Abstract
Introduction
Migraine is a recurrent, disabling neurological disorder with a substantial global disease burden. However, limited real-world data are available on the patient characteristics, treatment patterns, comorbidities, and economic burden of migraine in the United Arab Emirates (UAE). In this study, we evaluated the disease burden, comorbidities, treatment patterns, specialties involved in migraine diagnosis, and healthcare resource utilization (HCRU) and associated costs in patients with migraine in Dubai, UAE.
Methods
A retrospective, secondary database cohort study was conducted from 01 January 2014 to 31 March 2022 using the Dubai Real-World Database. Patients aged ≥ 18 years with at least one diagnosis claim for migraine with continuous enrollment during the study period were included. Patients were stratified into treatment sub-cohorts. Outcomes were evaluated in terms of clinical characteristics, comorbidities, specialists visited, treatment patterns, and HCRU.
Results
The study included 203,222 patients (mean age: 40 years), with male predominance (55.4%). About 13.4% of patients had specific cardiovascular comorbidities. Frequently prescribed drug classes were nonsteroidal anti-inflammatory drugs (84.4%), triptans (29.8%), and beta-blockers (12.8%), while only 1.0% of patients with migraine were prescribed newer medications like calcitonin gene-related peptide antagonists. General medicine was the most frequently visited specialty on the index date (51.5%). The all-cause and migraine-specific median gross costs during the 12-month post-index period were US $1252.6 (2.4–564,740.7) and US $198.1 (0–168,903.3) respectively, with maximum contribution from inpatients. The contribution of migraine-specific median costs to all-cause median costs was highest for the diagnosis-related group (64.9%), followed by consumables (35.2%), medications (32.0%), procedures (24.5%), and services (24.5%).
Conclusion
Migraine significantly impacts healthcare costs in the UAE. The role of newer therapies in migraine management should be explored to reduce the associated socioeconomic burden and improve patients’ quality of life.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40122-024-00634-1.
Keywords: Migraine, Epidemiology, Healthcare resource utilization, Treatment patterns, Comorbidity, e-Claims data study
Key Summary Points
| Why carry out this study? |
| Migraine is a neurological disorder with a substantial socioeconomic burden and high global prevalence, including in the United Arab Emirates (UAE). |
| The current treatment landscape for migraine is associated with unmet needs including patient dissatisfaction with the prescribed treatment, inadequate disease management and clinical outcomes, and increased healthcare resource utilization (HCRU) and pharmacy cost. |
| We conducted a retrospective e-claims database study to estimate the disease burden, comorbidities, treatment patterns, specialties involved in the diagnosis of migraine, and HCRU of patients with migraine in Dubai, UAE. |
| What was learned from the study? |
| This study demonstrated that cardiovascular comorbidities were more prevalent in the study population; nonsteroidal anti-inflammatory drugs, triptans, and beta-blockers constituted the most frequently prescribed treatments, while prescription of novel drugs such as calcitonin gene-related peptide antagonists was very low. |
| HCRU and costs due to inpatient visits, consumables, and medications were substantial in the study population during the post-index period. |
Introduction
Migraine, a recurrent, neurological disorder with debilitating symptoms, is a major public health concern. It is regarded as the second leading cause of disability globally, affecting around 15% of the population worldwide [1]. According to the 2016 Global Burden of Disease Study, approximately 1.04 billion people suffered from migraine and had high disability weight, with 45.1 million years of life lived with disability (YLD) [2]. Migraine prevalence is influenced by age, gender, and sociodemographic variables and often goes undiagnosed [3]. It is more prevalent among females 15–49 years of age [2]. A systematic review reported migraine prevalence of 2.6–32.0% in Arab countries, with females being more susceptible [4]. In the United Arab Emirates (UAE), migraine prevalence was found to be 1.56 million, with 0.76 million YLD [2].
Genetic factors and comorbid conditions are documented risk factors for migraine and augment disease severity [3]. A population-based study demonstrated the association of migraine with cardiovascular diseases, hypertension, diabetes, and hypercholesterolemia [5]. Neurological, psychiatric, and sleep disorders are more frequent among patients with migraine when compared with the general population [6, 7].
Migraine is associated with psychological effects, functional delays, activity impairment, and decreased productivity, leading to low health-related quality of life (QoL) [3, 8, 9]. Studies conducted using migraine-specific QoL questionnaires showed low QoL scores [8, 10]. The high Headache Impact Test (HIT-6) score (66.99 ± 6.79) and Migraine Disability Assessment Scale score (23.43 ± 12.32) obtained in a separate study also confirmed impaired migraine-specific QoL [11].
Treatment strategies for migraine include both acute and preventive medications for mitigating migraine attacks, alleviating pain, managing comorbid conditions, minimizing disabilities, and restoring function. Acute migraine treatment minimizes migraine attack symptoms and includes nonsteroidal anti-inflammatory drugs (NSAIDs), triptans, and emergency medications (intravenous metoclopramide, subcutaneous sumatriptan, dexamethasone, intravenous acetylsalicylic acid) [12, 13]. Preventive treatment reduces the frequency and intensity of migraine attacks and includes antiepileptic drugs, beta-blockers, antidepressants, serotonergic antagonists, calcium channel antagonists, angiotensin modulators, and nutrients and herbal products [14–16]. A study reported the highest prescription refills for NSAIDs, followed by triptans [17]. Apart from pharmacological agents, certain non-pharmacological approaches may be used for clinical benefits, including behavioral techniques, acupuncture, and noninvasive or invasive neuromodulation [18].
The current migraine treatment landscape is faced with certain unmet needs including dissatisfaction with the prescribed treatment as a result of adverse side effects or safety concerns (satisfaction with preventive and acute medications being 40.8% and 27.1%, respectively) [19]. Inadequately managed migraine poses a risk of medication overuse, headache, chronification, and healthcare resource utilization (HCRU) inefficiency [20, 21]. Thus, newer treatment modalities are being explored. Calcitonin gene-related peptide (CGRP), of the trigeminovascular system, is indicated as a key neuropeptide in migraine pathophysiology. Hence, pharmacological agents targeting this pathway might prove beneficial in migraine treatment [22]. While the use of first-generation CGRP antagonists—i.e., gepants—were limited due to hepatotoxicity, the newer-generation gepants (rimegepant, ubrogepant, and atogepant) were found to be tolerable, safe, and effective [23, 24]. Additionally, anti-CGRP monoclonal antibodies (erenumab, fremanezumab, galcanezumab, eptinezumab) have been approved by the US Food and Drug Administration and European Medicines Agency owing to their convincing safety and efficacy outcomes in clinical trials [24–29].
Besides a high clinical burden, the prevalence of migraine and migraine-related disabilities causes increased HCRU and pharmacy use [30, 31]. Along with direct costs (medical costs), indirect costs including productivity loss and comorbid conditions form the major share of migraine-associated economic burden [3, 6]. A retrospective analysis using a claims database demonstrated that direct all-cause healthcare costs (US $11,010 vs. US $4436) and indirect costs (US $11,294 vs. US $8945) were higher among patients with migraine than among patients without migraine [30]. The highest costs were attributable to procedure/imaging costs, followed by pharmacy costs [32]. In a retrospective study, for a 6-month follow-up period, the total cost for migraine treatment was €448.43 [33].
Despite holistic improvement throughout the migraine treatment landscape with the advent of newer treatment modalities, unsatisfactory clinical outcomes and unmet needs persist. Real-world studies on the characteristics, treatment patterns, comorbidities, and economic burden of migraine in the UAE are currently scarce. These data are paramount for developing tailored therapeutic regimens to reduce migraine-associated costs. Additionally, such data will benefit stakeholders, researchers, and policymakers by bridging knowledge gaps. Thus, this retrospective e-claims database study aimed to estimate the disease burden, comorbidities, treatment patterns, and specialties involved in the diagnosis of migraine, as well as assessing the HCRU of patients with migraine in Dubai, UAE.
Methods
Study Design
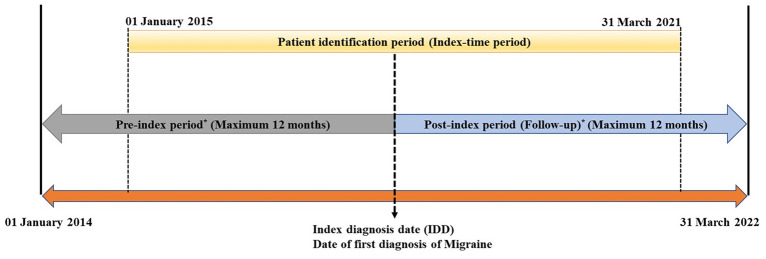
This retrospective, secondary database cohort study used insurance e-claims data from the Dubai Real-World Database (DRWD). Data were extracted for all patients with the first migraine claim available during the index period (01 January 2015–31 March 2021) and analyzed for the study period 01 January 2014–31 March 2022. The date of the first migraine claim available during the index period was termed the index date. The first migraine diagnosis date during the index period was considered the index diagnosis date (IDD). The 12-month period prior to and following the index date were termed the pre-index and post-index (follow-up) periods, respectively (Fig. 1).
Fig. 1.
Flowchart depicting study design. *Patients must have at least one claim (migraine/non-migraine) anytime during the 12-month pre-index period and the 12-month post-index (follow-up) period (a surrogate for continuous enrollment)
Data Sources
The DRWD e-claims database is the largest claims database in Dubai, covering approximately 100% of the population insured by Dubai private health insurance. It provides anonymized patient-level data for all insurance claims related to demographics, diagnoses, procedures (medical, surgical, and diagnostic), prescriptions, and other services. Nearly 89% of the Dubai population comprises expatriates covered by private insurance, while the remaining 11% (comprising the local Emirati population) are insured via public funding [34].
Study Population
The International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes were used to identify the study population (Table S1). The study included patients aged ≥ 18 years with at least one diagnosis claim (principal, secondary, and admitted) for migraine (as per ICD-10 CM codes) during the study period (01 January 2014–31 March 2022) and during the index period (01 January 2015–31 March 2021). Patients having continuous enrolment with at least one claim (migraine or non-migraine) during the 12-month pre-index and post-index periods were included. No explicit exclusion criteria were defined for patient selection; however, patients who did not satisfy the continuous enrolment criteria were excluded from the study.
Patients were divided into three sub-cohorts (or treatment cohorts) based on the medications they received (Table S2): patients with a migraine diagnosis code and prescribed preventive medications alone (preventive migraine), patients with a migraine diagnosis code and prescribed acute medications alone (acute migraine), and those with a migraine diagnosis code and prescribed both acute and preventive medications (preventive + acute migraine). For HCRU and associated cost evaluation, data on preventive migraine, acute migraine, and preventive + acute migraine sub-cohorts were analyzed.
Ethical Considerations
The study was observational in nature and involved analysis of previously collected anonymized structured data and did not impose any form of intervention. Hence, obtaining informed consent was not required for the study. Further, no institutional review board approval was required as it did not involve the collection, use, or transmission of individually identifiable data. The study adhered to the Health Insurance Portability and Accountability Act of 1996 to prevent disclosure of patient health information.
The dataset utilized for the present study was publicly inaccessible. This dataset was obtained from the Dubai Health Insurance Corporation (DHIC) after adhering to all the required legal procedures to acquire and utilize these anonymized patient claims data. Under the terms of the data sharing contract with DHIC, IQVIA received a legitimate and exclusive right to use certain components of the dataset as specified in the contract. IQVIA was given authorization to apply the data for conducting anonymized and uniform analyses and research projects pertaining to health insurance claim records. Additionally, IQVIA was allowed to distribute the results and insights from the anonymized and uniform dataset to its clients for research purposes involving health insurance claim information. IQVIA pledged to adhere to established procedures to maintain compliance with applicable regulations, including those related to data privacy.
Baseline Variables and Outcome Measures
Sociodemographic characteristics (age, gender, insurance plan, and nationality) were analyzed during the index period (01 January 2015–31 March 2021).
Number and percentage of new visits and repeat visits of the overall study population were analyzed per annum for the period 01 January 2015–31 December 2020.
Specific cardiovascular comorbidities (myocardial ischemia/infarction, cerebrovascular disease, peripheral vascular disease, arrhythmias, venous thromboembolism, congestive heart failure, pulmonary embolism, uncontrolled hypertension, and intestinal ischemia) were evaluated during the study period (01 January 2014–31 March 2022).
Specialty analysis was performed at the index date. During the post-index period, specialty analysis (type of specialty involved prior to neurologist visit) and average time (number of days) and time (in months) for neurologist consultation post other specialty visits since IDD were examined.
Treatment patterns, by drug class and prescribed lines of treatment (LOTs) for migraine, were assessed for the 12-month follow-up period.
During the 12-month post-index period, all-cause and migraine-specific HCRU were determined in the three individual sub-cohorts of patients with migraine (acute, preventive, and acute + preventive) and in the overall population. HCRU cost, by visit type (number of claims and cost for outpatients, inpatients, and emergency room [ER] visits) and activity type (number of claims and cost of medications, procedures, Healthcare Common Procedure Coding System (HCPCS) [consumables], and services), was assessed during the post-index period. Results for visit type and activity type have been presented as gross cost (sum of the amount paid by insurance and out-of-pocket expenditures by the patient) and net cost (amount paid by insurance provider), respectively. All costs were converted from Arab Emirates dirhams (AED) to United States dollars (USD), after adjusting for the purchasing power parity (PPP) conversion rate of 2.09 for the year 2023. Claims amounting to less than $0 were excluded from the analysis.
HCRU and associated median costs in the combined sub-cohort of patients with migraine diagnosed with specific cardiovascular comorbidities were evaluated during the follow-up period by visit type and activity type, as well as overall (without visit and activity split), and have been presented as gross costs and net costs, respectively. All costs were converted from AED to USD, after adjusting for the PPP conversion rate of 2.09 for year 2023. Claims less than US $0 were excluded from the analysis. Patient counts were not mutually exclusive.
Data Analysis
Descriptive analyses were performed to understand the qualitative and quantitative nature of the data collected and the characteristics of the target population. Study variables were analyzed for the overall population as well as the three sub-cohorts. HCRU and associated costs (all-cause and disease-specific) were analyzed for the three sub-cohorts without encounter and activity split. Number and percentage of new visits, repeat visits, HCRU, and costs (including patients with specific cardiovascular comorbidities by encounter or activity type) were analyzed for all patients with migraine. Continuous variables are reported as mean (and standard deviation) or median and interquartile range, where appropriate. Categorical variables are summarized as frequencies and percentages (n, %). Data are presented by sub-cohort, where appropriate.
Results
Demographics and Clinical Characteristics of Patients with Migraine
Of 451,983 patients, 203,222 were selected for the analysis and divided into five treatment cohorts: 105,158 (51.7%) patients with acute migraine; 8308 (4.1%) with preventive migraine; 30,314 (14.9%) with preventive + acute migraine; 98 (0.05%) patients undergoing non-pharmacological treatments; and 59,344 (29.2%) patients classified as others (having a migraine-related claim but having no related treatment defined in the medication list) (Table S3).
The mean age of the population of 65,982 (32.5%) patients with available demographic data was 40 years, and 45.6% were 35–44 years of age (Table 1). Overall, a higher proportion of males were affected (55.4%). Most patients (n = 47,775, 72.4%) opted for an enhanced insurance plan. Overall, patients were predominantly Indian (n = 27,303, 41.4%), Pakistani (n = 8174, 12.4%), or Filipino (n = 8003, 12.1%).
Table 1.
Patient demographic and clinical characteristics for the overall and sub-cohort population (sub-cohort 1: preventive, sub-cohort 2: acute, sub-cohort 3: preventive + acute)
| Baseline characteristics | Overall | Sub-cohort 1 | Sub-cohort 2 | Sub-cohort 3 | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Overall study population in the index period | 203,222 | 100% | 8304 | 4.1% | 105,158 | 51.7% | 30,314 | 14.9% |
| Patients with demographic data available (considered for further analysis) | 65,982 | 32.5% | 2552 | 30.7% | 34,635 | 32.9% | 10,164 | 33.5% |
| Patients with demographics data missing | 137,240 | 67.5% | 5756 | 69.3% | 70,523 | 67.1% | 20,150 | 66.5% |
| Mean age in years (SD) | 40 (9) | 43 (10) | 40 (8) | 41 (9) | ||||
| 18–24 | 1013 | 1.5% | 51 | 2.0% | 422 | 1.2% | 149 | 1.5% |
| 25–34 | 16,049 | 24.3% | 423 | 16.6% | 8840 | 25.5% | 2090 | 20.6% |
| 35–44 | 30,105 | 45.6% | 1073 | 42.0% | 16,012 | 46.2% | 4801 | 47.2% |
| 45–54 | 14,283 | 21.6% | 683 | 26.8% | 7238 | 20.9% | 2411 | 23.7% |
| 55–64 | 3745 | 5.7% | 244 | 9.6% | 1785 | 5.2% | 595 | 5.9% |
| 65+ | 787 | 1.2% | 78 | 3.1% | 338 | 1.0% | 118 | 1.2% |
| Gender | ||||||||
| Male | 36,580 | 55.4% | 1549 | 60.7% | 19,795 | 57.2% | 4970 | 48.9% |
| Female | 29,402 | 44.6% | 1003 | 39.3% | 14,840 | 42.8% | 5194 | 51.1% |
| Insurance plan | ||||||||
| Basic | 18,207 | 27.6% | 482 | 18.9% | 10,593 | 30.6% | 1919 | 18.9% |
| Enhance plan | 47,775 | 72.4% | 2070 | 81.1% | 24,042 | 69.4% | 8245 | 81.1% |
| Nationality | ||||||||
| India | 27,303 | 41.4% | 1167 | 45.7% | 14,247 | 41.1% | 4363 | 42.9% |
| Pakistan | 8174 | 12.4% | 263 | 10.3% | 4694 | 13.6% | 1166 | 11.5% |
| Philippines | 8003 | 12.1% | 170 | 6.7% | 4614 | 13.3% | 1002 | 9.9% |
| Egypt | 3779 | 5.7% | 176 | 6.9% | 1813 | 5.2% | 718 | 7.1% |
| Bangladesh | 1811 | 2.7% | 74 | 2.9% | 980 | 2.8% | 274 | 2.7% |
| Jordan | 1564 | 2.4% | 62 | 2.4% | 762 | 2.2% | 253 | 2.5% |
| Emirates | 1449 | 2.2% | 48 | 1.9% | 762 | 2.2% | 210 | 2.1% |
| Syria | 1200 | 1.8% | 62 | 2.4% | 553 | 1.6% | 234 | 2.3% |
| Britain | 1179 | 1.8% | 56 | 2.2% | 532 | 1.5% | 137 | 1.3% |
| Sri Lanka | 1140 | 1.7% | 30 | 1.2% | 614 | 1.8% | 182 | 1.8% |
| Nepal | 1070 | 1.6% | 37 | 1.4% | 626 | 1.8% | 141 | 1.4% |
| Lebanon | 1018 | 1.5% | 66 | 2.6% | 432 | 1.2% | 203 | 2.0% |
| Iran | 500 | 0.8% | 54 | 2.1% | 224 | 0.6% | 80 | 0.8% |
SD standard deviation
A patient can present in more than one Charlson Comorbidity Index (CCI) component; hence patient counts are not mutually exclusive
Number and Percentage of New Visits and Repeat Visits Among Patients with Migraine
A decrease in the percentage of new visits was observed each year, from 0.9% in 2015 to 0.8% in 2020 (Fig. 2). The percentage of repeat visits was recorded to be 0.9% and 0.9% in 2015 and 2020, respectively.
Fig. 2.
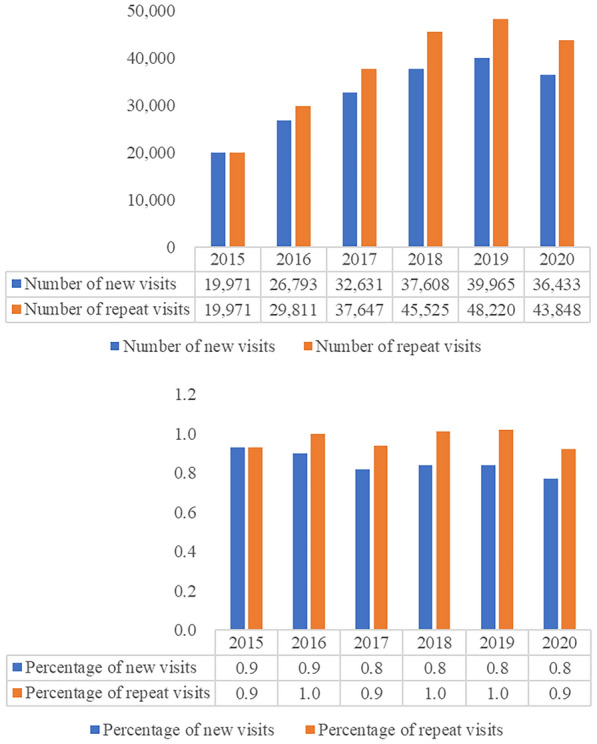
Comparison of number and percentage of new visits and repeat visits in patients with migraine
Cardiovascular Comorbidities in Patients with Migraine
Among 203,222 study patients, 27,148 (13.4%) had cardiovascular comorbidities during the study period. The ICD-10 codes used to define the different comorbidities have been elaborated in Table S4.
Increased prevalence of cardiovascular comorbidities in the preventive sub-cohort (22.1%; n = 1835/8304), followed by preventive + acute (18.5%; n = 5613/30,314), and acute (11.2%; n = 11,761/105,158) patients with migraine (Table S5). Overall, myocardial ischemia/infarction (n = 12,498, 6.1%), cerebrovascular disease (n = 8463, 4.2%), peripheral vascular disease (n = 5059, 2.5%), arrhythmias (n = 3498, 1.7%), and congestive heart failure (n = 2671, 1.3%) were the most frequently reported cardiovascular comorbidities during the study period (Tables S5 and S6). A similar trend was observed across individual sub-cohorts. It should be noted that patients were not mutually exclusive.
Treatment Patterns and Line of Treatment
During the 12-month post-index period, data relevant to treatment patterns were available for 141,447 (69.6%), 8177 (98.4%), 103,445 (98.4%), and 29,825 (98.4%) patients in the overall cohort, preventive migraine, acute migraine, and preventive + acute migraine sub-cohorts, respectively (Table 2). Overall, NSAIDs (n = 119,355, 84.4%), triptans (n = 42,138, 29.8%), and beta-blockers (n = 18,157, 12.8%) were the frequently prescribed drug classes. Patients in the acute migraine sub-cohort were primarily prescribed NSAIDs (n = 93,310, 90.2%) and triptans (n = 29,496, 28.5%), while beta-blockers (n = 3937, 48.1%) and anticonvulsants (n = 2878, 35.2%) were frequently prescribed for patients in the preventive migraine sub-cohort. In the preventive + acute migraine sub-cohort, the majority of the patients were prescribed NSAIDs (n = 26,045, 87.3%), beta-blockers (n = 14,218, 47.7%), triptans (n = 12,642, 42.4%), and anticonvulsants (n = 11,259, 37.8%).
Table 2.
Treatment pattern for the overall and sub-cohort population for the 12-month post-index period (sub-cohort 1: preventive, sub-cohort 2: acute, sub-cohort 3: preventive + acute)
| Overall | Sub-cohort 1 | Sub-cohort 2 | Sub-cohort 3 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Overall study population in the index period | 203,222 | 100 | 8304 | 4.1 | 105,158 | 51.7 | 30,314 | 14.9 |
| Treated Population in the 12-month follow-up period | 141,447 | 69.6% | 8177 | 98.4% | 103,445 | 98.4% | 29,825 | 98.4% |
| Treatment pattern | ||||||||
| NSAIDs | 119,355 | 84.4% | 0 | 0.0% | 93,310 | 90.2% | 26,045 | 87.3% |
| Triptans | 42,138 | 29.8% | 0 | 0.0% | 29,496 | 28.5% | 12,642 | 42.4% |
| Beta-blocker | 18,157 | 12.8% | 3937 | 48.1% | 2 | 0.0% | 14,218 | 47.7% |
| Anticonvulsants | 14,138 | 10.0% | 2878 | 35.2% | 1 | 0.0% | 11,259 | 37.8% |
| Tricyclic antidepressants | 5663 | 4.0% | 1444 | 17.7% | 1 | 0.0% | 4218 | 14.1% |
| Antiemetic | 4109 | 2.9% | 0 | 0.0% | 3122 | 3.0% | 987 | 3.3% |
| Stimulants | 2176 | 1.5% | 0 | 0.0% | 1793 | 1.7% | 383 | 1.3% |
| CGRP | 1451 | 1.0% | 215 | 2.6% | 0 | 0.0% | 1236 | 4.1% |
| Antihypertensive | 776 | 0.5% | 112 | 1.4% | 0 | 0.0% | 664 | 2.2% |
| Hypnosis | 594 | 0.4% | 0 | 0.0% | 269 | 0.3% | 325 | 1.1% |
| ACE inhibitors | 597 | 0.4% | 116 | 1.4% | 0 | 0.0% | 481 | 1.6% |
| Botox | 489 | 0.3% | 162 | 2.0% | 0 | 0.0% | 327 | 1.1% |
| Ca antagonist | 419 | 0.3% | 68 | 0.8% | 0 | 0.0% | 351 | 1.2% |
| Opioids | 360 | 0.3% | 0 | 0.0% | 182 | 0.2% | 178 | 0.6% |
| SSRI | 171 | 0.1% | 75 | 0.9% | 0 | 0.0% | 96 | 0.3% |
| Antidepressant | 67 | 0.0% | 28 | 0.3% | 0 | 0.0% | 39 | 0.1% |
| Ditans | 39 | 0.0% | 0 | 0.0% | 5 | 0.0% | 34 | 0.1% |
ACE angiotensin-converting enzyme; CGRP calcitonin gene-related peptide; NSAID nonsteroidal anti-inflammatory drug; SSRI selective serotonin reuptake inhibitor
Patients can present in more than one treatment; hence patient counts are not mutually exclusive
The LOT analysis showed that the treatments prescribed as LOT1 to patients with migraine were either monotherapies or combination therapies of NSAIDs, triptans, anticonvulsants, beta-blockers, Botox, and CGRP antagonists. In the post-index period, the most commonly prescribed LOT1 was NSAIDs (n = 7642), followed by triptans (n = 3374), NSAIDs + triptans (n = 2182), beta-blockers + NSAIDs (n = 1243), and anticonvulsants (n = 1194). Few patients received LOT2 and LOT3, possibly due to continuation of LOT1. No patient received a true LOT3.
Specialty Analysis
In the overall population, general medicine (n = 104,714, 51.5%) was the most frequently visited specialty at the index date, followed by neurology (n = 41,466, 20.4%), and internal medicine (n = 28,625, 14.1%) (Table 3). At the index date, 64.2% of patients (n = 67,466) in the acute migraine sub-cohort had the first consultation with general medicine practitioners, while 58.1% of patients (n = 4828) in the preventive migraine sub-cohort and 50.8% of patients (n = 15,413) in the acute + preventive sub-cohort had their first specialty consultation with a neurologist.
Table 3.
Specialty visited by overall population and sub-cohort population (sub-cohort 1: preventive, sub-cohort 2: acute, sub-cohort 3: preventive + acute)
| Overall | Sub-cohort 1 | Sub-cohort 2 | Sub-cohort 3 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Overall study population in the index period | 203,222 | 100 | 8304 | 4.1 | 105,158 | 51.7 | 30,314 | 14.9 |
| First-time visit specialty analysis | ||||||||
| Specialty | N | % | N | % | N | % | N | % |
| General medicine/family medicine | 104,714 | 51.5% | 1017 | 12.2% | 67,466 | 64.2% | 7743 | 25.5% |
| Neurology | 41,466 | 20.4% | 4828 | 58.1% | 10,655 | 10.1% | 15,413 | 50.8% |
| Internal medicine | 28,625 | 14.1% | 1064 | 12.8% | 14,358 | 13.7% | 4423 | 14.6% |
| ENT | 8637 | 4.3% | 615 | 7.4% | 4076 | 3.9% | 1402 | 4.6% |
| Ophthalmology | 6755 | 3.3% | 116 | 1.4% | 1624 | 1.5% | 386 | 1.3% |
| Unknown specialty | 5708 | 2.8% | 188 | 2.3% | 3238 | 3.1% | 759 | 2.5% |
| Emergency medicine | 2199 | 1.1% | 12 | 0.1% | 1611 | 1.5% | 312 | 1.0% |
| Obstetrics/gynecology | 1988 | 1.0% | 16 | 0.2% | 993 | 0.9% | 130 | 0.4% |
| Specialty patients visit prior to neurologist visit (12-month follow-up period) | ||||||||
| Patient visits other specialties prior to neurologist visit in 12-month follow-up period | 7910 | 3.9% | 278 | 3.3% | 2806 | 2.7% | 4178 | 13.8% |
| General medicine/family patient visits other specialties prior to neurologist visit in 12 months of follow-up | 4538 | 57.4% | 94 | 33.8% | 1748 | 62.3% | 2397 | 57.4% |
| Internal medicine | 1285 | 16.2% | 55 | 19.8% | 386 | 13.8% | 737 | 17.6% |
| ENT | 583 | 7.4% | 35 | 12.6% | 159 | 5.7% | 322 | 7.7% |
| Ophthalmology | 337 | 4.3% | 26 | 9.4% | 104 | 3.7% | 131 | 3.1% |
| Emergency medicine | 305 | 3.9% | 4 | 1.4% | 141 | 5.0% | 129 | 3.1% |
| Unknown specialty | 255 | 3.2% | 16 | 5.8% | 93 | 3.3% | 87 | 2.1% |
| Neurosurgery | 117 | 1.5% | 8 | 2.9% | 26 | 0.9% | 66 | 1.6% |
| Obstetrics/gynecology | 91 | 1.2% | 4 | 1.4% | 38 | 1.4% | 37 | 0.9% |
| Cardiology | 76 | 1.0% | 9 | 3.2% | 19 | 0.7% | 39 | 0.9% |
| Gastroenterology | 84 | 1.1% | 8 | 2.9% | 19 | 0.7% | 45 | 1.1% |
| Number of patients/days | ||||||||
| Number of patients | 7910 | |||||||
| Average number of days to neurologist visit | 65 days | |||||||
| Median number of days to neurologist visit | 21 days | |||||||
| Standard deviation | 88 days | |||||||
| Minimum number of days to neurologist visit | 1 day | |||||||
| Maximum number of days to neurologist visit | 364 days | |||||||
| Average number of days to neurologist visit | 65 days | |||||||
| Time to neurologist visit | ||||||||
| 1 month | 4428 | 56.0% | ||||||
| 2 months | 864 | 10.9% | ||||||
| 3 months | 583 | 7.4% | ||||||
| 4 months | 400 | 5.1% | ||||||
| 5 months | 327 | 4.1% | ||||||
| 6 months | 302 | 3.8% | ||||||
| 1 year | 1006 | 12.7% | ||||||
ENT ear, nose, throat
Patient counts are not mutually exclusive because patients might have visited multiple specialties on the first visit
The data pertinent to patients visiting other specialties prior to a neurologist in the 12-month post-index period were available for 3.9% of patients (n = 7910). The specialties visited prior to a neurologist consultation included general medicine (n = 4538, 57.4%), internal medicine (n = 1285, 16.2%), and ear, nose, throat (ENT) (n = 583, 7.4%). The average number of days taken to visit a neurologist was 65, and the number ranged from 1 to 364 days. More than half of the patients (n = 4428, 56.0%) visited a neurologist within a month (Table 3).
HCRU and Associated Costs
Overall HCRU and Associated Costs
HCRU and associated cost data related to combined sub-cohorts of patients with migraine (acute, preventive, and acute + preventive) were available for 143,780 patients with migraine during the 12-month post-index period including the index date (Table S7). The median all-cause and migraine-specific claims during the post-index period were 10.0 (1.0–323.0) and 2.0 (1.0–71.0), respectively. The percentage contribution of migraine-specific median healthcare cost to all-cause median healthcare cost was 20.7%. Among the three sub-cohorts, all-cause and migraine-specific claims were highest in the preventive + acute sub-cohort (13.0 [1.0–309.0] and 3.0 [1.0–71.0], respectively) with the migraine-specific median cost to all-cause median cost being 26.0%.
Likewise, the overall median all-cause and migraine-specific healthcare gross costs were US $1252.6 (2.4–564,740.7) and US $198.1 (0–168,903.3), respectively, with the highest in the preventive + acute sub-cohort (US $2030.6 [8.6–564,740.7] and US $424.8 [1.4–9443.5], respectively) (Table S7). Migraine-specific costs contributed 19.9% of the all-cause cost during the post-index period. The highest disease burden among the cohorts in terms of gross cost was in the preventive + acute migraine sub-cohort (27.3%).
HCRU and Associated Cost Based on Visit Type
During the post-index period, the median all-cause claims were highest for outpatient visits (10.0 [1.0–323.0]), followed by ER visits (2.0 [1.0–84.0]) and inpatient visits (1.0 [1.0–14.0]) (Table 4). Migraine-specific claims showed a similar trend, with the highest median claims for outpatient visits (2.0 [1.0–69.0]) followed by ER and inpatient visits.
Table 4.
Healthcare resource utilization and costs by visit type (12-month post-index period)
| Analysis of claims | |||
|---|---|---|---|
| Disease-specific | All-cause | Average disease burden | |
| Outpatient | |||
| N (patient counts) | 139,773 | 143,330 | 21.0% |
| Percentage of patients | 97.2% | 99.7% | |
| Total | 403,042 | 1,965,371 | |
| Mean | 2.9 | 13.7 | |
| Median | 2.0 | 10.0 | |
| Std | 2.7 | 12.6 | |
| Min | 1.0 | 1.0 | |
| Max | 69.0 | 323.0 | |
| Emergency | |||
| N (patient counts) | 10,301 | 23,575 | 58.1% |
| Percentage of patients | 7.2% | 16.4% | |
| Total | 18,205 | 71,684 | |
| Mean | 1.8 | 3.0 | |
| Median | 1.0 | 2.0 | |
| Std | 1.5 | 3.5 | |
| Min | 1.0 | 1.0 | |
| Max | 31.0 | 84.0 | |
| Inpatient | |||
| N (patient counts) | 1664 | 7754 | 88.5% |
| Percentage of patients | 1.2% | 5.4% | |
| Total | 1790 | 9428 | |
| Mean | 1.1 | 1.2 | |
| Median | 1.0 | 1.0 | |
| Std | 0.3 | 0.7 | |
| Min | 1.0 | 1.0 | |
| Max | 6.0 | 14.0 | |
| Gross cost (USD) | |||
| Outpatient | |||
| Total | 74,487,753.1 | 361,467,749.8 | 21.1% |
| Mean | 533.0 | 2522.0 | |
| Median | 180.4 | 1146.9 | |
| Std | 1267.5 | 4788.0 | |
| Min | 0.0 | 1.0 | |
| Max | 62,659.3 | 251,832.5 | |
| Emergency | |||
| Total | 3,924,199.0 | 18,379,427.3 | 48.9% |
| Mean | 380.9 | 779.4 | |
| Median | 278.0 | 423.9 | |
| Std | 414.4 | 1104.8 | |
| Min | 1.8 | 0.0 | |
| Max | 6926.3 | 22,016.7 | |
| Inpatient | |||
| Total | 13,189,105.3 | 80,992,938.8 | 75.9% |
| Mean | 7926.3 | 10,445.5 | |
| Median | 4496.7 | 5994.3 | |
| Std | 11,512.4 | 18,234.4 | |
| Min | 20.0 | 3.3 | |
| Max | 168,903.3 | 557,343.1 | |
USD United States dollars (US $)
Excluded the patients/claims having claim amount less than or equal to US $0 by each cohort
Gross cost = insurance paid amount + patient share
Implied PPP conversion rate (national currency per international dollar): https://www.imf.org/external/datamapper/PPPEX@WEO/OEMDC/ARE
The associated median all-cause cost was highest for inpatient visits (US $10,445.5 [3.3–557,343.1]), followed by outpatient (US $1146.9 [1.0–251,832.5]), and ER visits (US $423.9 [0–22,016.7]) (Figure S1 and Table 4). Similarly, the associated median disease-specific cost was also highest for inpatient visits (US $4496.7 [20.0–168,903.3]). Study analysis showed that migraine-specific median inpatient cost contributed 75.9% of all-cause inpatient cost during the post-index period.
HCRU and Associated Costs Based on Activity Type
The median all-cause claims were highest for medications (5.0 [1.0–262.0]), services (5.0 [1.0–145.0]), and procedures (4.0 [1.0–265.0]) during the post-index period (Table 5). Migraine-specific claims showed similar results, with the highest median claims for medications (1.0 [1.0–65.0]). Claims due to procedures (26.3%) and medications (26.2%) contributed maximally towards the average disease burden. However, the diagnosis-related group (DRG) contributed 89.5% towards the average disease burden.
Table 5.
Healthcare resource utilization and costs by activity type (12-month post-index period)
| Analysis of claims | Net cost (USD) | |||||
|---|---|---|---|---|---|---|
| Disease-specific | All-cause | Average disease burden | Disease-specific | All-cause | Average disease burden | |
| Medications | ||||||
| N (patient counts) | 141,769 | 143,490 | ||||
| Percentage of patients | 99% | 100% | ||||
| Total | 260,038 | 1,003,171 | 26.2% | 38,616,889.0 | 122,333,502.4 | 32.0% |
| Mean | 1.8 | 7.0 | 272.2 | 852.6 | ||
| Median | 1.0 | 5.0 | 65.6 | 319.6 | ||
| Std | 1.6 | 6.3 | 975.6 | 2805.7 | ||
| Min | 1.0 | 1.0 | 0.0 | 0.0 | ||
| Max | 65.0 | 262.0 | 56,229.7 | 247,424.9 | ||
| CPT (procedures) | ||||||
| N (patient counts) | 72,813 | 125,923 | ||||
| Percentage of patients | 51% | 88% | ||||
| Total | 110,661 | 726,626 | 26.3% | 25,891,564.1 | 182,397,377.5 | 24.5% |
| Mean | 1.5 | 5.8 | 355.5 | 1448.3 | ||
| Median | 1.0 | 4.0 | 91.4 | 484.7 | ||
| Std | 1.2 | 6.3 | 1095.7 | 3360.3 | ||
| Min | 1.0 | 1.0 | 0.3 | 0.5 | ||
| Max | 50.0 | 265.0 | 59,396.7 | 182,851.2 | ||
| HCPCS (consumables) | ||||||
| N (patient counts) | 3184 | 14,510 | ||||
| Percentage of patients | 2% | 10% | ||||
| Total | 4017 | 25,075 | 73.0% | 982,605.3 | 12,736,378.0 | 35.2% |
| Mean | 1.3 | 1.7 | 308.6 | 878.0 | ||
| Median | 1.0 | 1.0 | 14.8 | 191.4 | ||
| Std | 0.9 | 2.5 | 1469.9 | 3021.1 | ||
| Min | 1.0 | 1.0 | 0.0 | 0.0 | ||
| Max | 16.0 | 151.0 | 27,836.8 | 186,845.5 | ||
| Services | ||||||
| N (patient counts) | 109,001 | 135,038 | ||||
| Percentage of patients | 76% | 94% | ||||
| Total | 181,895 | 865,723 | 26.0% | 16,758,977.0 | 84,822,428.2 | 24.5% |
| Mean | 1.7 | 6.4 | 153.6 | 628.2 | ||
| Median | 1.0 | 5.0 | 57.4 | 212.0 | ||
| Std | 1.4 | 5.7 | 612.0 | 1850.2 | ||
| Min | 1.0 | 1.0 | 0.0 | 0.2 | ||
| Max | 38.0 | 145.0 | 78,589.0 | 225,702.9 | ||
| DRG | ||||||
| N (patient counts) | 131 | 833 | ||||
| Percentage of patients | 0.1% | 1% | ||||
| Total | 138 | 981 | 89.5% | 875,181.8 | 8,578,519.1 | 64.9% |
| Mean | 1.1 | 1.2 | 6680.9 | 10,298.6 | ||
| Median | 1.0 | 1.0 | 3996.2 | 7052.2 | ||
| Std | 0.2 | 0.6 | 9157.9 | 11,294.7 | ||
| Min | 1.0 | 1.0 | 0.0 | 0.0 | ||
| Max | 2.0 | 10.0 | 68,370.8 | 139,969.9 | ||
CPT Current Procedural Terminology; DRG diagnosis-related group; HCPCS Healthcare Common Procedure Coding System; USD United States dollars
Net cost = insurance paid amount
Excluded the claims having claim amount less than or equal to US $0
Implied PPP conversion rate (national currency per international dollar): https://www.imf.org/external/datamapper/PPPEX@WEO/OEMDC/ARE
The maximum median all-cause cost was incurred for procedures (US $484.7 [0.5–182,851.2]), followed by medications (US $319.6 [0–247,424.9]) and services (US $212.0 [0.2–225,702.9]). Migraine-specific findings suggested similar outcomes, with maximum cost associated with procedures (US $91.4 [0.3–59,396.7]). For DRG activity, the median all-cause claim was 1.0 (1.0–10.0), with the corresponding median cost being US $7052.2 [0–139,969.9]. DRG contributed around 64.9% towards all-cause cost. Study analysis showed that 32.0%, 24.5%, 24.5%, and 35.2% of all-cause HCRU cost of medications, procedures, services, and consumables, respectively, were related to migraine (Figure S2 and Table 5).
Overall HCRU and Associated Costs Among Combined Sub-Cohort of Patients with Migraine and Specific Cardiovascular Comorbidities
The disease-specific HCRU and associated costs among the combined sub-cohort of patients with migraine with specific cardiovascular comorbidities were analyzed for the 12-month post-index period. Most patients had myocardial ischemia/infarction (n = 5012), followed by cerebrovascular disease (n = 4119) and peripheral vascular disease (n = 1985).
The median migraine-specific cost was higher for patients with intestinal ischemia (US $1175.6 [43.1–12,613.9]), pulmonary embolism (US $1039.2 [8.6–126,246.9]), and cerebrovascular disease (US $647.8 [1.0–356,042.1]) than for patients with other specific cardiovascular comorbidities (Table S8).
HCRU and Associated Costs Based on Visit Type by Cardiovascular Comorbidity
The migraine-specific cost was analyzed among the combined sub-cohort of patients with migraine and specific cardiovascular comorbidities for inpatient, outpatient, and ER visits. For inpatient visits, the maximum median cost was observed for patients with arrhythmias (US $9698.6 [1300.0–89,921.5]) and congestive heart failure (US $9148.3 [1369.4–102,176.6]). The highest cost for outpatient visits was reported for patients with pulmonary embolism (US $804.8 [8.6–9903.3]) and venous thromboembolism (US $570.3 [4.8–19,845.9]). Similarly, migraine-specific costs due to pulmonary embolism (US $848.8 [130.1–6701.0]) and venous thromboembolism (US $747.8 [14.4–4915.8]) contributed the most towards ER visits (Table S9).
HCRU and Associated Costs Based on Activity Type by Cardiovascular Comorbidity
Analysis of migraine-specific costs among the combined sub-cohort of patients with specific cardiovascular comorbidities for activity type showed that the median net costs for medications and procedures were highest for patients with congestive heart failure (US $262.2 [0.5–14,233.5]) and intestinal ischemia (US $1255.5 [20.6–8687.6]) (Table S10). For consumables, the net cost was highest for patients with peripheral vascular disease (US $1987.6 [0.5–38,210.5]), while for services, patients with pulmonary embolism incurred the highest cost (US $31,389.7 [52.3–321,694.9]). For DRG-related activity, migraine-specific costs due to congestive heart failure (US $7599.0 [2049.8–89,921.5]) accounted for the maximum share in terms of net cost.
Discussion
Migraine has a considerable economic and societal burden and negatively impacts the QoL and productivity of affected individuals [1, 3, 16]. Despite this, the data on epidemiology, treatment patterns, and economic burden of migraine in the UAE are limited. Therefore, the current analysis evaluated patient demographics, comorbidity burden, treatment patterns, HCRU, and associated costs in patients with migraine in the UAE, using the DRWD.
The current study, contrary to earlier studies, showed a male preponderance in the prevalence of migraine [4, 35, 36]. This may be because the claims database primarily includes a male expatriate population (male to female expatriate ratio: 3:1) [37].
NSAIDs and triptans were commonly prescribed drugs for patients in the acute migraine sub-cohort, while beta-blockers constituted the major prescription drugs for patients in the preventive migraine sub-cohort, in accord with previous studies [17, 38]. Further, we noted the prescription of NSAIDs and triptans as monotherapy and combination therapy in the study population. Although triptans are widely used in acute migraine management in clinical practice, augmentation of therapy, with additional acute treatments, becomes essential when suboptimal responses are encountered.
In the current study, 13.4% (27,148 of total 203,222) of patients with migraine had cardiovascular comorbidity and many patients were prescribed triptans, which are contraindicated in patients with cardiovascular disease or risk factors. We would like to explain this observation considering a few limitations we had in our study analysis. We believe that this observation could be due to a large portion (55.4%) of the male population with migraine in our study. We also believe that since the mean age of the study population was 40 years, and notably 45.6% were 35–44 years of age, it may be that triptan was prescribed considering that the risk of cardiovascular adverse effects was lower in this younger cohort. The third limitation is that we are unable to quantify whether the cardiovascular risk was present before the prescription of triptan, or occurred after the triptan prescription. Therefore, we would need more focused studies to evaluate this potential relationship between triptan and cardiovascular risk.
Currently, the migraine treatment landscape is developing with the advent of novel drugs targeting CGRP or its receptor. These drugs effectively prevent migraine attacks with superior efficacy and safety profiles compared with conventional drugs [24, 39]. Moreover, they have substantially improved clinical outcomes and reduced the economic burden on patients with migraine [32, 40, 41]. Additionally, evidence from clinical trials and real-world studies have shown that anti-CGRP-monoclonal-antibodies (mAbs) are effective in reducing monthly migraine days, with a low discontinuation rate due to adverse events [42]. In an observational real-life study conducted in Spain on patients older than 65 years of age with migraine, the reduction in monthly migraine days after 6 months of treatment with anti-CGRP mAbs was 10.1 ± 7.3 days, with improved patient-related outcomes [43]. In yet another observational study, anti-CGRP mAbs proved to be effective in the treatment of patients with migraine (n = 155) who had failed at least three preventive medications. In the study, 51.6% of patients had ≥ 50% reduction in migraine days/month [44]. The prescription of novel therapies such as CGRP-targeting therapies was noted to be low in our study. It is therefore indispensable to understand the factors driving CGRP prescriptions or other newer drugs in the study population, despite more patients on premium than basic plans.
The clinical guidelines from the Dubai Health Authority include recommendations only for prophylactic or preventive treatment [16]. The recently published UAE consensus-based recommendations on the use of anti-CGRP mABs in the treatment of patients for prevention of migraine recommended anti-CGRP mAbs to be considered as a first-line treatment option for migraine prophylaxis. [45] Furthermore, the UAE consensus in 2024 on the acute management of migraine recommends the use of analgesics (primarily NSAIDs) and migraine-specific treatment with triptans, gepants, or ditans by following a stratified patient-centric care approach, considering the migraine severity, patient’s comorbidity, and contraindications to other medications [46].
In our study, the economic burden of migraine was considerable, accounting for 19.9% of all-cause costs incurred due to migraine. Additionally, the cardiovascular comorbidity analysis showed that significant costs were incurred when patients with migraine encountered specific cardiovascular comorbidities. Further, a higher HCRU burden was noted among patients with migraine due to inpatient department visits (81.3%) and medication costs (32.0%). In a real-world claims study, the mean pharmacy cost was found to be 1.8 times higher in patients with migraine than in those without migraine (US $22,429 vs. $13,166) [47]. In another retrospective, observational study, the mean direct and indirect all-cause healthcare costs were US $6575 and US $2350 higher in patients with migraine than in their non-migraine counterparts, respectively [30]. Reports suggest that preventive medication use significantly increased the total direct healthcare cost (US $50,274 ± 76,629) [32].
We now highlight a few limitations of the study. The study covered only the privately insured population of UAE (Dubai), i.e., the expatriate population; thus, the study results may not be generalizable to all patients with migraine. This study does not consider out-of-pocket expenses incurred by patients taking non-reimbursable over-the-counter medications or other alternative therapies. Information bias due to treatment pattern variability across providers could not be avoided. Migraine diagnosis date as per the database may not be the actual date of diagnosis, as patients may have been diagnosed earlier and made the claim later. The study did not collect medical history and pre-index medication data, leading to information bias. In addition, the database did not allow one-to-one mapping between diagnosis and medications/procedures/consumables. Thus, the data on disease-specific medications, HCRU, and associated costs were only suggestive that a specific medication or service was received that was possibly related to migraine. Hence, some of the reported migraine-specific medications, services, and costs may not be directly related to migraine. However, the study provides an in-depth analysis of the predominantly expatriate population covered by private insurance [34] and may prove beneficial for policymakers and aid in clinical decision-making.
Conclusion
Migraine has a substantial disease burden in the UAE and is associated with several comorbidities. Beta-blockers and anticonvulsants were frequently prescribed preventive treatments, while NSAIDs and triptans were common acute migraine therapies. Prescription of novel therapies (CGRP receptor antagonist, ditans) was low. Substantial HCRU and cost burden were primarily incurred for inpatient visits, medications, and procedural costs. Novel therapies, effective in reducing the frequency and intensity of migraine episodes and the socioeconomic burden are important factors in altering the migraine treatment landscape in the UAE. However, analysis of their role in migraine management is warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing/ Editorial Assistance
Medical writing and editorial support were provided by Dr. Kavitha Ganesha (Senior Consultant, IQVIA, India), Sharda Kandwal (Research Manager, IQVIA, India), and Bushra Nabi (Associate Consultant, IQVIA, India), and assistance with data analysis was provided by Ramya Mahendiran (IQVIA, India). No funding was provided for medical writing and editorial support since the associated people were full-time IQVIA employees (IQVIA conducted the study for Pfizer).
Author Contributions
All authors (Alessandro Terruzzi, Abubaker AlMadani, Suhail Al-Rukn, Mohamed Farghaly, Sara A. Dallal, Mostafa Zayed, Nora Vainstein, Mohamed Fathy, Anup Uboweja, Ashok Natarajan, Kumaresan Subramanyam, Badrinath C. Ramachandrachar, and Ali Aljabban) contributed to the study concept and design, data interpretation, and critical revision of the manuscript. Data acquisition and analysis was performed by Badarinath C. Ramachandrachar. Funding acquisition was contributed by Mostafa Zayed, Nora Vainstein, and Ali Aljabban. Project administration was performed by Badarinath C. Ramachandrachar and Ali Aljabban while Mostafa Zayed and Ali Aljabban contributed towards the manuscript supervision. All authors read and approved the final manuscript.
Funding
This study was funded by Pfizer Inc. Additionally, the Rapid Service and Open Access Fees are also being paid by Pfizer Inc.
Data Availability
All data generated or analyzed during this study are included in the main manuscript and the supplementary files.
Declarations
Conflict of Interest
Mostafa Zayed, Nora Vainstein, Mohamed Fathy, and Ali Aljabban are Pfizer employees. All other authors (Alessandro Terruzzi, Abubaker AlMadani, Suhail Al-Rukn, Mohamed Farghaly, Sara A. Dallal, Anup Uboweja, Ashok Natarajan, Kumaresan Subramanyam, and Badrinath C. Ramachandrachar) report no conflict of interest.
Ethical Approval
The study was observational in nature and involved analysis of previously collected anonymized structured data and did not impose any form of intervention. Hence, obtaining informed consent was not required for the study. Further, no institutional review board approval was required as it did not involve the collection, use, or transmission of individually identifiable data. The study adhered to the Health Insurance Portability and Accountability Act of 1996 to prevent disclosure of patient health information. The dataset utilized for the present study was publicly inaccessible. This dataset was obtained from the DHIC after adhering to all the required legal procedures to acquire and utilize this anonymized patient claims data. Under the terms of the data sharing contract with DHIC, IQVIA received a legitimate and exclusive right to use certain components of the dataset as specified in the contract. IQVIA was given authorization to apply the data for conducting anonymized and uniform analyses and research projects pertaining to health insurance claim records. Additionally, IQVIA was allowed to distribute the results and insights from the anonymized and uniform dataset to its clients for research purposes involving health insurance claim information. IQVIA pledged to adhere to established procedures to maintain compliance with applicable regulations, including those related to data privacy.
References
- 1.Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–76. [DOI] [PMC free article] [PubMed]
- 3.Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache J Head Face Pain. 2018;58(S1):17–32. [DOI] [PubMed] [Google Scholar]
- 4.El-Metwally A, Toivola P, AlAhmary K, Bahkali S, AlKhathaami A, Al Ammar SA, et al. The epidemiology of migraine headache in Arab countries: a systematic review. Sci World J. 2020;2020:4790254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M, et al. Migraine and cardiovascular disease: a population-based study. Neurology. 2010;74(8):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burch RC, Buse DC, Lipton RB. migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631–49. [DOI] [PubMed] [Google Scholar]
- 7.Buse DC, Reed ML, Fanning KM, Bostic R, Dodick DW, Schwedt TJ, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2020;21(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Ghadeer HA, AlSalman SA, Albaqshi FM, Alsuliman SR, Alsowailem FA, Albusror HA, et al. Quality of life and disability among migraine patients: a single-center study in AlAhsa, Saudi Arabia. Cureus. 2021;13(11): e19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doane MJ, Gupta S, Fang J, Laflamme AK, Vo P. The humanistic and economic burden of migraine in Europe: a cross-sectional survey in five countries. Neurol Ther. 2020;9(2):535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AlHarbi FG, AlAteeq MA. Quality of life of migraine patients followed in neurology clinics in Riyadh, Saudi Arabia. J Fam Commun Med. 2020;27(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradeep R, Nemichandra SC, Harsha S, Radhika K. Migraine disability, quality of life, and its predictors. Ann Neurosci. 2020;27(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelfand AA, Goadsby PJ. A neurologist’s guide to acute migraine therapy in the emergency room. Neurohospitalist. 2012;2(2):51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Öztürk V. Acute treatment of migraine. Noro Psikiyatr Ars. 2013;50(Suppl 1):S26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ailani J, Burch RC, Robbins MS. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–39. [DOI] [PubMed] [Google Scholar]
- 15.Zobdeh F, Ben Kraiem A, Attwood MM, Chubarev VN, Tarasov VV, Schiöth HB, et al. Pharmacological treatment of migraine: drug classes, mechanisms of action, clinical trials and new treatments. Br J Pharmacol. 2021;178(23):4588–607. [DOI] [PubMed] [Google Scholar]
- 16.DHA Telehealth clinical guidelines for virtual management of headache in adults—19. Health Policies and Standards Department, Health Regulation Sector (2021). Dubai Health Authority; 2021.
- 17.Roessler T, Zschocke J, Roehrig A, Friedrichs M, Friedel H, Katsarava Z. Administrative prevalence and incidence, characteristics and prescription patterns of patients with migraine in Germany: a retrospective claims data analysis. J Headache Pain. 2020;21(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puledda F, Shields K. Non-pharmacological approaches for migraine. Neurotherapeutics. 2018;15(2):336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim B-K, Chu MK, Yu SJ, Dell’Agnello G, Han JH, Cho S-J. Burden of migraine and unmet needs from the patients’ perspective: a survey across 11 specialized headache clinics in Korea. J Headache Pain. 2021;22(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombard L, Ye W, Nichols R, Jackson J, Cotton S, Joshi S. A real-world analysis of patient characteristics, treatment patterns, and level of impairment in patients with migraine who are insufficient responders vs responders to acute treatment. Headache. 2020;60(7):1325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipton RB, Munjal S, Buse DC, Alam A, Fanning KM, Reed ML, et al. Unmet acute treatment needs from the 2017 migraine in America symptoms and treatment study. Headache. 2019;59(8):1310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther. 2020;211: 107528. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ. New generation gepants: migraine acute and preventive medications. J Clin Med. 2022;11(6):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandervorst F, Van Deun L, Van Dycke A, Paemeleire K, Reuter U, Schoenen J, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan S, Olesen A, Ashina M. CGRP, a target for preventive therapy in migraine and cluster headache: systematic review of clinical data. Cephalalgia. 2019;39(3):374–89. [DOI] [PubMed] [Google Scholar]
- 26.Wang SJ, Roxas AA Jr, Saravia B, Kim BK, Chowdhury D, Riachi N, et al. Randomised, controlled trial of erenumab for the prevention of episodic migraine in patients from Asia, the Middle East, and Latin America: the EMPOwER study. Cephalalgia. 2021;41(13):1285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–32. [DOI] [PubMed] [Google Scholar]
- 28.Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J, et al. Eptinezumab for prevention of chronic migraine: a randomized phase 2b clinical trial. Cephalalgia. 2019;39(9):1075–85. [DOI] [PubMed] [Google Scholar]
- 30.Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700–14. [DOI] [PubMed] [Google Scholar]
- 31.Harris L, L’Italien G, Kumar A, Seelam P, LaVallee C, Coric V, et al. Real-world assessment of the relationship between migraine-related disability and healthcare costs in the United States. Headache. 2022;62(4):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster SA, Hoyt M, Ye W, Mason O, Ford JH. Direct cost and healthcare resource utilization of patients with migraine before treatment initiation with calcitonin gene-related peptide monoclonal antibodies by the number of prior preventive migraine medication classes. Curr Med Res Opin. 2022;38(5):653–60. [DOI] [PubMed] [Google Scholar]
- 33.Vo P, Swallow E, Wu E, Zichlin ML, Katcher N, Maier-Peuschel M, et al. Real-world migraine-related healthcare resource utilization and costs associated with improved vs. worsened/stable migraine: a panel-based chart review in France, Germany, Italy, and Spain. J Med Econ. 2021;24(1):900–7. [DOI] [PubMed] [Google Scholar]
- 34.UAEps. United Arab Emirates population statistics 2023. https://www.globalmediainsight.com/blog/uae-population-statistics/#:~:text=The%20UAE%20Population%20in%202022,in%202022%20is%208.92%20Million.
- 35.Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, et al. Migraine. Nat Rev Dis Primers. 2022;8(1):2. [DOI] [PubMed] [Google Scholar]
- 36.Faust E, Pivneva I, Yang K, Betts KA, Ahmed Z, Joshi S, et al. Real-world treatment profiles, clinical outcomes, and healthcare resource utilization of patients with migraine prescribed erenumab: a multicenter chart-review study of US headache centers. Neurol Ther. 2021;10(1):293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.UAE Population and Demographics. https://www.dubai-online.com/essential/uae-population-and-demographics. Accessed Apr 2023.
- 38.Gendolla A, Rauer N, Kraemer S, Schwerdtner I, Straube A. Epidemiology, demographics, triptan contraindications, and prescription patterns of patients with migraine: a German claims database study. Neurol Ther. 2022;11(1):167–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavridis T, Deligianni CI, Karagiorgis G, Daponte A, Breza M, Mitsikostas DD. Monoclonal antibodies targeting CGRP: from clinical studies to real-world evidence-what do we know so far? Pharmaceuticals (Basel). 2021;14(7):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipton RB, Brennan A, Palmer S, Hatswell AJ, Porter JK, Sapra S, et al. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;21(7):666–75. [DOI] [PubMed] [Google Scholar]
- 41.Tobin JA, Joshi S, Ford JH, Nichols RM, Foster SA, Ruff D, et al. Reductions in acute medication use and healthcare resource utilization in patients with chronic migraine: a secondary analysis of a phase 3, randomized, double-blind, placebo-controlled study of galcanezumab with open-label extension (REGAIN). J Med Econ. 2022;25(1):1030–8. [DOI] [PubMed] [Google Scholar]
- 42.Wang YF, Wang SJ. CGRP targeting therapy for chronic migraine-evidence from clinical trials and real-world studies. Curr Pain Headache Rep. 2022;26(7):543–54. [DOI] [PubMed] [Google Scholar]
- 43.Muñoz-Vendrell A, Campoy S, Caronna E, Alpuente A, Torres-Ferrus M, Nieves Castellanos C, et al. Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: a real-life multicentre analysis of 162 patients. J Headache Pain. 2023;24(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres-Ferrus M, Gallardo VJ, Alpuente A, Caronna E, Gine-Cipres E, Pozo-Rosich P. The impact of anti-CGRP monoclonal antibodies in resistant migraine patients: a real-world evidence observational study. J Neurol. 2021;268(10):3789–98. [DOI] [PubMed] [Google Scholar]
- 45.Alsaadi T, Kayed DM, Al-Madani A, Hassan AM, Terruzzi A, Krieger D, et al. Consensus-based recommendations on the use of CGRP-based therapies for migraine prevention in the UAE. Neurol Ther. 2023;12(6):1845–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsaadi T, Kayed DM, Al-Madani A, Hassan AM, Krieger D, Riachi N, et al. Acute treatment of migraine: expert consensus statements from the United Arab Emirates (UAE). Neurol Ther. 2024;13(2):257–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polson M, Williams TD, Speicher LC, Mwamburi M, Staats PS, Tenaglia AT. Concomitant medical conditions and total cost of care in patients with migraine: a real-world claims analysis. Am J Manag Care. 2020;26(1 Suppl):S3–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the main manuscript and the supplementary files.