Introduction
Glioma, a primary malignant tumor originating from glial cells, represents approximately 81% of intracranial malignant tumors. It is known for its high heterogeneity and generally poor prognosis [1–3]. Despite comprehensive treatment approaches, the prognosis for glioma remains grim due to its highly malignant nature [4]. Surgical intervention, primarily through routine craniotomy, has been the traditional treatment method, although it involves significant trauma and has long lacked an ideal approach. Conventional surgical treatments showed a high recurrence rate, necessitating supplementary postoperative radiotherapy and chemotherapy [5, 6].
Recent studies emphasize the critical role of postoperative radiotherapy, particularly intensity-modulated radiotherapy [7]. This technique offers precise targeting and dose concentration, effectively eliminating glioma while minimizing radiation exposure to surrounding healthy tissues [7]. Traditional imaging may lead to misinterpretations of therapeutic outcomes, such as pseudo-progression, where treatment may initially seem to worsen tumor imaging or symptoms, yet these can improve if the current treatment plan is maintained [8, 9].
Innovations in PET imaging with 11C or 18F-labeled choline (CHO) have shown promise in tumor diagnostics. CHO enters cells via high-affinity choline transporters, is phosphorylated by choline kinase, and integrated into phosphatidylcholine, reflecting the synthesis activity of the cell membrane system [10, 11]. CHO uptake is low in normal brain tissue but significantly higher in rapidly proliferating tumor cells. Several quantitative markers, such as maximum standardized uptake value (SUVmax), average standardized uptake value (SUVmean), metabolic tumor volume (MTV), total lesion CHO uptake (TLG), and the tumor-to-normal contralateral cortical activity ratio (T/N ratio), have proven crucial for correlating with glioma grading. These markers offer prognostic distinctions superior to those based on the World Health Organization (WHO) grading system [12, 13].
Utilizing 11C-CHO PET/CT imaging technology, type, location, and extent of tumors could be pinpointed more accurately. This method not only facilitates precise pre-surgical diagnoses and tumor boundary delineation but also provides insights into the tumor’s biological characteristics and invasiveness. Such detailed information is vital for crafting personalized treatment plans and for surgical planning, thereby optimizing surgical outcomes and minimizing risks. Postoperatively, 11C-CHO PET/CT imaging is invaluable for monitoring treatment response, evaluating residual tumors, assessing recurrence risks, and improving overall prognosis [14, 15].
This pilot study retrospectively analyzed 38 patients with recurrent glioma, as determined by 11C-CHO PET/CT imaging. The findings affirm the significant prognostic value of this imaging technology in assessing glioma outcomes and offer a reliable reference for prognosis evaluations in clinical settings.
Materials and methods
Participants
This study retrospective included participants have a histopathological confirmed diagnosis of glioma, have undergone prior treatment with surgery and/or radiotherapy at the Department of Neurosurgery, Affiliated Hospital of Inner Mongolia Medical University, from January 2019 to October 2021. PET Scans were performed post-operatively and during follow-up periods for monitoring. Patients presented elevated 11C-CHO uptake were retrospective recruited. Patients’ demographic and clinical information were collected. Ethical approval was granted by the ethics committee of Affiliated Hospital of Inner Mongolia Medical University, written informed consent was obtained from all patients before the imaging examinations. Patients with presence of brain metastases from other malignant tumors or non-glioma primary brain tumors were excluded.
PET imaging agent
The 11C-CHO agent was synthesized using a Sumitomo HM-20 S cyclotron and a GE TRACERLab FX-C chemical synthesizer, with HPLC purification by Shimadzu Corporation and TLC by Bioscan Corporation. The agent, provided by the Nuclear Medicine Department, exhibited a radioactive chemical purity of over 95%. All Participants were briefed about the procedure and signed informed consent forms before imaging.
Imaging procedure
After administering 10–15 mCi (370–555 Mbq) of 11C-CHO intravenously. A CT scan covered the skull top to base, using 140 kV, 110 mA, and a 5 mm slice thickness. PET imaging acquisition (ZOOM 2, slice thickness 5 mm) followed in three-dimensional mode within the same field of view (Biograph mCT Flow, SIEMENS, Erlangen, Germany) after 30–60 min. The PET images were corrected for attenuation using the CT data and then fused with the CT images.
Image analysis
The adjusted PET images were then processed using Euclid software (version1.0, Evomics Medical Technology Co., Ltd. Shanghai, China) for tumor delineation. Images were reviewed by two experienced nuclear medicine and CT specialists using a centralized reading approach using semi-automated 3D isocount volume-of-interest (VOI) tools to define tumor boundaries. Where necessary, a manual adjustment tool was used for slice-by-slice refinement when the automated tools failed to accurately define the tumor edges. Additionally, a reference VOI was set up in a mirror-image region to assist with tissue-background ratio (TBR) calculations. The system then automatically calculated the radioactive count for each ROI, determining the SUVmax, SUVmean, MTV, and TLG values.
Clinical and survival analysis
The associations between WHO grade, IDH mutation status, and imaging parameters were analyzed. Post-recurrence survival (PRS) was defined as the time between initiating PET imaging and date of death. Kaplan-Meier analysis was performed to study the prognostic value of PET-related parameters including SUVmax, SUVmean, MTV and TLG for PRS. The surv_cutpoint function of the survminer package in R (v0.4.9) was used to determine the optimal split point for continuous variables in the Kaplan Meier analysis. p < 0.05 was considered statistically significant. We have included additional analysis using Cox proportional hazards models to evaluate outcome.
Results
The cohort consisted of 38 patients with a median age of 48 years (range: 17–71). The patients’ gliomas were classified according to the World Health Organization (WHO) criteria, with seven patients having grade III gliomas and nine patients having grade IV gliomas. Patient demographics are summarized in Table 1. SUVmean (r = 0.79), SUVmax(r = 0.78), and TLG(r = 0.49), but not MTV(r = 0.21), were significantly associated with WHO grade (P < 0.01). However, none showed a significant relationship with IDH mutation status (P > 0.05).
Table 1.
Patient characteristics
| Characteristic | Values |
|---|---|
| Age (years) | Median:48(range,17–71) |
| Sex, n (%) | |
| Male | 23(60.5%) |
| Female | 15(39.5%) |
| Region of interest | |
| SUVmax | 2.783 ± 1.133(‾x ± s) |
| SUVmean | 1.508 ± 0.628(‾x ± s) |
| MTV | 6.824(9.835) Median (Interquartile range) |
| WHO grade, n (%) | |
| Grade II | 22(57.9%) |
| Grade III | 7(18.4%) |
| Grade IV | 9(23.7% ) |
| Biopsy | |
| IDH1 mutation | 17(44.7%) |
| MGMT methylation | 11(28.9%) |
| GFAP positive | 13(34.2%) |
Clinical and survival analysis
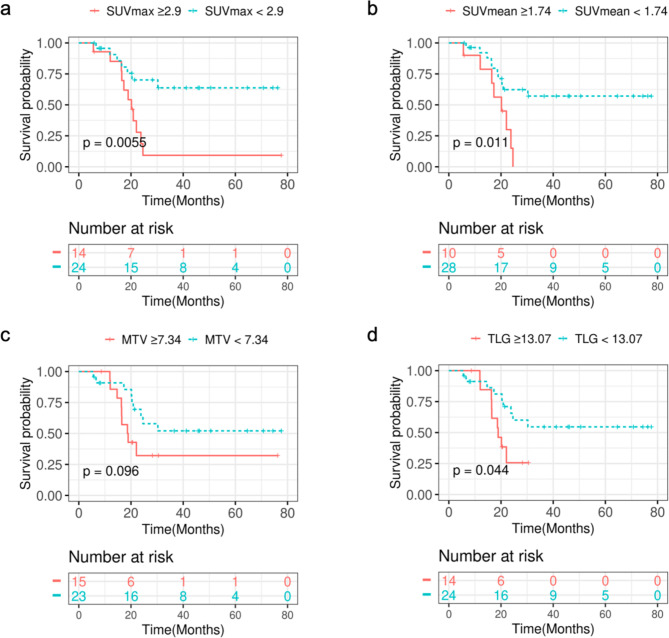
We divided patients into two groups based on WHO grade: a high-grade group (WHO grades III and IV) and a low-grade group (WHO grades I and II). In the high-grade group, patients with lower SUVmax, SUVmean, MTV, or TLG values had more favorable survival outcomes, with TLG showing a significant correlation with prognostic risk (p = 0.019). Similarly, in the low-grade group, lower SUVmax, SUVmean, MTV, or TLG values were associated with better survival outcomes. Specifically, MTV and TLG were significantly correlated with prognostic risk, with p-values of 0.034 and 0.023, respectively. (supplemental files). The Kaplan-Meier (KM) analysis revealed significant correlations between parameters such as the maximum SUVmax, SUVmean, and TLG with the prognostic risk, whereas the MTV result did not demonstrate significant association. Figure 1 provides illustrative examples of representative patients. Notably, patients exhibiting lower SUVmax, SUVmean, or TLG values exhibited more favorable survival outcomes, with respective p-values of 0.0055, 0.011, and 0.044, as depicted in Fig. 2.
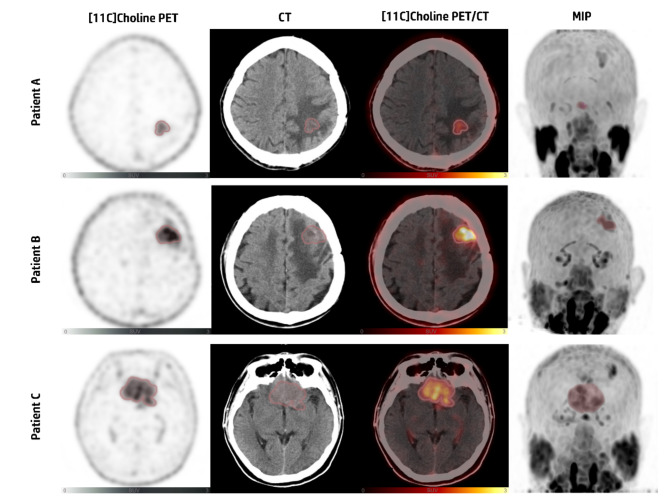
Fig. 1.
Patient A: a 41-year-old male diagnosed with mixed oligodendroglioma (WHO grade II), underwent surgery followed by radiotherapy. 11C-choline PET/CT imaging showed increased tracer uptake in the left frontal lobe (SUVmax=2.10; SUVmean=1.22; MTV = 4.88cm3). Follow-up: Alive, survival period of 38 months
Patient B: a 43-year-old male diagnosed with anaplastic oligodendroglioma (WHO grade III), underwent surgery followed by radiotherapy. 11C-choline PET/CT imaging showed increased tracer uptake in the left frontal lobe (SUVmax=5.28; SUVmean=3.17; MTV = 3.01 cm3). Follow-up: Deceased, survival period of 12 months
Patient C: a 57-year-old male diagnosed with glioblastoma (WHO grade IV), underwent surgery followed by radiotherapy. 11C-choline PET/CT imaging showed increased tracer uptake in bilateral frontal lobes and the genu of corpus callosum (SUVmax=3.59; SUVmean=1.97; MTV = 24.81 cm3). Follow-up: Deceased, survival period of 6 months
Fig. 2.
Kaplan-Meier curves of OS according to the patients’ baseline PET-related parameters derived from tumor lesions of whole brain
Cox proportional hazards models were utilized to evaluate the influence of various PET parameters on post-recurrence survival. The analysis revealed that lower SUVmax, SUVmean, and MTV values did not have a statistically significant association with improved survival outcomes, as indicated by hazard ratios showing a reduced risk of mortality (p value > 0.05). However, contrary to the Kaplan-Meier analysis, lower TLG values were not linked to poorer survival outcomes. (supplemental files).
Discussion
The findings of this retrospective analysis reinforce the substantial prognostic value of 11C-choline PET/CT imaging in managing recurrent glioma. By providing detailed insights into the metabolic activity of glioma cells, this study highlights the advantages of 11C-CHO PET/CT in facilitating more accurate clinical decision-making and treatment planning.
11C-CHO PET/CT imaging has provided distinct advantages over traditional imaging modalities, particularly in its ability to differentiate between tumor recurrence and radiation-induced changes such as pseudo-progression [8, 9]. This capability is crucial because it informs more accurate clinical decision-making and treatment planning. In this present study, by providing detailed insights into the metabolic activity of glioma cells, 11C-CHO PET/CT not only facilitates the identification and delineation of residual disease post-surgery which potentially including targeted radiotherapy, but also proves indispensable in postoperative assessments and long-term management.
The study’s findings also underscored the superior prognostic value of PET-related parameters such as SUVmax, SUVmean, and TLG. The correlation of these parameters with patient survival suggests that lower SUVmax, SUVmean, and TLG are associated with better survival outcomes. Interestingly, MTV did not show a significant correlation with PRS, indicating that the metabolic activity reflected by SUV and TLG might be more indicative of tumor aggressiveness than the volume measured alone.
These results have important implications for the clinical management of glioma. The ability of 11C-CHO PET/CT to provide quantitative and qualitative data enhances the WHO grading system, offering a more nuanced approach to patient stratification and personalized treatment planning. As the integration of 11C-CHO PET/CT in clinical settings continues to evolve, its potential to significantly improve patient outcomes becomes increasingly apparent. This in vivo approach ensures rigorous data collection and analysis, aiming to assess the prognostic value of 11C-CHO PET/CT imaging in glioma recurrence and to contribute significantly to personalized patient management strategies, but its short half-life limits availability to centers with on-site cyclotrons. FET PET/CT, with a longer half-life, is more accessible. However, 18F-FET may show lower uptake in low-grade gliomas, potentially underestimating residual disease.
11C-CHO PET/CT imaging is warranted in patients with suspected recurrent glioma to confirm recurrence for tailor personalized treatment plans, potentially including targeted radiotherapy based on the metabolic activity and extent of the tumor.
While the findings of this study demonstrate the substantial prognostic value of 11C-choline PET/CT imaging in managing recurrent glioma, several limitations should be acknowledged: The study is based on a relatively small sample size; larger, multicentric trials are needed. As a retrospective study, it is subject to inherent biases such as selection bias and information bias. While the study highlights the advantages of 11C-CHO PET/CT, it does not extensively compare this modality with other advanced imaging techniques such as MRI or other PET tracers.
Conclusion
This study demonstrated that 11C-CHO PET/CT are associated with post-recurrence survival, suggesting their utility in predicting patient outcomes effectively. Future studies should focus on validating these results in a larger cohort to establish standardized protocols that leverage the prognostic capabilities of 11C-CHO PET/CT imaging in managing glioma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- PET
Positron emission tomography
- CT
Computed tomography
- 11C-CHO
11C-Choline
- SUVmax
Maximum standardized uptake value
- SUVmean
Average standardized uptake value
- MTV
Metabolic tumor volume
- TLG
Total lesion CHO uptake
- T/N ratio
Tumor-to-normal contralateral cortical activity ratio
- WHO
World Health Organization
- VOI
Volume-of-interest
- TBR
Tissue-background ratio
- PRS
Post-recurrence survival
Authors contributions
GH: Data curation, Writing-Original draft. BT: Investigation, Writing-Original draft. SH: Formal analysis, Visualization. SW: Investigation. MH: Supervision. XL: Supervision, Writing - Review & Editing. XB: Methodology, Resources, Project administration. All authors read and approved the final manuscript.
Funding
This study was funded by the Inner Mongolia Autonomous Region Science and Technology Plan Project (201802111).
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All procedures involving human participants in this study were approved by the Institutional Review Board (Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University - Approval no. WZ20150014); the study protocol complied with the tenets of Declaration of Helsinki.
Consent for publication
Informed consent was obtained from all individual participants for publication of this study and accompanying images.
Conflict of interest
no conflict of interest.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Geng Hu and Bin Tian share the authorship.
Contributor Information
Xiang Li, Email: xiang.li@meduniwien.ac.at.
Xia Bai, Email: libaiqiangxia@163.com.
References
- 1.Xu H, Zhang A, Han X, et al. ITGB2 as a prognostic indicator and a predictive marker for immunotherapy in gliomas. Cancer Immunol Immunother. 2022;71(3):645–60. 10.1007/s00262-021-03022-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Q, Lin Y, Zhang W, He F, Xu Y, Chen Z. Bioinformatics analysis of LMAN1 expression, clinical characteristics, and its effects on cell proliferation and invasion in glioma. Brain Res. 2022;1789:147952. 10.1016/j.brainres.2022.147952 [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. 10.1007/s00401-016-1545-1 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 4.Vasilev A, Sofi R, Rahman R, Smith SJ, Teschemacher AG, Kasparov S. Using light for therapy of Glioblastoma Multiforme (GBM). Brain Sci. 2020;10(2):75. Published 2020 Jan 31. 10.3390/brainsci10020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roda D, Veiga P, Melo JB, Carreira IM, Ribeiro IP. Principles in the management of Glioblastoma. Genes (Basel). 2024;15(4):501. Published 2024 Apr 17. 10.3390/genes15040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol. 2013;15(1):4–27. 10.1093/neuonc/nos273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martín-Abreu C, Fariña-Jerónimo H, Plata-Bello J. Radiological and not clinical variables Guide the Surgical Plan in patients with Glioblastoma. Curr Oncol. 2024;31(4):1899–912. 10.3390/curroncol31040142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–61. 10.1016/S1470-2045(08)70125-6 [DOI] [PubMed] [Google Scholar]
- 9.Knudsen-Baas KM, Moen G, Fluge Ø, Storstein A. Pseudoprogression in high-grade glioma [published correction appears in Acta Neurol Scand Suppl. 2013;127(3):e17]. Acta Neurol Scand Suppl. 2013;(196):31–37. [DOI] [PubMed]
- 10.Giovannini E, Lazzeri P, Milano A, Gaeta MC, Ciarmiello A. Clinical applications of choline PET/CT in brain tumors. Curr Pharm Design. 2015;21(1):121–7. 10.2174/1381612820666140915120742 [DOI] [PubMed] [Google Scholar]
- 11.Alongi P, Vetrano IG, Fiasconaro E, et al. Choline-PET/CT in the Differential diagnosis between cystic Glioblastoma and Intraparenchymal Hemorrhage. Curr Radiopharm. 2019;12(1):88–92. 10.2174/1874471011666180817122427 [DOI] [PubMed] [Google Scholar]
- 12.Kong Z, Jiang C, Liu D, Chen W, Ma W, Cheng X, Wang Y. Quantitative features from CHO PET distinguish the WHO grades of primary diffuse glioma. Clin Nucl Med. 2021;46(2):103–10. 10.1097/RLU.0000000000003406 [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Liu D, Kong Z, et al. Prognostic value of Choline and other metabolites measured using 1H-Magnetic resonance spectroscopy in Gliomas: a Meta-analysis and systemic review. Metabolites. 2022;12(12):1219. Published 2022 Dec 5. 10.3390/metabo12121219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Kim D, Kim SH, Park MA, Chang JH, Yun M. The roles of 11 C-acetate PET/CT in predicting tumor differentiation and survival in patients with cerebral glioma. Eur J Nucl Med Mol Imaging. 2018;45(6):1012–20. 10.1007/s00259-018-3948-9 [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Zhu Y, Mu D, et al. Correlation of hypoxia as measured by fluorine-18 fluoroerythronitroimidazole (18F-FETNIM) PET/CT and overall survival in glioma patients. Eur J Nucl Med Mol Imaging. 2020;47(6):1427–34. 10.1007/s00259-019-04621-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.