Abstract
The absence of catalytic asymmetric methods for synthesizing chiral (hetero)bicyclo[n.1.1]alkanes has hindered their application in new drug discovery. Here we demonstrate the achievability of an asymmetric polar cycloaddition of bicyclo[1.1.0]butane using a chiral Lewis acid catalyst and a bidentate chelating bicyclo[1.1.0]butane substrate, as exemplified by the current enantioselective formal (3 + 3) cycloaddition of bicyclo[1.1.0]butanes with nitrones. In addition to the diverse bicyclo[1.1.0]butanes incorporating an acyl imidazole group or an acyl pyrazole moiety, a wide array of nitrones are compatible with this Lewis acid catalysis, successfully assembling two congested quaternary carbon centers and a chiral aza-trisubstituted carbon center in the pharmaceutically important hetero-bicyclo[3.1.1]heptane product with up to 99% yield and >99% ee.
Subject terms: Asymmetric synthesis, Synthetic chemistry methodology, Asymmetric catalysis
The absence of catalytic asymmetric methods for synthesizing chiral (hetero)bicyclo[n.1.1]alkanes has hindered their application in new drug discovery. Herein the authors report an enantioselective formal (3 + 3) cycloaddition of bicyclobutanes with nitrones using a chiral Lewis acid catalyst for the synthesis of hetero-bicyclo[3.1.1]heptane.
Introduction
The development of asymmetric catalytic reactions, as well as the introduction of new catalytic methods and strategies for preparing chiral molecules in enantioenriched forms, is crucial for drug discovery and innovation. This importance is underscored by the fact that over half of all pharmaceuticals currently in use are chiral compounds containing carbon stereocenters1.
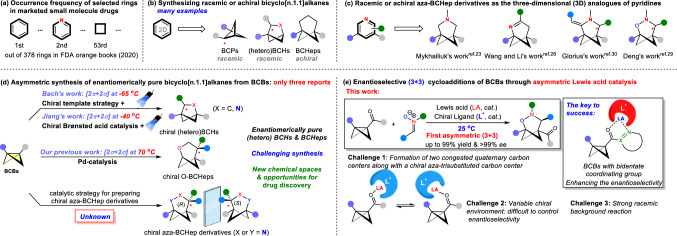
Recently, the substitution of planar aromatic rings with bridged bicyclic scaffolds has been increasingly acknowledged as a potent approach to enhance the physicochemical and pharmacokinetic properties of drug analogs2–19. 1,3-Disubstituted phenyl rings and N-heterocycles are ubiquitous structural motifs found in a variety of small-molecule drugs, with pyridines ranking as the second most prevalent in marketed drugs (Fig. 1a)20. In this context, Anderson21 and Uchiyama22 independently demonstrated that bridgehead-substituted bicyclo[3.1.1]heptanes (BCHeps), prepared via ring-opening reactions of [3.1.1]propellanes, are suitable bioisosteres for meta-substituted benzenes (Fig. 1b). Subsequently, Mykhailiuk documented that aza-BCHeps exhibit remarkable potential as pyridine bioisosteres23. Besides noncatalytic linear synthetic methods, several state-of-the-art catalytic strategies, including photocatalysis, boronyl radical catalysis, Lewis acid catalysis, and silver-promoted methods, have been developed by research groups led by Stephenson24, Molander25, Li26, Waser27, Wang28, Deng29, and Glorius30 for synthesizing BCHeps and aza-BCHeps through cycloadditions of bicyclo[1.1.1]pentanes (BCPs) or bicyclo[1.1.0]butanes (BCBs) (Fig. 1c)31–35.
Fig. 1. Outline of this work.
a Occurrence frequency of selected rings in marketed small molecule drugs. b Synthesizing racemic or achiral bicyclo[n.1.1]alkanes. c Racemic or achiral aza-BCHep derivatives as the three-dimensional (3D) analogs of pyridines. d Asymmetric synthesis of enantiomerically pure bicyclo[n.1.1]alkanes from BCBs. e Enantioselective (3 + 3) cycloadditions of BCBs through asymmetric Lewis acid catalysis.
In 2021, Baran and co-workers successfully achieved enantiopure BCP bioisosteres through SFC separation. Furthermore, their study revealed that substituting benzenoids with either R- or S-enantiomers of BCPs results in distinct drug bioactivities36. Consequently, the development of catalytic asymmetric synthesis techniques for (hetero)bicyclo[n.1.1]alkanes is not only highly desirable but would also significantly accelerate the creation of drugs with superior properties. While chirality transfer and bio-catalysis strategies have been used to synthesize chiral BCPs and BCHs respectively37,38, before the elegant asymmetric (3 + 2) cycloadditions of BCBs reported by Bach39 and Jiang40, the only asymmetric difunctionalization reactions within the BCB framework were limited to ring opening processes41,42. Notably, the strategies employed by Bach39 and Jiang40, focus exclusively on asymmetric photochemical cycloadditions of BCBs. Our group has recently achieved palladium-catalyzed enantioselective [2σ + 2σ] cycloadditions of BCBs with vinyl oxiranes to synthesize O-BCHeps (Fig. 1d)43.
Besides radical cycloadditions, polar cycloadditions of BCBs have been developed after Leitch’s pioneering research in Lewis acid catalysis44. Currently, the Lewis acid catalyzed (3+n) polar cycloadditions of BCBs can utilize ketenes45, aldehydes46, heteroarenes47,48, dienol ethers49, ynamides50 and imidazolidines51 as the cycloaddition partners. During the preparation of this manuscript, Deng pioneered the Eu(OTf)3-catalyzed cycloadditions of BCBs with nitrones29. This innovation resulted in the creation of a range of structurally unique and biologically intriguing 2-oxa-3-aza-BCHep skeletons, which show promise as bioisosteres for pyridines. Despite significant progress in this area, the enantioselective synthesis of (hetero)bicyclo[n.1.1]alkanes through Lewis acid catalysis is still unexplored and poses a significant challenge. A major challenge in achieving the asymmetric Lewis acid-catalyzed (3 + 3) cycloadditions of BCBs is identifying optimal ligand capable of overcoming the significant racemic background reaction29,52, thus facilitating the formation of two congested carbon centers together with a chiral carbon center with high enantioselective control53,54. In contrast to donor-acceptor cyclopropanes55–63, which feature two chelating, electron-withdrawing groups to form a stable chiral catalyst-substrate complex, achieving catalytic asymmetric reactions of BCBs with only a single electron-withdrawing group, leading to chiral environments with variable conformation, poses greater challenges. To address these challenges, we investigated the utilization of BCBs with bidentate coordinating groups and a chiral Lewis acid catalysis strategy in the asymmetric (3 + 3) cycloaddition of BCBs (Fig. 1e).
Results
Reaction optimization
Initially, BCB 1a and nitrone 2a were chosen as model substrates for reaction optimization (Table 1). Unfortunately, the model reaction showed low enantioselectivity (3aa, <40% ee) when subjected to zinc-Lewis acid/L1 or L2, as well as privileged chiral oxazoline ligands, including chiral bisoxazolines (BOX) and Py-BOX ligands (Supplementary Figs. 1,2 and Supplementary Table 1). The axially chiral binaphthyl-BOX L4 yielded promising results. Substituting 2a with benzyl-substituted nitrone 2b resulted in the desired 3ab with a 92% yield and 54% ee (entry 5). Since L4 exhibited better enantiomeric excess compared to the chiral spiro-ligand L3 (entry 3 versus 4), a series of bis(oxazolyl)binaphthyl ligands ((Ra,R,R)-L5-L7) were further examined, but no enhancement in enantioselectivity was observed. (Ra,S,S)-L5 was also examined, but it exhibited lower yield and ee compared to (Ra,R,R)-L5 (entry 9 versus 6). Recently, Xie and Guo developed two new tridentate nitrogen ligands, namely PyBPI and PyIPI ligands64–67. These ligands exhibited high levels of activity and enantioselectivity in Lewis acid-catalyzed asymmetric reactions. The enantioselectivity of the current reaction with PyBPI L8 was poor. However, the reaction using PyIPI L9 yielded 3ab in 87% ee (entry 10 versus 11). Next, we screened an array of commonly used metal-Lewis acids, including Ga(OTf)3, Eu(OTf)3, Cu(OTf)2, Ni(OTf)2 and Co(OTf)2 (entries 12-16). In contrast to other Lewis catalysts, Co(OTf)2 emerged as the optimal catalyst, enhancing enantioselectivity to 93% ee (entry 16). It is noteworthy that Ga(OTf)344 and Eu(OTf)329 previously employed in (3+n) cycloadditions of BCBs, exhibited significant racemic background reactions. Other solvents were also investigated, and CH2Cl2 was found to be superior. Next, PyIPI L10 − L12, which contain more sterically hindered amide substituents, were assessed (entries 17-20). All of these ligands further enhanced the enantioselectivity; The catalytic system comprising Co(OTf)2, L10, or L12 provides 3ab with a yield of over 90% and an 99% ee. Additionally, the catalyst loading could be reduced to 5 mol% almost without deterioration in yield and enantioselectivity (entry 18).
Table 1.
Optimization of the reaction conditionsa
 | ||||
|---|---|---|---|---|
| Entry | Lewis acid | Ligand | Yield (%)c | eed |
| 1 | Zn(ClO4)2·H2O | L1 | 83 | 18b,e |
| 2 | Zn(ClO4)2·H2O | L2 | 71 | 33b |
| 3 | Zn(ClO4)2·H2O | L3 | 86 | 11b |
| 4 | Zn(ClO4)2·H2O | L4 | 70 | 43b,e |
| 5 | Zn(ClO4)2·H2O | L4 | 92 | 54 f,e |
| 6 | Zn(ClO4)2·H2O | (Ra,R,R)-L5 | 99 | 53 f,e |
| 7 | Zn(ClO4)2·H2O | (Ra,R,R)-L6 | 99 | 27 f,e |
| 8 | Zn(ClO4)2·H2O | (Ra,R,R)-L7 | 89 | 0 f |
| 9 | Zn(ClO4)2·H2O | (Ra,S,S)-L5 | 85 | 30 f,e |
| 10 | Zn(ClO4)2·H2O | L8 | 91 | 27 f |
| 11 | Zn(ClO4)2·H2O | L9 | 65 | 87 f |
| 12 | Ga(OTf)3 | L9 | 99 | 0 f |
| 13 | Eu(OTf)3 | L9 | 90 | 2 f |
| 14 | Cu(OTf)2 | L9 | 89 | 65 f |
| 15 | Ni(OTf)2 | L9 | 95 | 80 f |
| 16 | Co(OTf)2 | L9 | 95 | 93 f |
| 17 | Co(OTf)2 | L10 | 98 | 99 f |
| 18 | Co(OTf)2 | L10 | 95 | 98 f,g |
| 19 | Co(OTf)2 | L11 | 99 | 97 f |
| 20 | Co(OTf)2 | L12 | 93 | 99 f |
a1a (0.1 mmol), 2a or 2b (0.12 mmol), Lewis acid (10 mol%) and ligand (12 mol%) in CH2Cl2 at room temperature for 24 h. b2a was used. cNMR yield with CH2Br2 as an internal standard. dThe enantiomeric excess (ee) of the product was determined by HPLC using a chiral stationary phase. eThe other enantiomer was obtained. f2b was used. gCo(OTf)2 (5 mol%) and L10 (6 mol%) were used.
Substrate scope
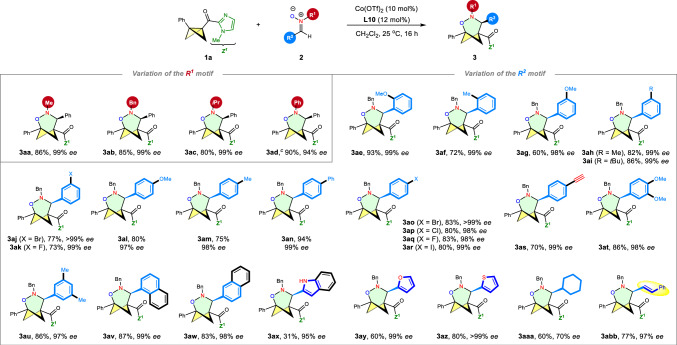
With the optimal conditions in hand, we next investigated the reactions of nitrones 2 bearing various R1 and R2 motifs with 1a in the presence of Co(OTf)2/L10 (Fig. 2). Nitrones with aliphatic R1 groups, which exhibited low reactivity with Deng’s racemic Eu(OTf)3 catalytic system29, yielded the corresponding enantiomerically pure cycloadducts with excellent yield (80–86%) and enantioselectivity (3aa-ac). The more reactive N-phenyl nitrone 2d resulted in a lower ee value (88% ee) compared to N-alkyl nitrones, possibly due to its stronger background reactions. Gratifyingly, the excellent ee of 3ad was reinstated by employing Co(OTf)2/L12 as the catalyst. The electronic properties of the α-aryl group on the nitrone had a slight impact on the enantioselectivity of the reaction (3ae-au). Asymmetric (3 + 3) cycloaddition with both electron-rich (methoxy group as in 3ae, 3ag and 3al) and electron-deficient (halides as in 3aj-ak and 3ao-ar) α-aryl nitrones showed outstanding enantioselectivity (97 ~ > 99% ee). In addition to α-aryl nitrones bearing functional groups in the para- and meta-positions, nitrones 2e-f with ortho-substituted phenyl groups also smoothly produced the desired cycloadducts (3ae-af) with 99% ee. Notably, an alkynyl group was found to be compatible (3as), further highlighting the functional-group tolerance of this reaction. Nitrones containing disubstituted aryl groups, such as 3at (3,4-diOMe) and 3au (3,5-diMe), or (α- or β-) naphthyl groups (3av-aw), at the R2 position, also yielded desired products in the range of 83–87% with 97–99% ee. Furthermore, heteroaryl groups such as 2-indolyl, 2-furyl, and 2-thienyl were well tolerated, resulting in the formation of 3ax, 3ay, and 3az with up to >99% ee. α-Alkyl nitrone 2aa which had not been compatible with Deng’s racemic catalytic system29, provided 3aaa in acceptable yield with 70% ee. Significantly, the asymmetric reaction between 1a and α-alkenyl nitrone 2bb exhibited remarkable enantioselectivity (97% ee).
Fig. 2. Scope I: variation of the nitrones.
a,b aReaction conditions: 0.2 mmol 1a, 1.2 equiv. 2, and 10 mol% Co(OTf)2/12 mol% L10 in 2.0 mL CH2Cl2 at 25 °C for 16 h. bIsolated yield. cL12 was utilized in place of L10.
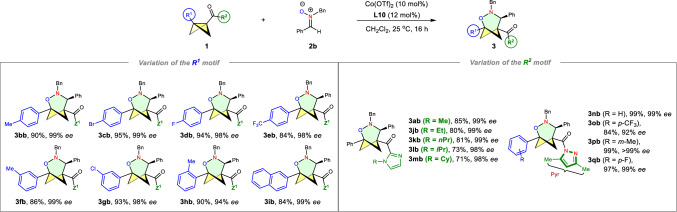
After the investigation of the nitrone scope, we studied the scope with respect to the BCBs (Fig. 3). The reaction tolerated alkyl and phenyl substituents at the phenyl moiety well (3bb-3hb). An array of BCBs bearing either electron-donating or electron-withdrawing groups on the para- (p-Me, p-Br, p-F, p-CF3, 3bb-eb), and meta-positions (m-Me, m-Cl, 3fb-gb) of phenyl rings (R1 group in BCB), participated smoothly in the reaction (84–95% yields, 98–99% ee). Additionally, BCB with o-tolyl group (3hb) and naphthyl-substituted BCB (3ib) also efficiently produced the desired cycloadducts with excellent enantioselectivity. Notably, the BCB 1e, containing a highly electron-withdrawing trifluoromethyl group, was previously considered incompatible with Glorius’s Lewis acid catalytic system46. However, it still yielded the corresponding cycloadducts (3eb versus 3bb) in good yield and selectivity. These results suggest that it follows the same pathway as Deng’s work, involving a concerted nucleophilic ring-opening mechanism of BCBs with nitrones29. Subsequently, we investigated the impact of N-substitution of 2-acyl imidazole in the current cycloadditions. The identity of the N-substituent (3jb-mb) had a minor effect on enantioselectivity (98–99% ee), but did influence the yield (71–85% yield). Moreover, we found that the Glorius’s BCB substrates with acyl pyrazole substituent instead of acyl imidazole could also be well accepted in the enantioselective cycloaddition with 2b, and the corresponding (3 + 3) cycloadducts were produced with up to 99% yield and >99% ee. However, in some cases, lower ee values were observed when using Glorius’s BCB compared to BCBs featuring a 2-acyl imidazole moiety (3ob versus 3eb). For instance, the (3 + 3) reaction of 2n with N-phenyl nitrone 2d yields the desired 3nd with 28% ee (Supplementary Fig. 5). In contrast, the cycloaddition of 1a and 2d produces 3ad with 94% ee under identical reaction conditions. Unfortunately, the employment of the methyl-substituted BCB delivered the corresponding product with low ee (52% ee, Supplementary Fig. 5).
Fig. 3. Scope II: variation of the BCBs.
a,b aReaction conditions: 0.2 mmol 1, 1.2 equiv. 2b, and 10 mol% Co(OTf)2/12 mol% L10 in 2.0 mL CH2Cl2 at 25 oC for 16 h. bIsolated yield.
Synthetic applications
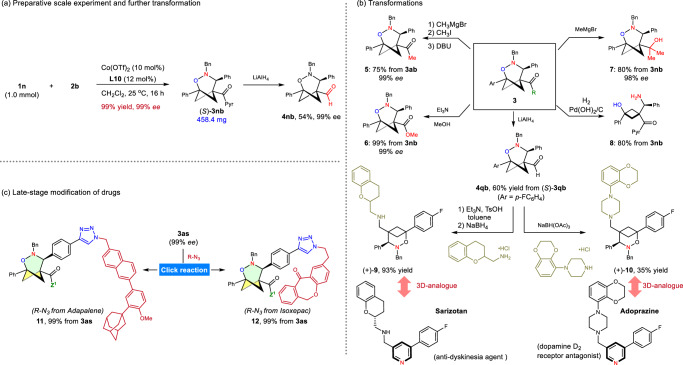
To demonstrate the synthetic utility of these chiral cycloadducts, several transformations of the cycloadducts were investigated (Fig. 4). The cycloaddition of 1n and 2b is scalable to 1.0 mmol under standard reaction conditions, yielding 3nb with 99% yield and 99% ee. The acyl pyrazole moiety of 3nb and 3qb was converted to an aldehyde group using LiAlH4, preserving its chirality (Fig. 4a). Moreover, in a two-step process, we integrated the chiral 2-oxa-3-azaBCHep 3qb unit into the structures of the anti-dyskinesia agent Sarizotan68 and dopamine D2 receptor antagonist Adoprazine69 by substituting the pyridine ring. These instances showcased the practical synthetic value of the chiral (3 + 3) cycloadducts. Cleavage of the imidazole moiety gave rise to the desired ketone 5 in 75% yield. The nucleophilic substitution or addition of 3nb smoothly produce enantiomerically enriched compounds 6 and 7, respectively. Cleaving the N-O bond in 3nb and deprotecting the benzyl group in the presence of H2 and Pd(OH)2/C produces functionalized cyclobutane 8 (Fig. 4b). Notably, including a terminal-alkyne group in cycloadduct 3as facilitates the incorporation of the chiral hetero-BCHep scaffold into bioactive compounds like Adapalene and Isoxepac through click reaction (Fig. 4c).
Fig. 4. Scale-up and derivatizations.
a Scale-up synthesis of 3nb. b Derivatization of the cycloadducts. c Late-stage modification of drugs.
Mechanistic studies
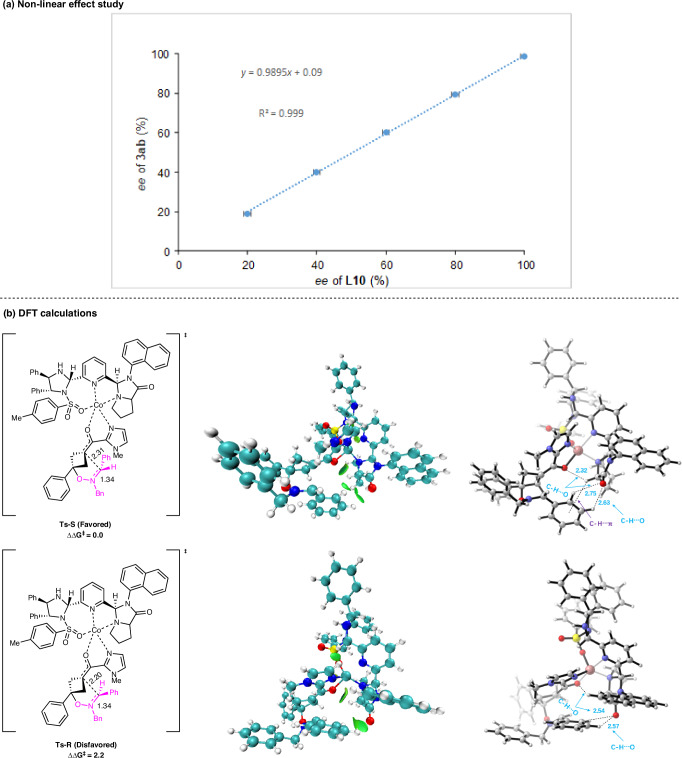
In order to gain insight into this transformation, a study was conducted to examine the correlation between the ee value of PyIPI-L10 and that of (S)-3ab (Fig. 5a). The study revealed a linear relationship, suggesting that an active catalyst/ligand being of a monomeric nature. Based on Deng29 and Xie&Guo’s work66,67, to unravel the origin of enantiocontrol, density functional theory (DFT) calculations were performed at PBE0/6-31 G(d)-SDD level of theory, using 1a and 2b as the model substrates along with the Co(II) − L12 chiral system. As shown in Fig. 5b, transition states Ts-S and Ts-R leading to both products were located, in which divalent Co coordinated with two nitrogen and one oxygen atoms of L12 as well as one oxygen and nitrogen atoms of 1a, generating a square pyramidal geometry. The difference between the two activation barriers of the intramolecular nucleophilic cyclization for the two enantiomers was 2.2 kcal/mol, consistent with the excellent enantioselectivity experimentally observed. Moreover, noncovalent interactions between reactant fragment and L12 in Ts-S and Ts-R were explored using independent gradient model based on Hirshfeld partition (IGMH) analysis70. Three pairs of C–H•••O interaction existed in both Ts-S and Ts-R. Notably, in the favored transition state Ts-S, imidazolidone of L12 engaged in C–H…π interaction with phenyl group of nitrone 2a, likely dominating the preference for the nucleophilic attack of the enolate on the Re-face of nitrone 2a to furnish the (S)-configuration of product 3ab.
Fig. 5. Non-linear effect study and DFT calculations.
a The relationship between ee values of ligand L10 and product 3ab. b Optimized structures of the enantio-determining transition state and IGMH analysis of the non-covalent interactions in Ts-S and Ts-R. The Gaussian 09 level of PBE0/6-31 G(d)-SDD was used. All distances are in angstrom. The relative free energies are in kcal mol−1.
Discussion
In conclusion, a strategy utilizing Lewis acid catalysis has been developed for the atom-economic and enantioselective synthesis of bridged bicyclic scaffolds from BCBs. By utilizing a chiral Co(II)/PyIPI catalyst and bidentate chelating BCB substrates, we can efficiently access enantioenriched pharmaceutically important hetero-bicyclo[3.1.1]heptane derivatives by varying the BCBs or nitrones used in the (3 + 3) cycloadditions. The acyl imidazole group or acyl pyrazole moiety in BCBs plays a crucial role in stereocontrol. The synthetic utility of this protocol has been further demonstrated in the concise synthesis of the analog of bioactive pyridine and the late-stage functionalization of drugs. Given the significance of chiral (hetero)bicyclo[n.1.1]alkane scaffolds as bioisosteres and the need for a new strategy for the asymmetric cycloaddition of BCBs, we anticipate that this approach will yield positive outcomes in both synthetic and medicinal chemistry.
Methods
General Procedure for the Enantioselective (3 + 3) Cycloadditions
Under an atmosphere of N2, to a 25 mL oven-dried Schlenk tube were added Co(OTf)2 (7.1 mg, 0.020 mmol) and L10 (17.1 mg, 0.024 mmol), followed by 2.0 mL of anhydrous CH2Cl2. The solution was stirred at 25 °C for 0.5 h, and then the BCBs 1 (0.20 mmol, 1.0 equiv) and nitrones 2 (0.24 mmol, 1.2 equiv) were added. Then the resulting mixture was stirred at room temperature for 16 h till full conversion of 1 by TLC analysis. After the solvent was removed under reduced pressure, the residue was directly subjected to a column chromatography purification using PE/EtOAc (4:1, v/v) as the eluent, to afford the desired product 3.
Supplementary information
Source data
Acknowledgements
We are grateful to the Fundamental Research Funds for the Central Universities, the National Natural Science Foundation of China (22471068 for J.-J. F), Natural Science Foundation of Hunan Province (2024JJ6126 for W.-B.W.) and the China Postdoctoral Science Foundation (2022M713667 for B.X., 2024M750865 for W.-B.W.) for financial support. 1H, 13C NMR spectra, single crystal X-ray diffraction and HRMS were performed at Analytical Instrumentation Center of Hunan University.
Author contributions
J.J.F. conceived the study. W.B.W. and X.C.Y. carried out the experiments and data analysis work. B.X. performed the computational studies. F.W. and H.X.H. synthesized the bicyclobutanes. X.Z. synthesized the nitrones. The paper was written by J.J.F. All authors contributed to discussions. W.B.W., B.X. and X.C.Y. contributed equally.
Peer review
Peer review information
Nature Communications thanks Shiyong Peng, Kewen Tang and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are available within this article and its Supplementary Information, which contains experimental details, characterization data, copies of NMR spectra and HPLC spectra for all new compounds, X-ray structural analysis, and DFT calculation data. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2345666 ((S)-3ao). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data of DFT calculation are also provided with this paper. All other data are available from the corresponding author upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wen-Biao Wu, Bing Xu, Xue-Chun Yang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-52419-x.
References
- 1.Ceramella, J. et al. A look at the importance of chirality in drug activity: some significative examples. Appl. Sci.12, 10909 (2022). 10.3390/app122110909 [DOI] [Google Scholar]
- 2.Mykhailiuk, P. K. Saturated bioisoteres of benzene: where to go next? Org. Biomol. Chem.17, 2839–2849 (2019). 10.1039/C8OB02812E [DOI] [PubMed] [Google Scholar]
- 3.Cuadros, S. et al. Light-driven synthesis and functionalization of bicycloalkanes, cubanes and related bioisosteres. Angew. Chem. Int. Ed.63, e202317333 (2024). 10.1002/anie.202317333 [DOI] [PubMed] [Google Scholar]
- 4.Semeno, V. V. et al. Bicyclo[m.n.k]alkane building blocks as promising benzene and cycloalkane isosteres: multigram synthesis, physicochemical and structural characterization. Chem. Eur. J.30, e202303859 (2023). 10.1002/chem.202303859 [DOI] [PubMed] [Google Scholar]
- 5.Shire, B. R. & Anderson, E. A. Conquering the synthesis and functionalization of bicy-clo[1.1.1]pentanes. JACS Au3, 1539–1553 (2023). 10.1021/jacsau.3c00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicy-clo[1.1.1]pentane. Synlett30, 1–11 (2019). 10.1055/s-0037-1610314 [DOI] [Google Scholar]
- 7.Cairncross, A. Blanchard Jr, E. P. Bicyclo[1.1.0]butane chemistry. II. cycloaddition reactions of 3-methylbicyclo[1.1.0]butanecarbonitriles. The formation of bicyclo[2.1.1]hexanes. J. Am. Chem. Soc.88, 496–504 (1966). 10.1021/ja00955a021 [DOI] [Google Scholar]
- 8.Wipf, P. & Walczak, M. A. A. Pericyclic cascade reactions of (bicy-clo[1.1.0]butylmethyl)amines. Angew. Chem. Int. Ed.45, 4172–4175 (2006). 10.1002/anie.200600723 [DOI] [PubMed] [Google Scholar]
- 9.Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature605, 477–482 (2022). 10.1038/s41586-022-04636-x [DOI] [PubMed] [Google Scholar]
- 10.Guo, R. et al. Strain-release [2π+2σ] cycloadditions for the synthesis of bicy-clo[2.1.1]hexanes initiated by energy transfer. J. Am. Chem. Soc.144, 7988–7994 (2022). 10.1021/jacs.2c02976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem.15, 535–541 (2023). 10.1038/s41557-023-01135-y [DOI] [PubMed] [Google Scholar]
- 12.Ren, H. et al. Ti-catalyzed formal [2π+2σ] cycloadditions of bicy-clo[1.1.0]butanes with 2-azadienes to access aminobicy-clo[2.1.1]hexanes. Org. Lett.26, 1745–1750 (2024). 10.1021/acs.orglett.4c00421 [DOI] [PubMed] [Google Scholar]
- 13.Xu, M. et al. Diboron(4)‐catalyzed remote [3+2] cycloaddition of cyclopropanes via dearomative/rearomative radical transmission through pyridine. Angew. Chem. Int. Ed.61, e202214507 (2022). 10.1002/anie.202214507 [DOI] [PubMed] [Google Scholar]
- 14.Liu, Y. et al. Pyridine-boryl radical-catalyzed [2π+2σ] cycloaddition of bicy-clo[1.1.0]butanes with alkenes. ACS Catal.13, 5096–5103 (2023). 10.1021/acscatal.3c00305 [DOI] [Google Scholar]
- 15.Kleinmans, R. et al. ortho-Selective dearomative [2π+2σ] photocycloadditions of bicyclic aza-arenes. J. Am. Chem. Soc.145, 12324–12332 (2023). 10.1021/jacs.3c02961 [DOI] [PubMed] [Google Scholar]
- 16.Reinhold, M., Steinebach, J., Golz, C. & Walker, J. C. L. Synthesis of polysubstituted bicyclo[2.1.1]hexanes enabling access to new chemical space. Chem. Sci.14, 9885–9891 (2023). 10.1039/D3SC03083K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho‐substituted benzenes. Angew. Chem. Int. Ed.59, 20515–20521 (2020). 10.1002/anie.202004183 [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y. et al. Titanium catalyzed [2σ+2π] cycloaddition of bicyclo[1.1.0]-butanes with 1,3-dienes for efficient synthesis of stilbene bioisosteres. Nat. Commun.15, 4374 (2024). 10.1038/s41467-024-48494-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woelk, K. J., Dhake, K., Schley, N. D. & Leitch, D. C. Enolate addition to bicyclobutanes enables expedient access to 2-oxo-bicyclohexane scaffolds. Chem. Commun.59, 13847–13850 (2023). 10.1039/D3CC04234K [DOI] [PubMed] [Google Scholar]
- 20.Shearer, J., Castro, J. L., Lawson, A. D. G., MacCoss, M. & Taylor, R. D. Rings in clinical trials and drugs: present and future. J. Med. Chem.65, 8699–8712 (2022). 10.1021/acs.jmedchem.2c00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature611, 721–726 (2022). 10.1038/s41586-022-05290-z [DOI] [PubMed] [Google Scholar]
- 22.Iida, T. et al. Practical and facile access to bicyclo[3.1.1]heptanes: potent bioisosteres of meta-substituted benzenes. J. Am. Chem. Soc.144, 21848–21852 (2022). 10.1021/jacs.2c09733 [DOI] [PubMed] [Google Scholar]
- 23.Dibchak, D. et al. General synthesis of 3-azabicyclo[3.1.1]heptanes and evaluation of their properties as saturated isosteres. Angew. Chem. Int. Ed.62, e202304246 (2023). 10.1002/anie.202304246 [DOI] [PubMed] [Google Scholar]
- 24.Harmata, A. S., Spiller, T. E., Sowden, M. J. & Stephenson, C. R. J. Photochemical formal (4+2)-cycloaddition of imine-substituted bicyclo[1.1.1]pentanes and alkenes. J. Am. Chem. Soc.143, 21223–21228 (2021). 10.1021/jacs.1c10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, Y. et al. Photochemical intermolecular [3σ+2σ]-cycloaddition for the construction of aminobicyclo[3.1.1]heptanes. J. Am. Chem. Soc.144, 23685–23690 (2022). 10.1021/jacs.2c11501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu, T. et al. Selective [2σ+2σ] cycloaddition enabled by boronyl radical catalysis: synthesis of highly substituted bicyclo[3.1.1]heptanes. J. Am. Chem. Soc.145, 4304–4310 (2023). 10.1021/jacs.2c13740 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen, T. V. T., Bossonnet, A., Wodrich, M. D. & Waser, J. Photocatalyzed [2σ+2σ] and [2σ+2π] cycloadditions for the synthesis of bicyclo[3.1.1]heptanes and 5- or 6-membered carbocycles. J. Am. Chem. Soc.145, 25411–25421 (2023). 10.1021/jacs.3c09789 [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y. et al. Pyridine-boryl radical-catalyzed [3π+2σ] cycloaddition for the synthesis of pyridine bioisosteres. ChemRxiv, 10.26434/chemrxiv-2024-n3wlw (2024).
- 29.Zhang, J., Su, J.-Y., Zheng, H., Li, H. & Deng, W.-P. Eu(OTf)3-catalyzed formal dipolar [4π+2σ] cycloaddition of bicyclo-[1.1.0]butanes with nitrones: access to polysubstituted 2-oxa-3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed.63, e202318476 (2024). 10.1002/anie.202318476 [DOI] [PubMed] [Google Scholar]
- 30.Liang, Y., Nematswerani, R., Daniliuc, C. G. & Glorius, F. Silver-enabled cycloaddition of bicyclobutanes with isocyanides for the synthesis of polysubstituted 3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed.63, e202402730 (2024). 10.1002/anie.202402730 [DOI] [PubMed] [Google Scholar]
- 31.Golfmann, M. & Walker, J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem.6, 9 (2023). 10.1038/s42004-022-00811-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly, C. B., Milligan, J. A., Tilley, L. J. & Sodano, T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci.13, 11721–11737 (2022). 10.1039/D2SC03948F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turkowska, J., Durka, J. & Gryko, D. Strain release-an old tool for new transformations. Chem. Commun.56, 5718–5734 (2020). 10.1039/D0CC01771J [DOI] [PubMed] [Google Scholar]
- 34.Walczak, M. A. A., Krainz, T. & Wipf, P. Ring-strain-enabled reaction discovery: new heterocycles from bicyclo[1.1.0]butanes. Acc. Chem. Res.48, 1149–1158 (2015). 10.1021/ar500437h [DOI] [PubMed] [Google Scholar]
- 35.Sujansky, S. J., Ma, X. Reaction paradigms that leverage cycloaddition and ring strain to construction bicyclic aryl bioisosteres from bicyclo[1.1.0]butanes. Asian J. Org. Chem. e202400045 (2024).
- 36.Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: Long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl Acad. Sci. USA118, e2108881118 (2021). 10.1073/pnas.2108881118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem.13, 950–955 (2021). 10.1038/s41557-021-00786-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harwood, L. A. et al. Selective P450BM3 hydroxylation of cyclobutylamine and bicyclo[1.1.1]pentylamine derivatives: underpinning synthetic chemistry for drug discovery. J. Am. Chem. Soc.145, 27767–27773 (2023). 10.1021/jacs.3c10542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Robichon, M. et al. Enantioselective, intermolecular [π2+σ2] photocycloaddition reactions of 2(1H)-quinolones and bicyclo[1.1.0]butanes. J. Am. Chem. Soc.145, 24466–24470 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Fu, Q. et al. Enantioselective [2π+2σ] cycloadditions of bicyclo[1.1.0]butanes with vinylazaarenes through asymmetric photoredox catalysis. J. Am. Chem. Soc.146, 8372–8380 (2024). 10.1021/jacs.3c14077 [DOI] [PubMed] [Google Scholar]
- 41.Shen, H.-C. et al. Iridium-catalyzed asymmetric difunctionalization of C–C σ-bonds enabled by ring-strained boronate complexes. J. Am. Chem. Soc.145, 16508–16516 (2023). 10.1021/jacs.3c03248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin, S.-L., Chen, Y.-H., Liu, H.-H., Xiang, S.-H. & Tan, B. Enantioselective synthesis of chiral cyclobutenes enabled by Brønsted acid-catalyzed isomerization of BCBs. J. Am. Chem. Soc.145, 21152–21158 (2023). 10.1021/jacs.3c06525 [DOI] [PubMed] [Google Scholar]
- 43.Zhou, J.-L. et al. Palladium-catalyzed ligand-controlled switchable hetero-(5+3)/enantioselective [2σ+2σ] cycloadditions of bicyclobutanes with vinyl oxiranes. J. Am. Chem. Soc.146, 19621–19628 (2024). 10.1021/jacs.4c01851 [DOI] [PubMed] [Google Scholar]
- 44.Dhake, K. et al. Beyond bioisosteres: divergent synthesis of azabicyclohexanes and cyclobutenyl amines from bicyclobutanes. Angew. Chem. Int. Ed.61, e202204719 (2022). 10.1002/anie.202204719 [DOI] [PubMed] [Google Scholar]
- 45.Radhoff, N., Daniliuc, C. G. & Studer, A. Lewis acid catalyzed formal (3+2)‐cycloaddition of bicyclo[1.1.0]butanes with ketenes. Angew. Chem. Int. Ed.62, e202304771 (2023). 10.1002/anie.202304771 [DOI] [PubMed] [Google Scholar]
- 46.Liang, Y., Paulus, F., Daniliuc, C. G. & Glorius, F. Catalytic formal [2π+2σ] cycloaddition of aldehydes with bicyclobutanes: expedient access to polysubstituted 2‐oxabicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed.62, e202305043 (2023). 10.1002/anie.202305043 [DOI] [PubMed] [Google Scholar]
- 47.Ni, D. et al. Intermolecular formal cycloaddition of indoles with bicy-clo[1.1.0]butanes by Lewis acid catalysis. Angew. Chem. Int. Ed.62, e202308606 (2023). 10.1002/anie.202308606 [DOI] [PubMed] [Google Scholar]
- 48.Tang, L. et al. Silver-catalyzed dearomative [2π+2σ] cycloadditions of indoles with bicyclobutanes: access to indoline fused bicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed.62, e202310066 (2023). 10.1002/anie.202310066 [DOI] [PubMed] [Google Scholar]
- 49.Nicolai, S., Waser, J. Lewis acid catalyzed [4+2] annulation of bicyclobutanes with dienol ethers for the synthesis of bicyclo[4.1.1]octanes. Chem. Sci. 15, 10.1039/D4SC02767A (2024). [DOI] [PMC free article] [PubMed]
- 50.Hu, Q.-Q. et al. Lewis acid catalyzed cycloaddition of bicyclobutanes with ynamides for the synthesis of polysubstituted 2-amino-bicyclo[2.1.1]hexenes. Angew. Chem. Int. Ed.63, e202405781 (2024). 10.1002/anie.202405781 [DOI] [PubMed] [Google Scholar]
- 51.Yang, L. et al. B(C6F5)3‑catalyzed formal (n + 3) (n = 5 and 6) cycloaddition of bicyclo[1.1.0]butanes to medium bicyclo[n.1.1]alkanes. Org. Lett.26, 4104–4110 (2024). 10.1021/acs.orglett.4c01219 [DOI] [PubMed] [Google Scholar]
- 52.Clementson, S., Radaelli, A., Fjelbye, K., Tanner, D. & Jessing, M. Strain-release driven cycloadditions for rapid construction of functionalized pyridines and amino alcohols. Org. Lett.21, 4763–4766 (2019). 10.1021/acs.orglett.9b01652 [DOI] [PubMed] [Google Scholar]
- 53.Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereo-centres. Nature516, 181–191 (2014). 10.1038/nature14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou, F. et al. Catalytic enantioselective construction of vicinal quaternary carbon stereocenters. Chem. Sci.11, 9341–9365 (2020). 10.1039/D0SC03249B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sibi, M. P., Ma, Z. & Jaspers, C. P. Enantioselective addition of nitrones to activated cyclopropanes. J. Am. Chem. Soc.127, 5764–5765 (2005). 10.1021/ja0421497 [DOI] [PubMed] [Google Scholar]
- 56.Kang, Y.-B., Sun, X.-L. & Tang, Y. Highly enantioselective and diastereoselective cycloaddition of cyclopropanes with nitrones and its application in the kinetic resolution of 2-substituted cyclopropane-1,1-dicarboxylates. Angew. Chem. Int. Ed.46, 3918–3921 (2007). 10.1002/anie.200604645 [DOI] [PubMed] [Google Scholar]
- 57.Young, I. S. & Kerr, M. A. A homo [3+2] dipolar cycloaddition: the reaction of nitrones with cyclopropanes. Angew. Chem. Int. Ed.42, 3023–3026 (2003). 10.1002/anie.200351573 [DOI] [PubMed] [Google Scholar]
- 58.Ahlburg, N. L., Hergert, O., Jones, P. G. & Werz, D. B. Donor-acceptor cyclopropanes: activation enabled by a single, vinylogous acceptor. Angew. Chem. Int. Ed.62, e202214390 (2023). 10.1002/anie.202214390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parsons, A. T., Smith, A. G., Neel, A. J. & Johnson, J. S. Dynamic kinetic asymmetric synthesis of substituted pyrrolidines from racemic cyclopropanes and aldimines: reaction development and mechanistic insights. J. Am. Chem. Soc.132, 9688–9692 (2010). 10.1021/ja1032277 [DOI] [PubMed] [Google Scholar]
- 60.Pirenne, V., Muriel, B. & Waser, J. Catalytic enantioselective ring-opening reactions of cyclopropanes. Chem. Rev.121, 227–263 (2021). 10.1021/acs.chemrev.0c00109 [DOI] [PubMed] [Google Scholar]
- 61.Wang, L. & Tang, Y. Asymmetric ring-opening reactions of donor-acceptor cyclopropanes and cyclobutanes. Isr. J. Chem.56, 463–475 (2016). 10.1002/ijch.201500094 [DOI] [Google Scholar]
- 62.Schneider, T. F., Kaschel, J. & Werz, D. B. A new golden age for donor–acceptor cyclopropanes. Angew. Chem. Int. Ed.53, 5504–5523 (2014). 10.1002/anie.201309886 [DOI] [PubMed] [Google Scholar]
- 63.Xia, Y., Liu, X. & Feng, X. Asymmetric catalytic reactions of donor–acceptor cyclopropanes. Angew. Chem. Int. Ed.60, 9192–9204 (2021). 10.1002/anie.202006736 [DOI] [PubMed] [Google Scholar]
- 64.Wang, X.-B. et al. Rational design of chiral tridentate ligands: bifunctional cobalt(II) complex/hydrogen bond for enantioselective Michael reactions. Org. Lett.24, 3861–3866 (2022). 10.1021/acs.orglett.2c01435 [DOI] [PubMed] [Google Scholar]
- 65.Xue, B.-Y., Hou, C.-Y., Wang, X.-B., Xie, M.-S. & Guo, H.-M. Asymmetric synthesis of 7-membered-ringbridged 3,4-fused tri-cyclic indoles via Friedel–Crafts alkylation/annulation. Org. Chem. Front.10, 1910–1914 (2023). 10.1039/D2QO01982E [DOI] [Google Scholar]
- 66.Wang, H.-X. et al. Design of C1-symmetric tridentate ligands for enantioselective dearomative [3+2] annulation of indoles with aminocyclopropanes. Nat. Commun.14, 2270 (2023). 10.1038/s41467-023-38059-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, X.-Y. et al. Cobalt-catalyzed asymmetric dearomative [3+2] annulation of quinolines, isoquinolines, and pyridines. ACS Catal.13, 11528–11540 (2023). 10.1021/acscatal.3c02546 [DOI] [Google Scholar]
- 68.Bottcher, H. et al. U.S. Patent US5767132, (1998).
- 69.McCreary, A. C. et al. SLV313 (1-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-4- [5-(4-fluoro-phenyl)-pyridin-3-ylmethyl]-piperazine monohydrochloride): a novel dopamine D2 receptor antagonist and 5-HT1A receptor agonist potential antipsychotic drug. Neuropsychopharmacology32, 78–94 (2007). 10.1038/sj.npp.1301098 [DOI] [PubMed] [Google Scholar]
- 70.Lu, T. & Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem.43, 539–555 (2022). 10.1002/jcc.26812 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within this article and its Supplementary Information, which contains experimental details, characterization data, copies of NMR spectra and HPLC spectra for all new compounds, X-ray structural analysis, and DFT calculation data. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2345666 ((S)-3ao). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data of DFT calculation are also provided with this paper. All other data are available from the corresponding author upon request. Source data are provided with this paper.