Abstract
Recent evidence from several investigators suggest that the human T-cell leukemia virus type 1 Tax oncoprotein represses the transcriptional activity of the tumor suppressor protein, p53. An examination of published findings reveals serious controversy as to the mechanism(s) utilized by Tax to inhibit p53 activity and whether the same mechanism is used by Tax in adherent and suspension cells. Here, we have investigated Tax-p53 interaction simultaneously in adherent epithelial (HeLa and Saos) and suspension T-lymphocyte (Jurkat) cells. Our results indicate that Tax activity through the CREB/CREB-binding protein (CBP), but not NF-κB, pathway is needed to repress the transcriptional activity of p53 in all tested cell lines. However, we did find that while CBP binding by Tax is necessary, it is not sufficient for inhibiting p53 function. Based on knockout cell studies, we correlated a strong genetic requirement for the ATM, but not protein kinase-dependent DNA, protein in conferring a Tax-p53-repressive phenotype.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent for a highly aggressive and fatal hematological T-cell malignancy known as adult T-cell leukemia (ATL) (17, 73, 100) and virus-associated neurodegenerative diseases (HTLV-1-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) 21, 68). Onset of ATL usually occurs after a prolonged asymptomatic latent period (20). HTLV-1-associated oncogenesis has been strongly linked to the expression of its regulatory protein Tax (16, 25, 26, 64, 74, 92). On the other hand, a direct correlation of HAM/TSP with HTLV-1 protein(s) has remained less clear.
Tax is a potent regulator of both viral and cellular gene expression (7, 11, 17, 19, 34, 35, 53, 84). It strongly activates transcription from the HTLV-1 long terminal repeat (LTR) as well as sequence-unrelated viral promoters such as the human immunodeficiency virus type 1 (HIV-1) LTR (85, 87). There is evidence that Tax can contact DNA indirectly (3, 22, 44, 50, 60, 67); however, it is well accepted that Tax regulates transcription primarily through protein-protein interactions with cellular transcription factors which include the activating transcription factor/cyclic AMP-responsive element-binding protein (ATF/CREB) (96), serum response factor (18), AP-1 (19, 35), and the NF-κB proteins (8, 14, 32, 61). Mechanistically, Tax facilitates the formation of ternary nucleoprotein complexes which include CREB and the pleiotropic cellular coactivator CREB-binding protein (CBP) (9, 23, 28, 29, 47, 66). It has been proposed that the formation of a Tax-CREB-CBP complex at the HTLV-1 LTR is essential for viral gene expression and viability (89, 98, 99, 102, 103).
Tax contributes pathologically to transformation and immortalization of T cells through dysregulation of cellular genes important for cell growth and cell cycle progression (31, 65). In this respect, several studies have proposed the ubiquitous cellular sentinel p53 as a significant target for the Tax oncoprotein (56). p53, a DNA-binding transcription factor, is a highly conserved tumor suppressor which induces cell cycle arrest at the G1-S junction and/or apoptosis in response to DNA damage from UV and γ radiation (1, 24, 45, 51, 88). Activation and inactivation of p53 have been proposed to be regulated through phosphorylation and dephosphorylation by various protein kinases, including ataxia telangectasia mutated (ATM) and DNA protein kinase (DNA-PK) (4, 12, 13, 39, 42, 63). In over half of human malignancies, genetic mutation in p53 leading to a loss of function has been identified (27, 52), suggesting that an in vivo p53 function is to suppress oncogenic transformation. Interestingly, HTLV-1-transformed cells generally contain unmutated wild-type p53 genes (91). This suggests that loss of p53 activity in ATL largely occurs through a mechanism other than genetic mutation.
Several recent studies have presented seemingly divergent thoughts as to how HTLV-1 Tax affects p53 function (60). Originally, it was demonstrated that Tax repressed transcription of the p53 gene (93). Subsequently, based on the finding that p53 binds CBP/p300 for function (80, 83), several investigators showed that Tax inhibited p53 activity through sequestration of the CBP coactivator (5, 90, 94). More recently, a novel DNA-PK-dependent phosphorylation mechanism (71) which was correlated with NF-κB activation (72) was proposed as one route through which Tax inactivates p53. These various observations are difficult to reconcile and suggest that additional details are needed to fully understand the interaction(s) between Tax oncoprotein and p53 tumor suppressor.
In examining Tax-p53 interaction, we have compared here the contributions from two signaling pathways, CREB/CBP and NF-κB, to the inhibition of p53 activity by Tax. We tested mutant Tax proteins which are either active or inactive in one or both of these pathways. Furthermore, using HTLV-2 Tax protein and Tax1 mutants which fail to bind CBP, we also investigated the hypothesis of direct coactivator competition for CBP between Tax and p53 as an explanation for functional suppression of the latter by the former. Last, we assayed DNA-PK and ATM knockout cell lines to examine the role of phosphorylation and the identity of the protein kinase(s) involved in Tax-mediated inactivation of p53. Our results indicate that Tax represses p53 function in a dose-dependent manner and that the ability of Tax to activate CREB and bind CBP is necessary, but not sufficient, for this repression. We found Tax inhibition of p53 activity to be independent of DNA-PK and to correlate with the intact presence of ATM. These findings contribute incrementally to further our understanding of how a viral oncoprotein, Tax, abrogates the activity of an important cellular tumor suppressor, p53.
MATERIALS AND METHODS
Cell lines.
Human cervical carcinoma (HeLa) and p53-negative human osteosarcoma (Saos-2) cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with l-glutamine, penicillin-streptomycin, and 10% fetal bovine serum. Ataxia telangectasia cells lines CRL 7201 and GM02052C were obtained from the American Type Culture Collection and the NIGM Human Genetic Mutant Cell Repository (Coriell Institute for Medical Research, Camden, N.J.), respectively, and were maintained in DMEM supplemented with l-glutamine, penicillin-streptomycin, and 20% fetal bovine serum. DNA-PKcs+/+ and DNA-PKcs−/− cells (46) were cultured in DMEM supplemented with l-glutamine, penicillin-streptomycin, and 20% fetal bovine serum. Jurkat cells were maintained in RMPI 1640 supplemented with l-glutamine, penicillin-streptomycin, and 10% fetal bovine serum.
Plasmids and transfections.
pU3RCAT (11), pG13CAT, pG13Luc, and p53 (human wild type) (gifts from B. Vogelstein) expression plasmids were previously described (41). Tax single amino acid mutants—S113A, S258A, S274A, and L320G—are indicated by the single-letter code for the amino acid to be changed, the position of the change, and the single-letter representation of the replacement amino acid. Tax mutants Δ2-58 and ΔStu have amino acids 2 through 58 deleted and deletion of all C-terminal amino acids downstream of residue 245 at the StuI site, respectively. Tax M1-45 contains the first 45 amino acids of the Tax open reading frame. Unless otherwise indicated, expression of all Tax mutants was driven by a cytomegalovirus (CMV) immediate-early promoter (85). TaxK88A and TaxL90A were gifts from C. Z. Giam. TaxM22 and TaxM47 were from Warner Greene (87). ATM expression plasmid pMAT1 was from Martin F. Lavin and was used in transfections as previously described (101).
HeLa or Saos-2 cells were seeded into six-well tissue culture dishes. Transfections were performed 24 h later, using Lipofectamine and Plus reagent (GIBCO-BRL) as described by the manufacturer. Jurkat cells were washed and resuspended in serum-free RMPI 1640 Resuspended cells, (0.5 ml; 107 cells per sample) were transfected by electroporation in Gene Pulsar cuvettes (0.4-cm electrode gap) manufactured by Bio-Rad. Electroporation was carried out in a Bio-Rad Gene Pulsar at 200 Ω, “EXT” capacitance, and 1.60 kV. Following electroporation, Jurkat cells were transferred to six-well tissue culture dishes, and complete RMPI 1640 was added to each well for a total volume of 5 ml. Typically, 5 × 106 HeLa and Saos-2 cells or 107 Jurkat cells were transfected with 1.0 μg of a reporter plasmid, 0.5 μg of p53 plasmid, 1 to 2 μg of Tax expression plasmid, and 0.2 μg of pCMV-β or 1 μg of pAD-β (CMV immediate-early or adenovirus major late promoter-driven β-galactosidase expression plasmid, respectively), used as a normalization control for transfection efficiency). pUC19 was added where needed to all transfections to equalize for the total amount of transfected DNA.
Caffeine treatment.
HeLa and Saos-2 cells were cotransfected with pG13Luc, p53, and Tax. At 24 h after transfection, caffeine was added to wells to a final concentration of 0.01 to 1.0 mM. Cells were harvested 48 h after transfection.
CAT, luciferase, and β-galactosidase assays.
For chloramphenicol acetyltransferase (CAT) assays, cells were harvested 48 h after transfection and lysed by three successive cycles of freezing and thawing. CAT activity was detected by thin-layer chromatographic separation of [14C]-chloramphenicol from its acetylated derivatives and quantitated by phosphorimaging (Fuji). Luciferase activity was measured 48 h after transfection. Cells were washed twice with 1× phosphate-buffered saline and then lysed in 1× lysis buffer (Promega). Luciferase assay substrate (Promega) was used according to the manufacturer's protocol, and activity was measured in an MGM Instruments Opticom II luminometer. Cell extracts used for CAT or luciferase assays were quantitated for β-galactosidase activity using Galacto-Star (Tropix) as described by the manufacturer. CAT and luciferase activities were normalized for transfection efficiency based on β-galactosidase readings.
Immunoblotting.
MT4 cells were cultured in RPMI 1640 containing 15% fetal calf serum. Then 108 cells were lysed with 500 μl of radioimmunoprecipitation assay buffer (1% Triton X-100, 0.05% bovine serum albumin, phenylmethylsulfonyl fluoride [100 μg/ml], 1% sodium deoxycholate, and 0.05% sodium dodecyl sulfate [SDS] in 0.01 M Tris-HCl [pH 8.0]–0.14 M NaCl) in an Eppendorf tube placed in a continuously rotating device in a cold room for 1 h; DNA was sheared using a 22-gauge needle. After centrifugation at 18,000 rpm (Eppendorf centrifuge model 5415C) for 10 min in the cold room, the pellet was saved and the supernatant was precleared by incubation with a preimmune antiserum followed by protein G-agarose for 2 h and then by centrifugation at 18,000 rpm (Eppendorf centrifuge model 5415C) in the cold room for 5 min. The precleared supernatant was incubated with monoclonal mouse anti-p53 antibody conjugated to agarose beads (Oncogene Science) for an additional 2 h in the cold room and then centrifuged to pellet beads. Beads were washed with radioimmunoprecipitation buffer four times, resuspended into SDS-sample buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol), boiled for 3 min, and resolved by electrophoresis in a 10% polyacrylamide gel (PAGE). The gel was transferred to a polyvinylidene difluoride membrane (Immobilion P; Millipore), which was reacted with rabbit polyclonal anti-Tax antibody (α-Tax) followed by visualization with chemiluminescence as suggested by the manufacturer (Tropix).
GST-Tax column chromatography.
HeLa nuclear extract was chromatographed in a glutathione S-transferase (GST)–Tax column containing purified Escherichia coli expressed GST-Tax fusion protein saturated onto glutathione-Sepharose beads (Pharmacia). After stepwise elutions with different concentrations of KCl salt buffer, each eluate was resolved by SDS-PAGE followed by immunoblotting.
Confocal microscopy.
Laser scanning immunofluorescent confocal microscopy was performed as described elsewhere (36). Two-color staining was achieved by using species-specific secondary antibodies (goat anti-mouse and goat anti-rabbit) conjugated to different fluorochromes (fluorescein and Texas red), with visualization using different filters and different wavelength laser emissions.
RESULTS
Tax represses p53 activity.
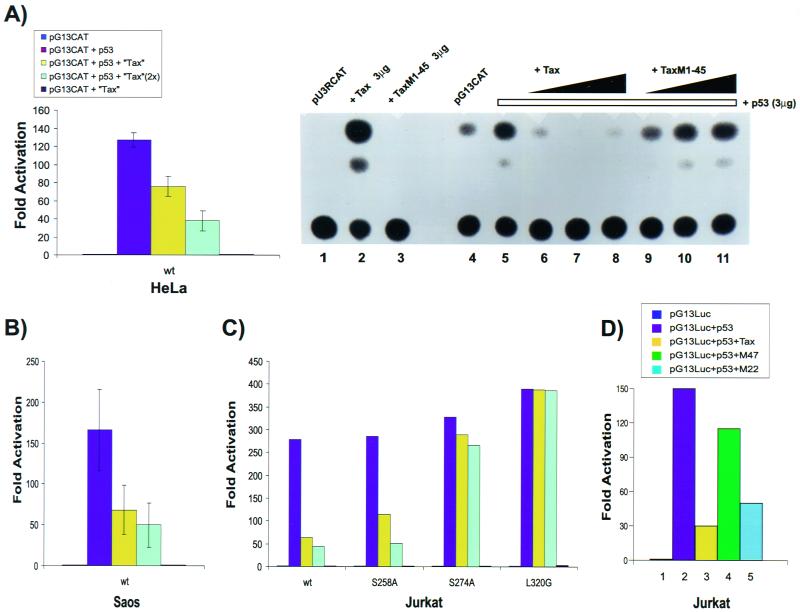
To explore the various hypotheses for Tax-p53 interaction, we first assessed the reported repressive activity of Tax on p53. We checked activities in three p53-negative cell lines, HeLa, Saos-2, and Jurkat. HeLa is phenotypically negative for p53 activity (82); in both Saos-2 and Jurkat cells, the p53 alleles are genotypically mutated. To test for expression, we introduced into cells either pG13CAT or pG13Luc reporter, containing 13 copies of a p53 consensus-binding site positioned upstream of the polyomavirus promoter attached to either a CAT or a luciferase cDNA, respectively. Cotransfection of pG13CAT or pG13Luc with wild-type p53 plasmid produced a 10- to 100-fold increase in activity over that basally expressed from pG13 reporter alone (Fig. 1). When wild-type Tax plasmid was introduced along with pG13CAT or pG13Luc and p53, Tax repressed in a dose-dependent manner p53 induction of pG13CAT or pG13Luc in HeLa (Fig. 1A), Saos-2 (Fig. 1B), and Jurkat (Fig. 1C and D) cells. This abrogation of p53 activity required functional Tax protein since a control transfection with the inactive Tax truncation mutant Tax M1-45 (Fig. 1A, lanes 9 to 11), Tax single-point mutants S274A and L320G (Fig. 1C; see also Fig. 3), and Tax double-point mutant M47 (Fig. 1D and reference 87) produced no significant inhibition.
FIG. 1.
Dose-dependent repression of p53 activity by HTLV-1 Tax. HeLa (A), Saos-2 (B), and Jurkat (C) cells were transfected with 1 μg of pG13CAT (or pG13Luc [Jurkat]), 0.5 μg of p53, and 1 or 2 (2×) μg of plasmids expressing either wild-type Tax or Tax mutants. Results are expressed as the mean fold activation with standard deviation. (A) Bar graph of Tax-p53 activity in HeLa cells (left) and representative results of CAT assays showing repression of p53 by Tax and lack of repression by a mutant of Tax that expresses amino acids 1 to 45 (Tax M1-45) (right). Lane 1, basal activity of the HTLV-1 LTR (5 μg of pU3RCAT); lane 2, activation of pU3RCAT by wild-type Tax (3 μg); lane 3, lack of activation of pU3RCAT by inactive Tax M1-45 (3 μg); lane 4, basal activity of pG13CAT (5 μg). Lanes 5 through 11 show pG13CAT (5 μg) with p53 (3 μg) and increasing amounts (1.0 to 5.0 μg) of either wild-type Tax (lanes 6 to 8) or mutant TaxM1-45 (lanes 9 to 11). (B and C) Bar graphs summarizing results for Soas and Jurkat cells. Jurkat cell were tested also using 1 or 2 (2×) μg of Tax mutants S258A, S274A, and L320G. TaxS258A is CREB+ NF-κB−; TaxL320G is CREB− NF-κB+; TaxS274A is CREB− NF-κB− (85). Dose-dependent repression of p53 activity by Tax in all transfections is based on normalization of transfection values to an internally cotransfected CMV–β-galactosidase plasmid (see Materials and Methods). (B and C) Same as for bar graph in panel A. (D) Transfections in Jurkat cells were performed as described above with 1 μg of pG13Luc, 3 μg of p53-expressing plasmid, and 1 μg of the indicated Tax or Tax mutant plasmid. Results represent average values from two independent transfections.
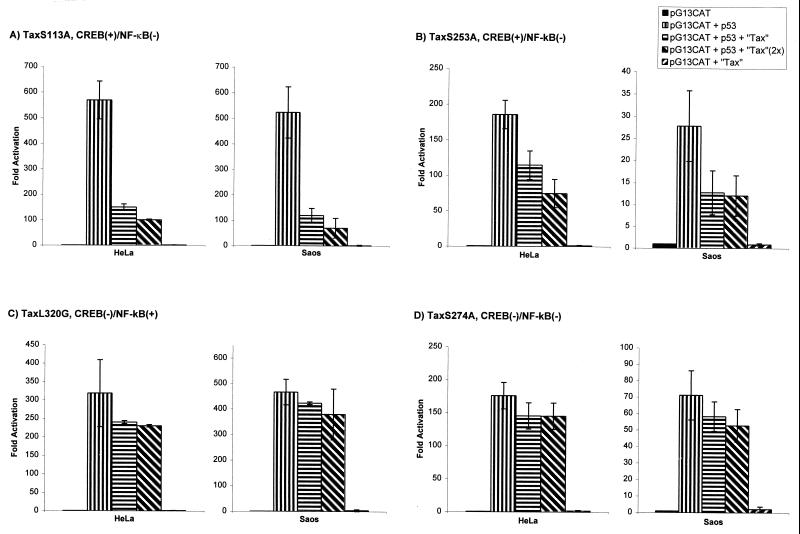
FIG. 3.
CREB+ NF-κB− but not CREB− NF-κB+ Tax mutants repress p53 transactivation. HeLa or Saos-2 cells were transfected with pG13CAT, p53, and TaxS113A (A), TaxS258A (B), TaxL320G (C), or TaxS274A (D). All results represent averages of at least three independent assays.
Interaction between Tax and p53.
In the majority of breast cancers (58) and neuroblastomas (59), wild-type p53 alleles are maintained. Interestingly, wild-type p53 proteins in these cells are found to be excluded from the nucleus. Hence, in these cancers, cytoplasmic sequestration, as opposed to gene mutation, is believed to be the p53-inactivating mechanism. Because most ATL cells also preserve wild-type alleles of p53 (91), we wondered if dislocation of wild-type p53 could explain the observed (Fig. 1) repressive activities of Tax. We next asked whether Tax might affect the normal nuclear localization of p53 in cells.
HeLa cells transfected with p53 alone, Tax alone, or both together were stained with α-p53 and α-Tax (Fig. 2A) and visualized by laser confocal microscopy. p53 and Tax were found in the nuclei of transfected cells, whether the cells were transfected with p53 and Tax plasmids individually or together. Thus, unlike findings from breast cancers and neuroblastomas, p53, rather than being sequestered in the cytoplasm, colocalized with Tax in the nucleus.
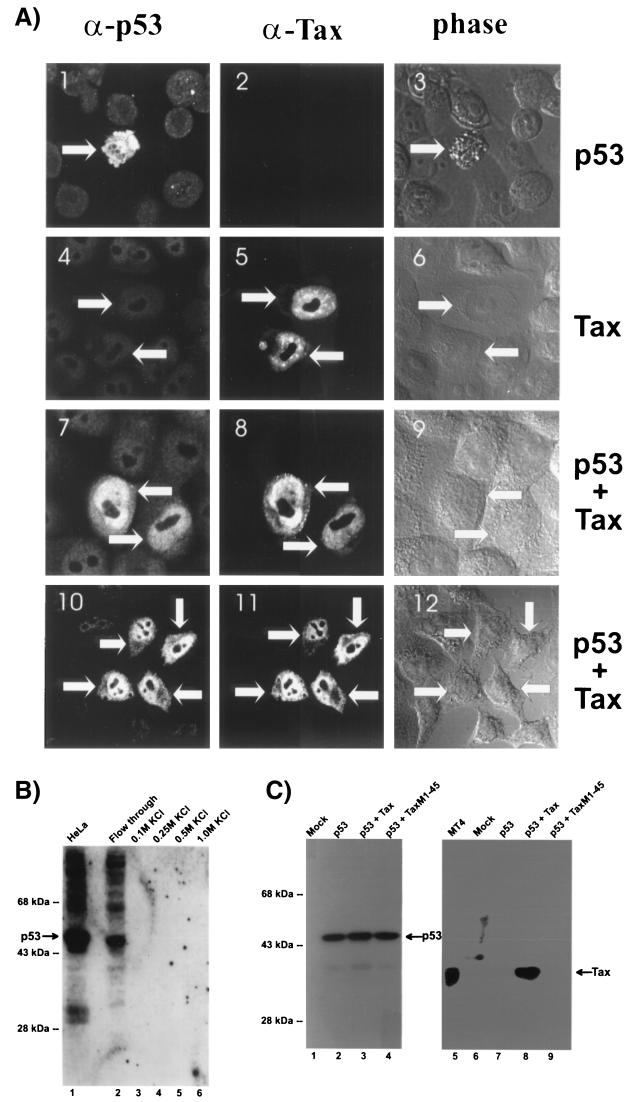
FIG. 2.
Characterization of Tax and p53 interaction. (A) Tax and p53 colocalize in the nucleus. HeLa cells were transfected individually with p53, Tax, or p53 plus Tax, as indicated, fixed with paraformaldehyde, and then stained simultaneously with mouse α-p53 (clone DO-1 from Santa Cruz Biotechnology) and rabbit α-Tax (first and second columns); morphology of cells was visualized by phase-contrast microscopy (phase). The same fields of cells viewed through different filters are shown in the four rows. Arrows point to corresponding cells within each row. Views shown are representative of duplicate preparations from three independent experiments. (B) Tax does not bind p53. HeLa cell nuclear extract was chromatographed onto a GST-Tax glutathione-Sepharose column and eluted with increasing concentrations of KCl (lanes 3 to 6). Each eluate, including the starting total HeLa extract (lane 1) and the column flowthrough (lane 2), was resolved by SDS-PAGE followed by immunoblotting with α-p53. (C) Tax does not stabilize de novo-synthesized p53. Saos-2 cells were mock transfected or transfected with p53, either alone or together with Tax or TaxM1-45, as indicated. Cells were harvested and resolved by SDS-PAGE. Lane 5 shows results with control MT4 cells. Lanes 1 through 4 were reacted with α-p53; lanes 5 to 9 were reacted with α-Tax.
Reproducibly, in multiple confocal images with different visual sectionings, we observed striking nuclear overlap of Tax and p53 (Fig. 2A, images 7 to 12). This raised the possibility that in vivo p53 and Tax might contact each other indirectly or directly. Although Tax has previously been shown to inactivate another important cellular checkpoint protein through direct binding (37), we, like others (5, 75, 90), found no in vitro evidence for physical interaction between p53 and Tax. No p53 was retained by a GST-Tax column when HeLa nuclear extract was serially chromatographed over this matrix (Fig. 2B). Similarly, Tax and p53 did not coimmunoprecipitate from cells (data not shown). Hence, we inferred that in vivo cross talk between Tax and p53 in the cell likely occurs through complex means.
Based on previous findings (75), we wondered whether in our experimental system a Tax-induced p53-stabilizing effect could be documented. We did indeed detect more p53 in HTLV-1-transformed MT2, MT4, and C816645 T cells than in nontransformed T cells (e.g., CCRF-CEM [K.-W. Yim, unpublished data). However, it was unclear whether this increased abundance was a long-term indirect consequence of HTLV-1 transformation or a direct result of Tax expression. To address this issue, we checked whether Tax affected the stability of de novo-synthesized p53 in an otherwise p53-null background. Saos-2 cells were transfected with p53 alone or together with either Tax or TaxM1-45 mutant; 48 h later, cell lysates were resolved by SDS-PAGE and immunoblotted in replicate (Fig. 2C). One set of membranes was reacted with α-p53; the second was reacted with α-Tax. Mock-transfected Saos-2 cell lysate served as a negative control for Tax, and MT4 lysate served as a positive control. In Soas-2 cells transfected with p53 plasmid, we found equivalent amounts of p53 regardless of whether Tax was (Fig. 2C, lane 3) or was not (lanes 2 and 4) expressed. Thus, under these conditions, Tax had little or no influence on the steady-state stability of de novo-synthesized p53. Our results agree with the findings of Akagi et al. (2), who found stabilizing effects of Tax on p53 on long-term selected cell lines but not on newly selected cells.
Ability of Tax mutants to repress p53 activity.
The above findings on Tax-p53 interaction suggest that complex intermediating events lead from the former to the latter. In this regard, we noted that among other activities, Tax induces transcription through two well-characterized pathways (i.e., activation of the HTLV-1 LTR through CREB and the HIV-1 LTR through NF-κB) (85, 87). Previously we described 47 Tax mutants based on relative abilities to induce expression of either the HTLV-1 LTR or the HIV-1 LTR (85). We tested six of these mutants, which segregated into three phenotypes (CREB+ NF-κB−, CREB− NF-κB+, and CREB− NF-κB−) for repression of p53 activity.
We found that expression of TaxS113A or TaxS258A, both CREB+ NF-κB− proteins, inhibited p53 transactivation (Fig. 3A and B) in a manner similar to that of wild-type CREB+ NF-κB+ Tax. On the other hand, TaxL320G, which is CREB− NF-κB+, did not repress p53 activity (Fig. 3C). These results are similar to those for Jurkat cells (Fig. 1C). In parallel assays, three CREB− NF-κB− Tax mutants, TaxS274A (Fig. 3D), TaxΔ2-58 (data not shown), and TaxΔStu (data not shown), also failed to repress p53 activity. Together with the findings for wild-type Tax (Fig. 1), these results indicate Tax's capacity to signal through CREB, but not NF-κB, as important for its repression of p53 activity.
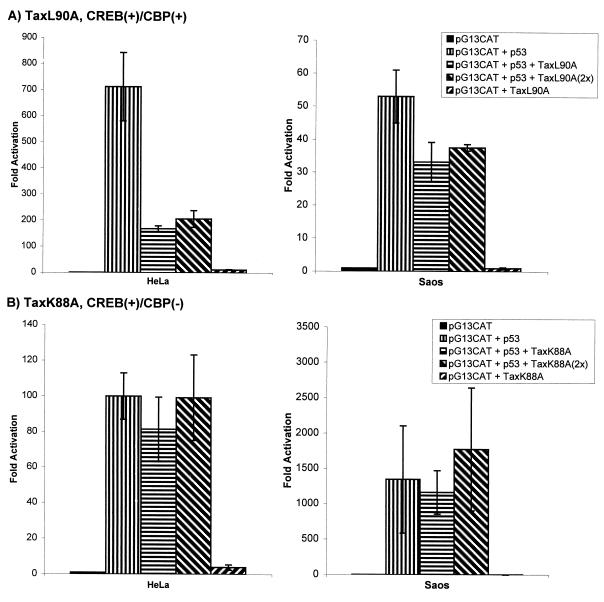
Several studies have linked Tax's binding to CBP with its function in the CREB pathway (9, 23, 28, 29, 47, 66). Tax mutants that are unable to bind CBP have recently been shown to be defective in transactivating the HTLV-1 LTR (29). To extend the finding that Tax signaling through CREB is important for its p53-repressing ability, we tested two additional Tax mutants, one (TaxL90A) competent for CBP binding and the other (TaxK88A) not. Expression of TaxK88A failed to repress p53 activity (Fig. 4B), whereas TaxL90A was repressive (Fig. 4A). These findings suggest that a CBP-binding component in Tax's activation through CREB correlates with p53 repression.
FIG. 4.
CBP-binding Tax mutant represses p53 transactivation. HeLa and Saos-2 cells were cotransfected as indicated with pG13CAT, p53, and either TaxL90A (A) or TaxK88A (B). L90A is competent for binding CBP, while K88A is not. All results represent averages of at least three independent assays.
HTLV-2 Tax does not repress p53.
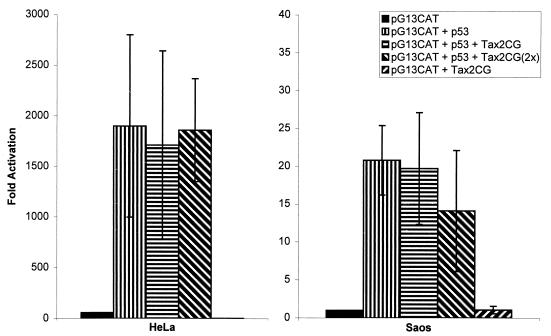
Unlike HTLV-1, the related virus HTLV-2 has not been linked to human malignancies (33, 55). HTLV-2, however, encodes a Tax protein (Tax2) which is highly homologous to HTLV-1 Tax (Tax1). Tax2 closely resembles Tax1 in its ability to activate either the HTLV-1 or the HTLV-2 LTR (86) and in signaling through the CREB and NF-κB pathways (77). Furthermore, based on the finding that the 81QRTSKTLKVLTPPIT95 stretch of amino acids in Tax1 (29) mediates binding to CBP, we noted that Tax2CG (from isolate CG 86) contains 14 of these 15 residues. Hence, it is highly probable, although not yet directly demonstrated, that the CBP-binding capacity of Tax1 would be conserved by Tax2CG. In view of in vivo differences between Tax1 and Tax2 regarding malignant transformation and their similarities regarding transcription, the activity (if any) of Tax 2CG on p53 could prove informative.
While Tax2CG and Tax1 are well conserved overall, it is notable that the former is shorter than the latter by 28 amino acids at the C terminus. As noted above, we had found that a C-terminally truncated Tax1 ΔStuI mutant failed to repress p53 activity (data not shown). We therefore wished to examine how the C-terminally truncated Tax2CG protein might behave functionally. When directly examined for effects on p53 Tax2CG was reproducibly without effect under cotransfection conditions in which Tax1 repressed p53 activity (Fig. 5).
FIG. 5.
HTLV-2 Tax does not repress p53 transactivation. HeLa and Saos-2 cells were transfected individually or in the indicated combinations with pG13CAT, p53, and Tax2CG. Bar graphs showing average fold activation from at least three independent assays with standard deviations are presented.
Tax repression of p53 correlates genetically with ATM.
We observed above that the ability of Tax1 to signal through CREB, ability to bind CBP, and its intact C terminus all contribute to its repression of p53 activity. How these discrete findings converge mechanistically remained unclear. One might surmise the involvement of multiple events through which a signal emanating from Tax ultimately impinges on p53. Indeed, a previous study had suggested that Tax influences DNA-PK phosphorylation of p53, implicating this event as one step in a repressive regulatory cascade (71).
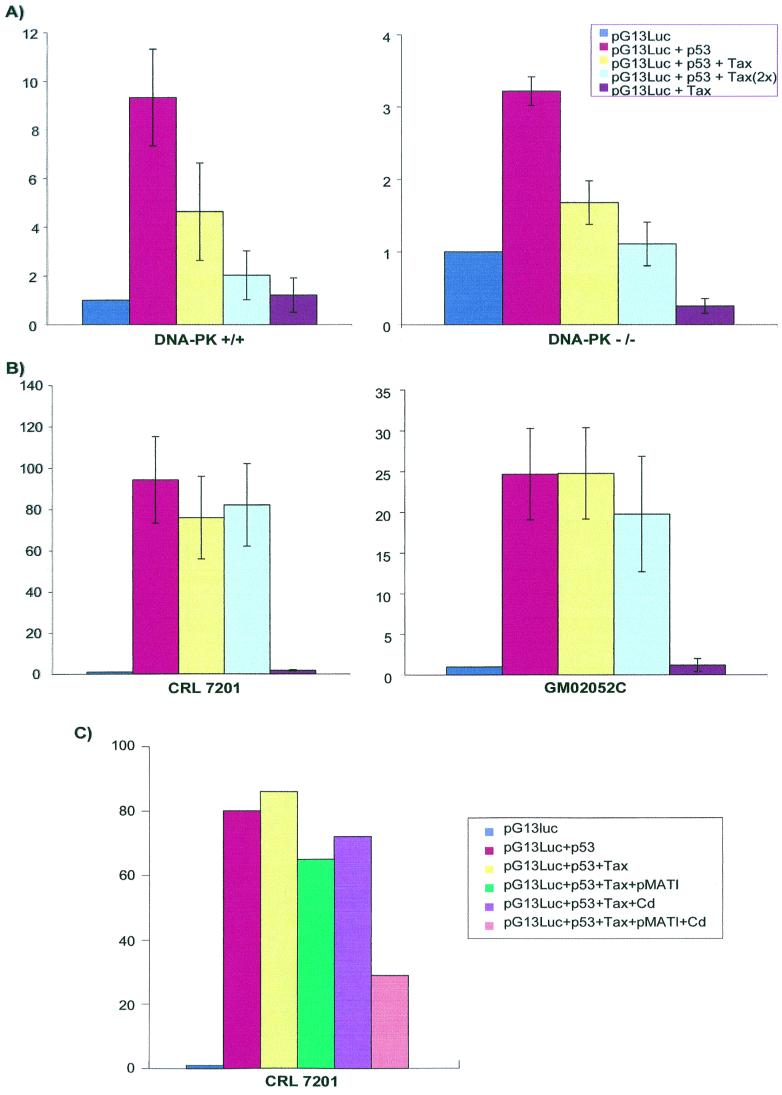
DNA-PK and the related protein ATM play significant roles in modulating p53 activity (12, 13, 69). Prompted by the findings of Pise-Masison et al., (70, 71), we wondered whether we could genetically link these kinases with functional observations on Tax and p53. First, the role of DNA-PK was investigated using paired cell lines that were either genotypically normal (DNA-PKcs+/+) or homozygously lacking the catalytic subunit of DNA-PK (DNA-PKcs−/−) (46). If intact DNA-PK were needed for Tax repression of p53, then one would expect a loss of this repressive phenotype in DNA-PKcs−/− compared to DNA-PKcs+/+ cells. Surprisingly, Tax repressed p53 activity comparably in DNA-PKcs−/− and DNA-PKcs+/+ settings, suggesting that this kinase is not required for a Tax p53-repressive phenotype (Fig. 6A). Next, two independent ATM−/− cells (CRL 7201 and GM02052C) were examined. Here, in marked contrast to the DNA-PK cells, we found that Tax's ability to repress p53 was absent in both ATM−/− cells (Fig. 6B). On the other hand, reconstitution of ATM expression in CRL 7201 cells by exogenous transfection with a cadmium-inducible ATM-expressing plasmid (pMAT1 101) restored the ability of Tax to repress p53 activity (Fig. 6C). Taken together, these results suggest a role for ATM, but not DNA-PK, in Tax p53 repression.
FIG. 6.
ATM, not DNA-PK, correlates with Tax repression of p53 activity. DNA-PKcs+/+ and DNA-PKcs−/− cells (A) and ATM-deficient cell lines CRL 7201 and GM02052C (B) were transfected individually or in the indicated combinations with pG13Luc, p53, or a plasmid expressing wild-type Tax. (C) Reconstitution of ATM in CRL 7201 recovered Tax repression of p53 activity. Transfections were as for panel B except that where indicated 10 μg of pMAT1 (87) plasmid was cotransfected, and cells were induced for 16 h with 5 μM CdCl2 (Cd). Results are averages from two independent transfections.
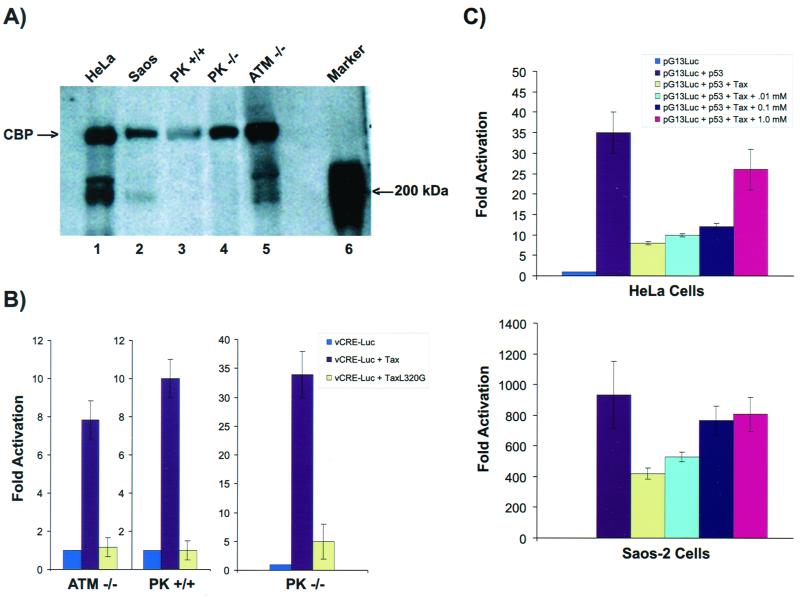
To better interpret our results and because genetically mutated cells can contain multiple changes, we performed additional control experiments to clarify the above findings. Initially, we wished to document that loss of p53 inhibition by Tax in ATM−/− cells was not a trivial consequence of a global defect in CBP and/or CREB signaling. To address these issues, we assayed first by Western blotting for the presence of CBP in HeLa, Soas-2, PK+/+, PK−/−, and ATM−/− cells. After normalizing for protein loading (based on Coomassie blue staining [not shown]), we concluded that all cells had nearly equivalent steady-state amounts of CBP (Fig. 7A). Next, we checked for preservation of Tax-CREB signaling in ATM−/−, DNA-PKcs−/−, and DNA-PKcs+/+ cells (Fig. 7B). In all three backgrounds, wild-type Tax, but not a CREB− NF-κB+ Tax L320G mutant, activated the CREB-responsive viral CRE-luciferase reporter (vCRE-Luc 95) comparably. Likewise, wild-type Tax, but not a CREB+ NF-κB− Tax S258A mutant, activated an NF-κB-responsive luciferase reporter competently, suggesting similar intactness of NF-κB signaling in these cells (data not shown). Finally, we wished to confirm through an independent approach the contribution of ATM to Tax's inhibition of p53 activity. Previous studies showed that caffeine can inhibit ATM activity (81). Using concentrations of caffeine which confer ATM inhibition, we treated ATM+/+ (HeLa and Soas-2) cells and examined whether repression of p53 by Tax would be affected. Consistent with results from ATM−/− cells, caffeine in HeLa and Soas-2 cells (Fig. 7C) abolished progressively the previously documented inhibition (Fig. 1) of Tax on p53.
FIG. 7.
Biological parameters in ATM and DNA-PKcs cells. (A) Detection of CBP protein in cells. Whole cell lysates from HeLa, Saos-2, DNA-PKcs+/+, DNA-PKcs−/−, and ATM−/− cells, as indicated, were resolved by SDS-PAGE (8% gel), transferred to a membrane, and then reacted with a specific antibody for CBP (Santa Cruz Biotechnology). Lane 6 shows a protein marker of 200 kDa. Based on normalization to Coomassie blue staining (not shown), nearly equivalent amounts of CBP are present in each cell line. (B) CREB pathway is intact in cells. ATM−/−, DNA-PKcs+/+, and DNA-PKcs−/− cells were transfected with vCRELuc and either Tax or TaxL320G. Luciferase activities were assayed. Intact activation of vCRELuc by Tax was observed in all cells. (C) Abrogation of Tax repression of p53 by caffeine. HeLa and Saos-2 cells were cotransfected with pG13Luc, p53, and Tax; 24 h after transfection, caffeine was added to cells to final concentrations from 0.01 to 1.0 mM. Cells were harvested 48 h after transfection. Tax-mediated repression of p53 activation of pG13Luc was progressively abolished with increasing concentrations of caffeine.
DISCUSSION
Tax is an HTLV-1-encoded oncoprotein whose expression is linked to cellular transformation (16, 25, 26, 64, 74, 76, 92). One unique characteristic of Tax is its ability to induce cellular DNA damage, leading to an impairment of genome integrity (37, 43, 54, 57, 68, 79, 86). From this perspective, Tax might be expected to interact strongly with the cellular “guardian of the genome,” p53 (48). Indeed, p53 is rapidly activated by cellular DNA damage and functions to enforce cell cycle arrest for purposes of repair or programmed cell death (51). Consistent with the rationale for Tax-p53 interaction, several laboratories have reported on the ability of HTLV-1 Tax to suppress the transcriptional activity of p53 (5, 60, 71, 72, 75, 89, 95).
In this study, we explored several discrepancies in the published literature regarding Tax-p53 interaction. For example, some investigators have suggested that a binding competition between Tax and p53 for coactivator CBP sufficiently explains the repressive activity of the former on the latter (5, 90, 95). However, others have argued for alternative mechanisms which invoke a phosphorylation-dependent signaling through DNA-PK and NF-κB (72). The results presented here reaffirm the necessity of Tax's ability to activate CREB (60) and to bind CBP (5, 90, 95) for its p53-repressive activity (Fig. 3 to 5). At the same time, our findings indicate clearly that CREB-signaling and CBP-binding activities are insufficient to fully account for p53 inhibition by Tax (Fig. 3 to 5). We inferred this latter conclusion based on the following observations. First, the Tax1-related protein Tax2CG failed to repress p53 activity (Fig. 5). Tax2CG largely conserves the CBP-binding domain of Tax1 (29) and, based on its similarity to Tax1 in CREB activation (77), would be expected to competently sequester CBP. Second, two Tax1 single-amino-acid point mutants L320G (Fig. 3C) and S274A (Fig. 3D Fig. 1), which have intact CBP-binding motifs (29) also failed to repress p53 activity. Third, wild-type Tax1 in ATM−/− settings where CREB signaling (Fig. 7B), NF-κB signaling (data not shown), and CBP protein (Fig. 7A) are intact nevertheless failed to inhibit p53 activity (Fig. 6B). Together, these findings support the inference that CBP binding is necessary but not sufficient for Tax1 to repress p53 function.
The observation that Tax2CG, unlike Tax1, does not repress p53 activity adds another piece of information about the in vivo biological differences between HTLV-1 and HTLV-2. Extant findings support a lack of correlation between HTLV-2 and human malignancies (33, 55). On the other hand, the linkage between HTLV-1 and ATL is unambiguous. To the extent that different properties of Tax1 and Tax2 might account for differences in the in transforming properties of the two HTLVs, it makes sense that the former, but not the latter, Tax protein subverts p53 function. In this regard, Tax2 differs from Tax1 in two other important respects: it is inefficient in promoting cellular DNA damage (86), and it cannot bind to the human homologue of the Drosophila disc large tumor suppressor protein, hDlg. Binding of hDlg (49, 90), induction of DNA damage (86), and partial contribution to p53 suppression (this report) all seemingly implicate the C terminus of Tax1, suggesting that differences in the C termini between Tax1 and Tax2 are important distinguishing features. However, such differences between Tax1 and Tax2 may not sufficiently explain variances in transformation, since in some model settings Tax2 does contribute to HTLV-dependent immortalization of T lymphocytes (78).
How mechanistically might one view Tax's inhibition of the transcriptional activity of p53? There is disagreement in the literature regarding this question. Whereas four groups have shown that CREB+ NF-κB− but not CREB− NF-κB+ Tax mutants repress p53 activity (5, 60, 89, 94), another group has presented strong evidence to the contrary (i.e., CREB− NF-κB+ but not CREB+ NF-κB− Tax proteins repress p53 72). It has been proposed that these divergent findings might be explained by the fact that the former results were largely derived from fibroblastic and epithelial cells whereas the latter were derived primarily from Jurkat lymphocytes (72). Our results here do not favor such an explanation. Indeed, in HeLa, Soas-2, and Jurkat lymphocytes (Fig. 1), we confirmed that CREB+ NF-κB− but not CREB− NF-κB+ Tax mutants repress p53 activity. We do not know the reasons for this difference; however, we should point out that our results are consistent with either CREB- and NF-κB-specific mutants constructed in our laboratory (85) or analogous mutants (Fig. 1D) constructed by Smith and Greene (87).
We do not fully understand how in our system CREB+ NF-κB− Tax mutants work to repress p53. However, based on findings from assays of ATM−/− cells (Fig. 6) and caffeine inhibition (Fig. 7), we suggest that one of the downstream mediators of Tax's effect is the ATM protein. The ATM protein, the deletion of whose gene is responsible for the human genetic disorder ataxia telangiectasia, has been shown to phosphorylate p53 at serine 15 in response to ionizing radiation (13). At this juncture, based on evidence from Ariumi et al. (5) that phosphorylation of p53 is not a major mechanism mediating Tax-inactivation of p53, we are uncertain as to whether a direct kinase activity from ATM transduces a Tax signal to p53. Indeed, how phosphorylation works as a general mechanism for regulating p53 function has recently been questioned by several investigators (6, 10). Thus, we do not exclude other indirect ATM-dependent but phosphorylation-independent mechanisms for regulating p53 activity (97) as contributing to our observations on Tax-p53 interaction.
ACKNOWLEDGMENTS
We thank K. Kibler, Y. Iwanaga, T. Kasai, and H. Iha for critical readings of manuscript, L. Lin for preparation of the manuscript, and D. J. Chen for discussions.
Work in the laboratory of K.-T.J. is supported in part by the AIDS Targeted Antiviral Program from the Office of the Director, NIH.
REFERENCES
- 1.Agarwal M L, Taylor W R, Chernov M V, Chernova O V, Stark G R. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Akagi T, Ono H, Tsuchida N, Simotohno K. Aberrant expression and function of p53 in T-cells immortalized by HTLV-I Tax1. FEBS Lett. 1997;406:263–266. doi: 10.1016/s0014-5793(97)00280-9. [DOI] [PubMed] [Google Scholar]
- 3.Altman R, Harrich D, Garcia J A, Gaynor R B. Human T-cell leukemia virus types I and II exhibit different DNase I protection patterns. J Virol. 1988;62:1339–1346. doi: 10.1128/jvi.62.4.1339-1346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki R, Fukumura R, Fujimori A, Taya Y, Shiloh Y, Kurimasa A, Burma S, Li G C, Chen D J, Sato K, Hoki Y, Tatsumi K, Abe M. Enhanced phosphorylation of p53 serine 18 following DNA damage in DNA-dependent protein kinase catalytic subunit-deficient cells. Cancer Res. 1999;59:3543–3546. [PubMed] [Google Scholar]
- 5.Ariumi Y, Kaida A, Lin J Y, Hirota M, Masui O, Yamaoka S, Taya Y, Shimotohno K. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene. 2000;19:1491–1499. doi: 10.1038/sj.onc.1203450. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft M, Kubbutat M H G, Vousden K H. Regulation of p53 function and stability by phosphorylation. Mol Cell Biol. 1999;19:1751–1758. doi: 10.1128/mcb.19.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 8.Beraud C, Sun S C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2403. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blattner C, Tobiasch E, Litfen M, Rahmsdorf H J, Herrlich P. DNA damage induced p53 stabilization: no indication for an involvement of p53 phosphorylation. Oncogene. 1999;18:1723–1732. doi: 10.1038/sj.onc.1202480. [DOI] [PubMed] [Google Scholar]
- 11.Brady J, Jeang K T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burma S, Kurimasa A, Xie G, Taya Y, Araki R, Abe M, Crissman H A, Ouyang H, Li G C, Chen D J. DNA-dependent protein kinase-independent activation of p53 in response to DNA damage. J Biol Chem. 1999;274:17139–17143. doi: 10.1074/jbc.274.24.17139. [DOI] [PubMed] [Google Scholar]
- 13.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 14.Caron C, Rousset R, Beraud C, Moncollin V, Egly J M, Jalinot P. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 1993;12:4269–4278. doi: 10.1002/j.1460-2075.1993.tb06111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmore L W, Hancock A R, Chang S F, Wang X W, Chang S, Callahan C P, Geller D A, Will H, Harris C C. Hepatitis B virus X protein and p53 tumor suppressor interaction in the modulation of apoptosis. Proc Natl Acad Sci USA. 1997;94:14707–14712. doi: 10.1073/pnas.94.26.14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchini G. Molecular mechanism of human T-cell leukemia/lymphotropic virus type 1 infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 17.Franklin A A, Nyborg J K. Mechanisms of Tax regulation of human T cell leukemia virus type 1 gene expression. J Biomed Sci. 1995;2:17–29. doi: 10.1007/BF02257921. [DOI] [PubMed] [Google Scholar]
- 18.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 19.Fujii M, Sassone-Corsi P, Verma I M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo R C. The first human retrovirus. Sci Am. 1986;255:88–98. doi: 10.1038/scientificamerican1286-88. [DOI] [PubMed] [Google Scholar]
- 21.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 22.Giam C Z, Xu Y L. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989;264:15236–15241. [PubMed] [Google Scholar]
- 23.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb T M, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 25.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type I X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris N, Brill E, Shohat O, Prokocimer M, Wolf D, Arai N, Rotter V. Molecular basis for heterogeneity of the human p53 protein. Mol Cell Biol. 1986;6:4650–4656. doi: 10.1128/mcb.6.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrod R, Kuo Y L, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam C Z. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem. 2000;275:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- 29.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropc virus type 1 Tax is responsible for the recruitment of coactivator CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinuma Y, Komoda H, Chosa T, Kondo T, Kohakura M, Takenaka T, Kikuchi M, Ichimaru M, Yunoki K, Sato I, Matsuo R, Takiuchi Y, Uchino H, Hanaoka M. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982;29:631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- 31.Hirai H, Fujisawa J, Suzuki T, Ueda K, Muramatsu M, Tsuboi A, Arai N, Yoshida M. Transcriptional activator Tax of HTLV-1 binds to the NF-kappa B precursor p105. Oncogene. 1992;7:1737–1742. [PubMed] [Google Scholar]
- 32.Hiscott J, Petropoulos L, Lacoste J. Molecular interactions between HTLV-I Tax protein and the NF-kappa B/kappa B transcription complex. Virology. 1995;214:3–11. doi: 10.1006/viro.1995.9960. [DOI] [PubMed] [Google Scholar]
- 33.Hjelle B, Chaney R. Sequence variation of functional HTLV-II Tax alleles among isolates from endemic population: lack of evidence of oncogenic determinant in Tax. J Med Virol. 1992;36:136–141. doi: 10.1002/jmv.1890360211. [DOI] [PubMed] [Google Scholar]
- 34.Jeang K T, Boros I, Brady J, Radonovich M, Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988;62:4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeang K T, Widen S G, Semmes IV O J, Wilson S H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 36.Jin D Y, Wang H L, Zhou Y, Chun A C S, Kibler K V, Hou Y D, Kung H F, Jeang K T. Hepatitis C virus core protein-induced loss of LZIP function correlates with cellular transformation. EMBO J. 2000;19:729–740. doi: 10.1093/emboj/19.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin D Y, Spencer F, Jeang K T. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 38.Kao S Y, Lemoine F J, Mariott S J. HTLV-1 Tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene. 2000;19:2240–2248. doi: 10.1038/sj.onc.1203559. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor M, Hamm R, Yan W, Taya Y, Lozano G. Cooperative phosphorylation at multiple sites is required to activate p53 in response to UV radiation. Oncogene. 2000;19:358–364. doi: 10.1038/sj.onc.1203300. [DOI] [PubMed] [Google Scholar]
- 40.Kashanchi F, Duvall J F, Kwok R P, Lundblad J R, Goodman R H, Brady J N. The coactivator CBP stimulates human T-cell lymphotrophic virus type I Tax transactivation in vitro. J Biol Chem. 1998;273:34646–34652. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- 41.Kern S E, Pietenpol J A, Thiagalingam A, Seymour A, Kinzler K W, Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992;256:827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- 42.Khanna K K, Keating K E, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller S P, Lavin M F. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 43.Kibler K V, Jeang K T. Taxing the cellular capacity for repair: human T-cell leukemia virus type 1, DNA damage, and adult T-cell leukemia. J Natl Cancer Inst. 1999;91:903–904. doi: 10.1093/jnci/91.11.903. [DOI] [PubMed] [Google Scholar]
- 44.Kimzey A L, Dynan W S. Identification of a human T-cell leukemia virus type I tax peptide in contact with DNA. J Biol Chem. 1999;274:34226–34232. doi: 10.1074/jbc.274.48.34226. [DOI] [PubMed] [Google Scholar]
- 45.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 46.Kurimasa A, Ouyang H, Dong L J, Wang S, Li X, Cordon-Cardo C, Li G C. Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:1403–1408. doi: 10.1073/pnas.96.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;18:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 48.Lane D P. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 49.Lee S S, Weiss R S, Javier R T. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large suppressor protein. Proc Natl Acad Sci USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenzmeier B A, Baird E E, Dervan P B, Nyborg J K. The tax protein-DNA interaction is essential for HTLV-I transactivation in vitro. J Mol Biol. 1999;291:731–744. doi: 10.1006/jmbi.1999.2969. [DOI] [PubMed] [Google Scholar]
- 51.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 52.Levine A J, Perry M E, Chang A, Silver A, Dittmer D, Wu M, Welsh D. The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer. 1994;69:409–416. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin H C, Dezzutti C S, Lal R B, Rabson A B. Activation of human T-cell leukemia virus type 1 tax gene expression in chronically infected T cells. J Virol. 1998;72:6264–6270. doi: 10.1128/jvi.72.7.6264-6270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majone F, Semmes O J, Jeang K T. Induction of micronuclei by HTLV-I Tax: a cellular assay for function. Virology. 1993;193:456–459. doi: 10.1006/viro.1993.1145. [DOI] [PubMed] [Google Scholar]
- 55.Martin M P, Biggar R J, Hamlin-Green G, Staal S, Mann D. Large granular lymphocytosis in a patient infected with HTLV. AIDS Res Hum Retroviruses. 1993;8:715–719. doi: 10.1089/aid.1993.9.715. [DOI] [PubMed] [Google Scholar]
- 56.Mesnard J M, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. . (Review.) [DOI] [PubMed] [Google Scholar]
- 57.Miyake H, Suzuki T, Hirai H, Yoshida M. Trans-activator Tax of human T-cell leukemia virus type 1 enhances mutation frequency of the cellular genome. Virology. 1999;253:155–161. doi: 10.1006/viro.1998.9500. [DOI] [PubMed] [Google Scholar]
- 58.Moll U E, Riou G, Levine A J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci USA. 1992;89:7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moll U E, LaQuaglia M, Benard J, Riou G. Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc Natl Acad Sci USA. 1995;92:4407–4411. doi: 10.1073/pnas.92.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mulloy J C, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi M V, Cara A, Nicot C, Giam C Z, Franchini G. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murakami T, Hirai H, Suzuki T, Fujisawa J, Yoshida M. HTLV-1 Tax enhances NK-kappa B2 expression and binds to the products p52 and p100, but does not suppress the inhibitory function of p100. Virology. 1995;206:1066–1074. doi: 10.1006/viro.1995.1029. [DOI] [PubMed] [Google Scholar]
- 62.Murphy E L, Hanchard B, Figueroa J P, Gibbs W N, Lofters W S, Campbell M, Goedert J J, Blattner W A. Modeling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 63.Nakagawa K, Taya Y, Tamai K, Yamaizumi M. Requirement of ATM in phosphorylation of the human p53 protein at serine 15 following DNA double-strand breaks. Mol Cell Biol. 1999;19:2828–2834. doi: 10.1128/mcb.19.4.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nerenberg M, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 65.Neuveut C, Jeang K T. HTLV-I Tax and cell cycle progression. Prog Cell Cycle Res. 2000;4:157–162. doi: 10.1007/978-1-4615-4253-7_14. . (Review.) [DOI] [PubMed] [Google Scholar]
- 66.Nicot C, Mahieux R, Opavsky R, Cereseto A, Wolff L, Brady J N, Franchini G. HTLV-I Tax transrepresses the human c-Myb promoter independently of its interaction with CBP or p300. Oncogene. 2000;19:2155–2164. doi: 10.1038/sj.onc.1203536. [DOI] [PubMed] [Google Scholar]
- 67.Nyborg J K, Dynan W S, Chen I S, Wachsman W. Binding of host-cell factors to DNA sequences in the long terminal repeat of human T-cell leukemia virus type I: implications for viral gene expression. Proc Natl Acad Sci USA. 1988;85:1457–1461. doi: 10.1073/pnas.85.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;ii:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 69.Piret B, Schoonbroodt S, Piette J. The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene. 1999;18:2261–2271. doi: 10.1038/sj.onc.1202541. [DOI] [PubMed] [Google Scholar]
- 70.Pise-Masison C A, Choi K S, Radonovich M, Dittmer J, Kim S J, Brady J N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pise-Masison C A, Rodonovich M, Sakaguchi K, Appella E, Brady J. Phosphorylation of p53: a novel pathway for p53 inactivation in human T-cell lymphotropic virus type 1-transformed cells. J Virol. 1998;72:6348–6355. doi: 10.1128/jvi.72.8.6348-6355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pise-Masison C A, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Du Guillerm C, Brady J N. Inactivation of p53 by human T-cell lymphotropic virus type 1 requires activation of the NF-κB pathway and is dependent on p53 phosphorylation. Mol Cell Biol. 2000;10:3377–3386. doi: 10.1128/mcb.20.10.3377-3386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reid R L, Lindholm P F, Mireskandari A, Dittmer J, Brady J N. Stabilization of wild-type p53 in human T-lymphocytes transformed by HTLV-I. Oncogene. 1993;8:3029–3036. [PubMed] [Google Scholar]
- 76.Rosin O, Koch C, Schmitt I, Semmes O J, Jeang K T, Grassmann R. A human T-cell leukemia virus Tax variant incapable of activating NF-kappaB retains its immortalizing potential for primary T-lymphocytes. J Biol Chem. 1998;273:6698–6703. doi: 10.1074/jbc.273.12.6698. [DOI] [PubMed] [Google Scholar]
- 77.Ross T M, Minella A C, Fang Z Y, Pettiford S M, Green P L. Mutational analysis of human T-cell leukemia virus type 2 Tax. J Virol. 1997;71:8912–8917. doi: 10.1128/jvi.71.11.8912-8917.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross T M, Pettiford S M, Green P L. The tax gene of human T-cell leukemia virus type 2 is essential transformation of human T lymphocytes. J Virol. 1996;8:5194–5202. doi: 10.1128/jvi.70.8.5194-5202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saggioro D, Majone F, Forino M, Turchetto L, Leszl A, Chieco-Bianch L. Tax protein of human T-lymphotropic virus type I triggers DNA damage. Leuk Lymphoma. 1994;3-4:281–286. doi: 10.3109/10428199409059600. [DOI] [PubMed] [Google Scholar]
- 80.Sang N, Avantaggiati M L, Giordano A. Roles of p300, pocket proteins, and hTBP in E1A-mediated transcriptional regulation and inhibition of p53 transactivation activity. J Cell Biochem. 1997;66:277–285. doi: 10.1002/(sici)1097-4644(19970901)66:3<277::aid-jcb1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 81.Sarkaria J N, Busby E C, Tibbetts R S, Roos P, Taya Y, Karnitz L M, Abraham R T. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 82.Scheffner M, Munger K, Byrne J C, Howley P M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scolnick D M, Chehab N H, Stavridi E S, Lien M C, Caruso L, Moran E, Berger S L, Halazonetis T D. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 84.Seiki M, Inoue J, Takeda T, Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986;5:561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Semmes O J, Jeang K T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 37 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semmes O J, Majone F, Cantemir C, Turchetto L, Hjelle B, Jeang K T. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology. 1996;217:373–379. doi: 10.1006/viro.1996.0126. [DOI] [PubMed] [Google Scholar]
- 87.Smith M R, Greene W C. Type I human T cell leukemia virus tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soussi T, Caron de Fromentel C, May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990;5:945–952. [PubMed] [Google Scholar]
- 89.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki T, Ohsugi Y, Uchida-Toita M, Akiyama T, Yoshida M. Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene. 1999;18:5967–5972. doi: 10.1038/sj.onc.1203008. [DOI] [PubMed] [Google Scholar]
- 91.Takemoto S, Trovato R, Cereseto A, Nicot C, Kislyakova T, Casareto L, Waldmann T, Torelli G, Franchini G. p53 stabilization and functional impairment in the absence of genetic mutation or the alteration of the p14(ARF)-MDM2 loop in ex vivo and cultured adult T-cell leukemia/lymphoma cells. Blood. 2000;95:3939–3944. [PubMed] [Google Scholar]
- 92.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uittenbogaard M N, Geibler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type 1 Tax protein. J Biol Chem. 1995;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 94.Van Orden K, Giebler H A, Lemasson I, Gonzales M, Nyborg J K. Binding of p53 to the KIX domain of CREB binding protein. A potential link to human T-cell leukemia virus, type I-associated leukemogenesis. J Biol Chem. 1999;274:26321–26328. doi: 10.1074/jbc.274.37.26321. [DOI] [PubMed] [Google Scholar]
- 95.Van Orden K, Yan J P, Ulloa A, Nyborg J K. Binding of the human T-cell leukemia virus Tax protein to the coactivator CBP interferes with CBP-mediated transcriptional control. Oncogene. 1999;18:3766–3772. doi: 10.1038/sj.onc.1202703. [DOI] [PubMed] [Google Scholar]
- 96.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 97.Waterman M J F, Stavridi E S, Waterman J L F, Halazonetic T D. ATM-dependent activation of p53 involves dephosphorylation and association with 14-2-2 proteins. Nat Genet. 1998;19:175–178. doi: 10.1038/542. [DOI] [PubMed] [Google Scholar]
- 98.Yin M J, Gaynor R B. HTLV-1 21 bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 99.Yin M J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang, Chen N P, Khanna K K, Scott S, Gatei M, Kozlov S, Watters D, Spring K, Yen T, Lavin M F. Isolation of full-length ATM cDNA and correction of the ataxia-telangectasia cellular phenotype. Proc Natl Acad Sci USA. 1997;94:8021–8026. doi: 10.1073/pnas.94.15.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao L J, Giam C Z. Interaction of the human T-cell lymphotrophic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]