Abstract
The gut microbiome has been studied in recent years due to its association with various pathological pathways involved in different diseases, caused by its structure, function, and diversity alteration. The knowledge of this mechanism has generated interest in the investigation of its relationship with ophthalmologic diseases. Recent studies infer the existence of a gut-eye microbiota axis, influenced by the intestinal barrier, the blood-retina barrier, and the immune privilege of the eye. A common denominator among ophthalmologic diseases that have been related to this axis is inflammation, which is perpetuated by dysbiosis, causing an alteration of the intestinal barrier leading to increased permeability and, in turn, the release of components such as lipopolysaccharides (LPS), trimethylamine oxide (TMAO), and bacterial translocation. Some theories explain that depending on how the microbiome is composed, a different type of T cells will be activated, while others say that some bacteria can pre-activate T cells that mimic ocular structures and intestinal permeability that allow leakage of metabolites into the circulation. In addition, therapies such as probiotics, diet, and fecal microbiota transplantation (FMT) have been shown to favor the presence of a balanced population of microorganisms that limit inflammation and, in turn, generate a beneficial effect in these eye pathologies. This review aims to analyze how the intestinal microbiome influences various ocular pathologies based on microbial composition and pathological mechanisms, which may provide a better understanding of the diseases and their therapeutic potential.
Keywords: gut-eye axis, ocular diseases, gut microbiota dysbiosis, ophthalmologic disorders, gut microbiome
Introduction and background
The microbiome denotes the entirety of microorganisms residing naturally within a specific environment, profoundly influencing physiological processes and overall health. Over the past two decades, advancements in microbiome research have accelerated due to techniques such as shotgun sequencing, which analyzes the entire genetic material of microbial communities; metatranscriptomics, which studies RNA transcripts to understand gene expression; and metaproteomics, which examines proteins to reveal metabolic processes and interactions. These methodologies enable in-depth analysis of microbial communities and their functions without the need for culturing, revealing critical insights into human health and disease mechanisms [1]. Exploring the microbiome's role in human health has transformed scientific inquiry, initially focused on cataloging microbial diversity; contemporary microbiome research has rapidly evolved toward elucidating functional dynamics and host interactions. This paradigm shift underscores profound advancements in understanding microbial mechanisms and their implications for disease pathogenesis and therapeutic strategies [2].
Dysbiosis, an imbalance in microbiota, is linked to inflammatory diseases through the disturbance of metabolic activities, the production of vital metabolites, and the regulation of immune responses, highlighting the microbiome's role in health maintenance and disease prevention [3]. There is already evidence that the microbiome plays a crucial role in ocular health, influencing physiological functions and interactions that contribute to maintaining ocular homeostasis and could offer avenues for therapeutic modulation in ocular diseases. Within specialized niches, the ocular microbiome itself also plays a crucial role in maintaining ocular health by contributing to immune regulation and protecting against pathogens [4-6]. Studies underscore the microbiome's influence on systemic immune responses in distant organs with mechanisms that include microbial translocation and metabolic by-products, highlighting the microbiome's pivotal role in shaping overall immune function [7]. Recent research using advanced technologies reveals the gut microbiota's significant role beyond gastrointestinal health, impacting ocular diseases such as autoimmune uveitis, retinopathies, dry eye, and glaucoma. Dysbiosis in Firmicutes and Bacteroidetes correlates with systemic inflammation and immune dysregulation in ocular pathology, suggesting potential therapeutic strategies through probiotics and dietary interventions [8].
This review explores the intricate relationship between the gut microbiome and diverse ocular diseases, focusing notably on retinal disorders alongside conditions such as uveitis, glaucoma, and dry eye.
Review
Gut microbiome and retinal diseases
Alterations in the microbiome of patients with retinopathies such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and some retinal dystrophies (RD) suggest the existence of a gut-retina axis. The axis is a novel concept whose dynamics are explained by the interaction between the blood-retinal barrier (BRB) and the intestinal barrier, derived from several factors such as dysbiosis and inflammatory response in the presence of ocular disease, which increases permeability, allowing excessive translocation of bacteria and intestinal components. Despite these known mechanisms, there are still areas of opportunity to determine how the relationship between the microbiome and different retinal pathologies works [9].
Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is an acquired degenerative retinopathy that causes central vision impairment resulting from a conjunction of neovascular and non-neovascular changes [10]. The microbiome's role in this disease's pathophysiology has been analyzed in a study of patients with new-onset neovascular AMD, and age- and sex-matched controls without AMD showed enrichment in species of the genera Anaerotruncus, Oscillibacter, Ruminococcus torques, and Eubacterium entries and a reduction in Bacteroides eggerthii [11,12]. On the other hand, another investigation highlights that subjects with AMD present an increased number of the genera Prevotella, Holdemanella, and Desulfovibrio, along with a decrease in the genera Dorea, Blautia, and Oscillospira. In addition, a metagenomic analysis performed on these patients implicated 72 important microbial pathways in AMD patients, showing enrichment in carotenoid biosynthesis, lipid metabolism, fatty acid biosynthesis, and bacterial chemotaxis [13].
The best-validated supplementation therapy for AMD treatment contains vitamins C and E, antioxidants, beta-carotene, copper, and zinc. The microbiome's composition may influence the bioavailability of these components. In addition, the production of some antioxidants, such as indole-3-propionic acid, is entirely dependent on the intestinal microflora, and zinc absorption can be influenced by zinc competition between the microbiomes of a host's gastrointestinal tract [14].
Diabetic Retinopathy
Diabetic retinopathy (DR) is one of the most prevalent metabolic and blinding eye diseases. Not all diabetics develop DR, which can be explained by individual factors such as the dysregulation of the intestinal microbiome in the human body, as it could trigger the development of inflammatory, metabolic, mental, and immune diseases. It is important to mention that hyperglycemia favors inflammation due to the increase in gram-negative bacteria that increase the production of lipopolysaccharides (LPS). LPS act because they can cross the blood-retinal barrier and induce imminent responses directly in retinal cells through Toll-like receptors 4 (TLR4), leading to an inflammatory response [15,16].
A Mendelian randomization study found that the prevalence of Ruminococcaceae and the eubacterium rectale group were risk factors for DR. Another alteration in the microbiota population was a decrease in Faecalibacterium, Bifidobacterium, and Lactobacillus in those who developed these complications and an increase in taxa such as Klebsiella. Faecalibacterium and Lactobacillus were inversely related to inflammation, hyperglycemia, and insulin resistance. It has also been observed that the consumption of probiotics containing Bifidobacterium and Lactobacillus decreases blood glucose in aids and glycosylated hemoglobin in patients with diabetes mellitus, so it is concluded that these genera, as they decrease, increase the risk of microvascular complications of the disease. These taxa could support the development of biomarkers that generate new treatments and prevention methods for DR [17].
When intestinal dysregulation exists, probiotic/prebiotic supplementation and fecal transplantation promote intestinal homeostasis. Another alternative is intermittent fasting, which is associated with a decrease in retinal complications by promoting increased production of tauroursodeoxycholic acid, which activates the Takeda G5 protein receptor as a retina neuroprotectant. This indicates that the maintenance of the intestinal microbiome has a protective effect on the retina, preventing the development of retinopathy [9].
Retinopathy of Prematurity
Retinopathy of prematurity (ROP) is an important cause of infant blindness, which is related to the intestinal microbiota among other factors. In premature infants, the microbiota can be altered and generate a dysbiosis that can lead to increased intestinal permeability and systemic inflammation. The flora of preterm neonates, as opposed to term neonates, is usually associated with an increase in Enterococcus and Staphylococcus, and a decrease in Bacteroides and Bifidobacterium, which is associated with intestinal infections and diseases [18,19].
The mechanism of this pathology is a negative regulation of vascular endothelial growth factor (VEGF) that is aggravated by a loss of maternal insulin-like growth factor (IGF-1), resulting in incomplete retinal vascularization. This alteration of IGF-1 is linked to an increase of Enterobacteriaceae species and Staphylococcus species that have been shown to interfere with vascular revascularization and repair [20]. Inflammation is also associated with ROP, which results in high short-chain fatty acid (SCFA) levels. SCFA results from the metabolism of certain intestinal bacteria [21]. Neonatal intermittent hypoxia (NIH), another risk factor, can generate oxidative stress and cause damage to the retina and intestinal dysbiosis, such as the depletion of Lactobacillus. This probiotic has been shown to have protective effects in retinopathy [18].
Inherited Retinal Dystrophies
Retinal dystrophies (RD) are a group of inherited disorders resulting from gene variants that play a fundamental role in retinal cell development, function, and maintenance [22]. The literature on the composition of the gut microbiota in RD is scarce, so much so that of the entire spectrum that exists, there are only studies related to retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA) [23]. RP can cause retinal degeneration and decreased vision, progressing to total blindness [24]. One study has analyzed the connection between RP and the gut microbiome composition using a mouse model of RP with retinal degeneration 10 (rd10) due to a nonsense mutation in the phosphodiesterase 6B (Pde6b) gene, which was compared to wild-type control mice. Differences in four species usually present in the healthy gut microbiome were observed, which were absent in rd10 mice (Rikenella, Muribaculaceae, Prevotellaceae UCG-011, and Bacilli species). At the same time, Bacteroides caecimuris was overrepresented in mice with RP but was absent in controls [25]. In other ways, mutations in the CRB1 gene can lead to the development of LCA, the earliest and most severe hereditary DR [26]. A study evaluating the relationship between the microbiome and mutated CRB1 gene demonstrated that the colon's outer blood-retinal barrier (BRB) and intestinal epithelial barrier are significantly impaired in mice carrying a CRB1 mutation. Disruption of permeable gut and retina leads to gut flora translocation from the lower gastrointestinal tract to the retina, resulting in bacterial-dependent retinal degeneration. Furthermore, it was shown that systemic bacterial depletion by the reintroduction of an LCA mouse model into an FG environment or after treatment with broad-spectrum antibiotics can successfully control this retinal damage [27].
Gut microbiome and other ocular diseases
Uveitis
Uveitis encompasses a heterogeneous group of intraocular inflammatory diseases consisting of inflammation of the uveal tract that can affect adjacent structures such as the retina or the optic nerve. It has been classified anatomically (anterior versus posterior), etiologically (infectious versus non-infectious), and pathogenically (autoimmune versus autoinflammatory). Autoimmune (non-infectious) uveitis represents a group that frequently manifests as anterior uveitis and is part of a systemic autoimmune syndrome affecting organs other than the eye, such as human leukocyte antigen (HLA)-B27-positive spondyloarthropathies, systemic sarcoidosis, Behçet's disease, and Vogt-Koyanagi-Harada disease [28].
Until now, no clinical studies have analyzed the direct relationship between uveitis and the microbiome. However, there is evidence of the association in autoimmune diseases that present uveitis within their clinical manifestations. In general, the immune dysregulation of these pathologies induces inflammation and tissue damage, a product of an aberrant activity of T cells, which may be modulated by the altered microbiome of these patients who have in common the depletion of butyrate-producing bacteria (BPB) and methanogens that lead to an altered intestinal barrier that allows the transference of immunostimulatory factors [28-31].
In the case of Behçet's disease, there is an increase in sulfate-reducing bacteria, Stenotrophomonas species, Actinomyces species, and Paraprevotella species [30]. On the other hand, in Vogt-Koyanagi-Harada disease, a type of panuveitis, patients present a mix of Bacteroides enterotype enriched with gram-negative bacteria, such as Bacteroides species, Paraprevotella species, Prevotella species, and Parabacteroides species, accompanied by a decrease in lactate-producing bacteria [31]. Another group of diseases that have been studied is HLA-B27 positive, of which spondyloarthropathies and inflammatory bowel disease stand out, whose inflammatory pathophysiological basis allows the translocation of bacteria and microbial products to the regional lymph nodes, as is the case of components of the cell walls of gram-negative bacteria, which binds to the Toll-like receptor 4, whose expression is increased in patients with active anterior uveitis. Another example of this is microbial peptides, such as those from Chlamydia trachomatis, which are antigens that bind to HLA-B27, which can, in turn, induce an immune response in target organs, resulting in uveitis [29].
Potential treatments for autoimmune uveitis with the microbiome as a therapeutic target include correcting intestinal dysbiosis with probiotics that supplement good immunosuppressive bacteria and antibiotics that eliminate bad proinflammatory bacteria. Complete restoration of the intestinal microbiome by fecal microbial transplantation is also possible. Another therapeutic possibility is the administration of bacterial metabolites, such as short-chain fatty acid (SCFA), to promote the expression of regulatory T cells and increase intestinal integrity. However, none of these treatments have yet been approved and are still under investigation [32].
Glaucoma
Glaucoma is a chronic progressive optic neuropathy involving retinal ganglion cells and is the leading cause of irreversible blindness worldwide. The risk factors for disease progression include intraocular pressure (IOP), vascular perfusion abnormalities, neurodegeneration, chronic inflammation, and oxidative stress [33].
Recently, a study has proposed that the intestinal microbiota plays an important role in developing glaucoma due to dysbiosis [34]. Such is the case of the untargeted association analysis of the intestinal microbiota within a British cohort, where it was found that glaucoma was negatively associated with the Mollicutes class, which was also negatively related to inflammatory bowel disease [35]. Furthermore, a study conducted in China on a cohort of patients with primary open-angle glaucoma (POAG) confirmed the correlation between gut microbiota and glaucoma. When comparing the composition of the gut microbiota in the cohort (30 patients with POAG and 30 healthy controls without POAG), it was found that Prevotellaceae, Escherichia coli, and Enterobacteriaceae were increased in patients with POAG. On the other hand, Megamonas and Bacteroides plebeius decreased [36].
In addition, the production of microbial metabolites such as trimethylamine (TMA) in the aqueous humor, but not betaine and trimethylamine oxide (TMAO), has been observed to be increased in patients with POAG. Bacterial toxins activate Toll-like receptor 4 (TLR4), an interconnected pathway shared between the ocular and intestinal microbiota. The binding of LPS to TLR4 activates signaling cascades that result in the production of innate effector responses and the initiation of an adaptive immune response [37].
Existing studies are still insufficient to determine the exact links between the intestinal microbiota and glaucoma, but promising results encourage further research in this area.
Dry Eye Disease
Dry eye is a chronic ocular surface disease caused by tear film instability and imbalances in the ocular surface microenvironment that result in various symptoms of discomfort and visual problems. It can be classified according to its etiology into primary dry eye and secondary dry eye. The pathophysiological mechanisms are not fully understood; however, it is known that an inflammatory vicious circle of the ocular surface is involved, together with a dysfunction of the meibomian glands, all influenced by various local and environmental factors [38]. Intriguingly, alterations in intestinal commensal bacteria can affect the immune status of the ocular surface as an imbalance in intestinal homeostasis occurs, which can cause pathogenic microorganisms to cross the intestinal mucosal barrier, releasing inflammatory factors and activating T and B lymphocytes. Lymphatic vessels transport the inflammatory by-products to distant tissues, including the ocular surface [39,40]. In the specific case of primary dry eye, there is a decreased abundance of the butyrate-producing bacterium Faecalibacterium, as well as reduced levels of Treg-inducing Clostridiales and Bacteroides, which play a role in suppressing the inflammatory response in Th17 cells [41]. Regarding dry eye secondary to Sjögren's syndrome, a decrease in the Firmicutes/Bacteroidetes ratio and a decrease in Bifidobacterium have been observed, suggesting that the disease's severity is related to the groups of bacteria involved [42].
Derived from the demonstrated influence of the microbiome on dry eye, a recent case-control study sought to determine if there is a benefit from probiotic and prebiotic consumption supported by evidence that the modulation of the gut microbiome also modulates proteins expressed by the lacrimal glands, with an increase in IL-10 and a decrease in IL-1β and IL-6. Their promising results indicate a beneficial impact of regular consumption of probiotics and prebiotics on symptoms and clinical signs of dry eye. However, future clinical studies are needed to investigate the benefits of probiotics for patients with Sjögren's syndrome further, as they were not included in the study [43,44].
Mechanisms linking the gut microbiome and ocular diseases
The processes in which the microbiome influences ocular diseases have been studied in recent times by different studies, some of which are summarized in Table 1. This has been shown to have an enormous impact on human metabolic mechanisms, especially on the immune system, as these cells are strategically located close to the host-microbiome interface [45,46]. The communication between the immune system and the microbiome is through synthesizing metabolites such as carbohydrates, amino acids, and bile acid metabolites, which are recognized by receptors in immune cells. As a result of this interaction, diverse responses train these cells for possible further infections with no commensal pathogens, enhancing barrier function and tolerance [47].
Table 1. Relevant studies that have been conducted on the role of the microbiome in ophthalmologic diseases since 2018.
AMD: age-related macular degeneration, HLA: human leukocyte antigen, POAG: primary open-angle glaucoma
| Authors | Study type | Disease | Findings |
| Peng et al. (2024) [27] | Case-control | Retinal dystrophy (Leber congenital amaurosis due to CRB1 gene mutation) | Mechanism connecting the gut and the eye: the principle of bacterial translocation-dependent retinal degeneration has implications that go beyond inherited retinal dystrophies. |
| Zhao et al. (2024) [48] | Systematic review and meta-analysis | Diabetic retinopathy | Association between alterations in the gut microbiome in type 2 diabetes and the development and progression of diabetic retinopathy: this suggests that restoring the homeostasis of the gut microbiome could be a potential way to prevent or treat diabetic retinopathy. |
| Morandi et al. (2024) [28] | Case-control | HLA-B27-associated non-infectious anterior uveitis | The development of uveitis is influenced by functional and compositional alterations of the intestinal microbiome. An increase in specific gram-negative bacteria and lipopolysaccharides may play a role in triggering inflammation and aberrant immune response in the eye. |
| Zhang et al. (2023) [49] | Case-control | AMD | AMD patients had different gut microbiota compared with healthy controls, and pathophysiology might be linked to changes in gut-related metabolic pathways. |
| Liu et al. (2023) [50] | Mendelian randomization | AMD | The order Rhodospirillales influenced AMD risk based on the gut-retinal axis. |
| Mao et al. (2023) [51] | Mendelian randomization | AMD | Causal relationship with microbiome gut taxa, including the Eubacterium oxidoreducens group, Faecalibacterium, Ruminococcaceae UCG-011, Anaerotruncus, and Candidatus Soleaferrea. These strains have the potential to serve as novel biomarkers. |
| Goodman et al. (2023) [52] | Case-control | Dry eye | Differences in the composition of the gut microbiome were found in individuals with predominantly early markers of Sjögren's syndrome compared to controls. |
| Liu et al. (2022) [15] | Mendelian randomization | Diabetic retinopathy | There is a possible causal relationship between some taxa of the gut microbiome and diabetic retinopathy, highlighting the association of the "gut-retina" axis and offering new insights into the mechanism of diabetic retinopathy. |
| Tavakoli et al. (2022) [43] | Case-control | Dry eye | The application of probiotics and prebiotics could be effective in the treatment of dry eye disease and suggests a possible alternative treatment. |
| Kutsyr et al. (2021) [25] | Case-control | Retinal dystrophy (retinitis pigmentosa) | Alterations in morphology and function of the rd10 mouse, an animal model of retinitis pigmentosa, demonstrate for the first time that retinal degenerative changes in neuronal and glial cells that occur in RP are concomitant with relevant changes in the gut microbiome. |
| Gong et al. (2020) [36] | Case-control | POAG | The first study that focused on the gut microbiome profile and its association with serum metabolites in patients with POAG. |
| Ye et al. (2020) [31] | Cohort | Uveitis | Distinct signature of the gut microbiome in patients with Vogt-Koyanagi-Harada, and they showed an exacerbating effect of this gut microbiome in experimental autoimmune uveitis. |
| Kalyana Chakravarthy et al. (2018) [53] | Cohort | Uveitis | The first study that demonstrated dysbiosis in the intestinal bacterial communities of patients with uveitis compared to healthy human subjects from a South Indian population. |
Evidence has indicated that an imbalance in the gut microbiome not only exerts an effect locally but also affects other locations, such as the ocular mucosa, which is known as the gut-eye axis [10,54]. One theory explains the relationship between the gastrointestinal tract and the eyes through the T-cell threshold model, which states that depending on how the microbiome is composed, a different type of T cells will be activated; other theories say that some bacteria can preactivate T cells that mimic ocular structures and intestinal permeability, allowing leakage of metabolites into the circulation. All these theories are not mutually exclusive; on the contrary, a combination of all may be the explanation for the gut-eye axis [55].
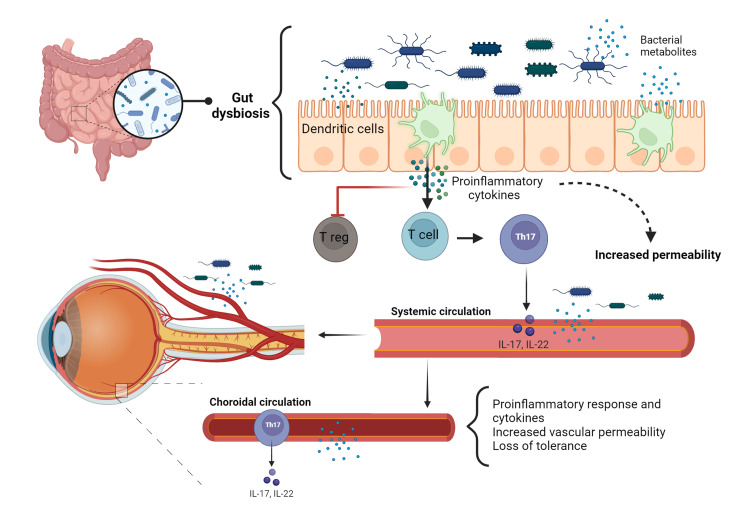
Dysbiosis in the gut with a high proportion of pathogenic bacteria activates dendritic cells, which secrete proinflammatory cytokines (interleukin (IL)-6, tumor necrosis factor-alpha (TNF-alpha), and IL-1B), leading to the activation of retina-specific T cells, such as proinflammatory T cells (Th17), and the inhibition of regulatory T cells. Once these T cells reach the eye, a cascade of proinflammatory processes such as secretion of proinflammatory cytokines, increased vascular permeability, loss of tolerance of the ocular microbiome, complement activation, and neovascularization is initiated [56]. Loss of intestinal barrier integrity and increased vascular permeability allow bacterial translocation and their products into the bloodstream, leading to passage across the blood-retinal barrier [57]. Once the ocular barrier has been breached, short-chain fatty acids (SCFAs) protect ocular structures by decreasing T-cell activity; however, studies have shown that trimethylamine oxide (TMAO), a metabolite produced by pathogenic bacteria associated with the development of vascular conditions such as age-related macular degeneration, can also travel through the systemic circulation and play a damaging role, inhibiting the eye's defense mechanisms (Figure 1) [58,59].
Figure 1. Mechanisms involved in the gut-eye axis.
Dysbiosis in the gut activates dendritic cells, which secretes proinflammatory cytokines (IL-6, TNF-alpha, and IL-1B), leading to the activation of specific retina T cells as proinflammatory T cells (Th17) and the inhibition of T-regulatory. Once these T cells reach the eye, they begin the secretion of proinflammatory cytokines, increased vascular permeability, loss of ocular microbiome tolerance, complement activation, and neovascularization. Also, TMAO, a metabolite produced by pathogen bacteria, is linked with the development of vascular conditions such as age-related macular degeneration; these bacteria and metabolites can travel through the systemic circulation and reach the eyes.
IL-6: interleukin-6, TNF-alpha: tumor necrosis factor-alpha, IL-1B: interleukin-1B, TMAO: trimethylamine oxide, IL-17: interleukin-17, IL-22: interleukin-22
Figure created with BioRender
All credits to Barbara Abreu Lopez
Current and potential therapeutic approaches
Probiotics and Prebiotics
Probiotics and prebiotics are increasingly known for their potential impact on the health of the gut microbiome, which extends to extraintestinal conditions. The gut microbiota plays a key role in immune modulation and the regulation of inflammation, crucial processes in the pathogenesis of previously mentioned ocular pathologies [60,61]. There is increasing evidence that the modulation of the gut microbiota by probiotics and prebiotics may be beneficial for ocular health, as they may influence the gut-eye axis, by reducing inflammation and oxidative stress [60,62,63].
Research indicates that certain strains of probiotics, such as Lactobacillus and Bifidobacterium, effectively reduce inflammation and support gut health. Combined with specific formulations, these strains have shown promise in addressing systemic inflammation, potentially benefiting ocular conditions. For instance, Lactobacillus rhamnosus GG has been studied for its ability to strengthen gut barrier function, which is crucial in preventing systemic inflammation that could impact the eyes [62]. Dietary interventions rich in prebiotics, such as fiber and resistant starches, promote the growth of beneficial gut bacteria and contribute to systemic anti-inflammatory effects. Prebiotics serve as food for probiotics, enhancing their effectiveness and supporting a healthy gut microbiome [63].
Impact of Diet on the Gut Microbiome and Ocular Health
Diet plays a significant role in shaping the gut microbiome. A diet high in prebiotics supports the growth of beneficial bacteria, producing short-chain fatty acids (SCFAs) that have anti-inflammatory properties. These systemic effects can help reduce inflammation in ocular tissues and improve overall eye health. To support gut and ocular health, it is recommended to increase the intake of dietary fibers from fruits, vegetables, whole grains, and fermented foods such as yogurt, kefir, and sauerkraut to provide natural probiotics. It is also recommended to reduce processed foods and sugar consumption, which can negatively affect the composition of the intestinal microbiota [63].
Fecal Microbiota Transplantation (FMT)
Fecal microbiota transplantation (FMT) involves the transfer of stool from a healthy donor to the gastrointestinal tract of a recipient. This procedure is primarily explored for gastrointestinal disorders but holds promise as a future therapeutic strategy for modulating systemic inflammation and potentially impacting ocular disease progression through microbiome alterations [64]. FMT has shown effectiveness in restoring healthy microbiota composition and reducing inflammation. Its application in ocular diseases is still under investigation, but preliminary studies suggest it could be beneficial in managing conditions such as AMD and DR by reducing systemic inflammation. The concept is based on the ability of FMT to reset the gut microbiome to a healthier state, thereby influencing systemic immune responses and potentially reducing ocular inflammation [44].
Drugs Involved in the Gut-Eye Axis
Based on the assumption that dysbiosis is implicated in the development of ophthalmologic diseases, the proposed therapeutic strategies seek to restore the microbiota [65]. One important strategy is prebiotics and probiotics, which together provide live microorganisms beneficial to the microbiome [66]. In addition, some drugs, such as antidiabetic drugs, have been shown to have an effect on the regulation of the microbiota. Such is the case of metformin, which increases the number of goblet cells and, therefore, mucin production, improving intestinal defenses. Liraglutide and glucagon-like peptide 1 (GLP-1) agonists improve parameters related to both glucose and lipid metabolism. Others such as dipeptidyl peptidase 4 inhibitors and sodium/glucose 2 inhibitors decrease the Firmicutes/Bacteroidetes ratio, which improves the action of GLP-1, decreasing inflammatory markers and endothelial dysfunction, demonstrating a decrease in the mechanisms involved in the pathogenesis of retinopathy [65,66].
Regarding topical or intraocular ophthalmologic drugs targeting the microbiota, there is still no evidence of their existence or development, due to the newness of this line of research and the fact that the pathogenic pathways are still under study.
Future Therapeutic Strategies
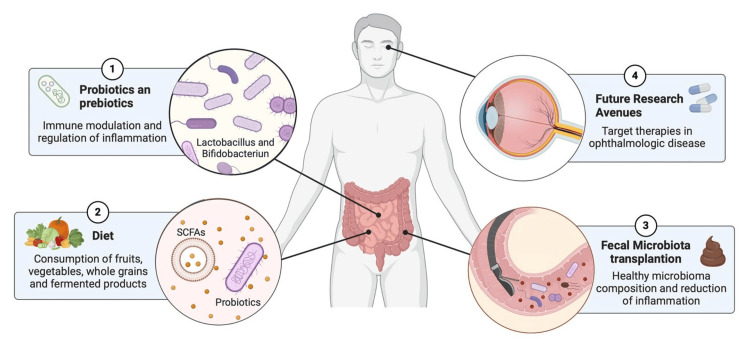
Future therapeutic strategies may capitalize on insights into the gut-retina axis to develop targeted interventions that address the complex interplay between gut microbiota composition, systemic inflammation, and ocular health [61-63]. Ongoing research explores advanced probiotic formulations, prebiotic-rich diets, and fecal microbiota transplantation (FMT) to manage and prevent ocular diseases (Figure 2). These therapies aim to harness a healthy gut microbiome's anti-inflammatory and immune-modulating effects, as they have been previously used in other inflammatory pathologies and have shown promising results [44].
Figure 2. Future therapeutic strategies that may influence the gut-eye axis.
This diagram illustrates the benefits of therapies aimed at modifying the microbiome. Probiotics and prebiotics such as Lactobacillus and Bifidobacterium modulate the immune response and decrease inflammation. Diets rich in fermented products and fiber from fruits, vegetables, and whole grains provide natural probiotics and SCFAs that have a positive effect on the microbiota. Fecal transplantation of microbiota from a healthy donor decreases inflammation in extraintestinal diseases and may have an important role in the treatment of eye diseases. Future research into these therapies, directed at ophthalmologic diseases, represents an opportunity to provide comprehensive management of patients with these diseases.
SCFAs: short-chain fatty acids
Figure created with BioRender
All credits to Anahi Rojas Huerta
New studies are examining how specific combinations of probiotics can more effectively reduce retinal inflammation and oxidative stress. In fact, cutting-edge probiotic formulations are being customized to bolster gut barrier function, diminish systemic inflammation, and enhance metabolic health, all of which play pivotal roles in sustaining ocular health [39].
Personalized Medicine Approaches
Personalized medicine approaches leveraging individual gut microbiome profiles to tailor probiotic therapies and dietary recommendations are gaining traction. These approaches aim to optimize ocular health outcomes by targeting the gut-retina axis and its implications in disease pathogenesis. However, recent insights suggest that alterations in the ocular microbiome could disrupt ocular homeostasis and elevate susceptibility to eye infections. The frequent use of antibiotics can lead to shifts in ocular microbiota and contribute to the emergence of antibiotic-resistant strains. By tailoring interventions to the unique microbiome composition of each patient, it may be possible to manage and prevent these diseases more effectively. By understanding and harnessing these interactions, researchers and ophthalmologic clinicians aim to pave the way for novel therapeutic modalities to prevent and manage ocular diseases. This underscores the importance of medical education guiding physicians to understand these mechanisms, thereby facilitating the implementation of innovative treatments and improving clinical outcomes [67].
Limitations
The relationship between the intestinal microbiota and its involvement in different ophthalmologic diseases is a topic of great interest. However, it is emerging, and many aspects are still not fully clarified, as is the case with specific pathogenic mechanisms. The pathways involved that have been mentioned are still not fully described, so a multiomic approach could be useful, as well as establishing the direct relationship of these mechanisms with regulatory pathways. The gut-eye axis is still under investigation, and much of the data still comes only from animal models. However, many of these show promising perspectives on the role of the microbiota both with protective effects and with a possible inflammatory association in the different regions of the eye. The microbiological agents and the different biomarkers involved in retinal diseases have not yet been fully and concretely elucidated, so the use of experimental research and applying sciences such as proteomics or sequencing may be beneficial to establish a relationship between them. In addition, the ability to identify microbiome profiles in a personalized way is still complex for current medical practice and represents a challenge in the comprehensive management of these patients. Some therapies, such as TMF, have a cautious use in clinical trials, as they are considered after failure of initial treatments, such as probiotics and prebiotics, since their metabolic impact is important and they require continuous patient monitoring.
Conclusions
This review highlights the interrelationship between the gut microbiome and ophthalmologic diseases, starting from the concept of the gut-eye axis, influenced by the different pathological pathways discovered. Future research should prioritize elucidating more mechanisms linking alterations in the gut microbiota to ocular inflammation. Understanding these interactions could reveal new biomarkers for disease susceptibility and treatment response, facilitating personalized therapies. Collaborative efforts merging microbiological and ophthalmological expertise are crucial for translating microbiome research into clinical practice. Integrating microbiome assessments into diagnostic algorithms can revolutionize disease management by enabling early detection and targeted interventions. It is essential to explore the bidirectional relationship between ocular health and gut microbiota dynamics to provide a comprehensive framework for managing chronic diseases and associated comorbidities. Leveraging microbiome knowledge will promote greater efficacy in managing ocular diseases and thus benefit patients' quality of life.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Jesus Lima Barrientos, Anahi Rojas Huerta
Acquisition, analysis, or interpretation of data: Jesus Lima Barrientos, Anahi Rojas Huerta, Angel Perez Mendoza, Barbara A. Abreu Lopez, Vanessa Pamela Salolin Vargas, Oxiris Yexalen Garcia Gonzalez, Mauricio A. Saldaña Ruiz, Edna Diarte, Angela Juliet Torijano Sarria
Drafting of the manuscript: Jesus Lima Barrientos, Anahi Rojas Huerta, Angel Perez Mendoza, Barbara A. Abreu Lopez, Vanessa Pamela Salolin Vargas, Oxiris Yexalen Garcia Gonzalez, Mauricio A. Saldaña Ruiz, Edna Diarte, Angela Juliet Torijano Sarria
Critical review of the manuscript for important intellectual content: Jesus Lima Barrientos, Anahi Rojas Huerta, Angel Perez Mendoza, Barbara A. Abreu Lopez, Vanessa Pamela Salolin Vargas, Oxiris Yexalen Garcia Gonzalez, Mauricio A. Saldaña Ruiz, Edna Diarte, Angela Juliet Torijano Sarria
Supervision: Jesus Lima Barrientos, Anahi Rojas Huerta, Angel Perez Mendoza
References
- 1.A brief history of microbial study and techniques for exploring the gastrointestinal microbiome. Sidebottom AM. Clin Colon Rectal Surg. 2023;36:98–104. doi: 10.1055/s-0042-1760678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Microbiome research outlook: past, present, and future. Gao Y, Li D, Liu YX. Protein Cell. 2023;14:709–712. doi: 10.1093/procel/pwad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The human microbiome and its impacts on health. Ogunrinola GA, Oyewale JO, Oshamika OO, Olasehinde GI. Int J Microbiol. 2020;2020:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The role of the gut microbiome in eye diseases. Zysset-Burri DC, Morandi S, Herzog EL, Berger LE, Zinkernagel MS. Prog Retin Eye Res. 2023;92:101117. doi: 10.1016/j.preteyeres.2022.101117. [DOI] [PubMed] [Google Scholar]
- 5.Relationship between the microbiome and ocular health. Cavuoto KM, Banerjee S, Galor A. Ocul Surf. 2019;17:384–392. doi: 10.1016/j.jtos.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Unveiling the gut-eye axis: how microbial metabolites influence ocular health and disease. Nguyen Y, Rudd Zhong Manis J, Ronczkowski NM, Bui T, Oxenrider A, Jadeja RN, Thounaojam MC. Front Med (Lausanne) 2024;11:1377186. doi: 10.3389/fmed.2024.1377186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gut microbiota and systemic immunity in health and disease. Lo BC, Chen GY, Núñez G, Caruso R. Int Immunol. 2021;33:197–209. doi: 10.1093/intimm/dxaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Microbiota and ocular diseases. Xue W, Li JJ, Zou Y, Zou B, Wei L. Front Cell Infect Microbiol. 2021;11:759333. doi: 10.3389/fcimb.2021.759333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The gut-retina axis: a new perspective in the prevention and treatment of diabetic retinopathy. Zhang H, Mo Y. Front Endocrinol (Lausanne) 2023;14:1205846. doi: 10.3389/fendo.2023.1205846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gut microbiota and age-related macular degeneration: a growing partnership. Lima-Fontes M, Meira L, Barata P, Falcão M, Carneiro Â. Surv Ophthalmol. 2022;67:883–891. doi: 10.1016/j.survophthal.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Age-related macular degeneration (AMD): pathogenesis and therapy. Nowak JZ. https://pubmed.ncbi.nlm.nih.gov/16845209/ Pharmacol Rep. 2006;58:353–363. [PubMed] [Google Scholar]
- 12.Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Devkota S, Wang Y, Musch MW, et al. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association of the intestinal microbiome with the development of neovascular age-related macular degeneration. Zinkernagel MS, Zysset-Burri DC, Keller I, et al. Sci Rep. 2017;7:40826. doi: 10.1038/srep40826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Lambert V, Lecomte J, Hansen S, et al. Nat Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 15.Causal effects of gut microbiota on diabetic retinopathy: a Mendelian randomization study. Liu K, Zou J, Fan H, Hu H, You Z. Front Immunol. 2022;13:930318. doi: 10.3389/fimmu.2022.930318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recent insights into the role of gut microbiota in diabetic retinopathy. Jiao J, Yu H, Yao L, Li L, Yang X, Liu L. J Inflamm Res. 2021;14:6929–6938. doi: 10.2147/JIR.S336148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Specific alterations of gut microbiota in diabetic microvascular complications: a systematic review and meta-analysis. Hong J, Fu T, Liu W, Du Y, Min C, Lin D. Front Endocrinol (Lausanne) 2022;13:1053900. doi: 10.3389/fendo.2022.1053900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gut microbiome and retinopathy of prematurity. Zhang JY, Greenwald MJ, Rodriguez SH. Am J Pathol. 2023;193:1683–1690. doi: 10.1016/j.ajpath.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Exploring the role of gut bacteria in health and disease in preterm neonates. Lee JK, Hern Tan LT, Ramadas A, Ab Mutalib NS, Lee LH. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17196963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The early gut microbiome could protect against severe retinopathy of prematurity. Skondra D, Rodriguez SH, Sharma A, Gilbert J, Andrews B, Claud EC. J AAPOS. 2020;24:236–238. doi: 10.1016/j.jaapos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Retinopathy of prematurity: metabolic risk factors. Fu Z, Nilsson AK, Hellstrom A, Smith LE. Elife. 2022;11 doi: 10.7554/eLife.80550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Extensive genic and allelic heterogeneity underlying inherited retinal dystrophies in Mexican patients molecularly analyzed by next-generation sequencing. Zenteno JC, García-Montaño LA, Cruz-Aguilar M, et al. Mol Genet Genomic Med. 2020;8 doi: 10.1002/mgg3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inherited retinal degenerations: current landscape and knowledge gaps. Duncan JL, Pierce EA, Laster AM, et al. Transl Vis Sci Technol. 2018;7:6. doi: 10.1167/tvst.7.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Retinitis pigmentosa: review of current treatment. Wang AL, Knight DK, Vu TT, Mehta MC. Int Ophthalmol Clin. 2019;59:263–280. doi: 10.1097/IIO.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 25.Retinitis pigmentosa is associated with shifts in the gut microbiome. Kutsyr O, Maestre-Carballa L, Lluesma-Gomez M, Martinez-Garcia M, Cuenca N, Lax P. Sci Rep. 2021;11:6692. doi: 10.1038/s41598-021-86052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Review and update on the molecular basis of Leber congenital amaurosis. Chacon-Camacho OF, Zenteno JC. World J Clin Cases. 2015;3:112–124. doi: 10.12998/wjcc.v3.i2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CRB1-associated retinal degeneration is dependent on bacterial translocation from the gut. Peng S, Li JJ, Song W, et al. Cell. 2024;187:1387–1401. doi: 10.1016/j.cell.2024.01.040. [DOI] [PubMed] [Google Scholar]
- 28.The gut microbiome and HLA-B27-associated anterior uveitis: a case-control study. Morandi SC, Herzog EL, Munk M, et al. J Neuroinflammation. 2024;21:120. doi: 10.1186/s12974-024-03109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Does the microbiome cause B27-related acute anterior uveitis? Rosenbaum JT, Lin P, Asquith M. Ocul Immunol Inflamm. 2016;24:440–444. doi: 10.3109/09273948.2016.1142574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A metagenomic study of the gut microbiome in Behcet's disease. Ye Z, Zhang N, Wu C, et al. Microbiome. 2018;6:135. doi: 10.1186/s40168-018-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altered gut microbiome composition in patients with Vogt-Koyanagi-Harada disease. Ye Z, Wu C, Zhang N, et al. Gut Microbes. 2020;11:539–555. doi: 10.1080/19490976.2019.1700754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Microbiome in immune-mediated uveitis. Rodríguez-Fernández CA, Iglesias MB, de Domingo B, et al. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23137020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaucoma and the brain: trans-synaptic degeneration, structural change, and implications for neuroprotection. Lawlor M, Danesh-Meyer H, Levin LA, Davagnanam I, De Vita E, Plant GT. Surv Ophthalmol. 2018;63:296–306. doi: 10.1016/j.survophthal.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 34.The role of the microbiota in glaucoma. Huang L, Hong Y, Fu X, Tan H, Chen Y, Wang Y, Chen D. Mol Aspects Med. 2023;94:101221. doi: 10.1016/j.mam.2023.101221. [DOI] [PubMed] [Google Scholar]
- 35.Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Jackson MA, Verdi S, Maxan ME, et al. Nat Commun. 2018;9:2655. doi: 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gut microbiota compositional profile and serum metabolic phenotype in patients with primary open-angle glaucoma. Gong H, Zhang S, Li Q, Zuo C, Gao X, Zheng B, Lin M. Exp Eye Res. 2020;191:107921. doi: 10.1016/j.exer.2020.107921. [DOI] [PubMed] [Google Scholar]
- 37.Glaucoma and the human microbiome. Chang CJ, Liu B, Liebmann JM, Cioffi GA, Winn BJ. J Glaucoma. 2024 doi: 10.1097/IJG.0000000000002448. [DOI] [PubMed] [Google Scholar]
- 38.Defining dry eye from a clinical perspective. Tsubota K, Pflugfelder SC, Liu Z, et al. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21239271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Microbiome and immune-mediated dry eye: a review. Watane A, Raolji S, Cavuoto K, Galor A. BMJ Open Ophthalmol. 2022;7 doi: 10.1136/bmjophth-2021-000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Can gut microbiota affect dry eye syndrome? Moon J, Yoon CH, Choi SH, Kim MK. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.What is the impact of microbiota on dry eye: a literature review of the gut-eye axis. Song J, Dong H, Wang T, et al. BMC Ophthalmol. 2024;24:262. doi: 10.1186/s12886-024-03526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gut dysbiosis is prevailing in Sjögren's syndrome and is related to dry eye severity. Moon J, Choi SH, Yoon CH, Kim MK. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0229029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The impact of probiotics and prebiotics on dry eye disease signs and symptoms. Tavakoli A, Markoulli M, Papas E, Flanagan J. J Clin Med. 2022;11 doi: 10.3390/jcm11164889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The effect of ophthalmic and systemic formulations of Latilactobacillus sakei on clinical and immunological outcomes of patients with dry eye disease: a factorial, randomized, placebo-controlled, and triple-masking clinical trial. Heydari M, Kalani M, Ghasemi Y, Nejabat M. Probiotics Antimicrob Proteins. 2024;16:1026–1035. doi: 10.1007/s12602-023-10079-1. [DOI] [PubMed] [Google Scholar]
- 45.Gut microbiome in retina health: the crucial role of the gut-retina axis. Scuderi G, Troiani E, Minnella AM. Front Microbiol. 2021;12:726792. doi: 10.3389/fmicb.2021.726792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Small molecule metabolites at the host-microbiota interface. Bishai JD, Palm NW. J Immunol. 2021;207:1725–1733. doi: 10.4049/jimmunol.2100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Immune regulation by microbiome metabolites. Kim CH. Immunology. 2018;154:220–229. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gut microbiome in diabetic retinopathy: a systematic review and meta-analysis. Zhao S, Yan Q, Xu W, Zhang J. Microb Pathog. 2024;189:106590. doi: 10.1016/j.micpath.2024.106590. [DOI] [PubMed] [Google Scholar]
- 49.Alterations of the intestinal microbiota in age-related macular degeneration. Zhang Y, Wang T, Wan Z, et al. Front Microbiol. 2023;14:1069325. doi: 10.3389/fmicb.2023.1069325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Exploring the effect of the gut microbiome on the risk of age-related macular degeneration from the perspective of causality. Liu K, Zou J, Yuan R, et al. Invest Ophthalmol Vis Sci. 2023;64:22. doi: 10.1167/iovs.64.7.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Causal effects of gut microbiota on age-related macular degeneration: a Mendelian randomization study. Mao D, Tao B, Sheng S, et al. Invest Ophthalmol Vis Sci. 2023;64:32. doi: 10.1167/iovs.64.12.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Case-control study examining the composition of the gut microbiome in individuals with and without immune-mediated dry eye. Goodman CF, Doan T, Mehra D, et al. Cornea. 2023;42:1340–1348. doi: 10.1097/ICO.0000000000003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dysbiosis in the gut bacterial microbiome of patients with uveitis, an inflammatory disease of the eye. Kalyana Chakravarthy S, Jayasudha R, Sai Prashanthi G, Ali MH, Sharma S, Tyagi M, Shivaji S. Indian J Microbiol. 2018;58:457–469. doi: 10.1007/s12088-018-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Role of microbiota in strengthening ocular mucosal barrier function through secretory IgA. Kugadas A, Wright Q, Geddes-McAlister J, Gadjeva M. Invest Ophthalmol Vis Sci. 2017;58:4593–4600. doi: 10.1167/iovs.17-22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A review of the role of the intestinal microbiota in age-related macular degeneration. Lin P, McClintic SM, Nadeem U, Skondra D. J Clin Med. 2021;10 doi: 10.3390/jcm10102072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The emerging role of gut microbiota in age-related macular degeneration. Xiao J, Zhang JY, Luo W, He PC, Skondra D. Am J Pathol. 2023;193:1627–1637. doi: 10.1016/j.ajpath.2023.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Influence of gut microbiota on eye diseases: an overview. Napolitano P, Filippelli M, Davinelli S, Bartollino S, dell'Omo R, Costagliola C. Ann Med. 2021;53:750–761. doi: 10.1080/07853890.2021.1925150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, et al. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sampieri-Cabrera R, Calderón-Martínez E. Zenodo. Published online. Zenodo; 2023. Signal transduction: from molecular to functional (Book in Spanish) [Google Scholar]
- 60.Gut microbiota and complications of type-2 diabetes. Iatcu CO, Steen A, Covasa M. Nutrients. 2021;14 doi: 10.3390/nu14010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The role of the microbiome in age-related macular degeneration: a review of the literature. Zisimopoulos A, Klavdianou O, Theodossiadis P, Chatziralli I. Ophthalmologica. 2021;244:173–178. doi: 10.1159/000515026. [DOI] [PubMed] [Google Scholar]
- 62.Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Hill C, Guarner F, Reid G, et al. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 63.Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Kang DW, Adams JB, Gregory AC, et al. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Role of fecal microbiota transplant in management of hepatic encephalopathy: current trends and future directions. Shah YR, Ali H, Tiwari A, et al. World J Hepatol. 2024;16:17–32. doi: 10.4254/wjh.v16.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gut microbiota: a potential therapeutic target for management of diabetic retinopathy? Alarcón Yempén RE, Venzel R, Paulino Campos MC, et al. Life Sci. 2021;286:120060. doi: 10.1016/j.lfs.2021.120060. [DOI] [PubMed] [Google Scholar]
- 66.Gut microbiota and metabolites in diabetic retinopathy: insights into pathogenesis for novel therapeutic strategies. Cai Y, Kang Y. Biomed Pharmacother. 2023;164:114994. doi: 10.1016/j.biopha.2023.114994. [DOI] [PubMed] [Google Scholar]
- 67.Sampieri-Cabrera R, Calderón-Martínez E. Zenodo. Published online. Vol. 2022. Zenodo; 2022. Biopsychosocial correlates in 21st century medical education: from theory to practice (Book in Spanish) [Google Scholar]