolysis product of 32P-labeled A36R protein (Fig. 2). Overexposure of the thin-layer plate revealed a trace amount of material that comigrated with the phosphothreonine marker (data not shown). However, no phosphotyrosine was detected by this procedure in either the A33R or the A36R protein.
FIG. 2.
Phosphoamino acid analysis of vaccinia virus A33R and A36R proteins. BS-C-1 cells were infected with vaccinia virus WR and labeled overnight with H332PO4. After cell lysis and incubation with α-A33R or α-A36RC, the proteins were resolved by SDS-PAGE and transferred to a membrane. The labeled protein was excised and hydrolyzed with HCl, and phosphoamino acid standards were added. Standards and sample were analyzed by two-dimensional thin-layer electrophoresis. Standards were visualized with ninhydrin, and the plates were autoradiographed. Positions of phosphoserine (Pser), phosphothreonine (Pthr), and phosphotyrosine (Ptyr) standards, as well as Pi and the origin (O), are marked.
The A36R protein is also phosphorylated on tyrosine.
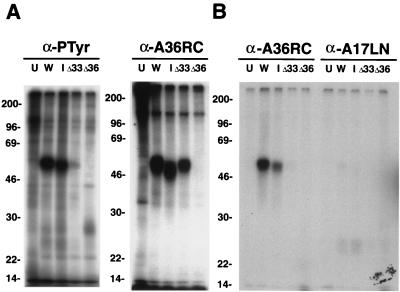
In a previous study, we had determined that the major phosphorylated amino acids in the vaccinia virus A17L IMV membrane protein were phosphothreonine and phosphoserine (2). Although phosphotyrosine was not abundant enough to detect by chemical analysis, its presence was established by using a phosphotyrosine antibody to precipitate 32P-labeled proteins. The phosphotyrosine antibody had also precipitated additional uncharacterized minor bands, one of which was approximately 50 kDa (2). Because phosphotyrosine antibodies exhibit some selectivity for neighboring amino acids, we tested several anti-phosphotyrosine antibodies and selected PY99 because it consistently precipitated a 32P-labeled 50-kDa protein from extracts of cells infected with vaccinia virus but not from control uninfected cells. We had noted that the A36R proteins from the WR and IHDJ strains had slightly different electrophoretic mobilities, and we took advantage of this to help with the identification. As shown earlier, α-A36RC precipitated a phosphorylated protein of 50 kDa from cells infected with WR or vΔA33R but not from vΔA36R-infected cells (Fig. 3A). However, a slightly faster migrating protein was obtained from cells infected with the IHDJ strain of vaccinia virus (Fig. 3A). When the 32P-labeled proteins from the same extracts were precipitated with the anti-phosphotyrosine antibody PY99 and analyzed by SDS-PAGE, a major band of approximately 50 kDa was detected in extracts from cells infected with WR and a slightly faster migrating one from cells infected with IHDJ (Fig. 3A). Moreover, the phosphotyrosine band was not discerned in lanes containing material from uninfected cells or from cells infected with vΔA36R, consistent with its being a product of the A36R gene (Fig. 3A). Interestingly, only a faint 50-kDa phosphotyrosine band was detected in extracts from cells infected with vΔA33R (Fig. 3A). This contrasted with the prominent 50-kDa band seen when 32P-labeled proteins from cells infected with vΔA33R were precipitated with α-A36RC (Fig. 3A), suggesting that A33R expression is important for tyrosine phosphorylation but not serine phosphorylation. A 25-kDa band, corresponding to tyrosine-phosphorylated A17L protein, was detected in some experiments but apparently was poorly recognized by the PY99 antibody (data not shown).
FIG. 3.
SDS-PAGE analysis of tyrosine-phosphorylated proteins from infected cells. BS-C-1 cells were uninfected (U) or were infected with vaccinia virus strain WR (W) or IHDJ (I) or with a recombinant vaccinia virus from which the A33R ORF (ΔA33) or the A36R ORF (ΔA36) had been deleted. Cells were then labeled with H332PO4. (A) Lysates were immunoprecipitated with either the anti-phosphotyrosine antibody PY99 or α-A36RC and were analyzed by SDS-PAGE. (B) An equivalent portion of the material immunoprecipitated by PY99 was eluted from the protein A-coupled antibody with cold phosphotyrosine and a high salt concentration. This postelution fraction was diluted and immunoprecipitated with either α-A36RC or antibody to the N-terminal region of A17L (anti-A17LN). The washed samples were analyzed by SDS-PAGE and autoradiography. The masses (in kilodaltons) and positions of migration of markers are indicated on the left.
Further experiments were performed to confirm that the 50-kDa protein that bound to the PY99 antibody contained phosphotyrosine and was the A36R protein. Extracts of 32P-labeled cells were bound to the PY99 antibody, complexed to protein A-Sepharose, and specifically eluted with phosphotyrosine. The proteins that were eluted with phosphotyrosine were immunoprecipitated with α-A36RC or an antibody to the A17L protein and were also analyzed by SDS-PAGE (Fig. 3B). Significantly, from cells infected with WR or IHDJ, the 32P-labeled 50-kDa species that was eluted from PY99 bound to α-A36RC, whereas virtually no signal was detected from cells infected with vΔA36R or vΔA33R (Fig. 3B). The anti-A17LN antibody served as a negative control; the 50-kDa phosphoprotein was not precipitated, although a faint 25-kDa band was detected in the lanes containing proteins from infected cells.
Differential regulation of serine and tyrosine phosphorylation of the A36R protein.
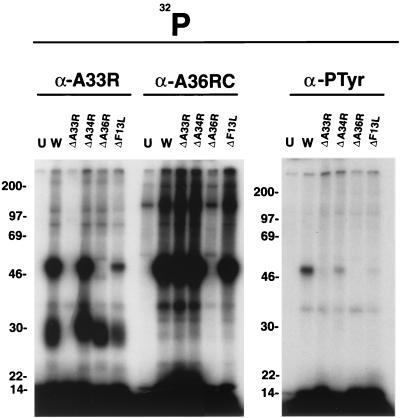
The difference between the total 32P labeling and the phosphotyrosine labeling of the A36R protein in cells infected with vΔA33R suggested that serine and tyrosine phosphorylation were regulated differently. To investigate this further, cells were infected with vΔF13L, a mutant with a defect in wrapping IMV that produces few EEV; vΔA34R, which produces few IEV but increased numbers of EEV; and vΔA33R, which accumulates incompletely wrapped IEV but increased numbers of EEV. Extracts of cells labeled with H332PO4 were immunoprecipitated with α-A33R or α-A36RC to determine serine phosphorylation, or with PY-99 to analyze tyrosine phosphorylation. α-A33R immunoprecipitated 32P-labeled 28-kDa proteins from cells infected with all mutants except vΔA33R and 32P-labeled 50-kDa proteins from cells infected with all mutants except vΔA33R and vΔA36R (Fig. 4). Thus, neither the A34R nor the F13L protein was needed for either the serine phosphorylation of the A36R protein or its association with the A33R protein. α-A36RC immunoprecipitated a phosphorylated 50-kDa protein from cells infected with all mutants except vΔA36R, indicating that none of the other EEV proteins were required for serine phosphorylation (Fig. 4). Quite different results were obtained, however, with the phosphotyrosine antibody PY99. A strongly labeled band was obtained only with cells infected with WR (Fig. 4). Except for vΔA36R, vΔA33R had the greatest defect, with no detectable phosphotyrosine-labeled 50-kDa band (Fig. 4). In addition, only faint bands were obtained from cells infected with vΔA34R or vΔF13L (Fig. 4).
FIG. 4.
SDS-PAGE analysis of proteins that were immunoprecipitated from lysates of cells infected with vaccinia virus deletion mutants. BS-C-1 cells were infected with vaccinia virus WR or the recombinant vaccinia virus vΔA33, vΔA34, vΔA36, or vΔF13L and then labeled with H332PO4 for 18 h. Lysates were immunoprecipitated with α-A33R, α-A36RC, or the phosphotyrosine antibody PY99 and then analyzed by SDS-PAGE and autoradiography. The masses (in kilodaltons) and migration positions of markers are indicated on the left.
Localization of the A36R protein in infected cells.
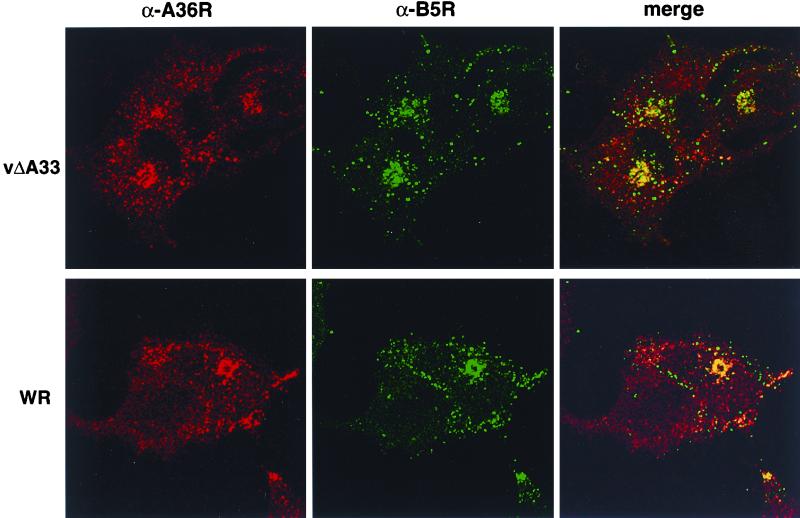
At this point, we considered the possibility that the A33R protein might be necessary for targeting the A36R protein to the IEV membrane, where tyrosine phosphorylation occurs. To investigate this question, we first compared the intracellular localization of the A36R protein in cells infected with WR or vΔA33R by confocal microscopy. Another EEV protein, B5R, served as a marker for Golgi and viral membranes. Cells were incubated with α-A36RC followed by a rhodamine-conjugated anti-rabbit IgG and with a MAb against B5R followed by fluorescein-conjugated anti-rat IgG. In the WR-infected cells, both antibodies gave bright juxtanuclear Golgi membrane staining as well as a pattern of punctate staining within the cytoplasm and bright areas at the tips of cells that may represent accumulations of IEV (Fig. 5). By merging the images of WR-infected cells, we observed considerable colocalization of the two proteins. In cells infected with vΔA33R, however, the colocalization was mainly limited to the Golgi regions (Fig. 5).
FIG. 5.
Localization of the A36R protein in infected cells by indirect immunofluorescence. HeLa cells grown on coverslips were infected with vaccinia virus WR or recombinant vΔA33R. Fixed, permeabilized cells were stained sequentially with α-A36RC (α-A36R) followed by tetramethyl rhodamine isocyanate (TRITC)-conjugated anti-rabbit antisera and with MAb 19C against B5R (α-B5R) followed by an FITC-conjugated anti-rat antibody. Fluorescence signals were collected independently using a Leica model TCS NT laser scanning confocal microscope.
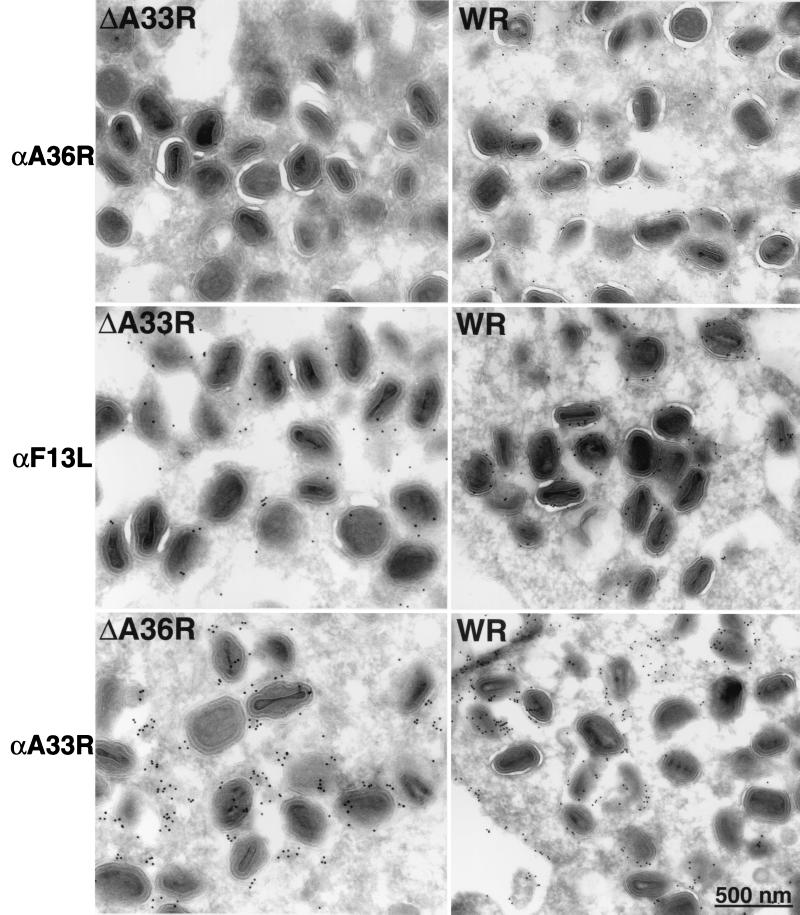
Higher-resolution studies were carried out by immunoelectron microscopy. In agreement with the immunofluorescence data, there was similar labeling of Golgi membranes by α-A36RC in cells infected with vΔA33R or WR (data not shown). In contrast, the labeling of the partially wrapped IEV in vΔA33R-infected cells was sparse, while that of IEV formed in WR-infected cells was more intense (Fig. 6). The specificity of this effect was demonstrated by the labeling of the partially wrapped IEV in ΔA33R-infected cells by an antibody to F13L (Fig. 6). Although the A33R protein appears to be necessary for incorporation of the A36R protein into IEV, the opposite was not true. There was a similar distribution of labeling with the α-A33R antibody on IEV formed in cells infected with WR or vΔA36R (Fig. 6).
FIG. 6.
Immunolabeling of IEV in infected cells. Thawed cryosections of BS-C-1 cells infected with WR, vΔA33R, or vΔA36R were labeled with α-A33R, α-F13L, or α-A36RC (α-A36R) followed by 10-nm protein A-gold.
We also noticed a difference in the labeling of the IEV membranes by antibodies to A36R and F13L in cells infected with WR. The latter appeared to label the two membranes similarly, whereas the former primarily labeled the outer membrane. This result is in agreement with recent data of van Eijl et al. (29), who reported, contrary to previous data, that the A36R protein is present in IEV but not in EEV. The latter result was confirmed by counting the number of gold grains on virus particles in thin sections of WR-infected cells incubated with anti-F13L, α-A33R, or α-A36RC (Table 1). With anti-F13L or α-A33R, similar percentages of IEV, CEV, and EEV were labeled, although the absolute numbers differed either because of the amount of protein or because of the avidity of the antibody. However, despite respectable labeling of IEV with α-A36RC, there was scarcely any labeling of CEV and EEV (Table 1). These data support the conclusion that the A36R protein is IEV specific, although biochemical studies are needed to exclude the possibility that the reactivity of the A36R protein was masked in the extracellular viral forms.
TABLE 1.
Distribution of proteins on wrapped virions
| Antibody | Resulta for:
|
||
|---|---|---|---|
| IEV | CEV | EEV | |
| F13L | 239/261 (92) | 238/261 (91) | 16/23 (70) |
| A33R | 142/261 (54) | 149/261 (57) | 12/23 (52) |
| A36R | 176/261 (67) | 16/261 (6) | 0/47 |
Expressed as the number of particle thin sections with gold grains (indicating the presence of the protein)/total number of particle thin sections (percent).
DISCUSSION
Previous studies from our laboratory and others had shown that the A33R, A34R, and A36R proteins are required for the formation of actin tails on the IEV (21, 24, 31, 33). Recent data, published during the course of the present investigation, suggested that the nucleation of actin tails involves the association of tyrosine-phosphorylated A36R protein with the adapter protein Nck and the recruitment of N-WASP (12). The role of the A33R proteins in this process, however, was unknown. Here we have shown that (i) the A33R and A36R proteins are phosphorylated predominantly on serine residues; (ii) serine phosphorylation of A33R and that of A36R occur independently of each other and do not require virus assembly; (iii) disulfide-bonded A33R dimers form a noncovalent complex with the A36R protein, and this also does not require virus assembly; (iv) tyrosine phosphorylation of the A36R protein is dependent on the presence of the A33R protein and is greatly reduced in the absence of the A34R and F13L EEV membrane proteins; (v) the A33R protein is necessary for the localization of the A36R protein but not the B5R or F13L protein in IEV membranes; and (vi) the A36R protein is not needed for the localization of the A33R protein in IEV membranes. Taken together, these data suggest that one function of the A33R protein is to guide the A36R protein to the IEV membrane, where tyrosine phosphorylation can occur. This does not, however, exclude the possibility that the A33R protein also has a more direct role in actin tail formation.
Our finding that the A36R protein is highly phosphorylated on serine residues was initially surprising, since the protein had been classified as a type 2 membrane protein with virtually no cytoplasmic tail (18). However, there is now agreement that the A36R protein is a type 1b protein with a long cytoplasmic tail but little or no extracellular domain (23). The low level of phosphorylation on tyrosine relative to serine is precisely what one would expect if phosphotyrosine were the signal for nucleation of actin tails. The bulk of the A36R protein, which is associated with the endoplasmic reticulum, the Golgi network, or IEV that have not yet acquired actin tails, would be phosphorylated on serine but not tyrosine. Recently, van Eijl et al. (29) reported that the A36R protein is targeted to the outer IEV membrane and is removed during fusion with the plasma membrane. We also noted that the antibody to the A36R protein did not label EEV and CEV, and labeling of IEV was predominantly observed on the outer IEV membrane. How this asymmetry is created is unclear, especially since the A33R and A36R proteins interact with each other even in the absence of morphogenesis. This immunogold labeling pattern suggested to van Eijl et al. (29) that actin tail formation occurs at the plasma membrane. A three-dimensional analysis of confocal images indicated that actin tails are mostly near the periphery of the cell, although it was not possible to ascertain whether they are all associated with the plasma membrane (E. J. Wolffe, unpublished data). The formation of actin tails at the plasma membrane would be consistent with evidence for the tyrosine phosphorylation of A36R by Src family kinases (12).
ACKNOWLEDGMENTS
We thank Norman Cooper for preparing cells and Owen Schwartz for assistance with the confocal microscopy.
REFERENCES
- 1.Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- 2.Betakova T, Wolffe E J, Moss B. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L protein kinase. J Virol. 1999;73:3534–3543. doi: 10.1128/jvi.73.5.3534-3543.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco R, Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulter E A, Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- 6.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 7.Derrien M, Punjabi A, Khanna R, Grubisha O, Traktman P. Tyrosine phosphorylation of A17 during vaccinia virus infection: involvement of the H1 phosphatase and the F10 kinase. J Virol. 1999;73:7287–7296. doi: 10.1128/jvi.73.9.7287-7296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan S A, Smith G L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992;66:1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earl P L, Moss B. Generation of recombinant vaccinia viruses. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing Associates & Wiley Interscience; 1991. pp. 16.17.1–16.17.16. [Google Scholar]
- 10.Engelstad M, Howard S T, Smith G L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 11.Engelstad M, Smith G L. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology. 1993;194:627–637. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- 12.Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 13.Grimley P M, Rosenblum E N, Mims S J, Moss B. Interruption by rifampin of an early stage in vaccinia virus morphogenesis: accumulation of membranes which are precursors of virus envelopes. J Virol. 1970;6:519–533. doi: 10.1128/jvi.6.4.519-533.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiller G, Jungwirth C, Weber K. Fluorescence microscopical analysis of the life cycle of vaccinia virus in chick embryo fibroblasts. Virus-cytoskeleton interactions. Exp Cell Res. 1981;132:81–87. doi: 10.1016/0014-4827(81)90085-9. [DOI] [PubMed] [Google Scholar]
- 15.Hiller G, Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985;55:651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiller G, Weber K, Schneider L, Parajsz C, Jungwirth C. Interaction of assembled progeny pox viruses with the cellular cytoskeleton. Virology. 1979;98:142–153. doi: 10.1016/0042-6822(79)90533-6. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs S N, Wolffe E J, Payne L G, Moss B. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol. 1992;66:7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkinson J E, Smith G L. Vaccinia virus gene A36R encodes a Mr 43–50 K protein on the surface of extracellular enveloped virus. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- 19.Payne L G. Significance of extracellular virus in the in vitro and in vivo dissemination of vaccinia virus. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 20.Payne L G, Norrby E. Presence of hemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976;32:63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- 21.Roper R, Wolffe E J, Weisberg A, Moss B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J Virol. 1998;72:4192–4294. doi: 10.1128/jvi.72.5.4192-4204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roper R L, Payne L G, Moss B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J Virol. 1996;70:3753–3762. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rottger S, Frischknecht F, Reckmann I, Smith G L, Way M. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J Virol. 1999;73:2863–2875. doi: 10.1128/jvi.73.4.2863-2875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanderson C M, Frischknecht F, Way M, Hollinshead M, Smith G L. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J Gen Virol. 1998;79:1415–1425. doi: 10.1099/0022-1317-79-6-1415. [DOI] [PubMed] [Google Scholar]
- 25.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sefton B M. Phosphoamino acid analysis, unit 13.3. In: Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T, editors. Current protocols in protein science. John Wiley & Sons, New York, N.Y. [Online.] 1997. [Google Scholar]
- 27.Stokes G V. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J Virol. 1976;18:636–643. doi: 10.1128/jvi.18.2.636-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traktman P, Liu K, DeMasi J, Rollins R, Jesty S, Unger B. Elucidating the essential role of the A14 phosphoprotein in vaccinia virus morphogenesis: construction and characterization of a tetracycline-inducible recombinant. J Virol. 2000;74:3682–3695. doi: 10.1128/jvi.74.8.3682-3695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Eijl H, Hollinshead M, Smith G L. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology. 2000;271:26–36. doi: 10.1006/viro.2000.0260. [DOI] [PubMed] [Google Scholar]
- 30.Wolffe E J, Isaacs S N, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67:4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolffe E J, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3904–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolffe E J, Moore D M, Peters P J, Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J Virol. 1996;70:2797–2808. doi: 10.1128/jvi.70.5.2797-2808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolffe E J, Weisberg A S, Moss B. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology. 1998;244:20–26. doi: 10.1006/viro.1998.9103. [DOI] [PubMed] [Google Scholar]