Abstract
Introduction
Multiple sclerosis (MS) is a chronic neurodegenerative disease that leads to impaired cognitive function and accumulation of disability, with significant socioeconomic burden. Serious unmet need in the context of managing MS has given rise to ongoing research efforts, leading to the launch of new drugs planned for the near future, and subsequent concerns about the sustainability of healthcare systems. This study assessed the changes in the Italian MS market and their impact on the expenditures of the Italian National Healthcare Service between 2023 and 2028.
Methods
A horizon-scanning model was developed to estimate annual expenditure from 2023 to 2028. Annual expenditure for MS was calculated by combining the number of patients treated with each product (clinical inputs) and the yearly costs of therapy (economic inputs). Baseline inputs (2020–2022) were collected from IQVIA® real-world data, while input estimation for the 5-year forecast was integrated with analog analyses and the insights of clinicians and former payers.
Results
The number of equivalent patients treated in 2028 in Italy was estimated at around 67,000, with an increase of 10% versus 2022. In terms of treatment pattern evolution, first-line treatments are expected to reduce their shares from 47% in 2022 to 27% in 2028, and Bruton tyrosine kinase inhibitors are expected to reach 23% of patient shares. Overall, expenditure for MS is estimated to decrease from €721 million in 2022 to €551 million in 2028, mainly due to losses of exclusivity and renegotiation of drug prices.
Conclusion
Despite the increase in the number of patients treated for MS and the launch of new molecules that will reach high market penetration, the model confirmed sustainability for the Italian National Healthcare Service.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00644-3.
Keywords: Multiple sclerosis, Costs, Forecast, Italian National Healthcare Service expenditure, BTKi, Treatment pattern
Key Summary Points
| Why carry out this study? |
| The launch of several new drugs in multiple sclerosis (MS) is expected in the near future, leading to concerns about sustainability for healthcare systems. |
| This study assessed expected changes in the MS landscape in Italy and their impact on expenditure by the Italian National Healthcare Service between 2023 and 2028. |
| What was learned from the study? |
| The number of equivalent MS patients treated in 2028 in Italy is estimated to increase by around 10% versus 2022. |
| First-line treatments currently in use are expected to progressively reduce their patient shares until 2028 in favor of treatments predominantly used as second line. |
| Despite the increase in the number of patients treated for MS and the launch of new innovative molecules, expenditure for MS is estimated to decrease from €721 million in 2022 to €551 million in 2028, mainly due to loss of exclusivity and renegotiation of drug prices. |
| The data collected to inform the model show long-term sustainability for the Italian National Healthcare Service and future availability of resources for new innovative treatment options. |
Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease affecting the brain and spinal cord [1, 2]. It is the most common non-traumatic disabling disease in young adults, affecting over 2 million people, with a worldwide prevalence ranging from 50 to 300 per 100,000 [1]. The mean incidence in Italy was estimated as 3.54 per 100,000, with a higher number in women than men (4.52 vs. 2.52) [3]. Both the incidence and prevalence of MS are increasing globally, although the underlying cause of this remains uncertain [1, 4].
MS can manifest clinically through several modalities, including relapsing–remitting MS (RRMS), secondary progressive MS (SPMS, further subdivided into active [aSPMS] and non-relapsing [NR-SPMS]), and primary progressive MS (PPMS) [5]. Collectively, RRMS and aSPMS are also called relapsing MS (RMS). The most frequent phenotype is RRMS, but it is estimated that 10% of patients convert to SPMS at 10 years, 50% at 20 years, and more than 90% at 30 years [6]. The subdivision of MS into different forms is the subject of ongoing scientific debate, given that emerging evidence suggests that the disease is a continuum characterized by a common underlying biology. The accumulation of disability, more or less evident, can occur from the onset and is driven by a process of smoldering neuroinflammation, which develops in parallel to the acute inflammation phenomena typical of the RRMS phase. Today, the previously mentioned descriptors of disease progression hold regulatory significance for defining the therapeutic applications of treatments, though this situation may be re-evaluated considering new scientific evidence [5, 7].
The severity of the disease has led to a continuous search for agents with new mechanisms of action to improve the efficacy of treatment [8, 9]. Currently, disease-modifying therapies (DMTs) are the cornerstone of treatment for MS, and the number of DMTs approved for MS has expanded in recent decades (Table 1) [10]. DMTs can induce a relevant change in the clinical course of MS by targeting a range of mechanisms such as immune cell suppression and depletion (B-cell-depleting therapies, e.g., ocrelizumab), immune modulation (e.g., interferons, fumarates), and enhanced immune cell sequestration (e.g., fingolimod) [10].
Table 1.
Currently approved treatments for MS in Italy
| Class | Active principle | Therapeutic indication | |||
|---|---|---|---|---|---|
| RMS | PMS | ||||
| RRMS | aSPMS | PPMS | NR-SPMS | ||
| Teriflunomide | teriflunomide | ✔ | |||
| Interferons | interferon beta-1a | ✔ | |||
| interferon beta-1b | ✔ | ✔ | |||
| peginterferon beta-1a | ✔ | ||||
| Glatiramer acetate | glatiramer acetate | ✔ | |||
| Fumarates | dimethyl fumarate | ✔ | |||
| S1P modulators | fingolimod | ✔ | |||
| siponimod | ✔ | ||||
| ponesimod | ✔ | ||||
| ozanimod | ✔ | ||||
| Anti-CD20 | ofatumumab | ✔ | |||
| ocrelizumab | ✔ | ✔ | |||
| Anti-CD52 | alemtuzumab | ✔ | |||
| Cladribine | cladribine | ✔ | |||
| Anti-alpha-4-integrin | natalizumab | ✔ | |||
The table shows, in order, class, active principle, and therapeutic indication as approved by the European Medicines Agency
RMS relapsing multiple sclerosis; RRMS relapsing–remitting multiple sclerosis; aSPMS active secondary progressive multiple sclerosis; PMS progressive multiple sclerosis; PPMS primary progressive multiple sclerosis; NR-SPMS non-relapsing secondary progressive multiple sclerosis
Despite the availability of different mechanisms of action, significant unmet needs still exist in the treatment of MS. Given the multifactorial pathogenesis of MS, none of the available DMTs is considered curative, as they are mainly aimed at controlling disease activity and reducing the rate of relapses [11]. Indeed, these drugs mainly target acute neuroinflammatory processes triggered by the migration of immune cells from the periphery into the central nervous system (CNS) and are largely ineffective when progression of the disease and accumulation of disability are driven by chronic neurodegeneration and smoldering inflammation [8]. Consequently, there are few to no treatment options that can satisfactorily modify the clinical course of the progressive forms of MS (i.e., aSPMS, PPMS, and NR-SPMS), which result in impaired cognitive function, accumulation of disability, reduced productivity, and significant limitations in daily activities.
The resulting socioeconomic burden has called for efforts to develop new therapeutic agents, as highlighted by the new products expected to be approved in the near future [8, 12–16]. In particular, according to information available as of November 2023, the launch of the monoclonal antibody ublituximab belonging to the α-CD20 class is expected in Italy, as it received marketing authorization from the EMA in May 2023 [17]. In addition, the Bruton tyrosine kinase inhibitors (BTKis) which are now in phase 3 studies will likely be launched in the Italian market by 2028 [18]. Acting as modulators of BTK, BTKis have the potential to influence both acute neuroinflammatory responses and chronic, compartmentalized neuroinflammatory processes, as well as neurodegeneration, by crossing the blood–brain barrier and targeting resident immune cells [8, 19]. The potency and selectivity of each BTKi are determined by their unique pharmacokinetic and pharmacodynamic profiles, leading to variations in their effectiveness and action [20, 21]. BTKis are also expected to have greater clinical efficacy than currently available treatment for progressive forms of MS [22]. In line with this, the BTKi tolebrutinib is under investigation as a potential treatment for NR-SPMS, a condition for which there are no approved therapies at present [23], as well as for PPMS, for which ocrelizumab is currently the only approved therapy [24].
The new launches expected in the MS therapeutic landscape will directly impact the economic sustainability of the National Healthcare Service, which in 2022 sustained total expenditure of more than €700 million for prescription of MS-related medications [25]. As MS currently ranks as the fifth largest category of per capita expenditure on direct purchases by public healthcare facilities (following oncologic drugs, immunosuppressants and immunomodulators, antidiabetics, and anticoagulants), careful forecasting of the economic impact of the expected changes is critical for ensuring the availability of resources to sustain innovation [25].
The present study integrated quantitative and qualitative sources to evaluate changes in treatment patterns and consequent forecasted expenditure for MS by the Italian National Healthcare Service between 2023 and 2028.
Methods
Expenditure Estimation
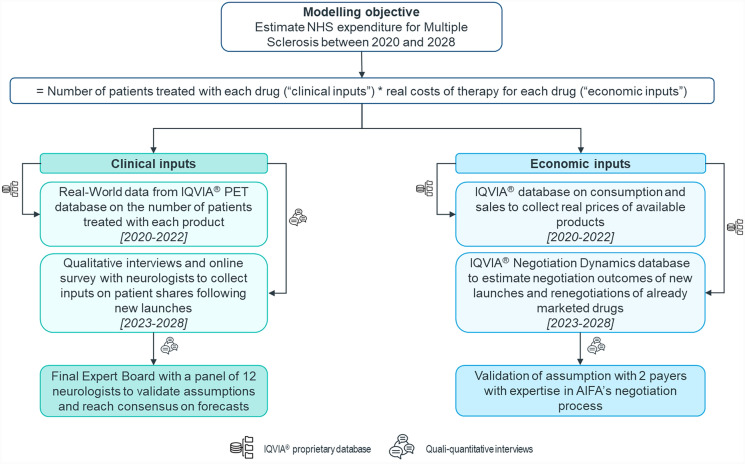
An Excel-based horizon-scanning model was developed to estimate the trend in annual expenditure from 2020 to 2028 for DMTs indicated for treating MS in Italy. Annual expenditure for MS was calculated by multiplying the number of patients receiving each specific treatment, defined as “clinical inputs”, by the yearly costs of each available therapy, referred to as “economic inputs” (Fig. 1).
Fig. 1.
Overview of the methodology and sources used to define and validate clinical (on the left) and economic (on the right) inputs of the model. All clinical and economic inputs were collected either via IQVIA®’s proprietary database or in qualitative/quantitative interviews with experienced neurologists and payers. All clinical inputs were validated by a panel of neurologists in a virtual expert board
Clinical and economic inputs resulted from the integration of quantitative data sources, which include three different IQVIA® proprietary databases (i.e., Patient Equivalent Tracker [PET], IQVIA® database on consumption and sales, and a database on negotiation dynamics), and qualitative sources represented by interviews with Italian experts, both clinicians (n = 16) and payers (n = 2). Baseline inputs (2020–2022) on the number of treated patients and real costs of therapy of DMTs were collected from real-world data, while input estimation for the 5-year forecast (2023–2028) required integration of analog analyses with the insights of clinicians and former payers to consider the impact of future events (i.e., new launches and losses of exclusivity) on the MS market.
No ethical committee approval was required for this study. All databases used in the study are property of IQVIA® and are accessible by its employees at any time.
Clinical Inputs
Baseline Input Collection (2020–2022)
Patients treated with DMTs between 2020 and 2022. The PET, an IQVIA® proprietary database, was used to extract real-world data on the number of patients with MS treated with each available drug for MS in Italy between 2020 and 2022. The PET database relies on posology claims, adjustment factors related to therapeutic efficacy, and any information captured through primary market research to provide a consistent measure of patients with MS (patient equivalents) currently undergoing treatment starting from units of consumption and sales volumes, therefore accounting for both untreated patients who eventually start a treatment for MS and patients who discontinue treatment during the year. A time series forecast algorithm is then used in the database to mitigate inconsistent months in which there is an abnormal behavior in product consumption, but not in the actual number of patients being treated.
Forecast Input Collection (2023–2028)
Stakeholder engagement and input validation. All clinical assumptions implemented in the model forecast (i.e., quantification of future treated population and patient share evolution) were validated with a representative panel of 16 experienced neurologists evenly distributed across Italy. In 2022, two rounds of interviews were conducted with a panel of four neurologists to collect preliminary inputs and validate assumptions developed by IQVIA® and Sanofi. In 2023, a computer-assisted quantitative survey was conducted with 12 neurologists to collect additional inputs and refine preliminary forecasts. The survey was developed by IQVIA® and Sanofi and conducted by IQVIA®, utilizing the Decipher survey platform for programming and hosting. Data collected from the survey were further validated with responders during a virtual expert board meeting in November 2023 to reach a final consensus on model projections and clinical inputs.
Quantification of future treated population. The total number of patients receiving MS treatment between 2023 and 2028 was determined by applying the compound annual growth rate (CAGR) of treated patients calculated for the baseline period (2020–2022) to forecast projections. This assumption allowed to estimate the future balance of patients undergoing treatment, including those starting and ending treatment in the same year. Manual adjustments were applied to account for clinician insights regarding the expected increase in the number of patients treated for NR-SPMS, following the availability of a drug specifically indicated for this condition, assuming that the launch of a product indicated for a form of MS for which no treatments are available would result in an increase in the number of treated patients.
Patient shares evolution. The model assumes that patient shares of already marketed drugs and drugs undergoing loss of exclusivity (LoE) remain stable unless impacted by new launches. Hence, all evidence collected from clinicians regarding the impact of new launches on the MS market were gathered to estimate the resulting variations in patient shares of currently marketed drugs.
Estimation of patient shares of new launches. Clinician inputs were collected to estimate the potential impact of new molecules on the MS market in terms of peak shares expected for new launches, time needed to reach the peak, and sources of business (defined as the molecules from which the new launches take market share). All inputs were collected according to clinicians’ perceptions of new drugs on the basis of data from clinical trials, if published, or other available scientific literature.
Having set 2024 as the threshold date for primary completion of phase III studies, among the BTKis under study only evobrutinib and tolebrutinib were included in the model (Table 2). Specific focus was developed to estimate the additional population eligible for treatment after the launch of new molecules for the NR-SPMS form.
Table 2.
Overview of phase III clinical trials of Bruton tyrosine kinase inhibitors: tolebrutinib, fenebrutinib, evobrutinib, and remibrutinib (source: ClinicalTrials.gov)
| Molecule | Clinical trial ID | Indication | Phase | Sponsor | Primary completion date |
|---|---|---|---|---|---|
| Tolebrutinib | NCT04410978 | RMS | III | Sanofi | 04/2024 |
| NCT04410991 | 04/2024 | ||||
| NCT04458051 | PPMS | 08/2024 | |||
| NCT04411641 | NR-SPMS | 08/2024 | |||
| Fenebrutinib | NCT04586010 | RMS | III | Roche | 10/2025 |
| NCT04586023 | 10/2025 | ||||
| NCT04544449 | PPMS | 01/2026 | |||
| Evobrutinib | NCT04338022 | RMS | III | Merck | 10/2023 |
| NCT04338061 | 10/2023 | ||||
| Remibrutinib | NCT05147220 | RMS | III | Novartis | 04/2026 |
| NCT05156281 | 04/2026 |
NR-SPMS non-relapsing secondary progressive multiple sclerosis; PPMS primary progressive multiple sclerosis; RMS relapsing multiple sclerosis
Estimation of penetration of generics and biosimilars. An analog analysis was performed to estimate the penetration of generics/biosimilars of drugs that will undergo loss of patent protection in the MS market by 2028 [26]. Penetration of generics/biosimilars in Italy was defined as the shares of days of therapy (DoT) of generics/biosimilars over the total DoT sold for the molecule according to the IQVIA® database on consumption and sales. To obtain reliable results for the MS context, analogs with an indication in a chronic therapeutic area and with the same reimbursement class and distribution channel as MS products were selected. In the case of biosimilars, those with a launch date after 2015 were included to consider the second wave of biosimilar launches [27].
Economic Inputs
Baseline Input Collection (2020–2022)
Cost of therapy of marketed DMTs. Annual costs of therapy of DMTs available on the market up to 2022 were calculated leveraging the IQVIA® database on consumption and sales data, which allows for the statistical elaboration of expenditure data from a hospital panel to estimate costs sustained by the National Healthcare Service for products commercialized in Italy. The elaborated price includes mandatory, negotiated, and tender discounts applied to ex-factory prices, while posology data are derived from the Summary of Product Characteristics for each product available on the EMA website.
Forecast Input Collection (2023–2028)
Stakeholder engagement and input validation. Price estimation assumptions implemented in the model forecast were validated with two former payers with extensive expertise in drug pricing negotiations and with knowledge of the Italian Medicines Agency (AIFA) procedures and decision-making process.
Price estimation. For molecules that will be launched on the market starting from 2024, the annual real cost of therapy was estimated based on the weighted average real cost of therapy of products in the same line of therapy at the time of the new drug’s expected reimbursement.
Price renegotiation of already marketed drugs. To ensure expenditure control, AIFA might ask manufacturers to renegotiate pricing conditions several times during each product’s life cycle. To account for this, an analog analysis was performed on an IQVIA®’s proprietary database on negotiation dynamics in Italy to estimate the timing and frequency of renegotiations, as well as resulting discounts following price renegotiations, of already marketed drugs between 2023 and 2028. The database on negotiation dynamics provides insights related to negotiation outcomes in Italy for products that received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) for their first indication starting from 2015. The database was leveraged to retrieve the average time between the first price negotiation and subsequent pricing renegotiations for products included in the panel, as well as level of additional discount requested by AIFA during the renegotiation.
Price erosion of molecules following LoE. In Italy, the introduction of generics/biosimilars triggers mandatory public tenders to guarantee the ability of the National Healthcare Service to purchase drugs at the lowest acquisition costs. Therefore, the introduction of generics and biosimilars induces relevant price cuts for originators. An estimation of tender price cuts of drugs that will undergo loss of patent protection in the MS market by 2028 was performed using the same analogs selected for the estimation of clinical inputs using IQVIA®’s databases. The IQVIA® database on consumption and sales was leveraged to assess historical trends in pricing dynamics of analogs to estimate potential prices and real costs of therapy for originators and generics/biosimilars following LoE.
Sensitivity Analysis
Given the uncertainties of some of the parameters considered in the analysis, a sensitivity analysis was conducted. The parameters that were varied include, among economic inputs, the discount at launch for new molecules, renegotiation discount, discount for biologics after LoE, discount for small molecules after LoE, and renegotiation frequency. The clinical parameters included in the analysis are generics penetration, biosimilars penetration, expected patient shares of ublituximab, tolebrutinib in RMS and PMS and evobrutinib, and the expected increase in treated patients for NR-SPMS. For all parameters, a variation of ± 20% of the baseline values was considered. The baseline parameter considered was the expenditure reduction between 2022 and 2028.
Results
Clinical Inputs
Baseline and Quantification of Future Treated Population
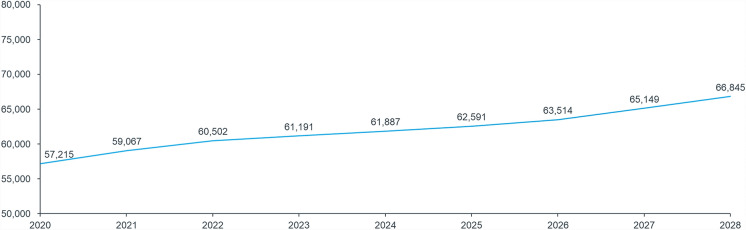
According to IQVIA®’s PET data, the number of patients with MS in treatment grew steadily between 2020 and 2022, from approximately 57,200 to approximately 60,500, with a resulting CAGR of 0.3%. In line with clinicians’ insights, CAGR was applied to projections between 2023 and 2028 (Fig. 2). An additional +4% increase in the number of treated patients was considered following the introduction of a specific treatment for patients with NR-SPMS in 2026, for whom currently no treatment is approved. Hence, in 2028, approximately 67,000 patients are expected to be treated for MS in Italy (Fig. 2).
Fig. 2.
Total population of treated patients, based on IQVIA® PET database (2020–2022) and estimates (2023–2028). Total growth was calculated according to CAGR and the increase in the number of patients following the availability of new treatments. Starting from 2026, the reimbursement of tolebrutinib for the treatment of NR-SPMS is expected to lead to a progressive increase the in total number of treated patients up to 4%
Patient Share Evolution
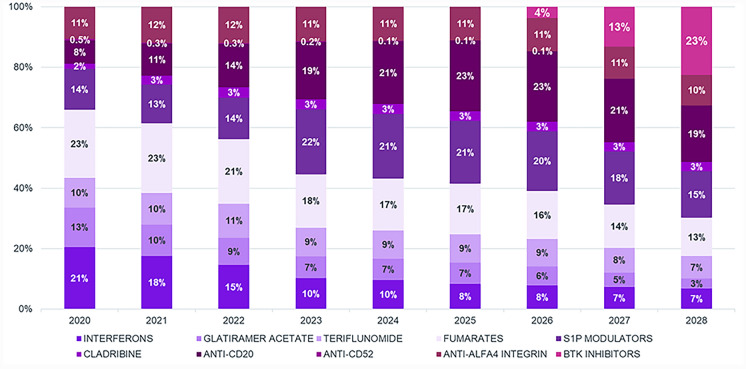
Clinical inputs on patient share evolution after new launches suggest that some first-line treatments (fumarates, teriflunomide, interferons) are expected to reduce their patient shares from 47% in 2022 to 27% in 2028 (Fig. 3). Other first-line injectables such as glatiramer acetate are expected to progressively reduce their patient shares, reaching 3% in 2028. (Fig. 3).
Fig. 3.
Patient shares of classes of products available for MS. Figure includes real-world data (2020–2022) as well as projections (2023–2028)
Among second-line treatments, anti-CD20s are expected to increase their patient shares by 5% (from 14% in 2022 to 19% in 2028), while sphingosine-1-phosphate modulators, despite an initial increase in their patient shares between 2023 and 2025 due to the recent introduction of several new molecules in this class (i.e., siponimod, ponesimod, and ozanimod), will ultimately maintain their patient shares in 2028 (from 14% in 2022 to 15% in 2028) (Fig. 3).
BTKis are expected to reach 23% of patient shares. Adoption of BTKis is expected to be consistent, given the innovative mechanism of action and high expectations of clinicians.
Analog Analysis
The analogs selected to estimate the expected penetration of generics over originators included entecavir for hepatitis B, bosentan for pulmonary hypertension, and atazanavir and darunavir for HIV (Supplementary Fig. 1). The analysis of total volumes sold for both originators and generics showed that generics reached an average market share penetration of 74% over originators after 2 years. Analogs selected to estimate the penetration of biosimilars over originators included etanercept and infliximab for autoimmune disease and rituximab for oncohematology and rheumatoid arthritis. The analysis showed that biosimilars reached an average market share penetration of 65% after 3 years (Supplementary Fig. 1).
Economic Inputs
All the implemented assumptions validated with payers are summarized in Table 3.
Table 3.
Overview of economic assumptions considered to build the model, resulting from the integration of data from IQVIA® databases and qualitative interviews with clinicians and former payers
| Category | Assumptions |
|---|---|
| Renegotiations of already marketed drugs |
• In the absence of an extension of indication, a 5% additional discount will be applied on top of the discount obtained at previous negotiation • With extension of indication, an 8% additional discount will be applied on top of the one obtained at previous negotiation • Renegotiations are assumed to happen every 3 years • No renegotiation for drugs that have not been renegotiated in the last 5 years |
| Tender discounts of generics/biosimilars |
• Branded small molecules: application of a maximum discount of 70% vs. ex-factory price, reached 2 years after LoE • Generics: application of a maximum discount of 89% vs. originator ex-factory price, reached one year after LoE • Biologics: application of a maximum discount of 41% vs. ex-factory price, reached 3 years after LoE • Biosimilars: application of a maximum discount of 72% vs. originator ex-factory price, reached 3 years after LoE |
| Negotiation of new launches |
• Ublituximab: same cost of therapy per year as 2L treatments weighted average at launch • Tolebrutinib RMS and tolebrutinib PMS: same cost of therapy per year as 2L treatments weighted average at launch • Evobrutinib: same cost of therapy per year as 2L treatments weighted average at launch |
LoE loss of exclusivity; PMS progressive multiple sclerosis; RMS relapsing multiple sclerosis
Price renegotiations of already marketed drugs. Based on analog analysis, renegotiations of already marketed drugs are expected to be scheduled every 3 years, with an additional discount of 5% applied to ex-factory prices. An 8% additional discount on ex-factory prices was considered in the case of indication extension. In line with payers’ insights, no renegotiations were considered for drugs that had not renegotiated in the last 5 years.
Price erosion of molecules following LoE. Based on analog analyses and payer consensus, it was estimated that the maximum price cut after LoE will reach 70% in 2 years for branded small molecules and 89% in 1 year for generics. In the case of biologics, the estimated maximum price cut will reach 41% after 3 years and biosimilars will reach a 72% discount in 3 years (Supplementary Fig. 2).
Expenditure Estimation
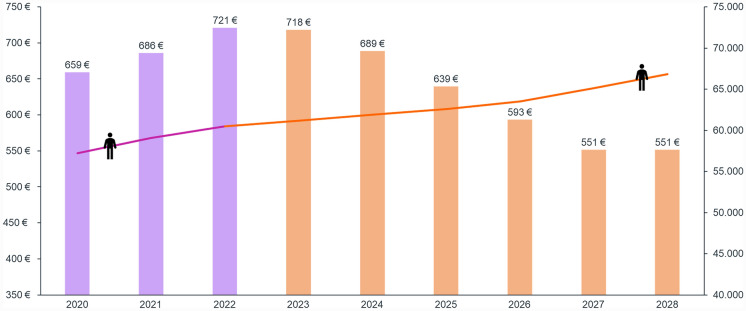
Combining clinical and economic inputs, expenditure for MS is expected to decrease from €721 million in 2022 to €551 million in 2028 (total variation between the two time periods: €170 million). The significant decrease in expenditure is expected to start from 2024 (€689 million), with a steady decrease up to 2028 despite the increase in total treated patients due to the foreseen introduction in 2026 of a drug specifically indicated for NR-SPMS (Fig. 4).
Fig. 4.
MS expenditure (on the left) and number of treated patients (on the right) between 2020 and 2028. The forecast includes the baseline period (in violet; 2020–2022) as well as projections (in orange; 2023–2028). All expenditure results are expressed as millions of euros (€)
The results of the sensitivity analysis indicate that the total expenditure reduction between 2022 and 2028 ranges from €182 million to €157 million versus the baseline. The greatest impact on expenditure is obtained by varying the inputs related to the discount at launch for new molecules (+7%; −7.6%), while all other parameters have an impact of less than 6% on the results (Supplementary Fig. 3).
Discussion
MS is a complex neurological disease characterized by increasing global prevalence and no cure. Although available DMTs are effective in controlling clinical relapses and delaying disease progression [28], the relevant unmet needs have driven continued research efforts, with the identification of new treatments and consequent impact on the MS market in the coming years. Indeed, the MS scenario is expected to undergo significant changes from both a clinical and economical perspective. While several innovative drugs are likely to enter the market soon, generics and biosimilars of other drugs will be launched. To better understand how these expected changes will affect the economic situation in Italy, we forecasted the expenditure of the Italian National Healthcare Service for reimbursed MS drugs between 2023 and 2028. To address this objective, we collected both qualitative insights from payers and expert clinicians in the MS field, and quantitative insights from IQVIA® databases. Together, these data sources provided a comprehensive overview of expected variations in the therapeutic landscape and their impact on expenditure associated with prescription drugs.
In line with literature data, our model suggests that the number of patients in Italy receiving treatment will increase from about 60,000 to almost 67,000 [1, 4]. This is driven by CAGR and by the launch of drugs that will also be indicated for progressive forms of MS (Table 1). In particular, the data collected suggest that the launch of the NR-SPMS drug (i.e., tolebrutinib) will increase the treated MS population by 4%. In addition, it was estimated that a drug for NR-SPMS will reach 10% of the current patient share, corresponding to patients with NR-SPMS who already receive treatment. Indeed, considering that the current scientific debate is heading towards the classification of the disease as a continuum between relapsing and progressive forms [5, 7], it is reasonable to assume that some patients are continuing treatment even when transitioning to a progressive phase, especially with the availability of drugs with high efficacy [29]. In this context, BTKis will provide a suitable alternative for treating a large proportion of patients who are currently receiving suboptimal treatment. Consequently, in light of their innovative mechanism of action, BTKis are considered highly promising agents for all forms of MS and are expected to reach 23% of patient shares in 2 years [22].
In contrast, first-line treatments (fumarates, teriflunomide, interferons) are expected to reduce their shares from 2023 to 2028, and other first-line injectables such as glatiramer acetate are also expected to progressively reduce their patient shares until 2028. The model’s estimations are in line with past trends and confirm expectations of a gradual shift from the use of drugs traditionally used in first-line treatment to more effective and innovative molecules. Indeed, the trend in the consumption of medicines for MS has shown an increase over the period 2014–2022 for those medicines predominantly used as second-line treatment, such as monoclonal antibodies and modulators of sphingosine-1-phosphate receptor, against a decrease in the category of interferons and glatiramer [25]. Of note, evidence in the literature suggests that in some countries (e.g., Sweden) the use of rituximab is rapidly increasing as an off-label treatment option for MS [30], and we therefore aimed at assessing trends in its use in Italy, also considering past discussions by the Italian Medicines Agency on its potential inclusion among drugs reimbursed for MS [31]. Although no further decision had been undertaken by the Italian Medicines Agency by November 2023, we collected clinicians’ opinions towards the potential use of the drug in MS in the event of a reimbursement by the NHS. The insights that we gathered indicated that rituximab is expected to gain low shares of patients in MS if reimbursed, as clinicians might consider more effective options including other treatments in the anti-CD20 class.
Despite the increasing number of patients and the launch of new drugs, the expenditure for MS is expected to decrease steadily, from €721 million in 2022 to €551 million in 2028. The main contributors to the decrease in net expenditure are the availability of generics/biosimilars which offer significantly larger discounts on both originator and generic/biosimilar products [32], and renegotiation of prices of some drugs that are already marketed.
Overall, the reduction in expenditure is projected to free up resources for prescribing newly available molecules, particularly BTKis [33]. In fact, BTKis are considered highly promising agents across the spectrum of MS due to their potential to target key pathophysiological features of the disease [22]. Furthermore, as they do not deplete B cells, they are likely not associated with an increased risk of infection in contrast to some of the therapies currently in use [22]. In addition to tolebrutinib and evobrutinib, other BTKis such as fenebrutinib and remibrutinib are under development for MS and, depending on the results of clinical trials, will likely be introduced after 2028. It will be of great interest to explore how these agents perform in various types of MS in terms of both effectiveness and safety. Understanding these differences is crucial and contributes to the anticipated significant market share for BTKis.
Study Limitations
All data used to perform this study were collected via interviews, surveys, and quantitative analysis on historical data from IQVIA® databases, as well as desk research of publicly available information, and are updated to November 2023. Hence, forecasts presented herein might undergo unforeseen changes due to variations in the clinical and economic landscape. Our predictions rely on assumptions inherent in the models used, which may not fully capture the complexity of real-world market dynamics and could introduce bias or inaccuracy into the predictions. More statistically accurate modeling approaches could help reduce uncertainties resulting from the modeling approach presented herein.
The study focused only on pharmacotherapy and did not consider cell-based therapies or other therapies such as aerobic exercise and lipoic acid, which may be beneficial in some patients.
In defining the clinical inputs to develop the model, we did not consider specific clinical concepts related to the varying efficacy of drugs available for MS. In particular, we did not account for the fact that some patients starting high-efficacy therapy are more likely to achieve no evidence of disease activity (NEDA) and might therefore discontinue treatment, influencing the final results.
Moreover, this analysis did not consider indirect costs or direct costs other than expenditure for prescription drugs. Given the primary objective of evaluating the expenditure sustained by the NHS for the prescription of reimbursed drugs and its evolution with variations in the treatment landscape, as well as the characteristics of the data sources used in the study, a broader evaluation of the impact of MS on other costs sustained by the NHS or resulting from MS-associated mortality/morbidity was not included in the analysis. An additional analysis considering indirect costs would be needed to provide a more comprehensive picture of the situation.
Lastly, given the number of participating clinicians, a statistical analysis of the collected data was not considered meaningful. Including a larger number of clinicians would improve the statistical power of the analysis. Nevertheless, we aimed to overcome this issue by basing our analysis on a well-structured methodology that combines qualitative insights with quantitative real-word data obtained from IQVIA®’s internal data assets. The experts participating in the study represented the Italian context well in terms of both expertise and geographical distribution. Their qualitative insights were collected during two rounds of interviews over a 2-year period, and then validated during a virtual expert board to reach a final consensus on inputs of the model. As a consequence of the methodology, the variability in responses was discussed by clinicians who approved the final values presented in the study.
Conclusion
To the best of our knowledge, this is the first study conducted to assess expected changes in MS pharmacotherapy, predicting expenditure and the evolution of the treatment landscape in MS, with a focus on Italian dynamics and peculiarities. In the coming years, the annual cost for first-line treatments for MS is expected to decrease and, despite the increase in the number of patients treated and the launch of BTKis, a decrease in MS drug expenditure should be observed, mainly due to LoEs and price renegotiations for currently reimbursed drugs. The model confirms sustainability for the Italian National Healthcare Service and offers valuable insights for payers and healthcare systems to navigate the evolving market landscape and enhance the management of patients with MS. Such insights will also enable clinicians to tailor treatment strategies on an individual basis, fostering positive economic outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
All authors contributed to the study conception and design, and to the material presented in this manuscript. Data collection and analysis were performed by Francesca Donnaloja, Francesco Berruto, Alessia Tettamanti, Gianluca Agostoni, Alberto Farina, and Margaret Mondino; Damiano Paolicelli, Raffaella Clerici, Marcello Moccia, Carla Tortorella, Davide Croce, and Massimo Riccaboni provided insights during the qualitative interviews; the survey was designed by Francesca Donnaloja, Francesco Berruto and Alessia Tettamanti and revised by Gianluca Agostoni; Giovanna Borriello, Elena Colombo, Emanuele D’Amico, Nicola De Rossi, Alessia Di Sapio, Giuseppe Fenu, Davide Maimone, Girolama A. Marfia, Paola Perini, Maria G. Piscaglia, Lorenzo Razzolini, and Elisabetta Signoriello filled in the survey and participated in the final Expert Board. All authors had equal opportunity to review and edit the manuscript and give approval prior to submission.
Funding
This study was funded by Sanofi S.r.l., Milan, Italy, which also funded the Rapid Service Fee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Damiano Paolicelli, Giovanna Borriello, Raffaella Clerici, Elena Colombo, Davide Croce, Emanuele D’Amico, Nicola De Rossi, Alessia Di Sapio, Giuseppe Fenu, Davide Maimone, Girolama A. Marfia, Marcello Moccia, Paola Perini, Maria G. Piscaglia, Lorenzo Razzolini, Massimo Riccaboni, Elisabetta Signoriello, Carla Tortorella received honoraria from Sanofi S.r.l. to participate in the study. Giovanna Borriello received fees for participating to Advisory Boards and travel compensations from Almirall, Biogen, Bristol-Myers Squibb, Johnson and Johnson, Merck, Novartis, Roche, and Sanofi. Elena Colombo reports travel expenses, speaker honoraria, and advisory board work from Alexion, Biogen, Bristol-Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi. Alessia Di Sapio received speaker and consulting personal compensation by Alexion, Alnylam, Amgen Biogen, Bristol-Myers Squibb, Janssen, Merck Serono, Novartis, Roche, and Sanofi and received reimbursement for attending conferences by Biogen, Merck Serono, Novartis, Roche, and Sanofi. Davide Maimone received honoraria for participating to Advisory Boards and lectures and received travel grants from Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, and Sanofi. Giuseppe Fenu received honoraria for consultancy or speaking from Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, and Sanofi. Marcello Moccia has received financial support by the MUR PNRR Extended Partnership (MNESYS no. PE00000006, and DHEAL-COM no. PNC-E3-2022-23683267), research grants from the ECTRIMS-MAGNIMS, the UK MS Society, and Merck, and honoraria from Biogen, Bristol-Myers Squibb, Ipsen, Janssen, Merck, Novartis, Roche, and Sanofi. Elisabetta Signoriello received travel grants and performed consultancy for Almirall, Biogen, Bristol-Myers Squibb, Novartis, Merck, Roche, Sanofi, and Teva. Carla Tortorella received travel funding and/or speaker honoraria from Alexion, Almirall, Biogen, Bristol-Myers Squibb, Horizon, Merck, Novartis, Roche, and Sanofi.
Ethical Approval
No ethical committee approval was required for this study. All databases used in the study are property of IQVIA® and are accessible by its employees at any time.
References
- 1.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4. 10.1212/WNL.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622–36. 10.1016/S0140-6736(18)30481-1 [DOI] [PubMed] [Google Scholar]
- 3.Niglio T, Mandolesi S, De Lucia O, d’Alessandro A. The incidence of the multiple sclerosis in Italy: 2011–2015. Clin Ter. 2022;173(5):453–7. [DOI] [PubMed] [Google Scholar]
- 4.Puthenparampil M, Perini P, Bergamaschi R, Capobianco M, Filippi M, Gallo P. Multiple sclerosis epidemiological trends in Italy highlight the environmental risk factors. J Neurol. 2022;269(4):1817–24. 10.1007/s00415-021-10782-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakimovski D, Bittner S, Zivadinov R, Morrow SA, Benedict RH, Zipp F, et al. Multiple sclerosis. Lancet. 2024;403(10422):183–202. 10.1016/S0140-6736(23)01473-3 [DOI] [PubMed] [Google Scholar]
- 6.Barzegar M, Najdaghi S, Afshari-Safavi A, Nehzat N, Mirmosayyeb O, Shaygannejad V. Early predictors of conversion to secondary progressive multiple sclerosis. Mult Scler Relat Disord. 2021;54: 103115. 10.1016/j.msard.2021.103115 [DOI] [PubMed] [Google Scholar]
- 7.Giovannoni G, Popescu V, Wuerfel J, Hellwig K, Iacobaeus E, Jensen MB, et al. Smouldering multiple sclerosis: the “real MS.” Ther Adv Neurol Disord. 2022;15:17562864211066752. 10.1177/17562864211066751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin M, Hersh CM. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener Dis Manag. 2023;13(1):47–70. 10.2217/nmt-2021-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–21. 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JH, Rempe T, Whitmire N, Dunn-Pirio A, Graves JS. Therapeutic advances in multiple sclerosis. Front Neurol. 2022;13: 824926. 10.3389/fneur.2022.824926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380-90 e2. 10.1016/j.amjmed.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauf M, Chinthapatla H, Dimri S, Li E, Hartung DM. Economic burden of multiple sclerosis in the United States: a systematic literature review. J Manag Care Spec Pharm. 2023;29(12):1354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blinkenberg M, Kjellberg J, Ibsen R, Magyari M. Increased socioeconomic burden in patients with primary progressive multiple sclerosis: a Danish nationwide population-based study. Mult Scler Relat Disord. 2020;46: 102567. 10.1016/j.msard.2020.102567 [DOI] [PubMed] [Google Scholar]
- 14.Dillon P, Heer Y, Karamasioti E, Muros-Le Rouzic E, Marcelli G, Di Maio D, et al. The socioeconomic impact of disability progression in multiple sclerosis: a retrospective cohort study of the German NeuroTransData (NTD) registry. Mult Scler J Exp Transl Clin. 2023;9(3):20552173231187810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurson-Doube J, Rijke N, Helme A, Baneke P, Banwell B, Viswanathan S, et al. Ethical use of off-label disease-modifying therapies for multiple sclerosis. Mult Scler. 2021;27(9):1403–10. 10.1177/13524585211030207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battaglia MA, Bezzini D, Cecchini I, Cordioli C, Fiorentino F, Manacorda T, et al. Patients with multiple sclerosis: a burden and cost of illness study. J Neurol. 2022;269(9):5127–35. 10.1007/s00415-022-11169-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EMA. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/briumvi. Accessed 15 Jan 2024.
- 18.Greenberg BM. Bruton’s tyrosine kinase inhibitors for multiple sclerosis treatment: a new frontier. Neurol Clin. 2024;42(1):155–63. 10.1016/j.ncl.2023.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Kramer J, Bar-Or A, Turner TJ, Wiendl H. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat Rev Neurol. 2023;19(5):289–304. 10.1038/s41582-023-00800-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker A, Martin EC, Mitchell DY, Grenningloh R, Bender AT, Laurent J, et al. Safety, tolerability, pharmacokinetics, target occupancy, and concentration-QT analysis of the novel BTK inhibitor evobrutinib in healthy volunteers. Clin Transl Sci. 2020;13(2):325–36. 10.1111/cts.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens TD, Smith PF, Redfern A, Xing Y, Shu J, Karr DE, et al. Phase 1 clinical trial evaluating safety, exposure and pharmacodynamics of BTK inhibitor tolebrutinib (PRN2246, SAR442168). Clin Transl Sci. 2022;15(2):442–50. 10.1111/cts.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider R, Oh J. Bruton’s tyrosine kinase inhibition in multiple sclerosis. Curr Neurol Neurosci Rep. 2022;22(11):721–34. 10.1007/s11910-022-01229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.clinicaltrials.gov. Nonrelapsing Secondary Progressive Multiple Sclerosis (NRSPMS) Study of Bruton's Tyrosine Kinase (BTK) Inhibitor Tolebrutinib (SAR442168) (HERCULES) (NCT04411641) 2023 [Available from: https://clinicaltrials.gov/study/NCT04411641.
- 24.clinicaltrials.gov. Primary Progressive Multiple Sclerosis (PPMS) Study of Bruton's Tyrosine Kinase (BTK) Inhibitor Tolebrutinib (SAR442168) (PERSEUS) (NCT04458051) 2024 [Available from: https://clinicaltrials.gov/study/NCT04458051.
- 25.AIFA. National report on medicines use in Italy. 2022. Available from: https://www.aifa.gov.it/documents/20142/2143103/Rapporto-OsMed-2022_EN.pdf. Accessed 27 Mar 2024.
- 26.Overview of the patent status of MS treatments 2022. Available at: https://www.msif.org/wp-content/uploads/2022/03/DMTs-patent-overview-March-22.pdf. Accessed 27 Mar 2024.
- 27.IQVIA. The impact of biosimilar competition in Europe 2022 [White paper]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2022.pdf.
- 28.Filippi M, Amato MP, Centonze D, Gallo P, Gasperini C, Inglese M, et al. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol. 2022;269(10):5382–94. 10.1007/s00415-022-11193-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayas A, Christ M, Faissner S, Klehmet J, Pul R, Skripuletz T, et al. Disease-modifying therapies for relapsing/active secondary progressive multiple sclerosis - a review of population-specific evidence from randomized clinical trials. Ther Adv Neurol Disord. 2023;16:17562864221146836. 10.1177/17562864221146836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berntsson SG, Kristoffersson A, Bostrom I, Feresiadou A, Burman J, Landtblom AM. Rapidly increasing off-label use of rituximab in multiple sclerosis in Sweden - Outlier or predecessor? Acta Neurol Scand. 2018;138(4):327–31. 10.1111/ane.12963 [DOI] [PubMed] [Google Scholar]
- 31.AIFA. Esiti Area Pre Autorizzazioni CTS 8, 9 e 10 Marzo 2023 2023 [Available from: https://www.aifa.gov.it/documents/20142/1835610/Esiti_CTS_del_8-9-10_marzo_2023_APA.pdf. Accessed 25 June 2024.
- 32.IQVIA. The Global Use of Medicines 2024: Outlook to 2028. 2024. Jan 16 2024.
- 33.Delvens. Multiple sclerosis (MS) market – trends forecast till 2030. 2023. Available at: https://www.delvens.com/report/multiple-sclerosis-ms-market. Accessed 27 Mar 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.