Abstract
Introduction
Atopic dermatitis (AD), with its hallmark symptoms of pruritus and skin lesions, often impairs patients’ quality of life. We assessed time spent with clear/almost clear skin and no/minimal itch during upadacitinib treatment versus placebo or dupilumab among patients with moderate-to-severe AD.
Methods
This analysis consisted of a post hoc analysis of Measure Up 1 (NCT03569293), Measure Up 2 (NCT03607422), and Heads Up (NCT03738397). Measure Up 1 and 2 were replicate, randomized, double-blind, placebo-controlled phase 3 studies with patients randomized (1:1:1) to once-daily oral upadacitinib 15 mg, upadacitinib 30 mg, or placebo for 16 weeks. Heads Up was a head-to-head, randomized, double-blind, double-dummy, phase 3b study with patients randomized (1:1) to upadacitinib 30 mg or subcutaneous dupilumab 300 mg for 24 weeks. Skin clearance was assessed with the Eczema Area and Severity Index (EASI) at baseline, weeks 1, 2, and 4, and every 4 weeks thereafter. Itch was assessed using the Worst Pruritus Numerical Rating Scale (WP-NRS) daily over 16 weeks and every 2 weeks thereafter to week 24 in Heads Up.
Results
This analysis included 1683 patients in Measure Up 1 and 2 and 673 patients in Heads Up. Through 16 weeks in Measure Up 1 and 2, patients receiving upadacitinib spent 9.8–13.4 times as many days with an EASI 90 response and 7.0–10.3 times as many days with a WP-NRS 0/1 response versus placebo. In Heads Up, patients receiving upadacitinib spent 2.0 and 1.7 times as many days through 16 and 24 weeks, respectively, with an EASI 90 response versus dupilumab. Through 16 and 24 weeks, patients receiving upadacitinib spent 3.0 and 2.6 times as many days, respectively, with a WP-NRS 0/1 response versus dupilumab.
Conclusions
Patients with moderate-to-severe AD spent more time with clear/almost clear skin and no/minimal itch with upadacitinib versus placebo or dupilumab.
Trial Registration
ClinicalTrials.gov identifier, Measure Up 1 (NCT03569293), Measure Up 2 (NCT03607422), Heads Up (NCT03738397).
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01242-9.
Keywords: Atopic dermatitis, Janus kinase inhibitors, Skin clearance, Itch response, Upadacitinib, Dupilumab
Key Summary Points
| Why carry out this study? |
| Atopic dermatitis (AD) adversely impacts quality of life; among patients with moderate-to-severe AD receiving treatment, higher rates of skin clearance and itch response have been associated with greater quality of life. |
| Upadacitinib, an oral selective Janus kinase inhibitor, and dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, have both demonstrated efficacy versus placebo in patients with moderate-to-severe AD. |
| Using data from the Measure Up 1, Measure Up 2, and Heads Up phase 3 clinical trials, this study evaluated the time patients spent with clear/almost clear skin and no/minimal itch while receiving upadacitinib compared with placebo or dupilumab. |
| What was learned from the study? |
| Patients with moderate-to-severe AD spent more time at higher skin clearance levels (as measured by Eczema Area and Severity Index response) and with no/minimal itch (as measured by Worst Pruritus Numerical Scale response) when treated with upadacitinib versus placebo (Measure Up 1 and 2 studies; upadacitinib 15 mg and upadacitinib 30 mg) or dupilumab (Heads Up study; upadacitinib 30 mg). |
Introduction
When treating patients with moderate-to-severe atopic dermatitis (AD), higher efficacy and itch responses are associated with greater quality of life. For example, patients who experience ≥ 75% improvement in the Eczema Area and Severity Index (EASI 75) are less satisfied than those who experience ≥ 90% improvement (EASI 90) [1]. Pruritus is a hallmark symptom of AD; itch intensity generally corresponds to disease severity [2]. Patients with greater pruritus improvement (e.g., a Worst Pruritus Numerical Rating Scale [WP-NRS] 0/1 response) are happier than those who experience less improvement in itch.[3].
Two common treatments approved for patients with moderate-to-severe AD include upadacitinib and dupilumab [4, 5]. Upadacitinib, an oral selective Janus kinase inhibitor, and dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, have both demonstrated efficacy versus placebo in itch reduction and skin clearance in patients with moderate-to-severe AD [6–8]. Despite the efficacy findings reported to date for upadacitinib and dupilumab, there remains a need to better understand the speed of onset, degree of response, and maintenance of response experienced by patients over time during treatment with these therapies.
Using data from the Measure Up 1, Measure Up 2, and Heads Up phase 3 clinical trials [8, 9], we sought to evaluate the time patients spent with clear/almost clear skin and no/minimal itch while receiving upadacitinib compared with placebo or dupilumab.
Methods
Study Design and Patients
Measure Up 1 (NCT03569293) and Measure Up 2 (NCT03607422) were replicate, multicenter, randomized, double-blind, placebo-controlled phase 3 studies comparing the efficacy and safety of upadacitinib in adults and adolescents (aged 12–75 years) with moderate-to-severe AD. Patients were randomized (1:1:1) to once-daily orally administered upadacitinib 15 mg, upadacitinib 30 mg, or placebo between 13 August 2018 and 23 December 2019 (Measure Up 1), and between 27 July 2018 and 17 January 2020 (Measure Up 2) [8]. Heads Up (NCT03738397) was a head-to-head, multicenter, randomized, double-blind, double-dummy, phase 3b study conducted between 21 February 2019 and 9 December 2020. Adult patients (aged 18–75 years) with moderate-to-severe AD were randomized (1:1) to once-daily orally administered upadacitinib 30 mg or subcutaneously administered dupilumab 300 mg every 2 weeks [9]. Detailed descriptions of these studies were reported previously [8, 9].
Study protocols, informed consent forms, and recruitment materials were approved by independent ethics committees or institutional review boards at each study site prior to patient enrollment. All studies were conducted in accordance with the International Council for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent.
Assessments
Skin clearance was assessed with Eczema Area and Severity Index (EASI) at baseline, weeks 1, 2, and 4, and every 4 weeks thereafter through 52 weeks in the Measure Up 1 and Measure Up 2 studies and through 24 weeks in the Heads Up study. Itch was evaluated using WP-NRS daily over 16 weeks, then at study visits thereafter (every 2 weeks to week 24 in the Heads Up study).
We evaluated the cumulative number of days and proportion of time each patient spent in response states. Skin clearance response states were based on improvements from baseline in EASI of 100%/≥ 90%/≥ 75%/≥ 50% (EASI 100/EASI 90/EASI 75/EASI 50, respectively); EASI 90–100 indicated a major clinical response of almost clear to clear skin.
Response states of no/minimal itch, defined as WP-NRS of 0/1 among patients with WP-NRS > 1 at baseline, and an itch-improved state, defined as WP-NRS improvement ≥ 4 from baseline among patients with WP-NRS ≥ 4 at baseline, were also assessed.
Statistical Analysis
In this post hoc analysis of the Measure Up 1 and Measure Up 2 studies (integrated data) and the Heads Up study, the cumulative number of days in a response state was summarized through weeks 4 and 16 for the Measure Up 1 and Measure Up 2 studies and through weeks 4, 16, and 24 for the Heads Up study or to study drug discontinuation, whichever occurred earlier. The proportion of time spent in a response state was the cumulative number of days in each response state out of the total number of days in the study period.
For EASI, response states between study visits were interpolated using modified last observation carried forward (mLOCF). In mLOCF, the last observation was not carried forward if a patient discontinued the study drug. For WP-NRS, the imputation method combined LOCF and nonresponder imputation (NRI). From baseline to week 16, LOCF was used for missing daily response status ≤ 7 days; if missing status lasted for > 7 days, NRI was used. After week 16 in the Heads Up study, the most recent previous nonmissing value was carried forward to impute missing data at later visits. If the patient discontinued the study drug, the last observation was not carried forward.
Results
Measure Up 1 and Measure Up 2 Studies
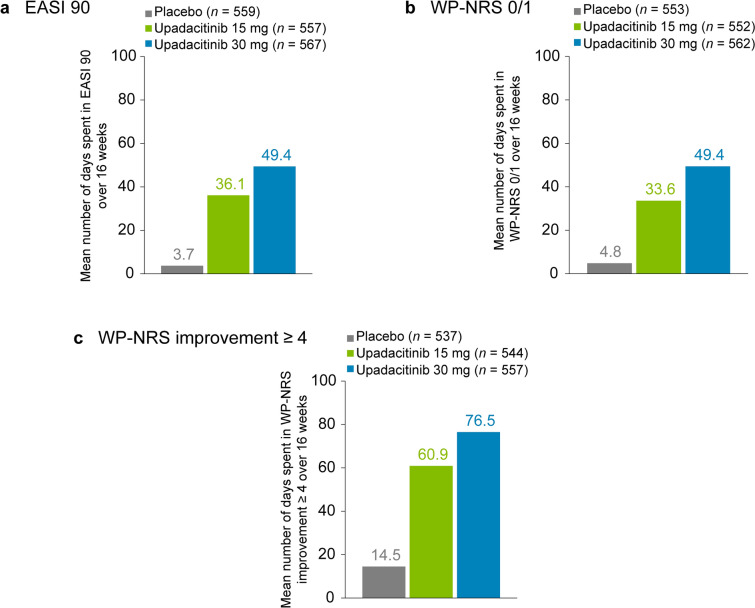
A total of 1683 patients were included in this analysis. Through 16 weeks, patients treated with upadacitinib 15 mg or upadacitinib 30 mg spent approximately 9.8 and 13.4 times as many days, respectively, with an EASI 90 response versus patients receiving placebo (Fig. 1a). Patients treated with upadacitinib 15 mg or upadacitinib 30 mg through 16 weeks spent approximately 7.0 and 10.3 times as many days, respectively, with a WP-NRS 0/1 response compared with those receiving placebo (Fig. 1b) and approximately 4.2 and 5.3 times as many days, respectively, with WP-NRS improvement ≥ 4 versus patients receiving placebo (Fig. 1c). See Fig. S1a–g for additional data through 16 weeks. Additionally, Fig. S2a–d shows greater time spent with WP-NRS 0/1 responses and WP-NRS improvement ≥ 4 through 4 weeks in patients treated with upadacitinib 15 mg or upadacitinib 30 mg compared with those receiving placebo.
Fig. 1.
Time spent in skin clearance and itch response states through 16 weeks of treatment with upadacitinib (Measure Up 1 and Measure Up 2 studies). a Mean number of days spent with an EASI 90 response. b Mean number of days spent with no/minimal itch for patients with WP-NRS > 1 at baseline, defined as a WP-NRS 0/1 response. c Mean number of days spent in an itch-improved state for patients with WP‑NRS ≥ 4 at baseline, defined as WP‑NRS improvement ≥ 4 from baseline. EASI 90 ≥ 90% improvement from baseline in the Eczema Area and Severity Index, WP-NRS Worst Pruritus Numerical Rating Scale
Heads Up Study
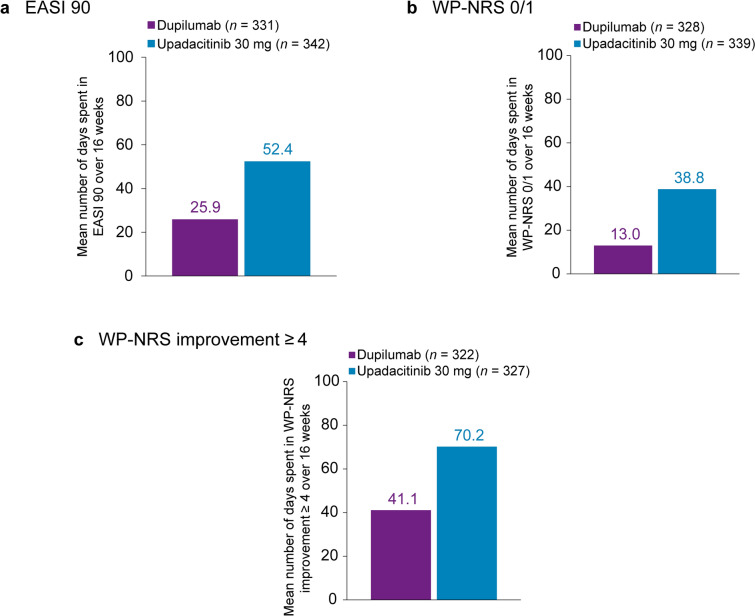
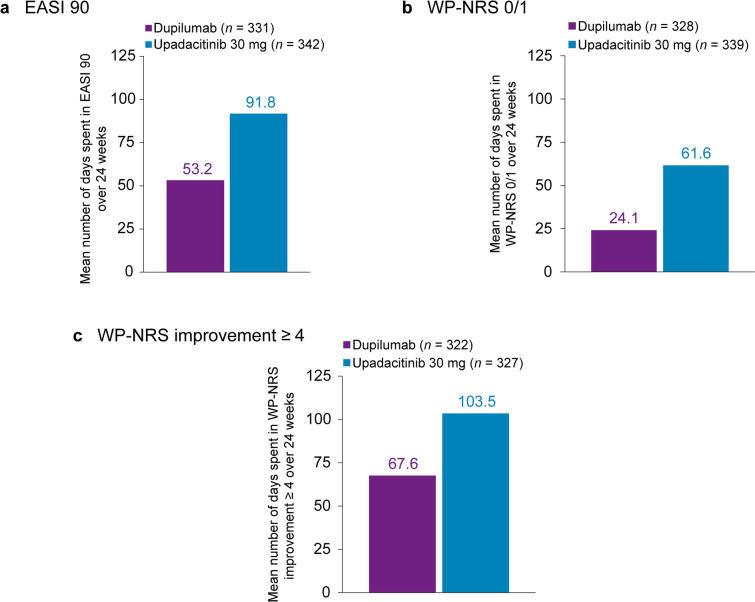
A total of 673 patients were included. Patients treated with upadacitinib 30 mg spent approximately 2.0 and 1.7 times as many days through 16 and 24 weeks, respectively, with an EASI 90 response versus patients receiving dupilumab (Figs. 2a and 3a). Through 16 and 24 weeks, patients receiving upadacitinib spent approximately 3.0 and 2.6 times as many days, respectively, with a WP-NRS 0/1 response compared with patients receiving dupilumab (Figs. 2b and 3b) and approximately 1.7 and 1.5 times as many days, respectively, with WP-NRS improvement ≥ 4 versus patients receiving dupilumab (Figs. 2c and 3c). See Figs. S3a–g and S4a–g for additional data through 16 and 24 weeks, respectively. Additionally, Fig. S5a–d presents data through 4 weeks showing more time spent with WP-NRS 0/1 responses and WP-NRS improvement ≥ 4 in patients treated with upadacitinib 30 mg versus dupilumab.
Fig. 2.
Time spent in skin clearance and itch response states through 16 weeks of treatment with upadacitinib versus dupilumab (Heads Up study). a Mean number of days spent with an EASI 90 response. b Mean number of days spent with no/minimal itch for patients with WP‑NRS > 1 at baseline, defined as a WP‑NRS 0/1 response. c Mean number of days spent in an itch‑improved state for patients with WP‑NRS ≥ 4 at baseline, defined as WP‑NRS improvement ≥ 4 from baseline. EASI 90 ≥ 90% improvement from baseline in the Eczema Area and Severity Index, WP-NRS Worst Pruritus Numerical Rating Scale
Fig. 3.
Time spent in skin clearance and itch response states through 24 weeks of treatment with upadacitinib versus dupilumab (Heads Up study). a Mean number of days spent with an EASI 90 response. b Mean number of days spent with no/minimal itch for patients with WP‑NRS > 1 at baseline, defined as a WP‑NRS 0/1 response. c Mean number of days spent in an itch‑improved state for patients with WP‑NRS ≥ 4 at baseline, defined as WP‑NRS improvement ≥ 4 from baseline. EASI 90 ≥ 90% improvement from baseline in the Eczema Area and Severity Index, WP-NRS Worst Pruritus Numerical Rating Scale
Discussion
Patients with moderate-to-severe AD spent more time at higher EASI response levels and with no/minimal itch when treated with upadacitinib versus placebo (Measure Up 1 and Measure Up 2 studies; upadacitinib 15 mg and upadacitinib 30 mg) or dupilumab (Heads Up study; upadacitinib 30 mg).
Patients with AD who experience rapid itch relief express greater satisfaction with their treatment [10]. In this study, itch severity, as measured by WP-NRS, was reported by patients on a daily basis (over 16 weeks). This provided data enabling investigation of itch reduction as early as week 4. Since only partial concordance between patients’ and clinicians’ pruritus-related concerns has been reported, the use of patient-reported outcomes is particularly valuable in assessing the holistic benefit of an AD treatment [11].
Reductions in AD severity (measured by EASI) and itch (measured by WP-NRS) are associated with increased work productivity and quality of life and decreased daily activity impairment [3, 12]. Patients with AD experience physical and psychosocial impairments that can impose a persistent life-long burden, termed cumulative life course impairment [13, 14]. Because the burdens of AD accumulate over time, the cumulative effects of treatment and long-term management are important. Evaluating the proportion of time patients experience improved AD symptoms with treatment, as opposed to assessing effectiveness at prespecified timepoints as is typical in clinical trials, supports consideration of the entire longitudinal patient journey in disease management and emphasizes the importance of long-term disease control.
Limitations
Findings were evaluated over relatively short periods (16 and 24 weeks). Upadacitinib and dupilumab were each evaluated as a monotherapy; however, in real-world practice, systemic and topical treatments are often combined. Although the study collected EASI data at specific weekly timepoints, this analysis was conducted on a scale of days. While the ensuing estimates may be mildly attenuated, comparisons among the treatments are not likely to be biased because all treatments were subject to this limitation.
Conclusions
Patients with moderate-to-severe AD spent more time with clear/almost clear skin and no/minimal itch when treated with upadacitinib compared with placebo or dupilumab. Our analysis of the cumulative time spent in the response states of clear/almost clear skin and no/minimal itch while receiving upadacitinib, dupilumab, or placebo provides a novel perspective on the lived treatment experience of patients and can inform treatment decision-making conversations between patients with AD and their clinicians.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AbbVie and the authors thank all the trial investigators and the patients who participated in these clinical trials.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Tara Rachinsky, PhD, and Akua Adu-Boahene, MD, MPH, of JB Ashtin, and funded by AbbVie.
Author Contributions
Andrew Blauvelt and Brian M. Calimlim had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Brian M. Calimlim, Wan-Ju Lee, and Yingyi Liu contributed to the study concept and design. Yingyi Liu contributed to statistical analysis. All authors contributed to the acquisition, analysis, or interpretation of data; critically reviewed the manuscript for important intellectual content; and approved the final version. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship.
Funding
AbbVie funded this study and participated in the study design, research, data collection, analysis, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Funding for the journal’s Rapid Service Fee was provided by AbbVie.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the USA and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home.”
Declarations
Conflict of Interest
Andrew Blauvelt has served as a speaker (received honoraria) for Lilly and UCB; has served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Celldex, CTI BioPharma, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, LEO Pharma, Lilly, Lipidio, Microbion, Merck, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Paragon, Pfizer, Q32 Bio, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor; has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Concert, Dermavant, DermBiont, Evelo, Evommune, Galderma, Incyte, Janssen, LEO Pharma, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, Takeda, UCB Pharma, and Ventyx; and owns stock in Lipidio and Oruka. Kilian Eyerich has received grants and personal fees from AbbVie; has received personal fees from Almirall, Bristol Myers Squibb, LEO Pharma, Lilly, Janssen, Novartis, UCB, and Sanofi; and has received grants from Lilly, LEO Pharma, Janssen, Novartis, and UCB. Alan D. Irvine has received personal fees as a consultant/speaker from AbbVie, Arena, Eli Lilly, LEO Pharma, Novartis, Pfizer, and Sanofi Regeneron. He has been an investigator for AbbVie and Regeneron/Sanofi. Marjolein de Bruin-Weller has served as a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Amgen, Aslan, Galderma, Janssen, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi‐Genzyme, and UCB. Shawn G. Kwatra has served as an advisory board member/consultant for AbbVie, Amgen, Arcutis, Aslan, Cara, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, LEO Pharma, Novartis, Pfizer, Regeneron, and Sanofi. He has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi. He is currently affiliated with the Department of Dermatology, University of Maryland School of Medicine, Baltimore, MD, and the Maryland Itch Center, University of Maryland School of Medicine, Baltimore, MD. Melinda Gooderham is or has been an investigator, adviser, and/or speaker for AbbVie, Akros, Amgen, AnaptysBio, Arcutis, Aristea, Bausch, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus BioSciences, Dermira, Dermavant, Galderma, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO, Lilly, Medimmune, Merck, Meiji, Moonlake, Nimbus, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi-Genzyme, Sun Pharma, UCB, and Ventyx. Brian Kim has served as a consultant for AbbVie, Almirall, Amagma, Argenx, AstraZeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim Corporation, Bristol Myers Squibb, Cara Therapeutics, Daewoong Pharmaceutical, Guidepoint Global, Janssen Pharmaceuticals, Incyte Corporation, Kiniksa Pharmaceuticals, LectureLinx, LEO Pharma, Lilly, Maruho, Novartis, OM Pharma, Pfizer, Sanofi Genzyme, Shaperon, Third Rock Ventures, and Trevi Therapeutics; is a stockholder of RecensMedical and Locus Biosciences; serves on the scientific advisory boards for Abrax Japan, Granular Therapeutics, RecensMedical, National Eczema Association, Cell Reports Medicine, and Journal of Allergy and Clinical Immunology; and holds a patent for the use of JAK inhibitors for chronic pruritus. Brian M. Calimlim, Wan-Ju Lee, Eliza M. Raymundo, Yingyi Liu, Sarah Ofori, and Andrew M. Platt are full-time employees of AbbVie Inc., and may hold AbbVie stock, stock options, and/or patents. Jonathan I. Silverberg has received honoraria as a consultant, advisory board member, and/or speaker for AbbVie, AObiome, Arcutis, Alamar, Amgen, Arena, Arcutis, Asana, ASLAN, Boehringer Ingelheim, BioMX, Biosion, Bodewell, Cara, Castle, Celgene, Connect, Dermavant, Dermira, Dermtech, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Lilly, Menlo, Novartis, Optum, Pfizer, RAPT, Regeneron, Sanofi-Genzyme, Shaperon, and Union. His institution has received grants from Galderma and Pfizer.
Ethical Approval
The Independent Ethics Committee or Institutional Review Board at each study site approved the study protocol, informed consent forms, and recruitment materials before patient enrollment. The studies were conducted in accordance with the International Conference for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent before screening.
Footnotes
Prior Presentation: Portions of these data were previously presented at the 31st European Academy of Dermatology and Venereology (EADV) Congress in Milan, Italy, and virtually on 7–10 September 2022; the International Society of Atopic Dermatitis (ISAD) meeting in Montreal, Canada on 17–19 October 2022; and the Revolutionizing Atopic Dermatitis (RAD) Conference virtually on 11 December 2022.
References
- 1.Reich K, de Bruin-Weller MS, Deleuran M, Calimlim BM, Chen N, Hu X, et al. Higher levels of response on clinical atopic dermatitis severity measures are associated with meaningful improvements in patient-reported symptom and quality of life measures: integrated analysis of three upadacitinib phase 3 trials. J Eur Acad Dermatol Venereol. 2023;37:1634–41. 10.1111/jdv.18995 [DOI] [PubMed] [Google Scholar]
- 2.Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136–43. 10.1056/NEJMra2023911 [DOI] [PubMed] [Google Scholar]
- 3.Ständer S, Bhatia N, Gooderham MJ, Silverberg JI, Thyssen JP, Biswas P, et al. High threshold efficacy responses in moderate-to-severe atopic dermatitis are associated with additional quality of life benefits: pooled analyses of abrocitinib monotherapy studies in adults and adolescents. J Eur Acad Dermatol Venereol. 2022;36(8):1308–17. 10.1111/jdv.18170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RINVOQ (upadacitinib). Prescribing information. AbbVie Inc.; 2023. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf. Accessed 18 Oct 2023.
- 5.DUPIXENT (dupilumab). Prescribing information. Sanofi-Aventis US LLC and Regeneron Pharmaceuticals, Inc.; 2023. https://www.regeneron.com/downloads/dupixent_fpi.pdf. Accessed 18 Oct 2023.
- 6.Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23. 10.1186/s41927-018-0031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48. 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 8.Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–68. 10.1016/S0140-6736(21)00588-2 [DOI] [PubMed] [Google Scholar]
- 9.Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–55. 10.1001/jamadermatol.2021.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg J, Gooderham M, Thyssen J, Pink A, Mansfield C, Lee W-J, et al. 419 Patient satisfaction with treatments for moderate-to-severe atopic dermatitis according to degree and speed of skin and itch improvements: results from a patient survey in the United States. Brit J Dermatol. 2023. 10.1093/bjd/ljad162.039. 10.1093/bjd/ljad162.039 [DOI] [Google Scholar]
- 11.Vakharia PP, Cella D, Silverberg JI. Patient-reported outcomes and quality of life measures in atopic dermatitis. Clin Dermatol. 2018;36(5):616–30. 10.1016/j.clindermatol.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Lio PA, Simpson EL, Han G, Soung J, Ball S, Sun L, et al. Improvement in sleep and itch and enhanced quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a phase 3 trial of baricitinib therapy. J Dermatolog Treat. 2022;33(4):2057–62. 10.1080/09546634.2021.1914308 [DOI] [PubMed] [Google Scholar]
- 13.von Stülpnagel CC, Augustin M, Düpmann L, da Silva N, Sommer R. Mapping risk factors for cumulative life course impairment in patients with chronic skin diseases—a systematic review. J Eur Acad Dermatol Venereol. 2021;35(11):2166–84. 10.1111/jdv.17348 [DOI] [PubMed] [Google Scholar]
- 14.Kimball AB, Gieler U, Linder D, Sampogna F, Warren RB, Augustin M. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24(9):989–1004. 10.1111/j.1468-3083.2010.03705.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the USA and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home.”