Abstract
Objectives
This cross-sectional study aimed to determine the prevalence, manifestation, and risk factors of pulmonary involvement in newly diagnosed, untreated rheumatoid arthritis (RA) and psoriatic arthritis (PsA) patients, and to evaluate the efficacy of various diagnostic tools in screening for pulmonary involvement.
Methods
Untreated, newly diagnosed patients with RA and PsA underwent an extensive multimodal diagnostic approach including clinical and laboratory assessment, pulmonary function tests, and chest radiography.
Results
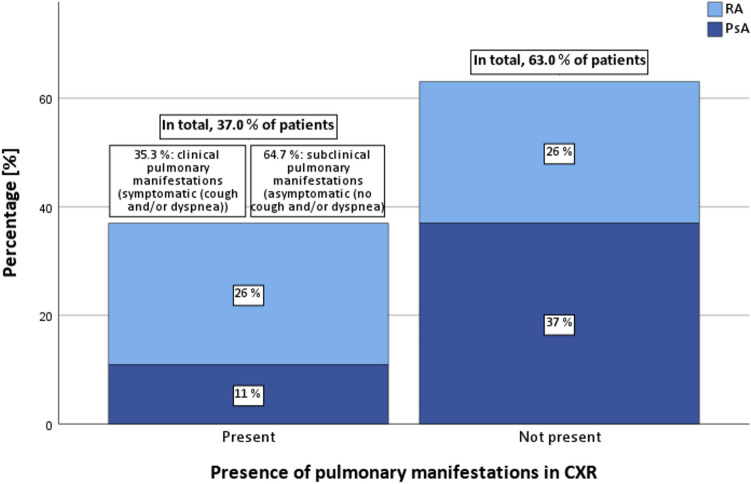
We recruited 50 arthritis patients (26 RA, 24 PsA) and 26 control subjects. Respiratory symptoms were found in 36.0 % of arthritis patients and 11.5 % of controls (p = 0.031). Pathologically reduced breathing width (< 3.0 cm) was significantly more common in arthritis patients (64.0 %) than in controls (23.1 %) (p < 0.001). Pulmonary function test results did not differ significantly between groups. Chest radiography revealed pulmonary involvement in 37.0 % of arthritis patients, higher in RA (50.0 %) than in PsA (22.7 %). Notably, only 35.3 % of arthritis patients with radiographic pulmonary involvement were symptomatic, with 64.7 % being asymptomatic. Radiographic pulmonary involvement was associated with advanced age (p = 0.002) and increased rheumatoid factor levels (p = 0.024).
Conclusion
Our research underscores the significant prevalence of largely asymptomatic pulmonary involvement in newly diagnosed RA and PsA patients. These findings highlight the importance of an early, multidisciplinary screening approach, particularly for high-risk individuals. Further large-scale studies are needed to develop comprehensive screening protocols to improve early detection and treatment of pulmonary involvement in arthritis.
Keywords: Rheumatoid arthritis; Psoriatic arthritis; Extra-articular manifestation; Pulmonary involvement; Imaging; Diagnostics, epidemiology
Introduction
Arthritic diseases, including rheumatoid arthritis (RA) and psoriatic arthritis (PsA) predominantly impact the musculoskeletal system. However, extra-articular manifestations significantly contribute to overall morbidity and mortality [1, 2]. Despite its importance, extra-articular manifestations remain under-explored, often leading to insufficient prevention, inadequate screening, and suboptimal management. Consequently, the European League Against Rheumatism (EULAR) recently emphasized the need for a structured assessment of extra-articular manifestations [3]. Notably, this call did not incorporate the evaluation of pulmonary involvement, despite the increased risk for arthritis patients to develop pulmonary impairment [4, 5]. Clinicians examining the relationship between arthritis and pulmonary involvement face substantial challenges. While respiratory symptoms often diverge from pulmonary function test (PFT) results or imaging findings [6], studies on pulmonary involvement in RA and PsA remain limited, with most being retrospective analyses.
RA, the most prevalent arthritis, is reported to affect between 0.4 and 1.1% of adults in developed countries [4]. Pulmonary involvement is one of the most common extra-articular manifestations in RA and is a leading cause of death among RA patients with an increased mortality exhibiting a threefold elevation [7–10]. It can be broadly categorised into interstitial lung disease in RA (RA-ILD) and non-RA-ILD [11].
RA-ILD is a chronic and progressive condition, representing the most prevalent type of pulmonary complication in patients with RA. Studies suggest that the lifetime risk of RA patients developing ILD lies between 6 and 15% [12–16]. It is characterized by heterogeneous damage to the lung interstitium, affecting not only the alveoli but also the pulmonary blood and lymph vessels. Such damage results in marked impairments to lung structure and function, manifesting as pulmonary fibrosis and restrictive lung disease [11, 15, 16]. The majority of RA-ILD cases present within ten years of diagnosis, with a smaller number predating articular symptoms [5, 16, 17]. RA-ILD patients have a shorter life expectancy and a higher risk of lung- or RA-related death than those without interstitial lung disease [18]. Concerning non-RA-ILD, various pulmonary mainfestations have been reported. Impairments include obstructive lung disease, organising pneumonia, pleural involvement, drug-induced lung disease, and rheumatoid pulmonary nodules [11].
In contrast to RA, the evidence of pulmonary involvement in PsA is less well-defined. Most studies have investigated the link between psoriasis and pulmonary symptoms rather than focusing specifically on PsA. This gap in the literature is particularly notable in newly diagnosed PsA patients, for whom no data currently exists. The few existing studies on pulmonary involvement in PsA have primarily evaluated pulmonary involvement over the course of the disease, with reported prevalence rates for pulmonary manifestations in PsA varying significantly across studies. A retrospective analysis that included psoriasis patients, including those with PsA, indicated a prevalence of 9.2% for pulmonary disease. In contrast, studies focusing exclusively on PsA patients have reported a prevalence range of 0.5–9.4% for pulmonary symptoms. Nonetheless, pulmonary involvement, particularly pneumonia and respiratory arrest due to chronic obstructive pulmonary disease, has been reported as the second leading cause of death among PsA patients.
In this cross-sectional study, we investigate the prevalence of pulmonary involvement in newly diagnosed, untreated RA and PsA patients. To our knowledge, this is the first study to systematically examine the prevalence and manifestation of pulmonary involvement in this specific patient cohort without the influence of any treatment. Utilizing a multimodal approach that combines clinical and laboratory assessments, chest radiography (CXR), and pulmonary function tests (PFT), our aim is to provide a clearer understanding of the baseline pulmonary status in RA and PsA patients, identify the potential for pulmonary manifestations predating articular symptoms and diagnosis, and pinpoint arthritis patients at elevated risk for pulmonary complications. Additionally, this study evaluates the efficacy of various diagnostic tools in screening for pulmonary involvement in RA and PsA, with the long term goal of establishing a screening algorithm for pulmonary involvement in arthritis.
Materials and methods
Patient characteristics
This cross-sectional study enrolled newly diagnosed and untreated RA and PsA patients from the Rheumatology Department at the University Hospital Bonn, Germany, between August 1, 2018, and August 31, 2022. Diagnoses were made by a board-certified rheumatologist. Inclusion criteria were based on the respective guidelines: ACR/European League Against Rheumatism criteria of 2010 for diagnosis of RA [23] and GRAPPA Recommendations of 2016 for PsA [24]. Participants were also required to have the necessary physical and mental capacity for study participation. Exclusion criteria included a prior diagnosis of another rheumatological disease. Additionally, individuals who had taken prednisolone or its equivalent at a dose greater than 5 mg per day for more than seven days prior to enrollment and patients who had been treated with disease-modifying antirheumatic for more than seven days prior to enrollment were excluded. Furthermore, patients with a history of medications known to adversely affect pulmonary status were excluded. Control subjects were recruited from the same department. These subjects were required to have no history of rheumatological conditions or corresponding symptoms and were not allowed to receive any rheumatologic or immunosuppressive medications. Exclusion criteria for control subjects included failing to meet any of the inclusion criteria or having an active infection that would interfere with the performance of pulmonary function tests. The control subjects were matched for age and gender with the included rheumatological patients.
Clinical and laboratory assessment
Demographic data and disease history for each patient were recorded. A standardised physical examination, including lung auscultation, chest excursion assessment, and breathing width analysis was conducted by a board-certified rheumatologist. The duration of arthritic symptoms and the Disease Activity Score in 28 joints using CRP (DAS28CRP) were recorded. Patients' history of smoking, pre-existing pulmonary diseases, medications and current symptoms were assessed. Arthirits patients displaying respiratory symptoms such as cough and/ or dyspnea were classified as symptomatic. Routine laboratory measurements were performed, including C-Reactive Protein (CRP), hematological counts, titer of rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA) and N-terminal pro-B-type natriuretic peptide level. In addition, a blood gas analysis was performed.
Functional assessment
Patients and control subjects underwent comprehensive PFT, six-minute walking test and transthoracic echocardiography. For PFT spirometry and body plethysmography were utilised. Vital capacity (VC), forced vital capacity (FVC), total lung capacity (TLC), forced expiratory volume during the first second of FVC (FEV1), Tiffeneau-index (FEV1/ FVC), and residual volume (RV) were assessed. Additionally, the diffusing capacity for carbon monoxide (DLCO) was determined using the single-breath technique. The PFT results were categorised as follows: (1) Obstructive disorder: FEV1/FVC ratio < lower limit of normal (LLN) and no restrictive patterns. (2) Restrictive disorder: TLC < LLN, and normal Tiffeneau-index. (3) Diffusional capacity disorder: DLCO < 60.0%. In six-minute walking test the total distance covered in six minutes was measured. Transthoracic echocardiography was conducted in a 2-dimensional, M-mode, and Doppler echocardiographic examination. The measurements recorded included: Cardiac dimensions, ejection fraction, functionality and morphology of the heart valves, presence of valvular regurgitation or stenosis, blood flow patterns and pericardial effusion.
Chest radiography imaging
All arthritis patients underwent CXR at the time of diagnosis, prior to the administration of any treatment. CXR were analysed by a board-certified radiologist from the Department of Radiology at the University Hospital Bonn. CXR findings were subsequently categorised into three categories: (1) Patients exhibiting no pathological findings. (2) Patients indicative of pulmonary involvement in association with their arthritis. (3) Patients presenting with other findings.
Statistical analysis
Using IBM SPSS 29.0 for Windows, the study was evaluated regarding the prevalence of clinical and subclinical pneumatological diseases. Following, this data was compared to potential risk factors and findings of several examinations. Continuous data were reported as mean (± SD) if normally distributed or as median (range) if skewed. Qualitative data were portrayed as frequency and percentage. In terms of metric data, the independent samples t-test or Mann–Whitney U test was used to analyse differences between the groups. A comparison of categorical data between different groups was tested with the Chi-square test or in the case of small numbers with Fisher’s exact test. A value of p < 0.05 was considered to be statistically significant.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and has been reviewed and approved by the ethics committee of the University Hospital Bonn, Germany (reference number: 209/18). Written informed consent was obtained from every patient prior to inclusion in the study.
Results
Patient characteristics
The study prospectively enrolled 50 arthritis patients, including 26 RA and 24 PsA cases, with a gender distribution of 27 males and 23 females. The control group consisted of 26 subjects, 12 males and 14 females. Significant age disparity was found regarding pulmonary abnormalities on CXR: 58.2 years (± 12.7) vs. 44.4 years (± 14.6) (p = 0.002). Detailed demographics and characteristics are shown in Table 1.
Table 1.
Patient demographics and clinical data
| Diagnosis | |||
|---|---|---|---|
| RA (n = 26) | PsA (n = 24) | Control subject (n = 26) | |
| Age [years] | |||
| Mean | 52.7 | 46.3 | 48.4 |
| Standard deviation | 16.1 | 12.8 | 11.9 |
| Sex | |||
| Female | 9 (34.6%) | 14 (58.3%) | 14 (53.8%) |
| Male | 17 (65.4%) | 10 (41.7%) | 12 (46.2%) |
| BMI [kg/m2] | |||
| Mean | 25.1 | 26.9 | 27.0 |
| Standard deviation | 4.1 | 5.3 | 4.1 |
| Smoking | |||
| Neversmoker | 17 (65.4%) | 14 (58.3%) | 19 (73.1%) |
| Eversmoker | 9 (34.6%) | 10 (41.7%) | 7 (26.9%) |
| Chronic cough | |||
| No | 16 (61.5%) | 19 (79.2%) | 24 (92.3%) |
| Yes | 10 (38.5%) | 5 (20.8%) | 2 (7.7%) |
| Dyspnea | |||
| No (NYHA I) | 20 (76.9%) | 19 (79.2%) | 24 (92.3%) |
| Yes (NYHA II +) | 6 (23.1%) | 5 (20.8%) | 2 (7.7%) |
| Previous pulmonary disease | |||
| No | 23 (88.5%) | 22 (91.7%) | 22 (84.6%) |
| Bronchial asthma | 2 (7.7%) | 1 (4.2%) | 1 (3.8%) |
| COPD | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) |
| Sarcoidosis | 0 (0.0%) | 1 (4.2%) | 2 (7.7%) |
Table 1 presents the epidemiological and disease-related characteristics of the study population, according to their rheumatological diagnosis
RA rheumatoid arthritis, PsA psoriatic arthritis, NYHA New York Heart Association, BMI Body Mass Index, COPD chronic obstructive pulmonary disease
Clinical and laboratory assessment
In our cohort, 36.0% of arthritis patients and 11.5% of control subjects displayed respiratory symptoms, classified as ‘symptomatic’ (p = 0.031). No significant differences were observed in lung auscultation (p = 0.544, data not shown) or thoracic excursion (p = 0.642) between arthritis patients and control subjects. A significantly diminished pathological breathing width (< 3.0 cm) was detected in 64.0% of arthritis patients compared to 23.1% in control subjects (p < 0.001). However, there was no significant association with radiographic pulmonary involvement (p = 0.210). CRP levels above 3 mg/l were found in 66.0% of arthritis patients, compared to 13.6% in control subjects (p < 0.001). Elevated CRP did not correlate significantly with radiographic pulmonary involvement (p = 0.117). Arthritis patients with radiographic pulmonary involvement showed a higher DAS28CRP (median 3.9, range 2.8) than those without (median 3.1, range 4.9) (p = 0.114). Increased RF levels (> 14 IU/ml) were found in 33.3% of arthritis patients (p = 0.026) and more frequent in subjects presenting with pulmonary manifestation on CXR (p = 0.024). ACPA levels exceeding 8 U/ml were found in 17.8% of arthritis patients, but not in control subjects (p = 0.095), without significant correlation to pulmonary anomalies on CXR (p = 1.000) (Tables 2, 3). Joint symptom duration, hematological counts, blood gas analyses and N-terminal pro-B-type natriuretic peptide level were not significantly associated with arthritis or radiographic pulmonary involvement (data not shown).
Table 2.
Manifestation of arthritis and functional and laboratory diagnostics
| Presence of rheumatic disease | |||
|---|---|---|---|
| RA/PsA (n = 50) | Control subject (n = 26) | Corr. (p value) | |
| Breathing width | |||
| Non-pathological (≥ 3 cm) | 18 (36.0%) | 20 (76.9%) | 0.362 (< 0.001*) |
| Pathological (< 3 cm) | 32 (64.0%) | 6 (23.1%) | |
| Chest excursion | |||
| Non-pathological (≥ 8 cm) | 29 (58.0%) | 17 (65.4%) | 0.071 (0.624) |
| Pathological (< 8 cm) | 21 (42.0%) | 9 (34.6%) | |
| Restrictive lung diseaseTLC < LLN | |||
| No | 34 (81.0%) | 19 (76.0%) | 0.059 (0.758) |
| Yes | 8 (19.0%) | 6 (24.0%) | |
| Obstructive lung disease FEV1/FVC < LLN | |||
| No | 42 (100.0%) | 25 (100.0%) | N/A |
| Yes | 0 (0.0%) | 0 (0.0%) | |
| Emphysema RV > 140% | |||
| No | 35 (85.4%) | 19 (76.0%) | 0.117 (0.512) |
| Yes | 6 (14.6%) | 6 (24.0%) | |
| Diffusing capacity for carbon monoxide | |||
| Non-pathological | 33 (84.6%) | 21 (91.3%) | 0.096 (0.698) |
| Pathological (DLCO < 60%) | 6 (15.4%) | 2 (8.7%) | |
| C-reactive protein (CRP) | |||
| Non-pathological | 17 (34.0%) | 19 (86.4%) | 0.435 (< 0.001*) |
| Pathological (> 3 mg/l) | 33 (66.0%) | 3 (13.6%) | |
| Rheumatoid factor (RF) | |||
| Non-pathological | 30 (66.7%) | 19 (95.0%) | 0.290 (0.026*) |
| Pathological (> 14 IU/ml) | 15 (33.3%) | 1 (5.0%) | |
| Anti-citrullinated peptide antibodies (ACPA) | |||
| Non-pathological | 37 (82.2%) | 20 (100.0%) | 0.242 (0.095) |
| Pathological (> 8 U/ml) | 8 (17.8%) | 0 (0.0%) | |
Table 2 illustrates the various diagnostic methods for functional assessment and the results of laboratory testing and their association with arthritis manifestation
RA rheumatoid arthritis, PsA psoriatic arthritis, CXR chest radiography, FVC forced vital capacity, LLN lower limit of normal, TLC total lung capacity, FEV1 forced expiratory volume during the first second of FVC, RV residual volume, N/A not applicable
Bold values indicate significance: *p < 0.05
Table 3.
Radiographic pulmonary involvement and functional and laboratory diagnostics
| Pulmonary involvement in CXR | |||
|---|---|---|---|
| Positive (n = 17) | Negative (n = 29) | Corr. (p value) | |
| Breathing width | |||
| Non-pathological (≥ 3 cm) | 4 (23.5%) | 13 (44.8%) | 0.208 (0.210) |
| Pathological (< 3c m) | 13 (76.5%) | 16 (55.2%) | |
| Chest excursion | |||
| Non-pathological (≥ 8 cm) | 9 (52.9%) | 16 (55.2%) | 0.022 (1.000) |
| Pathological (< 8 cm) | 8 (47.1%) | 13 (44.8%) | |
| Restrictive lung disease TLC < LLN | |||
| No | 14 (93.3%) | 18 (78.3%) | 0.198 (0.371) |
| Yes | 1 (6.7%) | 5 (21.7%) | |
| Obstructive lung disease FEV1/FVC < LLN | |||
| No | 15 (100.0%) | 23 (100.0%) | N/A |
| Yes | 0 (0.0%) | 0 (0.0%) | |
| Emphysema RV > 140% | |||
| No | 12 (80.0%) | 21 (95.5%) | 0.237 (0.283) |
| Yes | 3 (20.0%) | 1 (4.5%) | |
| Diffusing capacity for carbon monoxide (DLCO) | |||
| Non-pathological | 11 (78.6%) | 19 (90.5%) | 0.164 (0.627) |
| Pathological (DLCO < 60%) | 3 (21.4%) | 2 (9.5%) | |
| C-reactive protein (CRP) | |||
| Non-pathological | 3 (17.6%) | 12 (41.4%) | 0.237 (0.117) |
| Pathological (> 3 mg/l) | 14 (82.4%) | 17 (58.6%) | |
| DAS28CRP | |||
| Median (range) | 3.9 (2.8) | 3.1 (4.9) | 0.236 (0.114) |
| Rheumatoid factor (RF) | |||
| Non-pathological | 6 (40.0%) | 20 (76.9%) | 0.346 (0.024*) |
| Pathological (> 14 IU/ml) | 9 (60.0%) | 6 (23.1%) | |
| Anti-citrullinated peptide antibodies (ACPA) | |||
| Non-pathological | 12 (80.0%) | 21 (80.8%) | 0.009 (1.000) |
| Pathological (> 8 U/ml) | 3 (20.0%) | 5 (19.2%) | |
Table 3 illustrates the various diagnostic methods for functional assessment and the results of laboratory testing and their association with radiographic pulmonary manifestation
RA rheumatoid arthritis, PsA psoriatic arthritis, CXR chest radiography, FVC forced vital capacity, TLC total lung capacity, FEV1 forced expiratory volume during the first second of FVC, RV: residual volume, DA: disease activity, N/A not applicable
Bold values indicate significance: *p < 0.05
Evaluation smoking history, eversmoking rates were 34.6% for RA, 41.7% for PsA, and 26.9% for control patients status (p = 0.446). Regarding radiological pulmonary involvement, 41.2% of those with pulmonary abnormalities were eversmokers, compared to 34.5% of those without pulmonary abnormalities, also showing no significant association between smoking and pulmonary involvement (p = 0.755).
Functional assessment
PFT identified restrictive ventilatory disorders in 19.0% of arthritis patients (6/16 RA patients; 2/18 PsA patients) and 24.0% (6/19) of control subjects (p = 0.758). No obstructive patterns were observed. In arthritis patients, 14.6% exhibited emphysematous patterns with RV > 140%, compared to 24.0% in control subjects (p = 0.512). There was no significant association between change in RV and radiographic pulmonary involvement (p = 0.283). Impaired DLCO was not associated with either arthritis (p = 0.698) or radiographic pulmonary involvement (p = 0.627). No significant association was observed between radiographic pulmonary involvement and the following pulmonary function parameters: FVC (p = 0.344), TLC (p = 0.245), FEV1 (p = 0.740), Tiffeneau-index (p = 0.976), and DLCO (p = 0.654). Data for PFT and DLCO are depicted in Tables 2 and 3.
Transthoracic echocardiographic evaluations did not reveal any considerable cardiac abnormalities or their association to pulmonary impairment (ejection fraction p = 0.336, systolic pulmonary artery pressure p = 0.741, other data not shown).
The six-minute walking test results revealed no significant differences between arthritis patients and control subjects in terms of achieved walking distance, heart rate, respiratory rate, and oxygen saturation levels before and after the test. Similarly, no significant differences were observed in these parameters when considering radiographic pulmonary involvement (Table 4).
Table 4.
Presence of rheumatic disease and pulmonary involvement in chest radiography in six-minute walking test
| Presence of rheumatic disease | Pulmonary involvement in CXR | |||||
|---|---|---|---|---|---|---|
| RA/PsA (n = 50) | Control subject (n = 26) | p value | Positive (n = 17) | Negative (n = 29) | p value | |
| Walking distance [m] | ||||||
| Mean | 566.7 | 560.3 | 0.852 | 584.0 | 559.2 | 0.620 |
| Standard deviation | 142.8 | 106.3 | 147.3 | 152.8 | ||
| Heart rate before 6MWT [per min.] | ||||||
| Median | 75.0 | 81.0 | 0.551 | 74.1 | 74.9 | 0.841 |
| Range | 59.0 | 45.0 | 12.6 | 12.9 | ||
| Heart rate after 6MWT [per min.] | ||||||
| Mean | 91.7 | 96.9 | 0.330 | 93.1 | 89.8 | 0.587 |
| Standard deviation | 17.5 | 23.9 | 18.7 | 18.2 | ||
| Respiratory rate before 6MWT [per min.] | ||||||
| Median | 20.0 | 20.0 | 0.169 | 20.0 | 20.0 | 0.608 |
| Range | 15.0 | 12.0 | 9.0 | 12.0 | ||
| Respiratory rate after 6MWT [per min.] | ||||||
| Median | 21.0 | 22.5 | 0.066 | 21.0 | 21.5 | 0.942 |
| Range | 18.0 | 91.0 | 11.0 | 16.0 | ||
| sO2 before 6MWT [%] | ||||||
| Median | 97.0 | 98.0 | 0.098 | 96.0 | 97.0 | 0.247 |
| Range | 6.0 | 5.0 | 4.0 | 6.0 | ||
| sO2 after 6MWT [%] | ||||||
| Median | 97.0 | 97.0 | 0.734 | 97.0 | 98.0 | 0.117 |
| Range | 7.0 | 9.0 | 5.0 | 4.0 | ||
Table 4 illustrates the results of six-minute walking test and their association with manifestation of arthritis and radiographic pulmonary manifestation
RA rheumatoid arthritis, PsA psoriatic arthritis, CXR chest radiography, 6MWT six-minute walking test, sO2 oxygen saturation
*p < 0.05 was considered significant
Chest radiography imaging
Radiographic evidence of pulmonary involvement, detected via CXR, was observed in 37.0% of arthritis patients. The radiographic findings included:
Interstitial, fine reticular pattern, predominantly in lower lobe (63.2%)
Scarring changes in lung structure (15.8%)
Grouped calcifications (10.5%)
Pleural thickening (10.5%)
The remaining patients presented with either non-pathological CXR (58.7%) or nonspecific findings (4.3%). Pulmonary involvement on CXR was detected in 50.0% of RA and in 22.7% of PsA cases (p = 0.072). Among arthritis patients displaying radiographic evidence of pulmonary involvement on CXR, 35.3% were symptomatic, while 64.7% were asymptomatic (Fig. 1). Figure 2 depicts representative findings from the CXR imaging.
Fig. 1.
Radiographic Pulmonary Involvement and Clinical Symptoms. Figure 1 illustrates the relationship between the presence of radiographic pulmonary involvement and the presence of clinical symptoms. RA rheumatoid arthritis, PsA psoriatic arthritis, CXR chest radiography
Fig. 2.
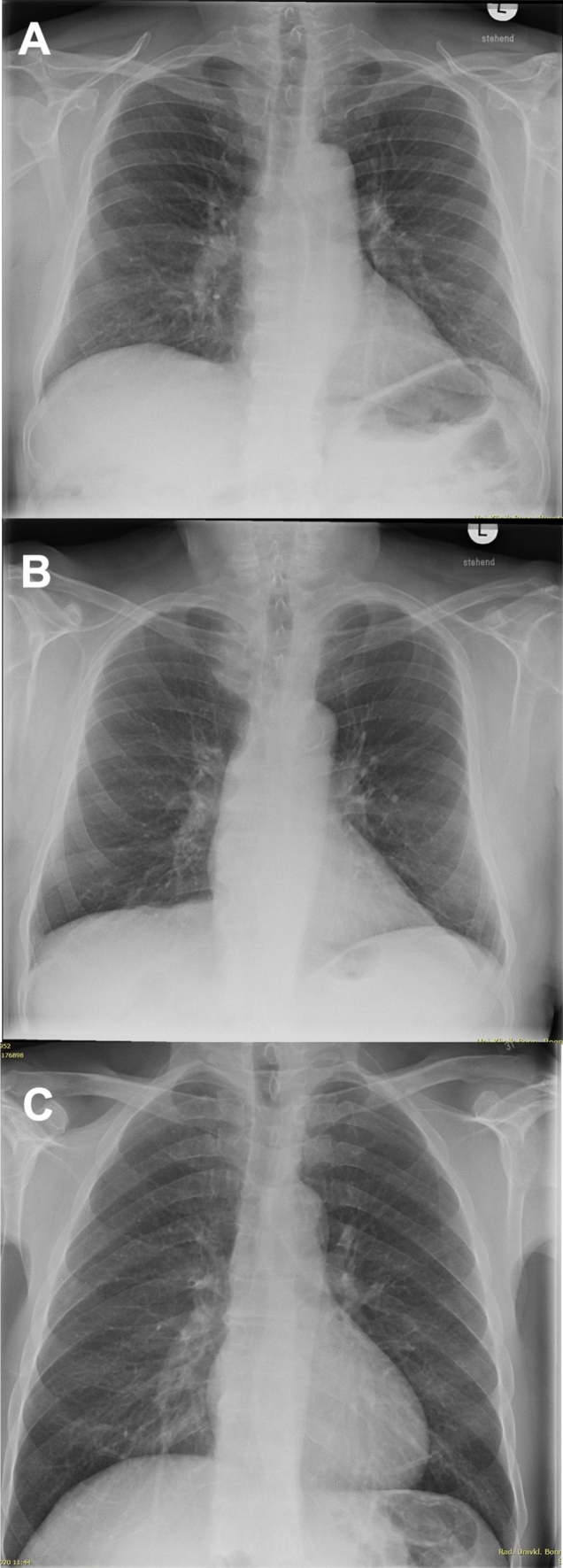
Findings in Chest Radiography. Figure 2 presents examples of typical findings in patients with arthritis in conventional posteroanterior chest radiography. A No pathological findings in a patient with rheumatoid arthritis, B Pleural scarring at the right costophrenic angle and fibrous strands in the lingular segment, interpreted as radiographic manifestations, in an asymptomatic patient with rheumatoid arthritis. C Irregular interstitial markings increase in the lung bases, interpreted as radiographic manifestations, in a symptomatic patient (cough) with rheumatoid arthritis
Discussion
This study is the first to examine the prevalence of pulmonary involvement in newly diagnosed, untreated RA and PsA patients using a multimodal approach. By incorporating clinical and laboratory assessments, CXR imaging, and PFTs, our aim was to not only investigate the prevalence and manifestation of pulmonary involvement in this specific patient cohort early in the course of the disease and without the influence of any treatment but also to evaluate the effectiveness of the applied diagnostic methods as screening tools. Our objective was to identify arthritis patients at higher risk for pulmonary involvement and, in the long run, contribute to an early screening algorithm for pulmonary involvement in arthritic diseases.
Pulmonary involvement in arthritis significantly deteriorates patient health, resulting in a diminished quality of life and compromised functional status [1]. Early detection and treatment can be crucial to reduce disease impact, emphasising the need for improved understanding of pulmonary involvement and subsequent screening diagnostic methods in RA and PsA. In this context, our study stands out due to its focus on newly diagnosed, untreated RA and PsA patients, which differs from most existing research that often examines patients with ongoing disease and treatment. By studying patients at the time of their first diagnosis, we eliminate potential confounders associated with prolonged disease, such as medication-induced pulmonary abnormalities and opportunistic infections commonly observed in advanced disease stages. This approach allows for the identification of pulmonary involvement mainly attributed to arthritis at disease onset, providing a clearer picture of the baseline pulmonary status in these patients.
In contrast, existing studies exploring the occurrence of pulmonary extra-articular manifestations in arthritis primarily focus on RA within the course of the disease. Prevalence data show considerable variation, attributed to differences in study design, cohort selection, disease definition and progression, and case detection methods. Additionally, determining the primary cause of pulmonary changes is challenging due to potential secondary causes of respiratory symptoms, including medication toxicity, cardiac conditions, and age-related diseases[25]. For example, while some reports indicate a prevalence of pulmonary involvement in RA as low as 10.0%[26], a study by Bilgici et al., utilising high-resolution computed tomography (HRCT) and PFT, identified abnormalities in up to 67.3% of cases [28]. As a result, a diagnostic algorithm for RA-ILD that includes HRCT for symptomatic patients, followed by a risk assessment of RA-ILD outcomes, has been proposed [27].
In our study, we found that 37.0% of patients showed radiographic pulmonary involvement in CXR. Further assessment of the different disease entities revealed a radiographic pulmonary involvement of 50.0% in RA and 22.7% in PsA patients. The lower prevalence of radiographic pulmonary involvement in our RA patients, compared to, for example, Bilgici et al.’s study [28], could be explained by several factors. Firstly, our study focused on patients at the time of their first diagnosis, thus eliminating potential confounders associated with prolonged disease, such as medication-induced pulmonary abnormalities [25] and opportunistic infections that are commonly observed in advanced disease stages. This allows for the identification of pulmonary involvement mainly attributed to arthritis at disease onset. Secondly, our choice of CXR as the diagnostic imaging tool, despite its lower sensitivity compared to HRCT (the gold standard for early or subclinical pulmonary involvement detection) [5], affects reported prevalence rates. Consequently, higher prevalence rates in studies using this latter method are expected. However, due to concerns about radiation exposure limiting the routine use of HRCT [5, 9], our study aimed to create a feasible screening algorithm for pulmonary involvement, utilising the more universally available and applicable CXR.
While the radiographic evidence of pulmonary involvement in RA aligns with previous research, our study shows a notably higher prevalence in PsA patients. This finding is especially significant given the limited existing studies on PsA and pulmonary involvement. Specifically, we found a 22.7% prevalence of radiographic pulmonary involvement in PsA patients, significantly higher than the previously reported range of 0.5–9.4% [20, 21]. Given that pulmonary involvement is the second leading cause of death in PsA patients [22], and considering that our detected prevalence surpasses existing rates even in early-stage disease, our results underscore the importance of heightened awareness and proactive screening in newly diagnosed and untreated PsA patients. However, the long-term progression of pulmonary involvement and the impact of treatment initiation warrant further investigation and will be subject to future studies.
When comparing the radiographic pulmonary involvement between RA and PsA patients, the higher prevalence in RA could be attributed to its distinct pathophysiological mechanisms. It is hypothesed that the lung could be more than an extra-articular manifestation site of RA and may actively contribute to the disease's etiology via protein citrullination [1, 29]. This theory speculates that RA-related autoimmunity could initiate at mucosal surfaces like the lung, leading to joint inflammation. Support for this hypothesis comes from studies indicating interstitial lung disease association with significantly elevated ACPA levels [5, 30]. Further evidence includes the shown increase in lung protein citrullination in smokers and individuals with a history of toxic inhalation, both recognised RA risk factors [31]. Consequently, RA pulmonary involvement may not simply be a secondary effect of joint inflammation. Instead, it could be an indicator of a “lung as a site of initiation” phenomenon, suggesting that pulmonary manifestations might precede arthritic symptoms. Although our study could not show a significant correlation between ACPA levels and pulmonary involvement in RA, possibly due to the limited RA cohort sample size, we propose that the lower pulmonary involvement observed in PsA could partly be due to less protein citrullination in the lungs compared to RA. This idea is further strengthened as there are no reports indicating the presence of interstitial lung disease in PsA [1].
Shifting our focus to functional assessment outcomes, the data showed no significant correlations between PFTs, six-minute walking test, and the presence of arthritis or radiographic pulmonary involvement. Crucial diagnostic parameters for RA-ILD, such as blood gas analysis and DLCO[32, 33], also did not correlate significantly with the manifestation of arthritis or pulmonary abnormalities as detected by CXR. A reason might be that early radiographic changes do not immediately translate to measurable deviations in functional tests. Thus, the design of our study, emphasizing newly diagnosed patients, could be a crucial factor. It seems plausible that the imaging abnormalities might emerge before detectable changes in PFTs readings become evident. Moreover, potential longitudinal changes in PsA patients, possibly due to suboptimal or delayed treatment, might only manifest in PFTs parameters at a later stage.
When comparing imaging findings with reported respiratory symptoms, we found that a significant proportion of patients across the arthritic diseases displayed abnormal CXR findings despite not reporting any respiratory symptoms. This is particularly interesting given the high fraction of our study population that reported respiratory symptoms. One could argue the reason, whether it is due to observation bias from the explicit querying of symptoms or the fact that a significant portion of our study was conducted during the COVID-19 pandemic. Nonetheless, only a third of the patients with abnormal CXR exhibited respiratory symptoms such as cough or dyspnea, leaving two-thirds of these cases subclinical and asymptomatic. The absence of a CXR would likely result in undiagnosed pulmonary involvement, potentially delaying appropriate monitoring and treatment. Our results align with previous studies that revealed significant imaging findings in RA, or impaired PFT results in RA and PsA, despite the absence of respiratory symptoms [28, 34]. These findings strengthen existing data, suggesting that the absence of respiratory symptoms in arthritis patients does not rule out pulmonary involvement. As an example, Kanat et al. reported an asymptomatic proportion of nearly 50.0%, which closely aligns with the 64.7% identified in our study [6]. Given the substantial number of patients without clinical symptoms, it is crucial to identify these asymptomatic high-risk individuals through suitable screening methods. Therefore, our study aimed not only to identify risk factors for pulmonary involvement in arthritis but also to contribute to an effective screening algorithm.
However, we recognize that the results of our exploratory, observational study are based on a small sample size, which limits the strength of our conclusions. Consequently, these recommendations should be considered initial guidelines rather than definitive diagnostic algorithm protocols. Within these guidelines, we recommend a comprehensive evaluation of identified risk factors such as age, DAS28CRP for disease activity assessment, and RF levels in all newly diagnosed RA and PsA patients based on our findings. Additionally, the presence of respiratory symptoms such as cough and dyspnea should be evaluated. Clinicians should remain vigilant for potential pulmonary involvement even in the absence of these symptoms.
While we observed potential associations between these risk factors and pulmonary involvement, our findings need to be validated through larger, multi-center studies to ensure their robustness and generalizability. Potentially due to the exploratory nature of our study and the lack of evidence to plan a study with an appropriate sample size, we were unable to validate previously recognized risk factors for pulmonary involvement, such as male gender, smoking status, and high-titer ACPA, in our patient cohort. Nevertheless, these factors should still be considered potential risk factors and need to be reassessed in the proposed larger, multi-center studies.
In our study, CXR emerged as the primary screening tool for potential pulmonary involvement, allowing for the identification of asymptomatic patients demonstrating radiographic pulmonary abnormalities, irrespective of respiratory symptoms. In case of abnormal CXR results, patients should undergo further functional assessment, including PFT with DLCO, to better characterise any detected pulmonary abnormalities on CXR. This can provide insight into the nature and severity of the pulmonary disorder. Despite not being used in our study, HRCT should be considered for further evaluation in patients with suggestive PFTs and CXR outcomes. The algorithm should be implemented within the framework of a multidisciplinary patient care approach involving rheumatologists, pulmonologists, and radiologists.
In conclusion, our study addresses a significant knowledge gap regarding pulmonary involvement in RA and PsA patients. We found that pulmonary involvement is a common extra-articular manifestation not only in RA but also in PsA at time of diagnosis, with many patients presenting no respiratory symptoms. Our results underscore the need to early screening high-risk patients with RA and PsA for pulmonary involvement at diagnosis. Even in the absence of respiratory symptoms, clinicians should be alert to indicators such as dyspnea, cough, high disease activity as measured by DAS28CRP, increased age, and elevated RF levels. Pulmonary involvement can be confirmed with a CXR, followed by supplementary diagnostic procedures like PFT with DLCO and possible HRCT to further characterise CXR-detected abnormalities. Our findings emphasise the need for a multidisciplinary approach to patient management. Implementing an international screening protocol could enable early detection and treatment of high-risk patients, possibly preventing disease progression. Further research, however, is required to track longitudinal changes in disease progression and determine if therapy initiation can reverse the observed imaging changes.
Limitations
Our study has several limitations that warrant discussion. The relatively small sample size restricts the generalizability of our findings. Although our study provides initial insights into pulmonary involvement in newly diagnosed, untreated RA and PsA patients, larger, multi-center studies are required to validate these results and enhance their applicability to the broader patient population. Additionally, longitudinal studies are needed to elucidate the progression of pulmonary abnormalities in these patients and offer more comprehensive insights into the functional implications of pulmonary involvement.
Furthermore, we did not exclude pre-existing pulmonary conditions, as we aimed to create a representative cohort reflective of real-world scenarios and detect pulmonary involvement predating articular symptoms. This approach may introduce bias. In particular, although we sought to identify pulmonary involvement related to arthritis, we did not systematically exclude other potential causes of respiratory symptoms, such as infections or other comorbid conditions. In cases where tuberculosis or other persistent infections were suspected, tests like the Quantiferon-TB test were conducted, but not routinely for all participants. However, given that tuberculosis infections have a negligible prevalence in Germany and other persistent infections in untreated patients are not highly probable, we consider tuberculosis or other infections a negligible moderating factor. Future studies should consider stratifying patients based on their pulmonary history to address this issue and include comprehensive differential diagnostics to ensure accurate attribution of respiratory symptoms. In this context, we acknowledge that HRCT scans, which were not utilized in our study, may offer a more detailed assessment of pulmonary involvement. Future research may incorporate HRCT to better characterize pulmonary abnormalities and refine the screening algorithm.
Author contributions
Conceptualization: VSS, LW, DS, SMP. Methodology: VSS, LW, DS, CB, SMP. Validation: VSS, LW, DS, CJB, CP, DK, CB, SMP, Formal analysis: VSS, LW, CB, SP. Investigation: VSS, LW, DS, CP, MW, DK, SMP. Resources: VSS, DS, MW, PB. Data curation: LW, CB. Writing—Original Draft: VSS, LW, SMP. Writing—Review & Editing: VSS, LW, DS, CJB, CP, MW, DK, CB. PB, SMP. Visualization: VSS, LW, DK, SMP. Supervision: VSS, DS. Project administration: VSS, DS. Funding acquisition: VSS.
Funding
Open Access funding enabled and organized by Projekt DEAL. This is an independent, investigator-initiated study supported by Boehringer Ingelheim, Germany. Boehringer Ingelheim has no role in the design, analysis or interpretation of the results in this study; Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy.
Data availability
Data are available on reasonable request.
Declarations
Conflict of interest
The authors report no disclosures relevant to the manuscript.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and has been reviewed and approved by the ethics committee of the University Hospital Bonn, Germany (reference number: 209/18). Written informed consent was obtained from every patient prior to inclusion in the study.
Consent to participate
Informed consent was obtained from all subjects before enrollment.
Patient consent for publication
Not applicable.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Valentin Sebastian Schäfer and Lone Winter have contributed equally to this work.
References
- 1.Kelly C, Iqbal K, Iman-Gutierrez L, Evans P, Manchegowda K (2016) Lung involvement in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 30:870–888. 10.1016/j.berh.2016.10.004 10.1016/j.berh.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 2.Jani M, Dixon WG, Matteson EL (2017) Management of the rheumatoid arthritis patient with interstitial lung disease. Lung Dis Rheum Arthritis. 10.1007/978-3-319-68888-6_9 10.1007/978-3-319-68888-6_9 [DOI] [Google Scholar]
- 3.Baillet A, Gossec L, Carmona L, de Wit M, van Eijk-Hustings Y, Bertheussen H et al (2016) Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 75:965–973. 10.1136/annrheumdis-2016-209233 10.1136/annrheumdis-2016-209233 [DOI] [PubMed] [Google Scholar]
- 4.West S (2018) Clinical overview of rheumatoid arthritis. In: Fischer A, Lee JS (eds) Lung disease in rheumatoid arthritis. Springer International Publishing, Cham, pp 1–18. 10.1007/978-3-319-68888-6_1 [Google Scholar]
- 5.Kristen Demoruelle M, Olson AL, Solomon JJ (2018) The epidemiology of rheumatoid arthritis-associated lung disease. Springer International Publishing, Cham, pp 45–58. 10.1007/978-3-319-68888-6_4 [Google Scholar]
- 6.Kanat F, Levendoglu F, Teke T (2007) Radiological and functional assessment of pulmonary involvement in the rheumatoid arthritis patients. Rheumatol Int 27:459–466. 10.1007/s00296-006-0234-0 10.1007/s00296-006-0234-0 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Shi Y, Wang X, Huang H, Ascherman D (2013) Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin Dev Immunol 2013:406927. 10.1155/2013/406927 10.1155/2013/406927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito AJ, Chu SG, Madan R, Doyle TJ, Dellaripa PF (2019) Thoracic manifestations of rheumatoid arthritis. Clin Chest Med 40:545–560. 10.1016/j.ccm.2019.05.003 10.1016/j.ccm.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biederer J, Schnabel A, Muhle C, Gross WL, Heller M, Reuter M (2004) Correlation between HRCT findings, pulmonary function tests and bronchoalveolar lavage cytology in interstitial lung disease associated with rheumatoid arthritis. Eur Radiol 14:272–280. 10.1007/s00330-003-2026-1 10.1007/s00330-003-2026-1 [DOI] [PubMed] [Google Scholar]
- 10.Morisset J, Lee JS (2018) Rheumatoid arthritis-associated interstitial lung disease. Springer International Publishing, Cham, pp 109–119. 10.1007/978-3-319-68888-6_8 [Google Scholar]
- 11.Dejcman D, Skowasch D, Pizarro C, Krause A, Thomas D, Schäfer VS (2021) Pulmonary manifestations of rheumatoid arthritis, psoriatic arthritis and peripheral spondyloarthritis: prevalence, diagnostic approach and treatment options. Curr Rheumatol Rev 17:17–28. 10.2174/1573397116666200905122757 10.2174/1573397116666200905122757 [DOI] [PubMed] [Google Scholar]
- 12.Turesson C, Jacobsson LTH (2004) Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol 33:65–72. 10.1080/03009740310004621 10.1080/03009740310004621 [DOI] [PubMed] [Google Scholar]
- 13.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL (2003) Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 62:722–727. 10.1136/ard.62.8.722 10.1136/ard.62.8.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH et al (2010) Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 62:1583–1591. 10.1002/art.27405 10.1002/art.27405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw M, Collins BF, Ho LA, Raghu G (2015) Rheumatoid arthritis-associated lung disease. Eur Respir Rev 24:1–16. 10.1183/09059180.00008014 10.1183/09059180.00008014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendstrup E, Møller J, Kronborg-White S, Prior TS, Hyldgaard C (2019) Interstitial lung disease in rheumatoid arthritis remains a challenge for clinicians. J Clin Med. 10.3390/jcm8122038 10.3390/jcm8122038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, Ellingsen T (2017) A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 76:1700–1706. 10.1136/annrheumdis-2017-211138 10.1136/annrheumdis-2017-211138 [DOI] [PubMed] [Google Scholar]
- 18.Johnson C (2017) Recent advances in the pathogenesis, prediction, and management of rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol 29:254–259. 10.1097/BOR.0000000000000380 10.1097/BOR.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 19.Feldman SR, Zhao Y, Shi L, Tran MH (2015) Economic and comorbidity burden among patients with moderate-to-severe psoriasis. J Manag Care Spec Pharm 21:874–888. 10.18553/jmcp.2015.21.10.874 10.18553/jmcp.2015.21.10.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peluso R, Iervolino S, Vitiello M, Bruner V, Lupoli G, Di Minno MND (2015) Extra-articular manifestations in psoriatic arthritis patients. Clin Rheumatol 34:745–753. 10.1007/s10067-014-2652-9 10.1007/s10067-014-2652-9 [DOI] [PubMed] [Google Scholar]
- 21.Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J, Sampalis JS (2011) Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol 30:877–885. 10.1007/s10067-011-1692-7 10.1007/s10067-011-1692-7 [DOI] [PubMed] [Google Scholar]
- 22.Wong K, Gladman DD, Husted J, Long JA, Farewell VT (1997) Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 40:1868–72. 10.1002/art.1780401021 10.1002/art.1780401021 [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO et al (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581. 10.1002/art.27584 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 24.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW et al (2016) Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 68:1060–1071. 10.1002/art.39573 10.1002/art.39573 [DOI] [PubMed] [Google Scholar]
- 25.Sathi N, Chikura B, Kaushik VV, Wiswell R, Dawson JK (2012) How common is methotrexate pneumonitis? A large prospective study investigates. Clin Rheumatol 31:79–83. 10.1007/s10067-011-1758-6 10.1007/s10067-011-1758-6 [DOI] [PubMed] [Google Scholar]
- 26.Yunt ZX, Solomon JJ (2015) Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am 41:225–236. 10.1016/j.rdc.2014.12.004 10.1016/j.rdc.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jani M, Hirani N, Matteson EL, Dixon WG (2014) The safety of biologic therapies in RA-associated interstitial lung disease. Nat Rev Rheumatol 10:284–294. 10.1038/nrrheum.2013.197 10.1038/nrrheum.2013.197 [DOI] [PubMed] [Google Scholar]
- 28.Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M (2005) Pulmonary involvement in rheumatoid arthritis. Rheumatol Int 25:429–435. 10.1007/s00296-004-0472-y 10.1007/s00296-004-0472-y [DOI] [PubMed] [Google Scholar]
- 29.Dai Y, Wang W, Yu Y, Hu S (2021) Rheumatoid arthritis-associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin Rheumatol 40:1211–1220. 10.1007/s10067-020-05320-z 10.1007/s10067-020-05320-z [DOI] [PubMed] [Google Scholar]
- 30.Aubart F, Crestani B, Nicaise-Roland P, Tubach F, Bollet C, Dawidowicz K et al (2011) High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol 38:979–982. 10.3899/jrheum.101261 10.3899/jrheum.101261 [DOI] [PubMed] [Google Scholar]
- 31.Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC et al (2015) Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med 191:1403–1412. 10.1164/rccm.201411-1950OC 10.1164/rccm.201411-1950OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR (2002) Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis 61:517–521. 10.1136/ard.61.6.517 10.1136/ard.61.6.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T, Zheng X-J, Liang B-M, Liang Z-A (2015) Clinical features of rheumatoid arthritis-associated interstitial lung disease. Sci Rep 5:14897. 10.1038/srep14897 10.1038/srep14897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daïen CI, Tubery A, Beurai-Weber M, Du Cailar G, Picot M-C, Jaussent A et al (2019) Relevance and feasibility of a systematic screening of multimorbidities in patients with chronic inflammatory rheumatic diseases. Joint Bone Spine 86:49–54. 10.1016/j.jbspin.2018.03.016 10.1016/j.jbspin.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 35.Fischer A, Lee JS (eds) (2018) Lung disease in rheumatoid arthritis. Springer International Publishing, Cham [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.