SUMMARY
Introduction
Scrub typhus is a mite-borne tropical febrile illness with high mortality if untreated. The presence of eschar is pathognomonic, but a wide range of frequencies of eschar positivity has been reported in Indian patients. Therefore, this systematic review and meta-analysis aimed to ascertain the frequency (overall and geographic region-wise) and anatomical distribution of eschar in scrub typhus in India.
Methodology
We searched articles in two databases using: [(scrub OR typhus OR Orientia) AND (eschar) AND (India)]. The articles were independently screened and critically appraised by two authors. The frequency and distribution of eschar in patients with scrub typhus were pooled using a random-effect model.
Results
After the title-abstract and full-text screening, 107 articles (34002 cases of scrub typhus) were finally included. The overall pooled proportion of eschar positivity was 28.5% (95% CI: 24.1 to 32.9%). The pooled eschar positivity varied from ≤12% in Haryana, Rajasthan, Madhya Pradesh, Punjab, and Meghalaya to ≥46% in Tamil Nadu and Tripura. The pooled proportion of eschar positivity in the ‘trunk’ (39.3%), ‘groin’ (23.8%), and ‘axilla’ (16.5%) was higher than in the ‘limbs’ (9.9%) and ‘head’ (11.3%).
Conclusion
Eschar is reported in less than a third of the patients with scrub typhus in India. Most eschars were in the groin, axilla, and the trunk. There is a need to create awareness amongst physicians of the need for thorough physical examination.
Keywords: Eschars, scrub typhus, India
INTRODUCTION
Scrub typhus, caused by the Orientia tsutsugamushi, a bacterium of the Rickettsiaceae family, has a high burden in Southeast Asia [1]. This acute febrile illness is transmitted through the bite of larval trombiculid mites, known as chiggers, and is associated with eschar development at the bite site. With increasing awareness, there has been a steady rise in the number of reported cases of scrub typhus from India [1]. Laboratory facilities with capacities to do serological tests [Immunofluorescence assay (IFA), Enzyme-linked immunosorbent assay (ELISA)] or molecular tests are required to confirm scrub typhus diagnoses. A simple physical examination can be fruitful in resource-limited settings where these tests are scarce [2]. Since several other febrile illnesses, such as dengue, malaria, leptospirosis, enteric fever, etc., can have similar presentations, the presence of eschar is useful in making an early confirmatory diagnosis [2].
The classical description of an eschar is a black necrotic lesion with an erythematous base [3]. Three eschar stages are described in the earliest scrub typhus reports [4]. The first stage is a small papule at the site of the mite bite. A shallow ulcer is formed with a red base in the second stage. The ulcer becomes a scab in the third stage, and the red base starts scaling. The scab is shed in two to three weeks. Most patients present in the second stage [4]. It must also be noted that an inexperienced examiner can rarely confuse the morphology of eschar with old scabs or clots. While phenotypic identification is enough for diagnosing scrub typhus in most cases, in cases of atypical appearance, polymerase chain reaction (PCR) assay from eschar material (biopsy or swab) has shown to be useful [5]. In a study of 20 patients in whom both swabs from eschar and whole blood were obtained for PCR, eschar was positive in 85% of the patients, while whole blood was positive in 25% [6]. On ultrastructural visualisation of the eschar biopsy, Orientia tsutsugamushi was found in the peripheral erythematous region but not the central necrotic region [7].
Studies worldwide report eschar at frequencies ranging from less than 10% to more than 90% [8]. There is a need to pool data on the frequency of eschar positivity from India systematically to understand the utility of eschar as a stand-alone tool for scrub typhus diagnosis. This will inform us of the need for better clinical decision-making tools and diagnostic tests. There is also a need to identify anatomical sites on the human body where eschar is most localized to educate busy clinicians on “Where to look for an eschar”. Therefore, this study aimed to ascertain the overall and geographic region-wise frequency and anatomical distribution of eschar in India.
METHODOLOGY
This systematic review and meta-analysis (SRMA) was registered with PROSPERO (Registration number CD42023483597) and has been reported according to the PRISMA guidelines [9]. We searched PubMed and Embase databases using an appropriate search string: [(scrub OR typhus OR Orientia) AND (eschar) AND (India)]. Articles in all languages from the two databases between 01.01.1900 and 16.11.2023 were included in the study. This SRMA included studies with individuals of any age and sex (including pregnant individuals) who were diagnosed with scrub typhus in India. Those studies that explicitly mentioned the presence or absence of eschar were included. We included randomized controlled trials, non-randomized clinical trials, analytical observational studies, and case series (≥20 cases). Studies on non-human subjects were not included. Case reports, case series (<20 cases), reviews, systematic reviews, conference abstracts and letters to the editor were excluded. After removing the duplicates, two authors (NG and KF) screened the titles and abstracts for eligibility. After the initial screening, the same two authors retrieved full-length articles for full-text screening. The conflicts were resolved by the third author (CB). Articles that met the eligibility criteria were included in the final analysis. Two authors independently appraised the articles using the standardized JBI critical appraisal checklist (CB and NG) [10]. The conflicts were resolved by the third author (TPK). Of the eight critical appraisal criteria, the exposure management criteria were not recorded as they were considered irrelevant to the current SR.
The following data were retrieved from the included articles to meet the study’s objective: author details, type of study, geographic region of India in which the study was conducted, details of microbiological diagnostics, age group of the included patients (adult or paediatric or both), number of included patients with confirmed scrub typhus and number of patients with eschar (overall and at each anatomical site).
For the quantitative synthesis, only studies which used diagnostic modalities with considerable specificity were included. Diagnostic modalities considered specific based on published literature were IFA, ELISA, ICT and PCR [11]. Weil-Felix test was not considered diagnostic of scrub typhus as previous studies show its poor diagnostic accuracy [12]. Those studies where serology was used for making a diagnosis, but the type of serology was not specified were also excluded. The anatomical distribution of eschar was also noted in the included studies. The anatomical location of eschar was categorized in the following headings: ‘axilla’ (includes axilla and infra-axillary location), ‘groin’ (includes inguinal region, genitals, peri-anal and buttocks), ‘trunk’ (includes shoulders, chest, breast, abdomen, back, trunk), ‘limbs’ (includes arm, forearm, hands, thighs, legs and feet), and ‘head’ (includes face, scalp, ears, peri-oral region and neck).
The data on frequency of eschar positivity in patients with scrub typhus were pooled using a random-effect model (Der Simonian and Laird), and the results were represented by a point estimate with a 95% confidence interval. The frequency of eschar positivity was calculated for different geographic regions (states and union territories) of India. Whenever two or more studies were reported from the same geographic regions, they were pooled using the random-effect model. The frequency of positivity (pooled or otherwise) in different regions was plotted on a map using the function available in Microsoft Excel. The frequencies of eschar in five different anatomical locations (axilla, groin, trunk, limbs and head) were similarly pooled, with the denominator being the total number of eschars in the study. The heterogeneity across studies was tested using the I2 test. The meta-analysis was performed using the open-source meta-analysis software (developed by Wallace et al.) that uses the R environment as the statistical engine and Python for the graphical user interface [13].
RESULTS
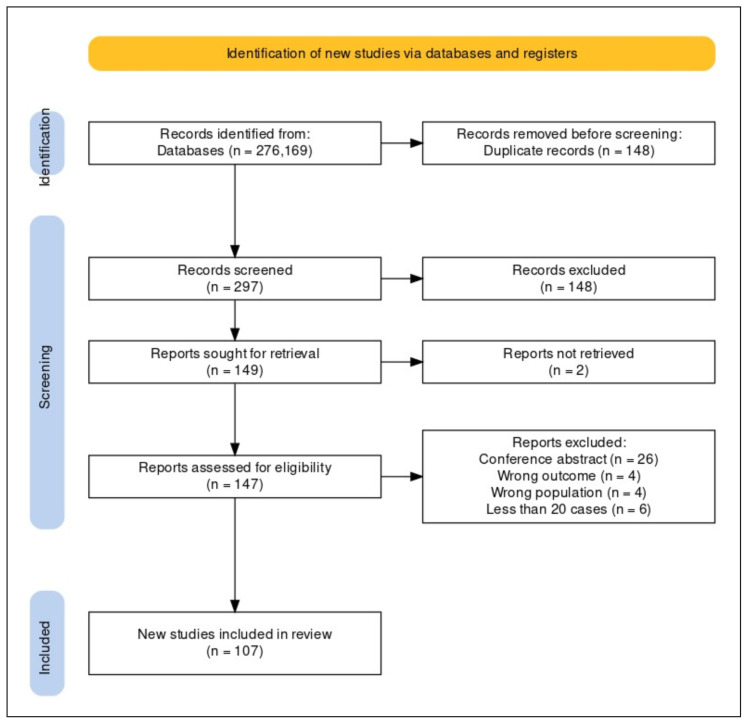
A total of 276 articles from Embase and 169 articles from PubMed were included for screening. After deleting 148 duplicates, 297 articles were included in the title-abstract screening. Out of these, 149 articles were selected for full-text screening. A total of 107 articles (34002 cases of scrub typhus) were finally included for data extraction and critical appraisal after excluding 42 articles [14–120]. The PRISMA flow chart was generated using the package developed by Haddaway et al. (Figure 1) [121]. All the included studies were published between 2010 and 2023. Most of the studies (n=57) were published in or after 2018.
Figure 1.
PRISMA flow diagram showing screening and final inclusion of the studies.
Using the JBI checklist, the criteria for inclusion and study subjects were described in all studies. Most studies did not identify confounding factors (n=91) and/or did not have strategies to deal with confounding (n=101), but these are unlikely to affect the primary objectives of this SRMA. Although outcomes were measured in the included studies, very few studies mentioned active strategies to search for eschars. The criteria for defining the disease (scrub typhus) were not mentioned in the five studies [18, 41, 42, 74, 116]. Studies that used only the Weil-Felix test (n=17) for diagnosis were excluded from the quantitative synthesis [17, 20, 24, 31, 40, 45, 53, 56, 63, 66, 73, 76, 79, 90, 92, 100, 118]. Additionally, two studies that included only patients with eschar were excluded from the prevalence of eschar estimation [65, 67]. IgM ELISA (n=81), ICT (n=10), IFA (n=6), and PCR (n=9) were the diagnostic modalities used in the included studies.
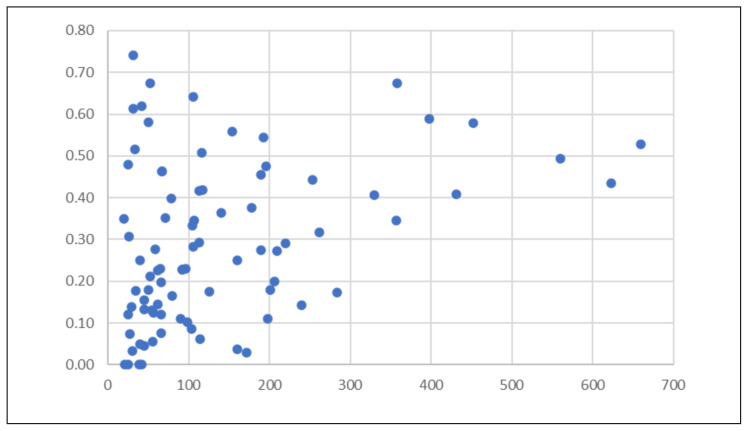
Of the 83 studies (n=11,471) included for the estimation of the prevalence of eschar, most studies were published in Tamil Nadu and Puducherry region (n=23) (Supplementary Table 1). Thirty-two studies focused entirely on the paediatric population. In contrast, 51 studies included adults or both adult and paediatric patients. The prevalence of eschar in patients ranged from 0 to 74% (Figure 2). Fifteen studies found the prevalence of eschar to be less than or equal to 10%, while only 14 found a prevalence of 50% or more.
Figure 2.
Frequency of eschar positivity in patients with scrub typhus (X-axis: number of scrub typhus patients, Y-axis: proportion of eschar positivity).
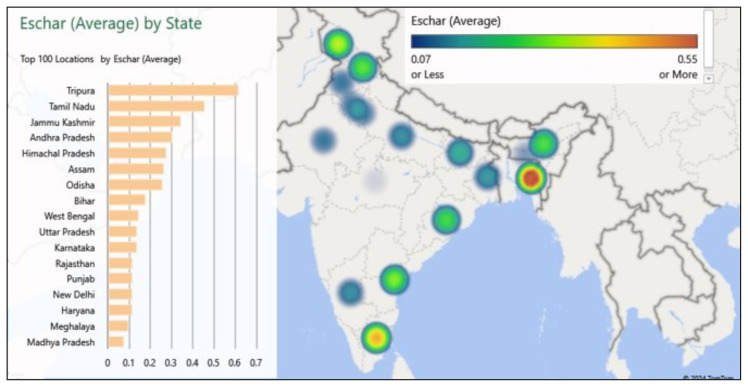
The pooled prevalence of the eschar was 28.5% (95% CI: 24.1 to 32.9) with high heterogeneity (I2-97.5%) (Supplementary Figure 1). The pooled frequency of eschar positivity in different geographic regions is summarized in Figure 3 and Supplementary Table 1. The forest plots for the pooled prevalence of geographic regions with two or more studies on eschar frequency are in the Supplementary Appendix (Supplementary Figures 2–11).
Figure 3.
Frequency of eschar positivity in different geographic regions (states and union territories) of India. (As the colour gradient moves from violet to red, the frequency of eschar positivity increases).
There was no significant difference when the pooled proportion of eschar positivity was calculated for retrospective [29.2% (95%CI: 21.2%–37.3%)] and prospective studies [28.2% (95%CI: 22.8%–33.5%)] separately (Supplementary Figure 12,13). When the frequency of eschar positivity was calculated separately for the paediatric population, it was not significantly different from the overall population [30.1% (95%CI: 22.5%–37.9%)] (Supplementary Figure 14).
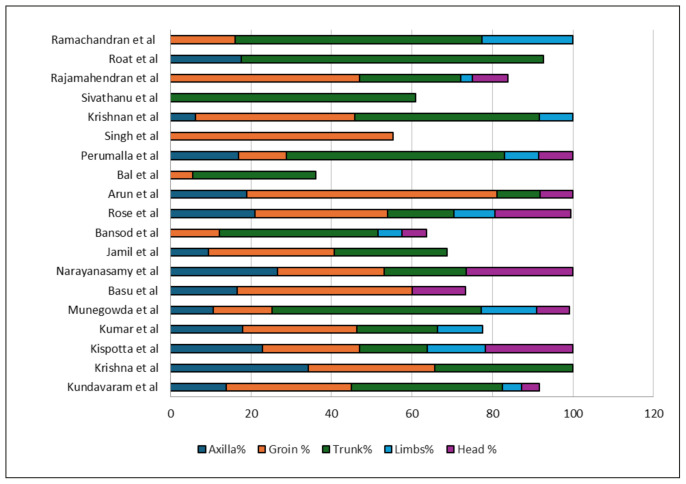
The pooled proportion of eschar in various anatomical sites was as follows: ‘trunk’ [39.3% (95% CI: 29.4%–49.3%)], ‘groin’ [23.8% (95%CI: 17.6%–30%)], ‘axilla’ [16.5% (95% CI: 13.4%–19.6%)], ‘head’ [11.3% (95%CI: 7.4%–15.3%)] and ‘limbs’ [9.9% (95% CI: 7–12.9%] (Supplementary Figures 15–19). The most common location of eschar in those studies that did not report the exact frequency is summarized in Supplementary Table 2. For studies that reported eschars in more than 20 participants, the relative frequency of eschar at various locations is summarized in Figure 4 [16, 19–21, 24, 25, 31, 33, 40, 41, 46, 49, 57, 62, 64–67, 76, 82, 85, 90–92, 105, 108, 111–113].
Figure 4.
Relative frequency of eschar at various locations in percentage in those studies that reported eschars in more than 20 participants. (Note-Some studies do not add up to 100% because of incomplete details or difficult categorization).
Most studies showed a high frequency of localization of eschar in the ‘groin’ and ‘trunk’. Of the 427 cases in the ‘groin’, where individual details of the location were available, most eschars were localized in the inguinal region (n=242, 57%), followed by perineal/anogenital region (n=167, 39%) and buttocks (n=18, 4%) [16, 19, 20, 21, 24, 25, 33, 40, 41, 49, 57, 64–67, 6, 82, 85, 92, 108, 113]. Of 403 patients with eschar on the ‘trunk’ with details of localization available, 222 (55%) were on the abdomen, 97 (24%) on the chest/breast and 84 (21%) on the back [16, 19, 21, 24, 25, 31, 33, 41, 46, 49, 57, 62–67, 76, 82, 85, 90, 105, 108, 111–113]. Many patients with eschar on the abdomen were localized around the umbilicus and the inframammary region (in women) [16, 19]. Of the 107 patients with details of localization of eschar on the ‘head’, 66 (61.6%) were on the neck, while 41 were on the scalp/face (38.3%) [16, 21, 24, 25, 40, 41, 49, 57, 64, 66, 67, 111, 113. In some studies, eschar on the ears and eyelids were also reported. [21, 41]. Four studies reported some patients with multiple eschars as well [40, 65–67].
DISCUSSION
In this SRMA, the frequency of eschar in confirmed scrub typhus cases ranged from 0 to 74%, with a pooled proportion of 29%. Some geographical disparities were observed within India, but not between age groups or study design. Most eschars were located on the trunk, groins and axilla. A previous SRMA by Yoo et al. that included studies till 2019 on the global prevalence of eschars reported that the prevalence of eschar in India was 33.3%. as opposed to a prevalence of 79% in East Asia and 52% in Oceania [122]. That SRMA concluded that the variation in eschar presence might be associated with differences in strains or mite species [122]. In India, the Kato-like and Karp-like genotype is common [123]. Additionally, previous studies have noted that eschar is challenging to spot in dark-skinned individuals, which might partially explain the low positivity in India [4]. One of the aims of the current SRMA is to explore the low eschar positivity in India further. More than 20 additional studies from India have been published since 2019 (the last date of inclusion for the previous SRMA) that explored the frequency and distribution of eschars in various regions of India. Additionally, the previous SRMA did not explore the geographic regional differences in eschar distribution in India.
The regional pooled eschar positivity varied from 12% or below in Haryana, Rajasthan, Madhya Pradesh, Punjab (including Chandigarh), and Meghalaya to 46% or above in Tamil Nadu (including Puducherry) and Tripura (Figure 3). In general, we noted that the frequency of eschar positivity in South Indian centres (Tamil Nadu, Andhra Pradesh) was higher than in other geographic regions. This could be because of their longer experience in diagnosing and managing scrub typhus, as evidenced by the number of reported studies [26, 35–37, 39, 43, 46, 56–58, 64, 86, 95, 98, 101, 107–109, 113]. Also, as evident from Figure 2, studies with a larger sample size (≥300 scrub typhus patients) generally had a higher frequency of eschar positivity than the average. It is also possible that cultural and regional differences in practices might have played a role in physicians’ reluctance to examine private areas of the body, such as genitals, where eschars are commonly located. Since all hospitals in India do not have temperature regulators, it can be assumed that a full body examination would be particularly difficult in colder regions. Since the hospital catchment area and population density vary from region to region, the consultation time per patient also varies. It is possible that eschar might be more frequently missed in a busy hospital. There is some variation in the prevalent genotypes in the different regions, which might have had an impact on the eschar positivity. In a multi-centric study, the Kato-like genotype was common in all regions, but North India and Northeast India also had Karp-like genotypes [123].
Since the larval stage of mites is not visible easily by the naked eye and the bite is painless, patients frequently miss these lesions. In the case of children, vigilant parents can often localize and report these lesions [40]. We postulated that eschar positivity in paediatric patients would be higher because of the vigilant parents and the ease of examination. However, we found no significant difference between adult and paediatric patients. Typical eschars are easy to identify, but they might get missed if the patient presents early with a non-specific lesion or presents late when the eschar has already healed [124]. It is also possible that previous exposure to the organism or use of antibiotics before presentation can impact the morphology of eschar [65]. Similarly, early eschar lesions may be easily missed by an inexperienced eye. An in-vitro study showed that intradermal inoculation produces eschar, but subcutaneous bite does not [124]. Therefore, the variation in the size of the proboscis of the mite may explain the absence of eschar [124].
Studies that included IgM ELISA often did not mention the cut-off they used for the diagnosis. Previous studies have shown that the cut-off optical density value in various regions is higher than the cut-off of 0.5 recommended by some guidelines [125]. Using a lower cut-off may have led to the inclusion of false positives, thereby decreasing the prevalence of eschar. We postulated that the proportion of eschars would be higher in prospective studies because we expected the search would be more thorough in a well-planned prospective study. However, the eschar positivity in India did not change significantly with the type of study (prospective or retrospective). Observational studies (both prospective and retrospective) often rely on documentation of eschar by the treating physicians. Poor documentation may have underestimated the frequency in both types of studies.
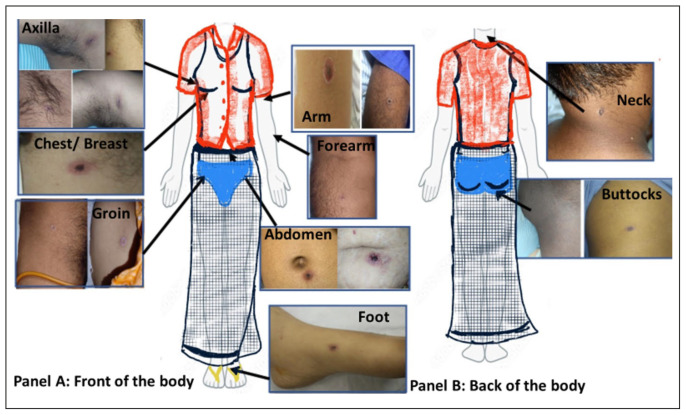
It is postulated that the chigger of the trombiculid bite latches on to the human and migrates up on a foreign object (at a speed of 2.2 inches per minute) till it finds resistance [3]. The site of the bite is usually at warm and moist locations where there is resistance, either due to the shape of the body (e.g. axilla, groin, inframammary region) or in regions where there is an artificial pressure point due to tight clothing (e.g. edges of the underwear) (Figure 5). The most common location of eschar in the SR was the ‘trunk’, with a pooled proportion of 39%. It is worth noting that many studies localized the eschar site around the inframammary region in women. It is also commonplace to see the eschar near the umbilicus, where traditional Indian clothing (Dhoti/lungi in men and saree in women) is tied [112]. Some studies reported eschar in unusual locations such as ears or eyelids. These regions should also be examined in patients with suspected scrub typhus.
Figure 5.
Representative image of a human body clothed in a representative Indian attire with eschar images (obtained from patients after consent) in different locations. Panel A shows the front of the body with eschars in the axilla, chest/breast, abdomen, groin and limbs. Panel B shows the back of the body with eschars on the neck and buttocks.
Limitations of the systematic review: We excluded case reports and case series with less than 20 cases to get the maximum number of studies with good-quality data. Of the included studies, very few studies had a primary objective of locating eschars, and the researchers often relied on the documentation of treating physicians. Although we tried to include studies that utilized a serological test of reasonable specificity, it was often not possible, as discussed earlier. Several studies combined the distribution of eschar in different regions (e.g. eschars in arm and axilla), which made it difficult to include all the available data for quantitative synthesis [113].
The study concluded that eschar is reported in less than a third of the patients with scrub typhus in India. Most eschars were located in the groin, axilla, around the umbilicus and inframammary region. There was a regional variation in the eschar positivity. In scrub typhus endemic areas, physicians need to be aware of the importance of a focused dermatologic examination of the trunk, groin and axilla of patients presenting with acute febrile illness. Other reasons for low eschar positivity among scrub typhus cases in certain regions deserve further study.
Supplementary Data
Forest plot showing the pooled prevalence of eschar in India.
Forest plot showing the pooled prevalence of eschar in Tamil Nadu and Puducherry.
Forest plot showing the pooled prevalence of eschar in Odisha.
Forest plot showing the pooled prevalence of eschar in Himachal Pradesh.
Forest plot showing the pooled prevalence of eschar in Rajasthan.
Forest plot showing the pooled prevalence of eschar in Karnataka.
Forest plot showing the pooled prevalence of eschar in West Bengal.
Forest plot showing the pooled prevalence of eschar in Andhra Pradesh and Telangana.
Forest plot showing the pooled prevalence of eschar in Punjab and Chandigarh.
Forest plot showing the pooled prevalence of eschar in Uttar Pradesh and Uttarakhand.
Forest plot showing the pooled prevalence of eschar in Meghalaya.
Pooled prevalence of eschar in retrospective studies.
Pooled prevalence of eschar in prospective studies.
Pooled prevalence in studies done exclusively in the paediatric population.
Pooled prevalence of eschar positivity in the axilla in those studies that reported
Pooled prevalence of eschar positivity in the groin in those studies that reported eschar location.
Pooled prevalence of eschar positivity in the trunk in those studies that reported eschar location.
Pooled prevalence of eschar positivity in limbs in those studies that reported eschar location.
Pooled prevalence of eschar positivity in head and neck in those studies that reported eschar location
Supplementary Table 1.
Geographic region-wise frequency of eschar patients with scrub typhus.
| State | Total Scrub | Number of studies | Prevalence |
|---|---|---|---|
| Tamil Nadu and Puducherry | 4191 | 23 | 46.10% |
| Andhra Pradesh and Telangana | 1466 | 5 | 31.10% |
| Odisha | 1260 | 11 | 26.40% |
| Himachal Pradesh | 893 | 6 | 28.50% |
| Karnataka | 847 | 5 | 14.20% |
| Uttar Pradesh and Uttarakhand | 726 | 9 | 13.60% |
| Rajasthan | 563 | 6 | 11.90% |
| West Bengal | 523 | 4 | 14.90% |
| Punjab and Chandigarh | 479 | 5 | 11.80% |
| Meghalaya | 168 | 2 | 10% |
| Madhya Pradesh | 104 | 1 | 8.60% |
| Assam | 58 | 1 | 27.50% |
| New Delhi | 56 | 1 | 12.50% |
| Bihar | 50 | 1 | 18% |
| Tripura | 42 | 1 | 61.90% |
| Haryana | 25 | 1 | 12% |
| Jammu Kashmir | 20 | 1 | 35% |
Supplementary Table 2.
Distribution and localization of eschar in studies where the exact frequency of positivity in each location was not mentioned.
| Sn | Authors | State | Eschar (n) | Distribution of eschar |
|---|---|---|---|---|
| 1 | Saha 2022 et al. | Tripura | 26 | Inguinal/peri-inguinal, axilla, below breast |
| 2 | Debnath 2021 et al. | Assam | 16 | Medial aspect of thigh, perineum, abdomen, armpit, upper back |
| 3 | Saluja 2019 et al. | Rajasthan | 6 | Groin, back and lower extremities |
| 4 | Dhar 2018 et al. | Odisha | 15 | Extremities, abdomen and thorax, groin and axilla, male genitalia |
| 5 | Patnaik 2017 et al. | Odisha | 12 | Axilla, abdomen, ear, groin, genitals |
| 6 | Shaikh 2017 et al. | Tamil Nadu | 105 | Groin, genitalia, axilla, chest |
| 7 | Chellamma 2016 et al. | Tamil Nadu | 7 | Abdomen, axilla, groin |
| 8 | Agarwal 2014 et al. | Andhra Pradesh | 20 | Abdomen, chest and neck |
| 9 | Aggarwal 2014 et al. | Haryana | 3 | Lower limbs |
| 10 | Bhat 2014 et al. | Uttarakhand | 14 | Groin and axilla |
| 11 | Stephen 2013 et al. | Pondicherry | 10 | Axilla, nipple, scrotum, forehead, shoulder, back, abdomen, leg, knee and foot |
| 12 | Palanivel 2012 et al. | TN | 31 | Axilla, genitalia, inguinal area |
| 13 | Vivekanandan 2010 et al. | Pondicherry | 23 | axilla, breast, groin |
| 14 | Varghese 2013 et al. | Tamil Nadu | 86 | groin, axilla, neck, breast folds |
| 15 | Abhilash 2015 et al. | Tamil Nadu | 52 | groin, genitalia, axilla, neck, inframammary folds |
| 16 | Verma 2021 et al. | Uttar Pradesh | 11 | abdomen, thighs and arms |
| 17 | Abhilash 2016 et al. | Tamil Nadu | 234 | groin, axilla, genitalia, neck, breast folds |
| 18 | Palanivel 2012 et al. | Tamil Nadu | 31 | axilla, genitalia, inguinal area |
| 19 | Bhargava 2016 et al. | Uttarakhand | 49 | neck, axilla, abdomen, inguinal and pubic regions |
| 20 | Sharma 2014 et al. | Rajasthan | 22 | axilla, breast, groin |
Footnotes
Authors contribution: NG and TPK contributed equally to the manuscript and should be considered as joint first authors.
Conflict of interest: None.
Funding: None to declare.
REFERENCES
- 1.Devasagayam E, Dayanand D, Kundu D, et al. The burden of scrub typhus in India: A systematic review. PLoS Negl Trop Dis. 2021;15(7):e0009619. doi: 10.1371/journal.pntd.0009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, Nischal N. Management of acute febrile diseases in limited resource settings: a case-based approach. Infez Med. 2020;28(1):11–16. [PubMed] [Google Scholar]
- 3.Irons EM, Armstrong HE. Scrub typhus in Dutch New Guinea. Ann Intern Med. 1947 Feb;26(2):201–20. doi: 10.7326/0003-4819-26-2-201. [DOI] [PubMed] [Google Scholar]
- 4.Willcox PHA. Mite typhus fever In Assam and Burma, 1944–1946. Trans R Soc Trop Med Hyg. 1948;42(2):171–189. [Google Scholar]
- 5.Lee SH, Kim DM, Cho YS, et al. Usefulness of Eschar PCR for Diagnosis of Scrub Typhus. J Clin Microbiol. 2006;44(3):1169–1171. doi: 10.1128/JCM.44.3.1169-1171.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. PLoS Negl Trop Dis. 2017;11(2):e0005397. doi: 10.1371/journal.pntd.0005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ro HJ, Lee H, Park EC, et al. Ultrastructural visualization of Orientia tsutsugamushi in biopsied eschars and monocytes from scrub typhus patients in South Korea. Sci Rep. 2018;8(1):17373. doi: 10.1038/s41598-018-35775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6(1):e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porritt K, Gomersall J, Lockwood C. JBI’s Systematic Reviews: Study selection and critical appraisal. Am J Nurs. 2014;114(6):47–52. doi: 10.1097/01.NAJ.0000450430.97383.64. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N, Chaudhry R, Kabra SK, et al. Comparative Evaluation of Serological and Molecular Methods for the Diagnosis of Scrub Typhus in Indian Settings. Jpn J Infect Dis. 2017;70(2):221–222. doi: 10.7883/yoken.JJID.2016.139. [DOI] [PubMed] [Google Scholar]
- 12.Janardhanan J, Trowbridge P, Varghese GM. Diagnosis of scrub typhus. Expert Rev Anti Infect Ther. 2014;12(12):1533–1540. doi: 10.1586/14787210.2014.974559. [DOI] [PubMed] [Google Scholar]
- 13.Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Stat Softw. 2012 Jun 30;49:1–15. [Google Scholar]
- 14.Sarangi R, Kabi S, Dhar SK, et al. A comparative study of clinical and laboratory profiles of scrub typhus in different age groups: our experience. Pediatr Pol. 2018;93(1):62–65. [Google Scholar]
- 15.Roychowdhury S, Majumder D, Mukhopadhyay P. A Menace without Specific Feature - Scrub Typhus a Reemerging Disease. J Assoc Physicians India. 2022;69(12):11–12. [PubMed] [Google Scholar]
- 16.Munegowda K, Nanda S, Varma M, et al. A prospective study on distribution of eschar in patients suspected of scrub typhus. Trop Doct. 2014;44(3):160–162. doi: 10.1177/0049475514530688. [DOI] [PubMed] [Google Scholar]
- 17.Loganathan S, Jaybhaye A, Dash N, et al. Acute respiratory distress syndrome in paediatric scrub typhus. Trop Doct. 2021;51(4):514–517. doi: 10.1177/00494755211029146. [DOI] [PubMed] [Google Scholar]
- 18.Ramasamy VP, Bollipo S, Vankudoth S, et al. An Emerging Infection “Scrub Typhus” - A detailed clinical profile and complications among children in a tertiary hospital. Indian J Public Health Res Dev. 2023;14(4):356–361. [Google Scholar]
- 19.Roat G, Singh R, Kumar S, et al. An observational study on clinical spectrum, diagnosis, complications and therapeutic outcomes on scrub typhus: an emerging rickettsial infection. Int J Acad Med Pharm. 2023;5(3):1796–1801. [Google Scholar]
- 20.Singh SI, Devi KP, Tilotama R, et al. An outbreak of scrub typhus in Bishnupur district of Manipur, India, 2007. Trop Doct. 2010;40(3):169–170. doi: 10.1258/td.2010.090468. [DOI] [PubMed] [Google Scholar]
- 21.Kispotta R, AK, PP KK, et al. Analysis of 262 Children with Scrub Typhus Infection: a single-center experience. Am J Trop Med Hyg. 2020;104(2):622–627. doi: 10.4269/ajtmh.20-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyagi S, Rahaengdale N, Shukla N, et al. Analysis of clinical and biochemical parameters in Scrub Typhus Patients in Central India. Intl J Pharm Clin Res. 2023;15(10):616–620. [Google Scholar]
- 23.Sharma R, Krishna VP, Manjunath, et al. Analysis of two outbreaks of Scrub Typhus in Rajasthan: a clinico-epidemiological study. J Assoc Physicians India. 2014;62(12):24–29. [PubMed] [Google Scholar]
- 24.Dass R, Deka NM, Duwarah SG, et al. Characteristics of pediatric scrub typhus during an outbreak in the North Eastern region of India: peculiarities in clinical presentation, laboratory findings and complications. Indian J Pediatr. 2011;78(11):1365–1370. doi: 10.1007/s12098-011-0470-5. [DOI] [PubMed] [Google Scholar]
- 25.Arun Babu T, Vijayadevagaran V, Ananthakrishnan S. Characteristics of Pediatric Scrub Typhus Eschar in South Indian Children. Pediatr Dermatol. 2017;34(2):124–127. doi: 10.1111/pde.13048. [DOI] [PubMed] [Google Scholar]
- 26.Abhilash K, Mannam PR, Rajendran K, et al. Chest radiographic manifestations of scrub typhus. J Postgrad Med. 2016;62(4):235–238. doi: 10.4103/0022-3859.184662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma SK, Gupta KK, Arya RK, et al. Clinical and biochemical profile of scrub typhus patients at a tertiary care hospital in Northern India. J Fam Med Prim Care. 2021;10(3):1459–1465. doi: 10.4103/jfmpc.jfmpc_1162_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivarajan S, Shivalli S, Bhuyan D, et al. Clinical and paraclinical profile, and predictors of outcome in 90 cases of scrub typhus, Meghalaya, India. Infect Dis Poverty. 2016;5(1):91. doi: 10.1186/s40249-016-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manjunath VG, Hedda S, Vijay Kumar GS, et al. Clinical features, laboratory findings and complications of scrub typhus in South Indian children. J Nepal Paediatr Soc. 2017;37(1):21–24. [Google Scholar]
- 30.Mohanty L, Dhanawat A, Gupta P, et al. Clinical manifestations and associated complications of scrub typhus in Odisha, India. J Clin Diagn Res. 2020;14(8):OC14–OC18. [Google Scholar]
- 31.Peesapati N, Rohit L, Sunitha S, Sivaram Pv. Clinical manifestations and complications of Scrub Typhus: A Hospital-based Study from North Andhra. J Assoc Physicians India. 2019;67(3):22–24. [PubMed] [Google Scholar]
- 32.Behera JR, Sahu SK, Mohanty N, et al. Clinical Manifestations and Outcome of Scrub Typhus in Infants From Odisha. Indian Pediatr. 2021;58(4):367–369. [PubMed] [Google Scholar]
- 33.Kumar R, Thakur S, Bhawani R, et al. Clinical Profile and Complications of Scrub Typhus: Hospital-Based Study in Sub-Himalayan Region. J Assoc Physicians India. 2016;64(12):30–34. [PubMed] [Google Scholar]
- 34.Bhattacharya PK, Murti VS, Jamil M, et al. Clinical profile and determinants of scrub typhus presenting with sepsis based on Sepsis-3 criteria. J Vector Borne Dis. 2020;57(4):307–313. doi: 10.4103/0972-9062.313963. [DOI] [PubMed] [Google Scholar]
- 35.Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39–43. doi: 10.1016/j.ijid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Ganesh R, Suresh N, Pratyusha LL, et al. Clinical profile and outcome of children with scrub typhus from Chennai, South India. Eur J Pediatr. 2018;177(6):887–890. doi: 10.1007/s00431-018-3143-9. [DOI] [PubMed] [Google Scholar]
- 37.Narayanasamy DK, Arun Babu T, Fredrick J, et al. Clinical profile and outcomes of pediatric scrub typhus associated with elevated hepatic transaminases. Indian J Gastroenterol. 2023;42(3):347–354. doi: 10.1007/s12664-023-01350-y. [DOI] [PubMed] [Google Scholar]
- 38.Nallasamy K, Gupta S, Bansal A, et al. Clinical Profile and Predictors of Intensive Care Unit Admission in Pediatric Scrub Typhus: A Retrospective Observational Study from North India. Indian J Crit Care. 2020;24(6):445–450. doi: 10.5005/jp-journals-10071-23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Premraj SS, Mayilananthi K, Krishnan D, et al. Clinical profile and risk factors associated with severe scrub typhus infection among non-ICU patients in semi-urban south India. J Vector Borne Dis. 2018;55(1):47–51. doi: 10.4103/0972-9062.234626. [DOI] [PubMed] [Google Scholar]
- 40.Basu S, Saha A, Sarkar S, et al. Clinical Profile and Therapeutic Response of Scrub Typhus in Children: A Recent Trend from Eastern India. J Trop Pediatr. 2019;65(2):139–146. doi: 10.1093/tropej/fmy027. [DOI] [PubMed] [Google Scholar]
- 41.Bansod YV, Aher AA, Bhole P, et al. Clinical Profile and Treatment Outcome in Scrub Typhus Patients in Central India. J Assoc Physicians India. 2021;69(9):11–12. [PubMed] [Google Scholar]
- 42.Goel T, Naveen, Krishna R, et al. Clinical profile of patients with scrub typhus; cross sectional study, at Maharishi Markandeshwar Medical College and Hospital, Solan. J Cardiovasc Res. 2023;14(2):1062–1069. [Google Scholar]
- 43.Palanivel S, Nedunchelian K, Poovazhagi V, et al. Clinical profile of scrub typhus in children. Indian J Pediatr. 2012;79(11):1459–1462. doi: 10.1007/s12098-012-0721-0. [DOI] [PubMed] [Google Scholar]
- 44.Sarangi R, Pradhan S, Debata NC, et al. Clinical profile of scrub typhus in children treated in a tertiary care hospital in eastern India. Pediatr Pol. 2016;91(4):308–311. [Google Scholar]
- 45.Chellamma P, Paramasivam S, Thayyil J, et al. Clinical profile of scrub typhus patients in a tertiary care hospital in Kerala. Indian J Public Health Res Dev. 2016;7(4):330–334. [Google Scholar]
- 46.Sivathanu S, Jayaraman A, Parthasarathy S, et al. Clinical profile, outcome, and atypical presentations of Scrub Typhus: experience from a tertiary care center in South India. J Pediatr Infect Dis. 2017;12(2):110–113. [Google Scholar]
- 47.Mondal M, Mandal PK, Basu R, et al. Clinical Spectrum and Laboratory Parameters in Scrub Typhus in Children: Experience from a Tertiary Care Centre in West Bengal, India. J Indian Med Assoc. 2023;121(10):23–27. [Google Scholar]
- 48.Dhar SK, Sarangi R, Samant S. IgM ELISA value for scrub typhus as a prognostic indicator: Our experience in pediatric and adult cases. Eur J Mol Clin Med. 2020;7(6):1236–1241. [Google Scholar]
- 49.Narayanasamy DK, Arunagirinathan AK, Kumar RK, et al. Clinico - Laboratory Profile of Scrub Typhus - An Emerging Rickettsiosis in India. Indian J Pediatr. 2016;83(12):1392–1397. doi: 10.1007/s12098-016-2171-6. [DOI] [PubMed] [Google Scholar]
- 50.Saha S, Bhaumik DP, Deb S. Clinico-demographic profile and outcome of Scrub Typhus in North Eastern State of India. J Indian Med Assoc. 2022;120(12):39–43. [Google Scholar]
- 51.Behera B, Biswal M, Das RR, et al. Clinico-epidemiological analysis of scrub typhus in hospitalised patients presenting with acute undifferentiated febrile illness: A hospital-based study from Eastern India. Indian J Med Microbiol. 2019;37(2):278–280. doi: 10.4103/ijmm.IJMM_19_147. [DOI] [PubMed] [Google Scholar]
- 52.Panda A, Kishore SV, Pradhan M, et al. Clinico-epidemiological and outcome of scrub typhus in paediatric patients: An observational study from Odisha, India. J Clin Diagn Res. 2021;15(7):SC1–SC4. [Google Scholar]
- 53.George T, Jakribettu R, Abraham S, et al. Clinico-hematological, treatment, and outcome profile for scrub typhus: Observations from a tertiary care center. J Appl Hematol. 2020;11(4):180–183. [Google Scholar]
- 54.Soorya RK, Sood M, Dhiman D. Clinicolaboratory Profile and Outcome of Serologically Confirmed Scrub Typhus among Children from Sub Himalayan Tribal District of India: A Hospital-based Cross-sectional Study. J Clin Diagn Res. 2022;10:18–19. [Google Scholar]
- 55.Singla D, Singh B, Ahire K, et al. Coagulation abnormalities in severe Scrub Typhus and their association with complications. J Assoc Physicians India. 2023;71(5):11–12. doi: 10.5005/japi-11001-0237. [DOI] [PubMed] [Google Scholar]
- 56.Rose W, Ghosh U, Punnen A, et al. Comparison of Scrub Typhus With and Without Meningitis. Indian J Pediatr. 2017;84(11):833–837. doi: 10.1007/s12098-017-2403-4. [DOI] [PubMed] [Google Scholar]
- 57.Rose W, Rajan RJ, Punnen A, et al. Distribution of eschar in pediatric Scrub Typhus. J Trop Pediatr. 2016;62(5):415–420. doi: 10.1093/tropej/fmw027. [DOI] [PubMed] [Google Scholar]
- 58.Shaikh IAA, Kundavaram PPA, Mitra S, et al. Does the presence of an eschar correlate with severity of scrub typhus infection? Indian J Med Sci. 2017;69(1):36–39. [Google Scholar]
- 59.Varma BCD, Dantuluri V, Premamrutha KSG, et al. Efficacy of oral doxycycline versus intravenous doxycycline for the treatment of uncomplicated Scrub Typhus in children: a prospective interventional study. J Clin Diagn Res. 2023;17(10) [Google Scholar]
- 60.Aggarwal HK, Jain D, Kaverappa V, et al. Emergence of scrub typhus in northern India: Experience from tertiary care hospital. Klimik Dergisi. 2014;27(1):6–11. [Google Scholar]
- 61.Patnaik S, Swain N, Sahoo B, et al. Emergence of scrub typhus in Odisha - A hospital based study. Ann Trop Med Public Health. 2017;10(3):636–640. [Google Scholar]
- 62.Tarai B, Sen P, Kanaujia R, et al. Epidemiological, clinical and genetic characterization of scrub typhus in patients presenting with acute febrile illness in New Delhi. Indian J Med Microbiol. 2022 Oct;40(4):552–556. doi: 10.1016/j.ijmmb.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Vanramliana, Pautu L, Lalmalsawma P, et al. Epidemiology of scrub typhus and other rickettsial infections (2018–22) in the hyper-endemic setting of Mizoram, North-East India. PLoS Negl Trop Dis. 2023;17(11):e0011688. doi: 10.1371/journal.pntd.0011688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perumalla SK, Paul S, Abhilash KPP, et al. Eschar and IgM ELISA in the diagnosis of scrub typhus. Indian J Med Microbiol. 2019;37(1):113–115. doi: 10.4103/0255-0857.264495. [DOI] [PubMed] [Google Scholar]
- 65.Ramachandran B, Subramaniam R, Latha N, et al. Eschar as a sensitive marker for Scrub Typhus diagnosis at the primary healthcare level. Indian J Public Health Res Dev. 2023;14(1):384–390. [Google Scholar]
- 66.Jamil M, Bhattacharya P, Mishra J, et al. Eschar in Scrub Typhus: A Study from North East India. J Assoc Physicians India. 2019;67(4):38–40. [PubMed] [Google Scholar]
- 67.Kundavaram AP, Jonathan AJ, Nathaniel SD, et al. Eschar in scrub typhus: A valuable clue to the diagnosis. J Postgrad Med. 2013;59(3):177–178. doi: 10.4103/0022-3859.118033. [DOI] [PubMed] [Google Scholar]
- 68.Mahajan SK, Sharma R, Singh B, et al. Is Hyperuricemia a Marker of Severity of Disease in Scrub Typhus? J Assoc Physicians India. 2022 Dec;69(12):11–12. [PubMed] [Google Scholar]
- 69.Debnath I, Das D, Difoesa B, et al. Myriad of Presentation of Scrub Typhus in a tertiary care hospital in North Eastern India - A prospective study. J Indian Med Assoc. 2021;119(12):19–24. [Google Scholar]
- 70.Mukhopadhyay S, Gupta R, Shukla S, et al. Once forgotten now re-emerging: Scrub Typhus Infection in pediatric patients from North West India. Cureus. 2023;15(8):e44044. doi: 10.7759/cureus.44044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stephen S, Kandhakumari G, Vinithra SM, et al. Outbreak of pediatric Scrub Typhus in South India: A preliminary report. J Pediatr Infect Dis. 2013;8(3):125–129. [Google Scholar]
- 72.Sethi S, Prasad A, Biswal M, et al. Outbreak of scrub typhus in North India: a re-emerging epidemic. Trop Doct. 2014;44(3):156–159. doi: 10.1177/0049475514523761. [DOI] [PubMed] [Google Scholar]
- 73.Vivekanandan M, Mani A, Priya YS, et al. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24–28. [PubMed] [Google Scholar]
- 74.Parasher V, Shaha S, Khatri R, et al. Pattern of admission and clinical outcome of scrub typhus patients admitted in PICU in Southern Rajasthan. Intl J Pharm Clin Res. 2022;14(2):223–228. [Google Scholar]
- 75.Mahajan SK, Raina R, Singh B, et al. Pattern of clinical presentation, laboratory findings and mortality risk among patients of Scrub Typhus in Western Himalayas. J Assoc Physicians India. 2016;64(3):26–30. [PubMed] [Google Scholar]
- 76.Krishnan R, Pillai RK, Elizabeth KE, et al. Pediatric scrub typhus in Southern Kerala: An emerging public health problem. Clin Epidemiol Glob Health. 2016;4(2):89–94. [Google Scholar]
- 77.Alam F, Ray A. Prediction of Scrub Typhus in Tertiary Care Centre of Northern Bihar. Intl J Pharm Clin Res. 2022;14(12):131–137. [Google Scholar]
- 78.Alam A, Agarwal P, Prabha J, et al. Prediction rule for Scrub Typhus meningoencephalitis in children: emerging disease in North India. J Child Neurol. 2020;35(12):820–827. doi: 10.1177/0883073820933148. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal VK, Reddy GKM, Krishna MR, et al. Predictors of scrub typhus: A study from a tertiary care center. Asian Pac J Trop Dis. 2014;4:S666–673. [Google Scholar]
- 80.Sharma R, Mahajan SK, Singh B, et al. Predictors of severity in Scrub Typhus. J Assoc Physicians India. 2019;67(4):35–38. [PubMed] [Google Scholar]
- 81.Karanth SS, Marupudi KC, Sama VPR, et al. Predictors of severity of scrub typhus in the Indian subcontinent. Asian Pac J Trop Dis. 2014;4:S674–678. [Google Scholar]
- 82.Tumbanatham A, Jayasingh K, Lokesh S, et al. Presenting features and clinical profile of scrub typhus fever cases in adult patients admitted in a tertiary care hospital in Puducherry, India. J Clin Diagn Res. 2020;14(1):OC01–OC05. [Google Scholar]
- 83.Lakshmi RMMVN, Dharma TV, Sudhaharan S, et al. Prevalence of scrub typhus in a tertiary care centre in Telangana, south India. Iran J Microbiol. 2020;12(3):204–208. [PMC free article] [PubMed] [Google Scholar]
- 84.Baidya A, Gunasekaran D, Dhodapkar R, et al. Prevalence, clinico-laboratory features, and the functional outcome of children with scrub typhus meningoencephalitis - a cohort study. J Trop Pediatr. 2022;68(5):fmac077. doi: 10.1093/tropej/fmac077. [DOI] [PubMed] [Google Scholar]
- 85.Bal M, Mohanta MP, Sahu S, et al. Ranjit M. Profile of pediatric Scrub Typhus in Odisha, India. Indian Pediatr. 2019;56(4):304–306. [PubMed] [Google Scholar]
- 86.Griffith M, Peter JV, Karthik G, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med. 2014;18(8):497–502. doi: 10.4103/0972-5229.138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arun Babu T, Narayanasamy DK, Jamir L. Prospective Study to Assess the Response to Therapy and Its Predictors in Children with Scrub Typhus. J Trop Pediatr. 2021;67(5):fmab087. doi: 10.1093/tropej/fmab087. [DOI] [PubMed] [Google Scholar]
- 88.Sinha P, Gupta S, Dawra R, et al. Recent outbreak of scrub typhus in North Western part of India. Indian J Med Microbiol. 2014;32(3):247–250. doi: 10.4103/0255-0857.136552. [DOI] [PubMed] [Google Scholar]
- 89.Bal M, Kar CR, Behera HK, et al. Scrub typhus associated acute kidney injury: An emerging health problem in Odisha, India. J Vector Borne Dis. 2021;58(4):359–367. doi: 10.4103/0972-9062.318318. [DOI] [PubMed] [Google Scholar]
- 90.Rajoor UG, Gundikeri SK, Sindhur JC, et al. Scrub typhus in adults in a teaching hospital in north Karnataka, 2011–2012. Ann Trop Med Public Health. 2013;6(6):614–617. [Google Scholar]
- 91.Bhat NK, Dhar M, Mittal G, et al. Scrub typhus in children at a tertiary hospital in north India: Clinical profile and complications. Iran J Pediatr. 2014;24(4):387–392. [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar M, Krishnamurthy S, Delhikumar CG, et al. Scrub typhus in children at a tertiary hospital in southern India: clinical profile and complications. J Infect Public Health. 2012;5(1):82–88. doi: 10.1016/j.jiph.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Kumar Jana J, Krishna Mandal A, Gayen S, et al. Scrub Typhus in children: a prospective observational study in a tertiary care hospital in Eastern India. Cureus. 2023;15(7):e41976. doi: 10.7759/cureus.41976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agrawal A, Parida P, Rup AR, et al. Scrub Typhus in Paediatric Age Group at a Tertiary Care Centre of Eastern India: Clinical, Biochemical Profile and Complications. J Fam Med Prim Care. 2022;11(6):2503–2506. doi: 10.4103/jfmpc.jfmpc_1464_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rajan SJ, Sathyendra S, Mathuram AJ. Scrub typhus in pregnancy: Maternal and fetal outcomes. Obstet Med. 2016;9(4):164–166. doi: 10.1177/1753495X16638952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loomba V, Mani A, John M, et al. Scrub typhus in Punjab: an acute febrile illness with multisystem involvement. Trop Doct. 2014;44(3):152–155. doi: 10.1177/0049475514522226. [DOI] [PubMed] [Google Scholar]
- 97.Masand R, Yadav R, Purohit A, et al. Scrub typhus in rural Rajasthan and a review of other Indian studies. Paediatr Int Child Health. 2016;36(2):148–153. doi: 10.1179/2046905515Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 98.Varghese GM, Janardhanan J, Trowbridge P, et al. Scrub typhus in South India: clinical and laboratory manifestations, genetic variability, and outcome. Int J Infect Dis. 2013;17(11):e981–987. doi: 10.1016/j.ijid.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 99.Bhargava A, Kaushik R, Kaushik RM, et al. Scrub typhus in Uttarakhand & adjoining Uttar Pradesh: Seasonality, clinical presentations & predictors of mortality. Indian J Med Res. 2016;144(6):901–919. doi: 10.4103/ijmr.IJMR_1764_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Viswanathan S, Muthu V, Iqbal N, et al. Scrub typhus meningitis in South India--a retrospective study. PLoS One. 2013;8(6):e66595. doi: 10.1371/journal.pone.0066595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abhilash KP, Gunasekaran K, Mitra S, et al. Scrub typhus meningitis: An under-recognized cause of aseptic meningitis in India. Neurol India. 2015 Mar;63(2):209–214. doi: 10.4103/0028-3886.156282. [DOI] [PubMed] [Google Scholar]
- 102.Oberoi A, Varghese SR. Scrub typhus-an emerging entity: a study from a tertiary care hospital in North India. Indian J Public Health. 2014;58(4):281283. doi: 10.4103/0019-557X.146299. [DOI] [PubMed] [Google Scholar]
- 103.Bhat NK, Pandita N, Saini M, et al. Scrub Typhus: A clinico-laboratory differentiation of children with and without meningitis. J Trop Pediatr. 2016;62(3):194–199. doi: 10.1093/tropej/fmv097. [DOI] [PubMed] [Google Scholar]
- 104.Bhat NK, Dhar M, Mittal G, et al. Scrub typhus: A common rickettsial disease emerging in a new geographical region of north India. J Pediatr Infect Dis. 2014;9(2):93–99. [Google Scholar]
- 105.Takhar RP, Bunkar ML, Arya S, et al. Scrub typhus: A prospective, observational study during an outbreak in Rajasthan, India. Natl Med J India. 2017;30(2):69–72. [PubMed] [Google Scholar]
- 106.Mittal V, Singh P, Shukla S, et al. Scrub typhus: An under-reported and emerging threat - hospital based study from central and eastern Uttar Pradesh, India. J Vector Borne Dis. 2021;58(4):323–328. doi: 10.4103/0972-9062.318311. [DOI] [PubMed] [Google Scholar]
- 107.Chrispal A, Boorugu H, Gopinath KG, et al. Scrub typhus: An unrecognized threat in South India - Clinical profile and predictors of mortality. Trop Doct. 2010;40(3):129–133. doi: 10.1258/td.2010.090452. [DOI] [PubMed] [Google Scholar]
- 108.Krishna MR, Vasuki B, Nagaraju K. Scrub typhus: audit of an outbreak. Indian J Pediatr. 2015;82(6):537–540. doi: 10.1007/s12098-014-1664-4. [DOI] [PubMed] [Google Scholar]
- 109.Hazra D, Fernandes J, Nekkanti A, et al. Scrub typhus: Clinical presentation and severity. Curr Med Issues. 2020;18(2):111–114. [Google Scholar]
- 110.Saluja M, Vimlani H, Chittora S, et al. Scrub typhus: Epidemiology, clinical presentation, diagnostic approach, and outcomes. J Indian Acad Clin Med. 2019;20(1):15–21. [Google Scholar]
- 111.Mahajan A, Jasrotia DS, Charak RS, et al. Scrub typhus: Jammu outbreak-2009. JK Sci. 2010;12(2):98–101. [Google Scholar]
- 112.Pathania M, Amisha, Malik P, et al. Scrub typhus: Overview of demographic variables, clinical profile, and diagnostic issues in the sub-Himalayan region of India and its comparison to other Indian and Asian studies. J Fam Med Prim Care. 2019;8(3):1189–1195. doi: 10.4103/jfmpc.jfmpc_124_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajamahendran P, Barathiraja P, Thenmozhi A, et al. Scrubbing out scrub typhus: insights from a tertiary care hospital in Tamil Nadu. Int J Acad Med Pharm. 2023;5(4):726–730. [Google Scholar]
- 114.Ramyasree A, Kalawat U, Rani ND, et al. Seroprevalence of Scrub typhus at a tertiary care hospital in Andhra Pradesh. Indian J Med Microbiol. 2015;33(1):68–72. doi: 10.4103/0255-0857.148381. [DOI] [PubMed] [Google Scholar]
- 115.Mondal T, Sarkar A, Rahaman J, et al. A study of scrub typhus in a medical college hospital in West Bengal, India. Biomedicine. 2022;42(5):1091–1093. [Google Scholar]
- 116.Inamdar S, Thunga G, Acharya R, et al. Study of clinical characteristics and treatment pattern of scrub typhus in tertiary care hospital. J Pharm Sci Res. 2013;5(5):107–110. [Google Scholar]
- 117.Kusagur VB, Manasa KB. Study of clinical features of rickettsial fever in pediatric age group. Int J Acad Med Pharm. 2022;4(4):648–651. [Google Scholar]
- 118.Sharma SR, Masaraf H, Lynrah KG, et al. Tsutsugamushi Disease (Scrub Typhus) Meningoencephalitis in North Eastern India: A Prospective Study. Ann Med Health Sci Res. 2015;5(3):163–167. doi: 10.4103/2141-9248.157486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhar SK, Kabi S, Das C, et al. Clinical spectrum of scrub typhus in a tertiary care hospital at eastern India. Asian J Pharm Clin Res. 2018;11(5):351–354. [Google Scholar]
- 120.Gulati S, Chunduru K, Madiyal M, et al. Validation of a clinical risk-scoring algorithm for scrub typhus severity in South India. Indian J Crit Care Med. 2021;25(5):551–556. doi: 10.5005/jp-journals-10071-23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haddaway NR, Page MJ, Pritchard CC, et al. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18(2):e1230. doi: 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoo JS, Kim D, Choi HY, et al. Prevalence rate and distribution of eschar in patients with scrub typhus. Am J Trop Med Hyg. 2022;106(5):1358–1362. [Google Scholar]
- 123.Varghese GM, Janardhanan J, Mahajan SK, et al. Molecular Epidemiology and Genetic Diversity of Orientia Tsutsugamushi from Patients with Scrub Typhus in 3 Regions of India. Emerg Infect Dis. 2015;21(1):64–69. doi: 10.3201/eid2101.140580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lewthwaite R, Savoor SR The Typhus Group of Diseases in Malaya. Part VII: The Relation of Rural Typhus to the Tsutsugamushi Disease (with Special Reference to Cross-immunity Tests) Br J Exp Pathol. 1936;17(6):448–460. [Google Scholar]
- 125.Gupta N, Chaudhry R, Thakur CK. Determination of Cutoff of ELISA and Immunofluorescence Assay for Scrub Typhus. J Glob Infect Dis. 2016;8(3):97–99. doi: 10.4103/0974-777X.188584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot showing the pooled prevalence of eschar in India.
Forest plot showing the pooled prevalence of eschar in Tamil Nadu and Puducherry.
Forest plot showing the pooled prevalence of eschar in Odisha.
Forest plot showing the pooled prevalence of eschar in Himachal Pradesh.
Forest plot showing the pooled prevalence of eschar in Rajasthan.
Forest plot showing the pooled prevalence of eschar in Karnataka.
Forest plot showing the pooled prevalence of eschar in West Bengal.
Forest plot showing the pooled prevalence of eschar in Andhra Pradesh and Telangana.
Forest plot showing the pooled prevalence of eschar in Punjab and Chandigarh.
Forest plot showing the pooled prevalence of eschar in Uttar Pradesh and Uttarakhand.
Forest plot showing the pooled prevalence of eschar in Meghalaya.
Pooled prevalence of eschar in retrospective studies.
Pooled prevalence of eschar in prospective studies.
Pooled prevalence in studies done exclusively in the paediatric population.
Pooled prevalence of eschar positivity in the axilla in those studies that reported
Pooled prevalence of eschar positivity in the groin in those studies that reported eschar location.
Pooled prevalence of eschar positivity in the trunk in those studies that reported eschar location.
Pooled prevalence of eschar positivity in limbs in those studies that reported eschar location.
Pooled prevalence of eschar positivity in head and neck in those studies that reported eschar location
Supplementary Table 1.
Geographic region-wise frequency of eschar patients with scrub typhus.
| State | Total Scrub | Number of studies | Prevalence |
|---|---|---|---|
| Tamil Nadu and Puducherry | 4191 | 23 | 46.10% |
| Andhra Pradesh and Telangana | 1466 | 5 | 31.10% |
| Odisha | 1260 | 11 | 26.40% |
| Himachal Pradesh | 893 | 6 | 28.50% |
| Karnataka | 847 | 5 | 14.20% |
| Uttar Pradesh and Uttarakhand | 726 | 9 | 13.60% |
| Rajasthan | 563 | 6 | 11.90% |
| West Bengal | 523 | 4 | 14.90% |
| Punjab and Chandigarh | 479 | 5 | 11.80% |
| Meghalaya | 168 | 2 | 10% |
| Madhya Pradesh | 104 | 1 | 8.60% |
| Assam | 58 | 1 | 27.50% |
| New Delhi | 56 | 1 | 12.50% |
| Bihar | 50 | 1 | 18% |
| Tripura | 42 | 1 | 61.90% |
| Haryana | 25 | 1 | 12% |
| Jammu Kashmir | 20 | 1 | 35% |
Supplementary Table 2.
Distribution and localization of eschar in studies where the exact frequency of positivity in each location was not mentioned.
| Sn | Authors | State | Eschar (n) | Distribution of eschar |
|---|---|---|---|---|
| 1 | Saha 2022 et al. | Tripura | 26 | Inguinal/peri-inguinal, axilla, below breast |
| 2 | Debnath 2021 et al. | Assam | 16 | Medial aspect of thigh, perineum, abdomen, armpit, upper back |
| 3 | Saluja 2019 et al. | Rajasthan | 6 | Groin, back and lower extremities |
| 4 | Dhar 2018 et al. | Odisha | 15 | Extremities, abdomen and thorax, groin and axilla, male genitalia |
| 5 | Patnaik 2017 et al. | Odisha | 12 | Axilla, abdomen, ear, groin, genitals |
| 6 | Shaikh 2017 et al. | Tamil Nadu | 105 | Groin, genitalia, axilla, chest |
| 7 | Chellamma 2016 et al. | Tamil Nadu | 7 | Abdomen, axilla, groin |
| 8 | Agarwal 2014 et al. | Andhra Pradesh | 20 | Abdomen, chest and neck |
| 9 | Aggarwal 2014 et al. | Haryana | 3 | Lower limbs |
| 10 | Bhat 2014 et al. | Uttarakhand | 14 | Groin and axilla |
| 11 | Stephen 2013 et al. | Pondicherry | 10 | Axilla, nipple, scrotum, forehead, shoulder, back, abdomen, leg, knee and foot |
| 12 | Palanivel 2012 et al. | TN | 31 | Axilla, genitalia, inguinal area |
| 13 | Vivekanandan 2010 et al. | Pondicherry | 23 | axilla, breast, groin |
| 14 | Varghese 2013 et al. | Tamil Nadu | 86 | groin, axilla, neck, breast folds |
| 15 | Abhilash 2015 et al. | Tamil Nadu | 52 | groin, genitalia, axilla, neck, inframammary folds |
| 16 | Verma 2021 et al. | Uttar Pradesh | 11 | abdomen, thighs and arms |
| 17 | Abhilash 2016 et al. | Tamil Nadu | 234 | groin, axilla, genitalia, neck, breast folds |
| 18 | Palanivel 2012 et al. | Tamil Nadu | 31 | axilla, genitalia, inguinal area |
| 19 | Bhargava 2016 et al. | Uttarakhand | 49 | neck, axilla, abdomen, inguinal and pubic regions |
| 20 | Sharma 2014 et al. | Rajasthan | 22 | axilla, breast, groin |