Abstract
Cladribine tablets (CladT), like alemtuzumab, acts as an immune reconstitution therapy. However, CladT is administered orally (alemtuzumab is given by infusion) and without the potential for serious side effects that limit the therapeutic use of alemtuzumab in multiple sclerosis (MS). Treatment with CladT, given initially as short courses of treatment 1 year apart, provides years of freedom from MS disease activity in responders to treatment. The appearance of mild or moderate MS disease activity after the initial 2 years of treatment may prompt careful follow-up or a further course of CladT, depending on the nature of the activity and individual circumstances. The appearance of severe MS disease activity requires a switch to an alternative high-efficacy disease-modifying treatment (DMT). The accumulating data from CladT-treated people with MS in real-world studies, including those with follow-up durations extending for years beyond the initial treatment, have demonstrated long-term freedom from MS disease activity in a good proportion of patients. This clinical experience has also confirmed that treatment with CladT is generally safe and well tolerated. The best time to prescribe a high-efficacy DMT is the subject of debate, with evidence that earlier versus later use of such agents may provide more effective long-term protection from disability progression. High-efficacy DMTs have traditionally been reserved for use in people with MS and high disease activity on presentation or breakthrough disease on one or more DMTs, as per the current product labels. The latest evidence from real-world studies suggests that CladT is effective and safe in DMT-naïve patients, including those with shorter disease duration.
Keywords: Relapsing multiple sclerosis, Cladribine tablets, Immune reconstitution therapy, Disease-modifying therapy
Key Summary Points
| Cladribine tablets (CladT) acts as an oral immune reconstitution therapy for the management of relapsing multiple sclerosis (MS). |
| Early treatment with CladT provides years of freedom from MS activity in responders to this therapy without the need for further treatment beyond the initial 2-year course. |
| Real-world data show CladT to be an effective, safe, convenient and cost-effective treatment option for relapsing MS. |
| MS disease reactivation may need further CladT (mild or moderate reactivation) or a switch to an alternative high-efficacy disease-modifying therapy (severe reactivation). |
Introduction
The introduction of several new high-efficacy disease-modifying therapies (DMTs) for the management of relapsing–remitting multiple sclerosis (RRMS) in recent years has revolutionised the management of this disease. High-efficacy DMTs can be stratified according to their mechanism of action. Anti-trafficking agents, e.g. sphingosine-1-phosphate (S1P) inhibitors (fingolimod, ozanimod, ponesimod and siponimod) and natalizumab, and anti-CD20 B cell-depleting agents (ocrelizumab, ublituximab and ofatumumab) are given continuously, with dosing intervals up to 6-monthly. These agents induce continuous suppression of the immune system, with potential increase in risk of opportunistic infections and malignancy.
Immune reconstitution therapies (IRT) are also classified as high-efficacy DMTs [1–3]. Exemplified by cladribine tablets (CladT) and alemtuzumab, they are given as short courses of treatment at the beginning of the first 2 years of therapy. The mechanistic process of IRT has been described as the “three Rs”: reduction, repopulation and reconstitution [4]. Rapid, but transient, decreases occur in circulating levels of B cells (especially) and T cells after each treatment course (reduction), followed by recovery of lymphocyte levels over a period of months, with no effect on the innate immune system (repopulation) [4, 5]. Subsequent qualitative alterations in the function of immune system (reconstitution) are believed to underlie the prolonged freedom from disease activity observed in responders to IRT, which far outlasts effects on immune cell counts and may last for years following the second course of treatment [1–5].
This review will focus on the therapeutic use of CladT: the therapeutic use of alemtuzumab has been limited by significant safety concerns, including cardiovascular toxicity and a high incidence of autoimmune disorders including hypothyroidism and type 1 diabetes [6]. IRT is a relatively new concept in MS care, and our understanding of its therapeutic use is still evolving. In particular, real-world studies are adding to the evidence base, especially with regard to longer-term efficacy and safety. This article summarises the latest expert practical recommendations on the use of CladT in the management of RMS in several countries within the Gulf Cooperation Council (GCC), based on the latest real-world evidence.
This narrative review is based on previously conducted studies and the clinical expertise of the authors in treating patients with RMS. No new clinical studies were performed by the authors. No patient-specific efficacy or safety data were reported. Therefore, institutional review board (IRB)/ethics approval was not required.
When Should a High-Efficacy DMT be Prescribed?
CladT is classified within the “high efficacy” group of DMTs [7, 8]. Debate continues regarding the relative merits of two approaches to the pharmacologic management of MS. Firstly, the escalation approach involves administration of a first-line (“platform”) DMT, such as interferon-beta, dimethyl fumarate, teriflunomide or glatiramer acetate, with escalation using a high-efficacy DMT in case of breakthrough disease [9]. The escalation approach has the advantage of facilitating the long-term administration of immunomodulatory therapy (certainly in the case of the interferons), rather than administration of immunosuppressive DMTs. However, treatment escalation usually occurs only following a clinical relapse or appearance of significant new MRI activity. Recent studies have shown that most of the damage to the central nervous system (CNS) in MS occurs early and independently of relapses, raising the possibility of loss of brain reserve and increased risk of future disability during an apparently successful course of treatment [10, 11].
These and other observations have prompted the study of potential benefits of high-efficacy DMTs early in the course of MS [9]. A real-world study from Norway showed that more people with MS achieved no evidence of disease activity (NEDA) if they received a high-efficacy DMT as their first pharmacologic treatment (68% in year 1 and 52% in year 2 of treatment), compared with platform DMTs (36% and 19%, respectively) [12]. The odds ratio for achieving NEDA during year 1 with early high-efficacy treatment was 3.9 (95% CI 2.4–6.1; p < 0.001), and using high-efficacy DMT as third-line therapy was as effective as continued platform therapy. Analysis of registry data showed that early versus later use of high-efficacy DMTs (≤ 2 years from MS diagnosis) was associated with a lower mean Expanded Kurtze Disability Status Scale (EDSS) score (2.3 vs. 3.5) 10 years after diagnosis [13]. Similarly, starting with a high-efficacy versus platform DMT almost halved the risk of disability worsening during 4 years of follow-up [14]. Finally, data from the Swedish and Danish national registries data revealed the consequences of different MS management strategies in Denmark, where 7.6% of patients initiated treatment with a high-efficacy DMT, and neighbouring Sweden, where 34.5% did so [15]. Early use of high-efficacy DMTs in Sweden was associated with significantly less disability worsening and lower risk of reaching EDSS 3 or 4.
In clinical practice, the early use of high-efficacy DMTs has been limited to patients with more severe disease at presentation, in line with the labels of these agents. For example, the European Summary of Product Characteristics for CladT includes the following indication, “for the treatment of adult patients with highly active relapsing multiple sclerosis (MS) as defined by clinical or imaging features”, without further definition [16]. It is important to note, however, the following:
The current European guideline for MS management supports the early use of a highly active DMT for patients with high disease activity on presentation [17].
The 2023 revision of the Middle East North Africa Committee for Treatment and Research in Multiple Sclerosis (MENACTRIMS) treatment guidelines goes further, including high-efficacy DMTs as a treatment option for “moderately active” MS, in addition to the other categories of “highly active” and “rapidly evolving aggressive” MS [18].
Prescribing in the GCC region, where the authors of this article practise, is only guided, not restricted, by the European and US drug labels [19].
These concepts have guided our recommendations on the therapeutic use of CladT in our region.
Current Evidence for the Use of CladT in the Treatment of Multiple Sclerosis
The Pivotal, Randomised CLARITY Study
The introduction of CladT into MS care was based on the results of the randomised CLARITY study [20]. Briefly, 1326 people with RRMS (with at least one relapse in the previous year and EDSS < 5.5 at baseline) were randomised to receive one of two doses of oral CladT (cumulative dose over 2 years of 3.5 mg/kg or 5.25 mg/kg) or placebo, given as short (4–5 day) courses separated by 4 weeks, administered at baseline and 1 year later. At 2 years, compared with placebo, CladT 3.5 mg/kg reduced the annualised relapse rate (0.14 vs. 0.33, p < 0.001), increased the proportion of patients who were relapse free (79.7% vs. 60.9%, p < 0.001) and reduced the risk of 3-month progression of disability (hazard ratio 0.67 [95% CI 0.48–0.93], p = 0.02).
At the end of 2 years, patients were rerandomised in a 2-year extension study to receive two additional courses of CladT (total additional cumulative dosage 3.5 mg/kg) or placebo [21]. Re-treatment with CladT at the beginning of years 3 and 4 did not provide further efficacy, compared with no active treatment during this period (placebo) [21]. This had important implications for the therapeutic application of CladT, as its European label states that “Following completion of the 2 treatment courses, no further cladribine treatment is required in years 3 and 4”.
The most common side effects noted in the randomised phase of CLARITY were lymphopenia (21.6% for CladT vs. 1.8% for placebo) and herpes zoster reactivation (8 patients vs. no patient, respectively). The relatively high incidence of lymphopenia was partly a result of the study design, and some flexibility has been introduced into the administration schedule for the second year treatment course (Table 3) [1, 16, 20–24].
Table 3.
Summary of selected commonly arising safety issues relevant to the therapeutic use of CladT in the routine management of relapsing MS
| Issue | Summary of available evidence |
|---|---|
| Lymphopenia | The absolute lymphocyte count (ALC) should be normal before initiation of cladribine tablets, and ≥ 800 cells/mm3 before initiating CladT treatment in year 2. The relatively high incidence of higher-grade lymphopenia in the CLARITY pivotal trial (although > 90% of cases of Grade 3 and 4 lymphopenia in that study had resolved to Grade 0–1 by the end of its extension phase) resulted from a rigid administration schedule which required administration of the second-year course of CladT irrespective of the ALC. As per the current label, the second year course of CladT can be delayed by up to 6 months to allow recovery of the lymphocytes [16] |
| Infections and vaccinations |
Screening for latent infections (especially tuberculosis and hepatitis B and C) is required prior to any administration of CladT for MS to avoid risk of reactivation; delay CladT administration until infections have been treated/resolved] [1, 3, 22] An adequate humoral response to vaccination, including for Covid-19, has been observed in people with relapsing MS during treatment with cladribine tablets [23, 24]. Herpes zoster infection during treatment with CladT has been associated mainly with low lymphocyte counts (< 200/mm3): vaccination against zoster and other potential infections (including tuberculosis in areas where this disease is endemic) is advised for patients previously unexposed to these infections. In general, vaccinate 4–6 weeks before the administration of CladT [21] |
| Malignancy | A higher incidence of malignancy was observed in a pooled clinical trial population receiving vs. not receiving CladT [21]. However, these concerns have largely abated as accumulating real-world evidence and comparisons of malignancy rates in CladT-treated patients with general populations indicate no excess risk of malignancy with CladT [1, 23]. CladT is contraindicated for people with active malignancy [21] |
Recommendations here have been paraphrased for conciseness: refer to the full labelling [21] before prescribing
Real-World Evaluations of CladT in People with RMS
A systematic review published in 2023 summarised the real-world experience with CladT up to that time [25]. Data from 20 real-world studies demonstrated a low rate of discontinuation of (or switch from) CladT during the first year of treatment (2–7% of patients across these studies). Breakthrough disease prompting discontinuation of CladT was seen most often in patients who had received previous treatment with a DMT, especially anti-trafficking agents (natalizumab or S1P inhibitors).
Table 1 summarises the results of recent real-world studies from the GCC region, which were mostly published or presented after the publication of the 2023 review (most of these data have been published as abstracts to date) [26–36]. Treatment with CladT was associated with marked reductions in indices of disease activity (relapse rates, disability progression, NEDA) [26]. The mean follow-up duration was short in most studies, as CladT is a relatively new addition to the MS treatment armamentarium; however, in the study by Hassan et al., efficacy was maintained in a substantial proportion of patients up to 5 years after treatment initiation [28]. Figure 1 summarises the main outcomes of this study. Inshasi et al. showed that no rebound activity occurred in patients initiating CladT following withdrawal of fingolimod or natalizumab [33].
Table 1.
Recent real-world studies of cladribine tablets in people with MS in the Gulf region
| Ref. (location) | N | Previously DMT-naïve | Overview of main findings |
|---|---|---|---|
| [26] (UAE) | 88 | 37% | 30/88 had taken the 2nd year treatment course. One relapse after 2 months, persistent MRI activity in one patient at the end of year 1. One discontinued for pregnancy. No EDSS progression. No Grade 4 lymphopenia (N = 1 for Grade 3, N = 2 for Grade 2, N = 72 for Grade 1) |
| [27] (UAE) | 69 | 29% | Mean 1.7 months follow-up. ARR reduced from 0.52 to 0.03. EDSS improved in 5, was stable in 55 and worsened in 1. 89.7% were relapse free, 91.2% were free from new/enlarged T2 lesions, 90% achieved NEDA-3 |
| [28] (UAE) | 19 | 21% | Mean follow-up 2.1 years. ARR reduced from 0.84 to 0.08, relapse free increased from 36.8% to 89.5%. 76% achieved NEDA-3, including all DMT-naïve patients, 80% of 2nd line, 86% of 3rd line and 33% of 4th line. 5.2% reported Grade 3 lymphopenia (no Grade 4) |
| [29] (UAE) | 68 | 26% | ARR reduced from 0.4 at baseline to 0.0–0.11 in years 1–5. 75% with ≥ 1 year of follow-up achieved NEDA-3 (incl. 92% of patients with MS disease activity in year 1). EDSS improved in 23%, was stable in 63% and declined in 13%. 7.2% reported Grade 3 lymphopenia (no Grade 4) |
| [30] (Qatar) | 54 | 46% | Average 30 months of follow-up. ARR reduced from 0.9 to 0.06; proportion relapse free increased from 29.6% to 88.9%. Similar MS and clinical MRI outcomes in previously DMT-naïve and in switchers from platform or high-efficacy DMTs. Five reported Grade 3 lymphopenia (no Grade 4) |
| [31] (Kuwait) | 123a | 100% | Observational study of 123 people with MS and high disease activity given CladT, alemtuzumab or ocrelizumab as their first DMT. Across treatments, ARR was 1.00–1.13 at baseline and 0.07–0.13 after treatment. Similar proportions across groups were relapse free (88–93%) or achieved NEDA-3 (84–90%). CDP was observed in 6.9% (ocrelizumab), 3.1% (CladT) and 0% (alemtuzumab); p < 0.28. Rates of AE were similar (18–25%) |
| [32, 33] (UAE) | 13 | 0% | Post hoc analysis from the CLUE studyb in 13/55 patients with MS and previously high MS disease activity who had previously received natalizumab or fingolimod and switched because of side effects (N = 5), non-adherence (N = 2), decision to start IRT (N = 2), JCV seropositivity (N = 4, all natalizumab). No rebound MS activity occurred, and there was no Grade 3 or 4 lymphopenia |
| [34] (Kuwait) | 200c | 45% | For CladT/ocrelizumab groups, EDSS improved in 7%/7%, was stable in 78%/82%, and declined in 11%/15%. CDP occurred in 7% of each group. AE occurred in 18.0%/19.4% |
| [35] UAE | – | – | Model-based budget impact analysis over a 5-year time horizon for MS care with vs. without use of CladT (UAE healthcare system perspective, comparators were DMF, fingolimod, natalizumab, ocrelizumab and alemtuzumab) for people with MS and high MS disease activity. Model inputs included total number of patients, market shares, and costs associated with drug administration, monitoring, AE management, and relapse therapy, from the literature and/or from interviews with local experts. Incorporation of CladT into MS care reduced overall budget by 3.9% assuming CladT accounted for 33% of the DMT market by year 5. Complete replacement of other DMTs by CladT resulted in a 23.1% budget reduction |
| [36] (Kuwait) | – | – | Similar analysis to that shown above, this time from the perspective of the healthcare system in Kuwait. Projected budget savings were 4.8% for incorporation of CladT into MS care and 28.5% for replacement of other DMTs with CladT |
ARR annualised relapse rate, CDP confirmed disability progression, CladT cladribine tablets, DMF dimethyl fumarate, DMT disease-modifying therapy, EDSS Expanded Kurtze Disability Status Scale, IRT immune reconstitution therapy, JCV John Cunningham virus, MS relapsing multiple sclerosis, N total number of patients included, NEDA-3 3-point no evidence of disease activity (no new relapses, no new MRI activity, no disability progression), UAE United Arab Emirates
a58 received ocrelizumab, 32 received alemtuzumab, 32 received CladT
bPreviously conducted observational study of patient satisfaction with treatment with CladT [28]
cAll had highly active MS (72 received CladT, 128 received ocrelizumab)
Fig. 1.
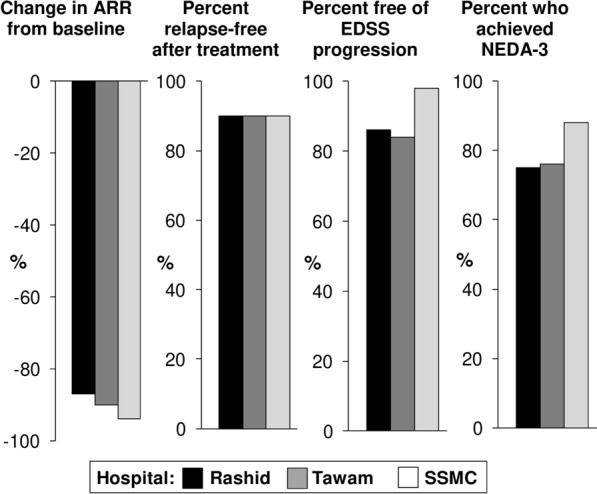
Main results from a 5-year real-world analysis of cladribine tablets in people with relapsing multiple sclerosis from three hospitals in the United Arab Emirates. 156 patients were analysed (68 at Rashid Hospital, Dubai, 19 at Tawam Hospital, Abu Dhabi and 69 at Sheikh Shakhbout Medical City (SSMC; Abu Dhabi). Annual relapse rate (ARR) at baseline was 0.4, 0.8 and 0.5, respectively. EDSS a high-efficacy DMT (≤ 2 years from MS diagnosis) was associated with a lower mean Expanded Kurtze Disability Status Scale, NEDA-3 3-point no evidence of disease activity. Drawn from data presented in Ref. [29]
Two pharmacoeconomic studies demonstrated budgetary savings compared with other high-efficacy DMTs when CladT was used in patients with highly active disease [35, 36]. Finally, Abdelmoneim et al. evaluated the impact of treatment with various high-efficacy DMTs on time requirements of healthcare professional (time needed in the clinic, laboratory, pharmacy, and for administration of the treatment) [37]. The administration of CladT tied up healthcare resources for less time compared with other high-efficacy DMTs (Fig. 2). The differences between DMTs were driven mainly by infusions requiring longer times for administration, and longer (but variable) pharmacy time needed for dimethyl fumarate (DMF), fingolimod, anti-CD20 agents and alemtuzumab (Fig. 2).
Fig. 2.
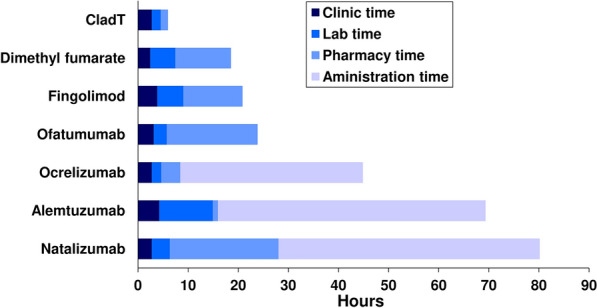
Average of estimated time consumed by healthcare professionals to administer disease-modifying therapies in Qatar, Kuwait and Bahrain. Experts in MS care in one tertiary MS centre each in Qatar, Kuwait and Bahrain estimated the time associated with therapeutic use of a disease-modifying therapy (as shown) required in the clinic, laboratory, and pharmacy, and for administration to the patient. Mean values of estimates are shown
Current Expert Opinion on Use of CladT in MS Care
Comparisons with Other Disease-Modifying Therapies
Randomised, head-to-head comparisons of high-efficacy DMTs are lacking, and comparisons between these agents must rely on real-world evidence. One study from Kuwait evaluated the effects of CladT, ocrelizumab or alemtuzumab in 123 treatment-naïve highly active patients with MS (Table 1) [31]. Across all treatment groups, there were similar and substantial reductions in the annual relapse rate (from 1.00–1.13 at baseline to 0.07–0.13 after treatment) and similar proportions of patients who were relapse free (88–93%) or achieved NEDA-3 (84–90%). Rates of confirmed disability progression did not differ significantly between groups. A study from the international MSBase registry showed that treatment with CladT was associated with a longer time to treatment switch or discontinuation compared with other orally administered DMTs (teriflunomide, DMF or fingolimod) [8]. However, the follow-up period on treatment was short (around 1 year).
The therapeutic profiles of available DMTs were compared using expert opinion, and the results of this exercise are described here. Experts rated the efficacy, tolerability and safety of DMTs commonly used for MS care in the GCC region (Fig. 3). Natalizumab, anti-CD20 and CladT were rated as most effective for controlling MS disease activity, followed by DMF and fingolimod and then the platform therapies. Platform therapies, ocrelizumab and CladT were rated similarly as the safest DMTs, with lower ratings for natalizumab, fingolimod and (especially) alemtuzumab. CladT was rated as the DMT with the highest tolerability, with interferon-beta the least well tolerated. This expert opinion consensus was consistent with the results of the CLUE study that demonstrated high levels of patient satisfaction with CladT treatment in patients residing in GCC countries: average scores for the validated Treatment Satisfaction Questionnaire for Medication 14 (v. 1.4) were 76.2 (95% CI 71.6–80.7) for effectiveness and 94.2 (95% CI 91.0–97.3) for tolerability (each out of a possible maximum 100) [28]. High average ratings for ease of use of CladT (87.4 [95% CI 83.7–91.0]) and overall treatment satisfaction (77.8 [95% CI 73.0–82.6]) were also reported in this study [32].
Fig. 3.
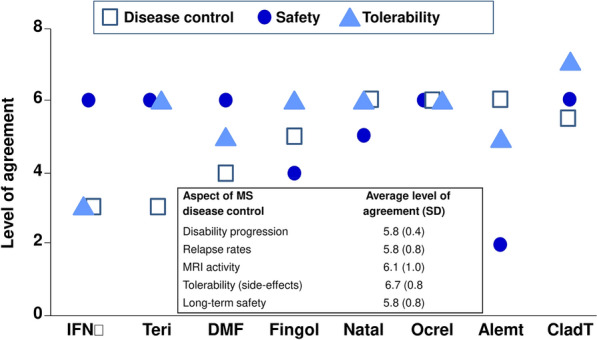
Expert perceptions of efficacy and safety of disease-modifying treatments (DMTs) for relapsing multiple sclerosis (MS). Chart shows the overall ratings of MS disease control, safety and tolerability for interferon-beta (INFβ), dimethyl fumarate (DMF), fingolimod (Fingol), natalizumab (natal), ocrelizumab (ocrel), alemtuzumab (alemt) and cladribine tablets (CladT). Inset: Experts from Kuwait (N = 7) rated each aspect of each DMT as shown (higher ratings denoted higher efficacy (MS disease control) or better overall safety); averages of these ratings are shown). This exercise did not include glatiramer acetate which is not available for prescription in most countries in the Gulf Cooperation Council region
Proposed Algorithm for Use of CladT in MS Care
Figure 4 provides an overview of recommendations for management of patients with MS who received treatment with CladT.
Fig. 4.
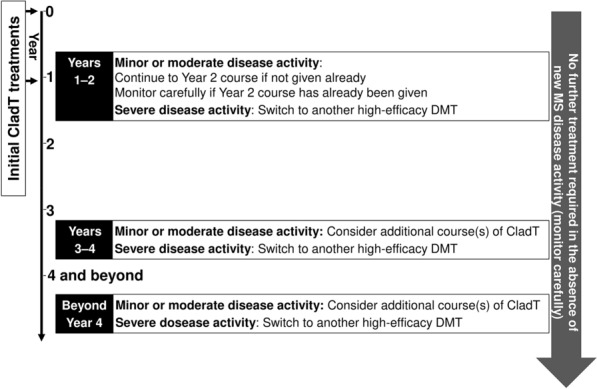
Overview of recommendations for long-term management of patients with MS who received treatment with CladT. “Year 1” extends from the first dose of the initial treatment course to the first dose of the second treatment course, 1 year later (assuming sufficient recovery of the absolute lymphocyte count, see text and Table 3). “Year 2” is the year following the first dose of the second, annual treatment course. “Year 3”, “Year 4”, etc., reflect successive 12-month periods thereafter. CladT cladribine tablets, DMT disease-modifying therapy, MS multiple sclerosis
Years 1 and 2
The management of patients with CladT from first administration should follow its label, whereby two courses of treatment are given at the beginning of years 1 and 2. It is important to note that the full efficacy of CladT is only achieved with the full 2-year course [38]. Accordingly, it may not be necessary to switch to an alternative DMT in the event of mild or moderate MS disease activity during year 1. The exception to this would be in rare situations where disease activity is paradoxically increased, in which case we recommend switching to another high-efficacy DMT.
Years 3 and 4
The European and US labels for CladT provide no guidance on the management of patients who develop recurrence of disease activity beyond year 2 of treatment. Experience with alemtuzumab, the other currently available IRT, shows that further treatment beyond year 2 can be beneficial [39]. Administering additional courses of CladT after 2 years does not appear to have adverse safety consequences: in the extension to the CLARITY study, comparison of patients who received two annual courses of CladT followed by two annual courses of placebo with patients who received four annual courses of CladT showed comparable rates of adverse events (76% vs. 80%, respectively) and serious adverse events (16% vs. 13%, respectively). There was an excess of lymphopenia and discontinuation due to lymphopenia in the re-treated cohort, which was in part due to re-treatment irrespective of the lymphocyte count, which is not consistent with the current label and practice, as discussed above. A reanalysis of the data based on the current label for re-treatment showed a significant decrease in the rate of severe lymphopenia [40]. Additional courses of CladT beyond 2 years are therefore likely to be beneficial and safe in patients with reactivation of MS disease activity at this time [21]. Such an approach has also been supported by other international expert consensus groups [3, 8, 18, 25, 41–43].
We recommend that no additional treatment is required in the absence of renewed disease activity, as per the product label. New mild disease activity, whether clinical relapses or new lesions on MRI, should prompt further course(s) of CladT (ensuring the absolute lymphocyte count is above 800 cells/mm3), or switch to an alternative DMT. A severe relapse at this time should prompt switching to other high-efficacy DMTs rather than administering another course of CladT.
Beyond Year 4
Observational data (summarised above) suggest there is a good chance of maintaining freedom from disease activity beyond 4 years, although more data are required here. Patients that are stable beyond year 4 should be followed carefully with regular monitoring, with special attention to cognitive functions, fatigue, urinary symptoms and patient-reported outcomes. Such patients are considered “cladribine responders” and therefore further course(s) of CladT may be considered in the event of new clinical or radiologic disease.
Use of CladT in Subgroups of People with MS
Older Patients
The IRT approach may also be appropriate for older patients for whom DMTs acting via continuous immunosuppression may increase the risk of infections. The efficacy of CladT does not appear to diminish above 45 years of age [44].
Patients Who are Planning a Pregnancy
The prolonged period free of MS disease activity seen in responders to IRT provides an opportunity to carry a pregnancy uncomplicated by concomitant treatment with a continuously applied DMT [43, 44]. However, any planned pregnancy would need to be delayed by 6 months following the last course of CladT [45, 46].
Switching to CladT from Other DMTs
Table 2 contains recommendations on suitable washout periods for patients switching to CladT from other DMTs. Patients treated with interferons, glatiramer acetate or DMF do not need a washout period between cessation of these agents and initiation of CladT. The only exception would be severe Grade 3 lymphopenia on DMF, which will require a longer washout period. The washout periods following administration of other DMTs reflect a compromise between allowing the effects of earlier DMTs to dissipate and providing protection against the possibility of resurgent MS disease activity. This is especially important for preventing rebound MS disease activity after withdrawing anti-trafficking agents (S1P inhibitors and natalizumab), where prompt initiation of CladT (no more that 4 weeks after withdrawing anti-trafficking agent) takes precedence over the usual careful monitoring of recovery of lymphocyte counts to normal levels before treatment. The accelerated washout procedure may be useful in facilitating recovery of lymphocytes in a patient who previously received teriflunomide. A washout period of 6 months is optimal following withdrawal of anti-CD20 agents, although again this can be shortened (to 2–3 months) in the setting of highly active MS.
Table 2.
Recommended washout periods disease-modifying therapies (DMTs) before initiation of treatment with cladribine tablets (CladT)
| Drug | Waiting period before switching | Comments |
|---|---|---|
| IFN, GA, DMF | No washout period | CladT can be initiated as soon as a normal lymphocyte count has been established (in case of severe Grade III lymphopenia on DMF a longer washout period is needed) |
| Teriflunomide | 4 weeks | In case of an emergency, serum levels of teriflunomide should be assessed. Accelerated elimination of the drug can also be considered before switching to cladribine tablets. This is done by using cholestyramine, either 4 g TID or 8 g TID, or charcoal 50 g BID for 11 days. However, this can cause severe constipation and tolerability issues or may be challenging to procure |
| Fingolimod | 4 weeks | Physicians do not have to wait for the lymphocyte count to reach 1000 mm−3 following withdrawal of S1P inhibitors or natalizumab, as this can increase the risk of rebound MS activity |
| Natalizumab | 4 weeks | |
| Ocrelizumab | 6 months | If a patient has highly active MS, they can be switched to CladT 2–3 months after stopping ocrelizumab |
| Ofatumumab | 3 months |
Based on expert opinion
BID twice daily, DMF dimethyl fumarate, GA glatiramer acetate, IFN interferons, TID three times daily
Common Safety Issues Arising During Treatment with CladT
Strategies for optimising the safety of CladT, with regard to common side effects and management of/vaccination for opportunistic infections, have been summarised in the product literature [21] and in previous articles (including from the perspective of healthcare delivery in the Middle East) [1, 22] and are not considered in detail within the current review. Table 3 summarises this information, along with a summary of current evidence to support a low risk of malignancy with CladT [1, 16, 20–24].
Conclusions
Real-world observational studies of people with MS treated with CladT have demonstrated long-term freedom from disease activity in a good proportion of patients. This clinical experience has also confirmed that treatment with CladT is generally safe and well tolerated. The use of high-efficacy DMTs such as CladT has traditionally been reserved for use in treatment-naïve patients with highly active MS or patients with breakthrough disease on one or more previous DMTs. Accumulating evidence that CladT is effective and safe in DMT-naïve patients with shorter disease duration may support the use of this agent in treatment-naïve patients with moderately or highly active disease as recommended in the updated MENACTRIMS treatment guidelines [17].
Acknowledgements
Medical Writing and Editorial Assistance
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial assistance (funded by Merck Serono Middle East FZ-Ltd, an affiliate of Merck KGaA).
Author Contributions
Bassem Yamout, Raed Alroughani, Jihad Inshasi, Samar Farouk, Amir Boshra were for conceptualisation and overall direction of the article. Amir Boshra oversaw resources and administration. Bassem Yamout, Raed Alroughani, Jihad Inshasi, and Amir Boshra oversaw writing of original draft. Bassem Yamout, Raed Alroughani, Jihad Inshasi, Samar Farouk, Fatema Abdulla, Namareq Y Al-Jarki, Abdulla Alasmi, Sarmad Al Fahad, Jaber Alkhabouri, Khalid Al-Saffar, Beatrice Benedetti, Beatriz Canibano, Dirk Deleu, Ali Hassan, Pournamy Sarathchandran, Ahmed Shatila, Mohammad Abouelnaga, Mona Thakre, Miklos Szolics, Amir Boshra: participated in reviewing and editing. The co-authors of the article remained in control of its content throughout.
Funding
Merck Serono Middle East FZ-Ltd, Dubai, UAE, an affiliate of Merck KGaA (CrossRef Funder ID: 10.13039/100009945) funded editorial support and the Rapid Service Fee.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
This article arose from discussions at meetings funded by Merck Serono Middle East FZ Ltd, an affiliate of Merck KGaA. Additionally: Dr Khalid Al Saffar is engaged as a speaker with pharmaceutical companies and is a member of advisory committees of Abbott, Novartis, Lilly, Pfizer, Merck, Sanofi, Janssen, Bayer, Biogen, Julfar, Alhikma and GlaxoSmithKline. Dr Pournamy Sarathchandran received speaker honoraria from Merck, Novartis, Roche, and Dr Ahmed Osman Shatila reports honoraria for lectures (Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Boehringer Ingelheim, Biologix) and for advisory boards (Sanofi-Genzyme, Roche, Novartis, Pfizer, Biologix); educational conferences travel and registration and hotel accommodation has been sponsored by Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Biologix. Dr Amir Boshra is an employee of Merck Serono Middle East FZ Ltd, Dubai, UAE, an affiliate of Merck KGaA. Dr Raed Alroughani received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. Dr Samar Farouk received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. Dr Bassem Yamout has received honoraria for lectures and advisory boards from Bayer, Biogen, Genpharm, Merck, Novartis, and Sanofi, and has received research grants from Bayer, Biogen, Merck, Novartis, and Pfizer. Dr Dirk Deleu has served on Advisory Boards of Merck, Novartis, Biologix, Roche and Sanofi and is a member of MENACTRIMS. Dr Beatriz Canibano has received travel, speaker and consultant honoraria from Merck, Novartis, Biologix, Roche and Sanofi. Dr Abdullah Al-Asmi received honoraria for serving on scientific advisory boards from Merck, Novartis, Roche, and Sanofi, and also received travel reimbursement from, Biologix, Sanofi, Merck, Roche, Bayer and Novartis. Drs Jihad S Inshasi, Sarmed Alfahad, Ali Hassan, Miklos Szolics, Jaber Alkhabouri, Beatrice Benedetti, Fatema Abdulla, Dr Namareq Alarji, Mohammad Abouelnaga, and Mona Thakre reported no other dualities of interest.
Ethical Approval
This narrative review is based on previously conducted studies and the clinical expertise of the authors in treating patients with RMS. No new clinical studies were performed by the authors. No patient-specific efficacy or safety data were reported. Therefore, institutional review board (IRB)/ethics approval was not required.
References
- 1.AlSharoqi IA, Aljumah M, Bohlega S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? a narrative review. Neurol Ther. 2020;9:55–66. 10.1007/s40120-020-00187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord. 2019;28(12):1756286419836913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sèze J, Suchet L, Mekies C, et al. The place of immune reconstitution therapy in the management of relapsing multiple sclerosis in France: an expert consensus. Neurol Ther. 2023;12:351–69. 10.1007/s40120-022-00430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther. 2022;11:571–95. 10.1007/s40120-022-00339-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–74. 10.1016/j.msard.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency. Lemtrada. Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada. https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada. Accessed Jan 2024.
- 7.Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf region expert opinion. Neurol Ther. 2019;8:13–23. 10.1007/s40120-019-0129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spelman T, Ozakbas S, Alroughani R, et al. Comparative effectiveness of cladribine tablets versus other oral disease-modifying treatments for multiple sclerosis: results from MSBase registry. Mult Scler. 2023;29:221–35. 10.1177/13524585221137502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edan G, Le Page E. Escalation versus induction/high-efficacy treatment strategies for relapsing multiple sclerosis: which is best for patients? Drugs. 2023;83:1351–63. 10.1007/s40265-023-01942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller J, Cagol A, Lorscheider J, et al. Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: a systematic review. JAMA Neurol. 2023;80:1232–45. 10.1001/jamaneurol.2023.3331 [DOI] [PubMed] [Google Scholar]
- 11.Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80:151–60. 10.1001/jamaneurol.2022.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonsen CS, Flemmen HØ, Broch L, et al. Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol. 2021;12:693017. 10.3389/fneur.2021.693017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–16. 10.1016/S1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 14.Buron MD, Chalmer TA, Sellebjerg F, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology. 2020;95:e1041–51. 10.1212/WNL.0000000000010135 [DOI] [PubMed] [Google Scholar]
- 15.Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78:1197–204. 10.1001/jamaneurol.2021.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summary of Product Characteristics for Mavenclad® (cladribine tablets). https://www.medicines.org.uk/emc/product/8435/smpc. Accessed Jan 2024.
- 17.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. 10.1177/1352458517751049 [DOI] [PubMed] [Google Scholar]
- 18.Yamout B, Al-Jumah M, Sahraian MA, et al. Consensus recommendations for diagnosis and treatment of multiple sclerosis: 2023 revision of the MENACTRIMS guidelines. Mult Scler Relat Disord. 2024;83: 105435. 10.1016/j.msard.2024.105435 [DOI] [PubMed] [Google Scholar]
- 19.Alroughani R, Inshasi J, Al-Asmi A, et al. Expert consensus from the Arabian Gulf on selecting disease-modifying treatment for people with multiple sclerosis according to disease activity. Postgrad Med 2020;132:368–376. [DOI] [PubMed]
- 20.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–26. 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
- 21.Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24:1594–604. 10.1177/1352458517727603 [DOI] [PubMed] [Google Scholar]
- 22.Clavelou P, Castelnovo G, Pourcher V, et al. Expert narrative review of the safety of cladribine tablets for the management of relapsing multiple sclerosis. Neurol Ther. 2023;12:1457–76. 10.1007/s40120-023-00496-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mimpen M, Kreiter D, Kempkens T, Knippenberg S, Hupperts R, Gerlach O. Humoral immune response after SARS-CoV-2 vaccination in cladribine-treated multiple sclerosis patients. Vacc X. 2024;16: 100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inshasi J, Alroughani R, Al-Asmi A, et al. Expert consensus and narrative review on the management of multiple sclerosis in the Arabian Gulf in the COVID-19 era: focus on disease-modifying therapies and vaccination against COVID-19. Neurol Ther. 2021;10:539–55. 10.1007/s40120-021-00260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oreja-Guevara C, Brownlee W, Celius EG, et al. Expert opinion on the long-term use of cladribine tablets for multiple sclerosis: systematic literature review of real-world evidence. Mult Scler Relat Disord. 2023;69: 104459. 10.1016/j.msard.2022.104459 [DOI] [PubMed] [Google Scholar]
- 26.Thakre M, Inshasi J. Real world experience of oral immune reconstitution therapy (cladribine) in the treatment of multiple sclerosis in the United Arab Emirates. Mult Scler J. 2020;26 (S3):185 (Abstract).
- 27.Samad HA, Riachi N, Barakat A, Shatila S. Sustained no evidence of disease activity over 4 years for patients treated with cladribine tablets: cohort study from Sheikh Shakbout Medical Center, Abu Dhabi. Mult Scler Relat Disord. 2023:80:105305 (Abstract).
- 28.Hassan AM, Hassan AM, Aldhuhoori A, et al. Early use of cladribine tablets impact on disease control in patients with relapsing multiple sclerosis: cohort study from Tawam Hospital, UAE. Mult Scler Relat Disord. 2023;80:105311 (Abstract).
- 29.Inshasi J, Alshamali SA, Al Madani A. Real world experience for cladribine tablets in management of relapsing multiple sclerosis in UAE: Cohort study from Rashid Hospital, Dubai. Mult Scler Relat Disord. 2023;80:105320 (Abstract).
- 30.Baniamer YZ, Deleu D, Ibrahim F, Garcia Canibano B. Effectiveness and safety results from real world experience for cladribine tablets in management of relapsing multiple sclerosis in Qatar- 30 months follow up. Mult Scler Relat Disord 2023;80:105197 (Abstract).
- 31.Alroughani R, Al-Hashel J, Ahmed SF. Early initiation of highly efficacy disease modifying therapies in naïve relapsing remitting multiple sclerosis patients reduce disease activity and disability. Mult Scler Relat Disord. 2023;80:105326 (Abstract).
- 32.Inshasi J, Farouk S, Shatila A, et al. Multicentre observational study of treatment satisfaction with cladribine tablets in the management of relapsing multiple sclerosis in the Arabian Gulf: the CLUE study. Neurol Ther. 2023;12:1309–18. 10.1007/s40120-023-00497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inshasi J, Al Roughani R, Al Shamali SA, Shatila A, Szolics M, Benedetti B. Rebound of multiple sclerosis disease activity after switching from fingolimod or natalizumab to cladribine tablets: real world experience from the Arabian Gulf. Mult Scler Relat Disord. 2023;80:105335 (Abstract).
- 34.Alroughani R, Al-Hashel J, Alshwaf, et al. Ocrelizumab and cladribine had similar EDSS stability in patients with highly active multiple sclerosis. Mult Scler Relat Disord 2023;80:105330 (Abstract).
- 35.Inshasi J, Yamout B, Farghali M, et al. Budget impact analysis for introduction of cladribine tablets as a treatment for high disease active relapsing multiple sclerosis in UAE. Value Health. 2022;25(Issue 1, Suppl):S105 (Abstract).
- 36.Alroughani R, Ahmed SF, Abokoura A, et al. Economic analysis for introduction of cladribine tablets as a treatment for relapsing multiple sclerosis patients with high disease activity in Kuwait. MS Relat Disord. 2022;59:103627 (Abstract).
- 37.Abdelmoneim MS, Garcia Canibano B, Farouk S, Abdulla F, Boshra A, Alroughani R. Time requirements of healthcare professionals for managing disease-modifying therapies for multiple sclerosis in Qatar, Kuwait and Bahrain. Mult Scler Relat Disord. 2023;80:105317 (Abstract).
- 38.De Stefano N, Sormani MP, Giovannoni G, et al. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: the CLARITY and CLARITY Extension studies. Mult Scler. 2022;28:111–20. 10.1177/13524585211010294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coles AJ, Achiron A, Traboulsee A, et al. Safety and efficacy with alemtuzumab over 13 years in relapsing-remitting multiple sclerosis: final results from the open-label TOPAZ study. Ther Adv Neurol Disord. 2023;16:17562864231194824. 10.1177/17562864231194823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook S, Comi G, Giovannoni G, et al. Rates of lymphopenia in years 1–4 in patients with relapsing multiple sclerosis treated annually with cladribine tablets. Abstract 039 at the 2018 meeting of the Australian & New Zealand Association of Neurologists (ANZAN). J Neurol Neurosurg Psych. 2018;89:A16. https://jnnp.bmj.com/content/89/6/A16.2. Accessed Mar 2024.
- 41.Habek M, Drulovic J, Brecl Jakob G, et al. Treatment with cladribine tablets beyond year 4: a position statement by southeast European multiple sclerosis centers. Neurol Ther. 2023;12:25–37. 10.1007/s40120-022-00422-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centonze D, Amato MP, Brescia Morra V, et al. Multiple sclerosis patients treated with cladribine tablets: expert opinion on practical management after year 4. Ther Adv Neurol Disord. 2023;16:17562864231183220. 10.1177/17562864231183221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meuth SG, Bayas A, Kallmann B, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets beyond year 4. Expert Opin Pharmacother. 2022;23:1503–10. 10.1080/14656566.2022.2106783 [DOI] [PubMed] [Google Scholar]
- 44.Giovanonni G, Rammohan K, Cook S, et al. Cladribine tablets 35 mg/kg is efficacious in patients aged above and below 45 years with relapsing multiple sclerosis in the Clarity Study. MS Relat Disord. 2018;26:P262. 10.1016/j.msard.2018.10.097 [DOI] [Google Scholar]
- 45.Al Jumah M, Al Malik Y, AlKhawajah NM, et al. Family planning for people with multiple sclerosis in Saudi Arabia: an expert consensus. Mult Scler Int. 2021;2021:6667006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alroughani R, Inshasi J, Al-Asmi A, et al. Disease-modifying drugs and family planning in people with multiple sclerosis: a consensus narrative review from the Gulf region. Neurol Ther. 2020;9:265–80. 10.1007/s40120-020-00201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


