Abstract
IMPORTANCE:
In-hospital cardiac arrest (IHCA) is a significant public health burden. Rates of return of spontaneous circulation (ROSC) have been improving, but the best way to care for patients after the initial resuscitation remains poorly understood, and improvements in survival to discharge are stagnant. Existing North American cardiac arrest databases lack comprehensive data on the post-resuscitation period, and we do not know current post-IHCA practice patterns. To address this gap, we developed the Discover In-Hospital Cardiac Arrest (Discover IHCA) study, which will thoroughly evaluate current post-IHCA care practices across a diverse cohort.
OBJECTIVES:
Our study collects granular data on post-IHCA treatment practices, focusing on temperature control and prognostication, with the objective of describing variation in current post-IHCA practice.
DESIGN, SETTING, AND PARTICIPANTS:
This is a multicenter, prospectively collected, observational cohort study of patients who have suffered IHCA and have been successfully resuscitated (achieved ROSC). There are 24 enrolling hospital systems (23 in the United States) with 69 individual enrolling hospitals (39 in the United States). We developed a standardized data dictionary, and data collection began in October 2023, with a projected 1000 total enrollments. Discover IHCA is endorsed by the Society of Critical Care Medicine.
INTERVENTIONS, OUTCOMES, AND ANALYSIS:
The study collects data on patient characteristics including pre-arrest frailty, arrest characteristics, and detailed information on post-arrest practices and outcomes. Data collection on post-IHCA practice was structured around current American Heart Association and European Resuscitation Council guidelines. Among other data elements, the study captures post-arrest temperature control interventions and post-arrest prognostication methods. Analysis will evaluate variations in practice and their association with mortality and neurologic function.
CONCLUSIONS:
We expect this study, Discover IHCA, to identify variability in practice and outcomes following IHCA, and be a vital resource for future investigations into best-practice for managing patients after IHCA.
Keywords: cardiac arrest, neurologic recovery, prognostication, temperature management
KEY POINTS
Current State of the Clinical Problem
Practice variation in temperature control and prognostication after in-hospital cardiac arrest (IHCA) is not incompletely understood. There are no multicenter North American databases that collect granular data on post-IHCA practices.
Rationale for the Study Design
We developed a multicenter, prospective, observational cohort with the intent to identify variation in post-IHCA practices and better understand current practice across a diverse population.
Which Domain(s) (i.e., Design, Regulatory Processes, Data Sources, or Analytic Methods) Contain Novelty/Innovation, and How
This study is novel in that it collects granular data on post-IHCA practices.
In-hospital cardiac arrest (IHCA) is a major burden to public health, occurring over 300,000 times each year in the United States (1, 2). Rates of return of spontaneous circulation (ROSC) have improved over the past decades, from 42.7% in 2000 to 54.1% in 2009 (3), and as high as 70% for certain age groups by 2016 (4). However, rates of post-ROSC survival have not shown such rapid improvement, with rates of around 20% in 2007 (5), and 25% in 2017 (6). The slower improvements in post-ROSC survival, as compared with the improvements in ROSC, underscore a key gap in cardiac arrest care: the strategies, procedures, and approaches essential for post-arrest survival and neurologic recovery are not fully understood. Indeed, the updated International Liaison Committee on Resuscitation (ILCOR) consensus statement on knowledge gaps and clinical research priorities for cardiopulmonary resuscitation identifies the post-cardiac arrest period as a critical priority area. The consensus statement concluded that reducing post-arrest hospital mortality for all cardiac arrests admitted to an ICU from the current rate of about 60% to 50% would save one additional life for every ten patients admitted to an ICU (7). The ILCOR consensus statement focuses on two post-arrest practices: physiologic post-arrest targets including temperature control, and prognostication in comatose cardiac arrest survivors.
DATA AND EVIDENCE GAP
Existing cardiac arrest databases are not equipped to answer detailed questions about post-IHCA care. Our project, “Discover In-Hospital Cardiac Arrest (Discover IHCA),” is designed to fill this gap. This report describes Discover IHCA, a novel multicenter collaboration endorsed by the Society of Critical Care Medicine (SCCM) Discovery Network aimed at characterizing post-IHCA practices, specifically focusing on temperature control and prognostication. The network established by this study and the data collected will be highly useful in studying practice variation during the post-IHCA period. Information on the Discover IHCA research collaborative can be accessed through the SCCM website (https://sccm.org/Research/Discovery-Research-Network/Discovery-Research-Collaboratives/Discover-IHCA-Project).
Temperature control, the deliberate manipulation of core body temperature, encompasses hypothermic temperature control, normothermic temperature control, and temperature control with fever prevention (8). Controlling a patient’s temperature after cardiac arrest is thought to be essential because hyperthermia is associated with worse neurologic outcomes after cardiac arrest (9). Time appropriate, evidence-based prognostication is necessary to inform families and avoid premature decision-making on recovery or withdrawal of life-sustaining therapies. The best way to implement these practices is not completely understood (1).
A systematic review of global cardiac arrest and sudden cardiac death registries by Paratz et al (10), which searched up to December 10, 2018, identified 49 cardiac arrest registries. Only seven of these registries track arrests in the United States and Canada (Supplemental Table 1, http://links.lww.com/CCX/B396). Of note, the majority of these North American cardiac arrest registries (6/7) collect data only on patients who had out-of-hospital cardiac arrest (OHCA). These two types of cardiac arrest (IHCA and OHCA) encompass fundamentally different patient populations, with different arrest etiology, comorbidities, time to treatment, and available interventions (1, 11). The only registry that enrolls IHCA does not have detailed information about the post-arrest period.
DISCOVER IHCA SUMMARY AND STUDY DESIGN
Discover IHCA is a multicenter, prospective observational study designed and conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (Supplemental Table 2, http://links.lww.com/CCX/B396) and is registered as an observational study (NCT06207201). The study is primarily descriptive, exploring variation in usual practices following IHCA. No specific post-arrest practices have been recommended to the participating hospital systems. Data collection is being conducted over a limited time frame, as has been done in other successful studies conducted through the SCCM Discovery platform (12, 13). Enrollment is currently underway, with 24 participating hospital systems. The project is endorsed by the Discovery Critical Care Research Network of the SCCM and data collection began in October 2023. Details of the study are summarized in Supplemental Table 3 (http://links.lww.com/CCX/B396).
The study aims to characterize practice variations that may impact patient outcomes after IHCA, as well as differences in hospital characteristics that are associated with practice variation and outcomes. The focus of Discover IHCA is on temperature control and prognostication practices for patients who have achieved ROSC. Temperature control and prognostication are important components of care unique to critical care of the post-IHCA patient, and the best approach to implementing these interventions remains poorly understood. The data elements were designed to capture information pertaining to the most recent ILCOR (2022) (8) and American Heart Association (AHA) (2020 and 2023) (14, 15) recommendations for temperature control and prognostication. The 2023 AHA guidelines recommend selecting and maintaining a constant temperature between 32°C and 37.5°C for at least 24 hours for patients that do not follow commands after ROSC. The guidelines stipulate that it is reasonable to actively prevent fever for these patients after the initial temperature control period (14). The most recent ILCOR consensus recommends active fever prevention for at least 72 hours for the same population (8). To prognosticate, treatment teams must implement multiple modalities to improve decision-making (15). We used the 2020 AHA recommendations to inform our data collection on prognostication as they are the most recent guidelines to comprehensively define recommended prognostication modalities (the 2023 update did not discuss prognostication). Data capture for prognostication was also informed by the 2021 European Resuscitation Council and European Society of Intensive Care Medicine 2021 guidelines on post-resuscitation care (16).
Society of Critical Care Medicine Discovery Network
Discover IHCA is endorsed by the SCCM Discovery Critical Care Research Network. Discovery is a critical care research network from SCCM that promotes collaborative research to improve outcomes for critically ill patients (17). The network launched in 2016 (18) and has supported several successful projects (a full list of programs and publications can be found through the SCCM website) (19). The network offers resources such as data storage, data management, data analysis, central institutional review board (IRB) services, and project management. Discover IHCA has used several of these resources, including data storage through a Research Electronic Data Capture (REDCap) Cloud database hosted by SCCM and project management.
Consortium Formation
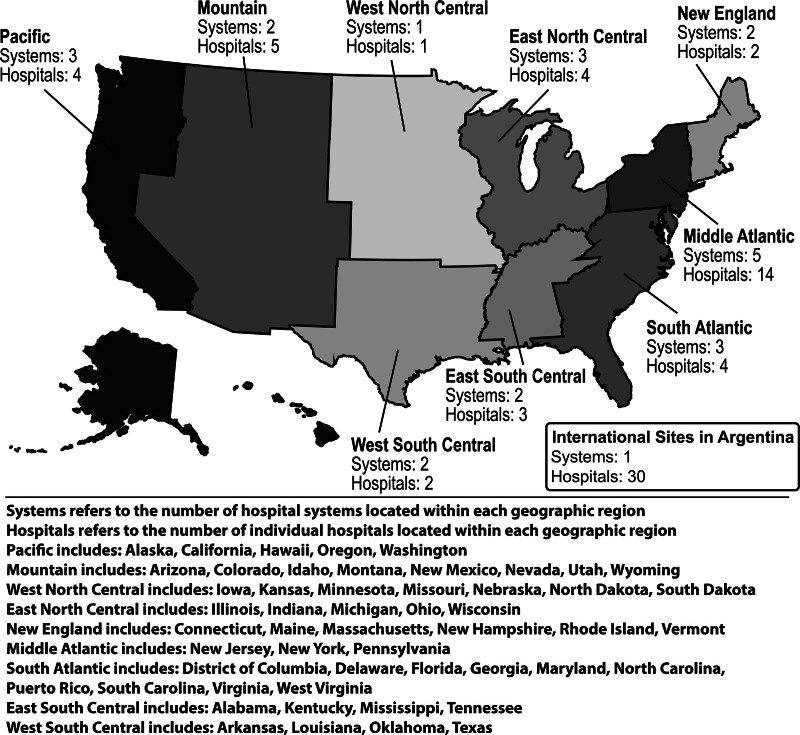
Initial site recruitment for the project began at the SCCM Discovery meeting, where the project was first presented in the fall of 2022. The project was endorsed by the SCCM Discovery Network at that time, and interested hospital systems involved in the network joined the collaboration. A second round of site recruitment occurred through other existing networks, word-of-mouth, and information about the project on the SCCM website (20). All participating hospitals obtained local IRB approval with waiver of informed consent and formalized data use agreements with SCCM. The final study consortium included 24 hospital systems (23 U.S. hospital systems) with a total of 69 individual hospitals (39 individual U.S. hospitals) enrolling patients and collecting data. The 30 international hospitals that joined this research collaborative are all independent hospitals in Argentina, not all of them are expected to enroll patients, and the overall enrollment pace (enrollments from all of the participating international hospitals combined) has been similar to other hospital systems participating in the study. Although the international hospitals are not under a single hospital system, we have grouped them together for the purpose of their description here due to the enrollment pace and anticipated number of enrollments. Figure 1 displays the geographic region of hospital systems. The majority of participating hospital systems are large, academic, tertiary care centers serving urban populations; hospital system characteristics can be seen in Supplemental Table 4 (http://links.lww.com/CCX/B396).
Figure 1.
Geographic region of participating hospital systems. This figure shows the number of participating hospital systems, and individual hospitals, within each geographic region.
Data Elements
This database was constructed to compile meaningful and granular information about post-IHCA care across multiple hospital systems. Discover IHCA collects completely de-identified data in compliance with the National Institutes of Health’s recommended HIPAA Privacy Rule (21). The inclusion and exclusion criteria are displayed in Table 1, and the rationale for these criteria is described in Supplemental Table 3 (http://links.lww.com/CCX/B396); all inclusion and exclusion criteria were chosen based on expert consensus by the Discover IHCA executive committee, and agreed upon by participating hospital systems. Abstractors are responsible for narrative review of the medical record to determine if a patient meets the inclusion and exclusion criteria. Sessions were held with participating hospital systems to review the inclusion and exclusion criteria and the workflow for data abstraction before the initiation of the data collection period.
TABLE 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|---|
| Adult (≥ 18 yr old) patients |
| Patients with a cardiac arrest (a lack of palpable pulse or perfusing cardiac activity) while admitted to the hospital |
| Patients admitted to a ward (telemetry or nontelemetry) or an ICU or who are admitted but still in the ED waiting for a hospital bed |
| Patients who achieved ROSC (defined as > 20 min of sustained spontaneous circulation) OR were placed on extracorporeal CPR with chest compressions ongoing |
| Patients survived for 6 hr after ROSC |
| Exclusion Criteria |
| Cardiac arrest in non-inpatients (e.g., outpatients, visitors) |
| Patients whose CPR starts outside of the hospital |
| Nonindex arrests (arrests that are not the patient’s first arrest during the hospital admission; this also excludes patients who were initially admitted for an out-of-hospital cardiac arrest) |
| Patients suffering in-hospital cardiac arrest in the operating room or post-anesthesia care unit |
| Patients with cardiac arrest after arriving to an ED but before being evaluated and admitted to the hospital |
| Cardiac arrests lasting < 2 min (i.e., chest compressions performed < 2 min) |
| Cardiac arrests where the patient is transitioned to comfort focused care within 6 hr of ROSC |
CPR = cardiopulmonary resuscitation, ED = emergency department, ROSC = return of spontaneous circulation.
The REDCap Cloud Case Report Form is designed to capture baseline patient information, limited pertinent intra-arrest information, and granular post-arrest details and outcomes. See the entire Case Report Form with Data Dictionary in Supplemental Document 1 (http://links.lww.com/CCX/B396). In brief, baseline information for patients is captured by the data collection forms “Demographics and Admission Data” and “Pre-Arrest (Baseline Data).” These sections encompass the demographic information for patients, pre-hospital functional status, chronic and acute medical conditions, location of the arrest, and organ support at the time the arrest starts. It also collects pre-arrest frailty for enrolled patients, using the clinical frailty scale (22). The database collects pertinent intra-arrest information, including the day (relative to the day of hospital presentation) and time when the arrest resuscitation was initiated, the initial rhythm, the presumed etiology of the arrest, and whether extracorporeal life support was initiated during the arrest.
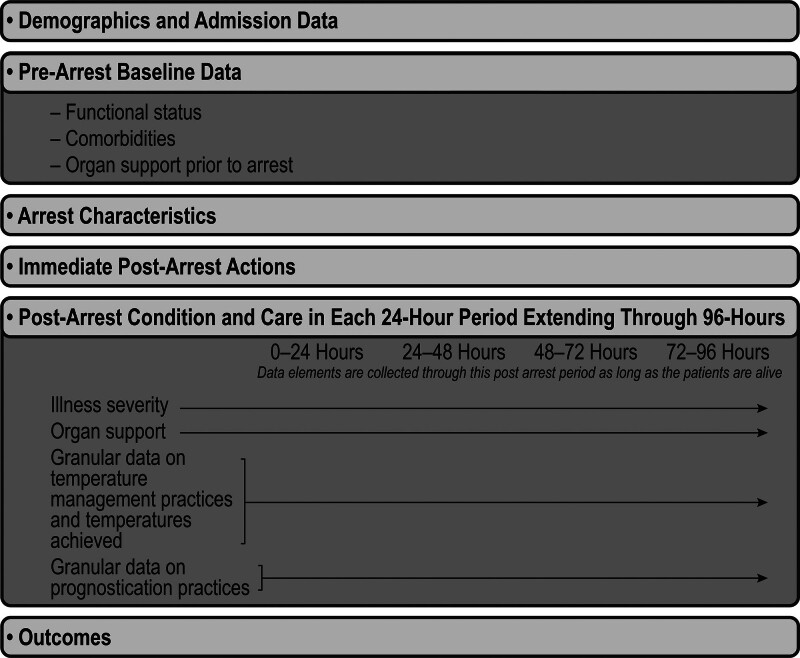
Granular post-arrest data are then collected for the first 96 hours after ROSC. The collection form “Post-Arrest” concerns data about immediate disposition, and interventions initiated in the immediate post-arrest period. Then, the database collects data in 24-hour intervals (0–24 hr after ROSC, 24–48 hr after ROSC, and so on), through the first 96 hours after ROSC. For patients meeting the inclusion and exclusion criteria, the data collection period starts at the time of first sustained ROSC, which is the start of the first 24-hour period. For each 24-hour period across those 4 days, abstractors collect granular data on the following three domains: 1) illness severity and organ support (including pertinent laboratory values); 2) temperature control practices (temperature targets, temperature monitoring modalities, methods employed for temperature control, and the actual temperatures achieved for the patient); 3) prognostication practices (neurologic examinations, neurologic testing including biochemical testing, neuroelectrophysiologic testing, invasive intracranial monitoring, and cross-sectional imaging, consultants, code status, and documentation of the anticipated prognosis. These data elements incorporate previously published definitions informed by the Utstein criteria (23, 24) and are informed by the AHA Emergency Cardiovascular Care Committee and ILCOR Consensus on Science with Treatment Recommendations.
Lastly, information is abstracted on patient outcomes, including mortality, organ support, and functional status. Neurologic function is obtained both through the Cerebral Performance Category score and the modified Rankin Scale, both of which are commonly used measures for neurologic function after cardiac arrest (25–27). Figure 2 visually displays the data collection domains for each patient.
Figure 2.
Data elements collected for each patient in the Discover In-Hospital Cardiac Arrest (Discover IHCA) study. This figure visually displays the structure of the data elements collected in the Discover IHCA study.
Data Collection
Data elements are abstracted from the electronic health records or medical records at each participating site by investigators and entered into the REDCap Cloud database hosted by the SCCM. All cardiac arrests identified at each hospital system are screened for inclusion and exclusion criteria during the data collection period. Those that meet appropriate criteria are identified for data abstraction and enrolled. Collection of outcome data is truncated at 60 days post-cardiac arrest (post-ROSC), with those still alive and in the hospital at 60 days post-ROSC being considered discharged alive. The duration of the data collection period was chosen to allow for 1000 enrollments, based on preliminary survey data on the number of IHCA at each participating site. The goal of 1000 enrollments was selected to collect a large enough cohort to explore variations in care and perform risk adjustments as necessary, while taking into account pragmatic considerations regarding the burden of data abstraction.
Data Governance, Research, and Reporting
Hospital systems will have access to their own Discover IHCA data for process improvement and for internal pilot data. Investigators participating in the study, as well as outside researchers applying to access the data, will have the opportunity to propose ancillary studies. Proposals for ancillary studies, including statistical plans, will be reviewed by the Discover IHCA executive committee, and abstracts and publications for ancillary studies will again be reviewed by the Discover IHCA executive committee before submission. Details on the process for ancillary proposals are available through SCCM. Discover IHCA data will also be available to participating sites as preliminary data for future projects and grants, after a request to use the data has been approved by the executive committee. Finally, the Discover IHCA leads will publish the primary findings of the study once the data collection period is complete and the data has been cleaned and analysis has been performed. Statistical analyses will depend on the research question, the planned methods, and the final distribution of the data, and will be described in subsequent manuscripts presenting our results. Where appropriate, statistical consideration and adjustment for hospital site and hospital characteristics will be applied. As a supplement to Discover IHCA, we plan to conduct an evaluation of post-cardiac arrest protocols at the hospital systems participating in Discover IHCA. Through the completion of this project, we hope to foster additional investigations into crucial areas of post-IHCA practices and outcomes.
LIMITATIONS
Some variable definitions were altered slightly after data collection was initiated; this was done for clarity based on feedback from early data abstraction. Since this is an observational study, resulting data should not be used to determine causal inference. Participation in Discover IHCA was voluntary, and it is possible that hospital systems interested in participation have innately better adherence to published guidelines, limiting generalizability; however, this is a limitation of any voluntary registry. Although sites were not asked to alter their post-IHCA treatment protocols in any way during the study period, or to provide any specific interventions, it is possible that the increased focus on post-IHCA care due to participation in the study could alter treatment practices. There were also changes to AHA guidelines that occurred during the study period, as they were published in January 2024. Changes to the guidelines may alter post-IHCA practice during the months of data collection.
FUTURE DIRECTIONS
We expect Discover IHCA to find variability in clinical care for patients following IHCA. Where there is variability in care there are often avenues for improvement, areas of clinical equipoise, and even opportunities for novel insights. Variations in practice identified through Discover IHCA will also be of use as preliminary data to inform the design of future clinical trials. Discover IHCA has assembled a group of investigators who are interested in IHCA and committed to future collaboration. Our hope is that Discover IHCA will serve as a jumping-off point for critical future investigations and trials on IHCA.
ACKNOWLEDGMENTS
The Society of Critical Care Medicine’s Discovery, the Critical Care Research Network DISCOVER In-Hospital Cardiac Arrest (DISCOVER IHCA): Investigator Group collaborators are as follows: Devin Videlefsky, MD (Bronx Center for Critical Care Outcomes and Resuscitation Research, Division of Critical Care Medicine, Montefiore Medical Center, Bronx, NY), Michelle Ng Gong, MD, MS (Bronx Center for Critical Care Outcomes and Resuscitation Research, Division of Critical Care Medicine, Montefiore Medical Center, Bronx, NY), Maneesha Bangar, MD (Bronx Center for Critical Care Outcomes and Resuscitation Research, Division of Critical Care Medicine, Montefiore Medical Center, Bronx, NY), Rishi Malhotra, MD (Bronx Center for Critical Care Outcomes and Resuscitation Research, Division of Critical Care Medicine, Montefiore Medical Center, Bronx, NY), John H. Lee, MD (Center for Resuscitation Science, Beth Israel Deaconess Medical Center, Boston, MA; Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, MA), Matthew E. Prekker, MD (Division of Pulmonary, Allergy, and Critical Care Medicine, Hennepin County Medical Center, Minneapolis, MN), Kenneth W. Dodd, MD (Division of Pulmonary, Allergy, and Critical Care Medicine, Hennepin County Medical Center, Minneapolis, MN), Ivan Alfredo Huespe, MD, MS (Hospital Italiano de Buenos Aires, Ciudad Autónoma de Buenos Aires, Buenos Aires, Argentina), Judith Sagardia, MD, MS (Hospital Nacional Profesor Alejandro Posadas, El Palomar, Argentina), Alicia Roxana Gira, MD (Hospital Universitario Austral, Pilar, Argentina), Eleonora Cunto, MD (Hospital de Infecciosas Francisco Javier Muñiz, Ciudd Autónoma de Buenos Aires, Buenos Aires, Argentina), Pascual Valdez, MD, PhD (Hospital General de Agudos Dalmacio Vélez Sarsfield, Ciudad Autónoma de Buenos Aires, Buenos Aires, Argentina), Kevin W. Gibbs, MD (Section of Pulmonary, Critical Care, Allergy and Immunologic Disease, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, NC), Dustin Norton, MD (Section of Pulmonary, Critical Care, Allergy and Immunologic Disease, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, NC), John P. Gaillard, MD (Section of Pulmonary, Critical Care, Allergy and Immunologic Disease, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, NC), Taylor Wachs, DO (Section of Pulmonary, Critical Care, Allergy and Immunologic Disease, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, NC), Oscar J. L. Mitchell, MD, MSCE (Center for Resuscitation Science, Department of Emergency Medicine, University of Pennsylvania, Philadelphia, PA; Division of Pulmonary, Allergy, and Criticial Care, Department of Medicine, University of Pennsylvania, Philadelphia, PA), Laura Faiver, MD (Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA), Jonathan Tam, MD (Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA; Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA), Christopher Hansen MD, MPH (Division of Pulmonary and Critical Care Medicine, Lahey Hospital and Medical Center, Burlington, MA), Lenka Craigova, MD (Department of Anesthesiology, Division of Critical Care, University of Wisconsin School of Medicine & Public Health, Madison, WI), Vijay Krishnamoorthy, MD (Division of Critical Care Medicine, Department of Anesthesiology, Duke University School of Medicine, Durham, NC), Sujatha Cumaran, MD, MS (Division of Critical Care Medicine, Department of Anesthesiology, Duke University School of Medicine, Durham, NC), Kelly Ivins-O’Keefe, MD (Division of Critical Care Medicine, Department of Anesthesiology, Duke University School of Medicine, Durham, NC), Stephanie C. DeMasi, MD (Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN), Ithan D. Peltan, MD, MS (Pulmonary Division, Department of Medicine, Intermountain Medical Center, Murray, UT), Carolyn Klippel (Pulmonary Division, Department of Medicine, Intermountain Medical Center, Murray, UT), Michelle Adamson, MSN, RN (Pulmonary Division, Department of Medicine, Intermountain Medical Center, Murray, UT), Darrin Applegate (Pulmonary Division, Department of Medicine, Intermountain Medical Center, Murray, UT), Lars-Kristofer N. Peterson, MD (Division of Critical Care, Cooper University Health Care, Camden, NJ; Cooper Medical School of Rowan University, Camden, NJ), Nafisa Wadud, DO (Cooper Medical School of Rowan University, Camden, NJ), Reine Fowajuh, MD (Department of Medicine, Cooper University Health Care, Camden, NJ), Jared Ward, DO, MPH (Division of Critical Care, Cooper University Health Care, Camden, NJ; Cooper Medical School of Rowan University, Camden, NJ), Awab Khan, DO (Department of Medicine, Inspira Medical Center Vineland, Vineland, NJ), Clifford Chang, MD (Department of Emergency Medicine, Inspira Medical Center Vineland, Vineland, NJ), Heath D. White, DO, MS (Division of Pulmonary, Critical Care and Sleep Medicine, Baylor Scott and White Medical Center, Baylor College of Medicine, Temple, TX), Braden Anderson, DO (Division of Pulmonary, Critical Care and Sleep Medicine, Baylor Scott and White Medical Center, Baylor College of Medicine, Temple, TX), Jose Pena, MD (Division of Pulmonary and Critical Care Medicine, Department of Medicine, Oregon Health and Science University School of Medicine, Portland, OR), Edvinas Pocius (Division of Pulmonary and Critical Care Medicine, Department of Medicine, Oregon Health and Science University School of Medicine, Portland, OR), Genesis Briceno, MD (Division of Pulmonary and Critical Care Medicine, Department of Medicine, Oregon Health and Science University School of Medicine, Portland, OR), Omar Mehkri, MD, MBA (Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland, OH), Talha Saleem, MD (Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland, OH), Mohammed Al Tameemi (Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland, OH), Alex Bui, MD (Department of Anesthesiology, Valleywise Health Medical Center, Creighton University School of Medicine, Phoenix, AZ), Erica Whetten, DNP, APRN, AGACNP-BC (Department of Anesthesiology, Valleywise Health Medical Center, Creighton University School of Medicine, Phoenix, AZ), Vincent Chan, MD (Korman Lung Center, Thomas Jefferson University, Philadelphia, PA), Timothy Crisci, MD (Korman Lung Center, Thomas Jefferson University, Philadelphia, PA), Nathaniel Rosal, DO (Korman Lung Center, Thomas Jefferson University, Philadelphia, PA), Ahna Weeks, MD (Department of Emergency Medicine, University of Washington, Seattle, WA), Kelly Stewart, MD (Department of Emergency Medicine, University of Washington, Seattle, WA), Feona Kennedy (Harborview Medical Center, Seattle, WA), Anthony Martinez, MD (Ascension Saint Agnes Hospital, Baltimore, MD), Pathik Patel, MD (Ascension Saint Agnes Hospital, Baltimore, MD), Rachel Hammer, PA-C (Ascension Saint Agnes Hospital, Baltimore, MD), Greggory Davis, PhD (Our Lady of the Lake Regional Medical Center, Baton Rouge, LA; Louisiana State University Health Sciences Center, Emergency Medicine Residency Program, Baton Rouge Campus, Baton Rouge, LA), Micah Klumpp, PhD, CCC-A, F-AAA (Our Lady of the Lake Regional Medical Center, Baton Rouge, LA; Louisiana State University Health Sciences Center, Emergency Medicine Residency Program, Baton Rouge Campus, Baton Rouge, LA), Trinity Howard (Our Lady of the Lake Regional Medical Center, Baton Rouge, LA; Louisiana State University Health Sciences Center, Emergency Medicine Residency Program, Baton Rouge Campus, Baton Rouge, LA).
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Jacob Vine, Email: cobyvine@gmail.com.
Katherine M. Berg, Email: kberg@bidmc.harvard.edu.
Saiara Choudhury, Email: saiarachoudhury.md@gmail.com.
Mariana Vaena, Email: mariana.vaena@hospitalitaliano.org.ar.
Jordan E. Nogle, Email: jnogle@wakehealth.edu.
Saleem M. Halablab, Email: saleem.halablab@pennmedicine.upenn.edu.
Aarthi Kaviyarasu, Email: Aarthi.Kaviyarasu@Pennmedicine.upenn.edu.
Jonathan Elmer, Email: elmerjp@upmc.edu.
Gabriel Wardi, Email: gwardi@health.ucsd.edu.
Conor Crowley, Email: Conor.P.Crowley@lahey.org.
Micah T. Long, Email: mtlong@wisc.edu.
J. Taylor Herbert, Email: james.herbert@duke.edu.
Brittany D. Bissell Turpin, Email: brittanydbissell@gmail.com.
Michael J. Lanspa, Email: michael.lanspa@imail.org.
Adam Green, Email: green-adam@cooperhealth.edu.
Shekhar A. Ghamande, Email: shekhar.ghamande@bswhealth.org.
Akram Khan, Email: khana@ohsu.edu.
Siddharth Dugar, Email: dugars@ccf.org.
Aaron M. Joffe, Email: aaron_joffe@dmgaz.org.
Michael Baram, Email: mloew1@lsuhsc.edu.
Cooper March, Email: marchcm@uw.edu.
Nicholas J. Johnson, Email: nickj45@uw.edu.
Alexander Reyes, Email: alexander.reyes1@ascension.org.
Krassimir Denchev, Email: kdenchev@wayne.edu.
Michael Loewe, Email: mloew1@lsuhsc.edu.
Ari Moskowitz, Email: amoskowitz@montefiore.org.
REFERENCES
- 1.Andersen LW, Holmberg MJ, Berg KM, et al. : In-hospital cardiac arrest: A review. JAMA 2019; 321:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators: Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019; 12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, Nallamothu BK, Spertus JA, et al. ; American Heart Association Get with the Guidelines–Resuscitation Investigators: Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012; 367:1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiberg S, Holmberg MJ, Donnino MW, et al. ; American Heart Association’s Get With The Guidelines®-Resuscitation Investigators: Age-dependent trends in survival after adult in-hospital cardiac arrest. Resuscitation 2020; 151:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandroni C, Nolan J, Cavallaro F, et al. : In-hospital cardiac arrest: Incidence, prognosis and possible measures to improve survival. Intensive Care Med 2007; 33:237–245 [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart Disease and Stroke Statistics-2018 update: A report from the American Heart Association. Circulation 2018; 137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 7.Kleinman ME, Perkins GD, Bhanji F, et al. : ILCOR scientific knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care: A consensus statement. Resuscitation 2018; 127:132–146 [DOI] [PubMed] [Google Scholar]
- 8.Wyckoff MH, Greif R, Morley PT, et al. ; Collaborators: 2022 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: Summary from the basic life support; advanced life support; pediatric life support; neonatal life support; education, implementation, and teams; and first aid task forces. Resuscitation 2022; 181:208–288 [DOI] [PubMed] [Google Scholar]
- 9.Zeiner A, Holzer M, Sterz F, et al. : Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med 2001; 161:2007–2012 [DOI] [PubMed] [Google Scholar]
- 10.Paratz ED, Rowsell L, Zentner D, et al. ; Australian UCDP Registry: Cardiac arrest and sudden cardiac death registries: A systematic review of global coverage. Open Heart 2020; 7:e001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskowitz A, Holmberg MJ, Donnino MW, et al. : In-hospital cardiac arrest: Are we overlooking a key distinction? Curr Opin Crit Care 2018; 24:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qadir N, Bartz RR, Cooter ML, et al. ; Severe ARDS: Generating Evidence (SAGE) Study Investigators: Variation in early management practices in moderate-to-severe ARDS in the United States: The severe ARDS: Generating evidence study. Chest 2021; 160:1304–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JT, Roberts R, Fazzari MJ, et al. ; VOLUME-CHASERS Study Group and Society of Critical Care Medicine Discovery Network: Variation in fluid and vasopressor use in shock with and without physiologic assessment: A multicenter observational study. Crit Care Med 2020; 48:1436–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perman SM, Elmer J, Maciel CB, et al. ; American Heart Association: 2023 American Heart Association focused update on adult advanced cardiovascular life support: An update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2024; 149:e254–e273 [DOI] [PubMed] [Google Scholar]
- 15.Panchal AR, Bartos JA, Cabañas JG, et al. ; Adult Basic and Advanced Life Support Writing Group: Part 3: Adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020; 142:S366–S468 [DOI] [PubMed] [Google Scholar]
- 16.Nolan JP, Sandroni C, Böttiger BW, et al. : European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med 2021; 47:369–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society of Critical Care Medicine: Discovery, the Critical Care Research Network. 2021. Available at: https://www.sccm.org/Research/Discovery-Research-Network. Accessed December 20, 2023 [Google Scholar]
- 18.Walkey AJ, Kumar VK, Harhay MO, et al. : The Viral Infection and Respiratory Illness Universal Study (VIRUS): An international registry of coronavirus 2019-related critical illness. Crit Care Explor 2020; 2:e0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Society of Critical Care Medicine: Publications. 2021. Available at: https://www.sccm.org/Research/Discovery-Research-Network/Discovery-Activities/Publications. Accessed December 20, 2023 [Google Scholar]
- 20.Society of Critical Care Medicine: Discover IHCA Project. 2023. Available at: https://sccm.org/Research/Discovery-Research-Network/Discovery-Activities/Discover-IHCA-Project. Accessed December 20, 2023 [Google Scholar]
- 21.U.S. Department of Health and Human Services: Health Information Privacy. 2015. 45 CFR 164.501, 164.508, 164.512(i). Available at: https://www.hhs.gov/hipaa/for-professionals/special-topics/research/index.html. Accessed December 28, 2023 [DOI] [PubMed] [Google Scholar]
- 22.Church S, Rogers E, Rockwood K, et al. : A scoping review of the Clinical Frailty Scale. BMC Geriatr 2020; 20:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs I, Nadkarni V, Bahr J, et al. ; International Liaison Committee on Resuscitation: Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation 2004; 110:3385–3397 [DOI] [PubMed] [Google Scholar]
- 24.Cummins RO, Chamberlain D, Hazinski MF, et al. : Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: The in-hospital “Utstein style.” American Heart Association. Ann Emerg Med 1997; 29:650–679 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen N, Wetterslev J, Cronberg T, et al. ; TTM Trial Investigators: Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013; 369:2197–2206 [DOI] [PubMed] [Google Scholar]
- 26.Lascarrou JB, Merdji H, Le Gouge A, et al. ; CRICS-TRIGGERSEP Group: Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med 2019; 381:2327–2337 [DOI] [PubMed] [Google Scholar]
- 27.Jennett B, Bond M: Assessment of outcome after severe brain damage. Lancet 1975; 1:480–484 [DOI] [PubMed] [Google Scholar]