Abstract
Introduction
Treating plaque psoriasis (PsO) with guselkumab (GUS) promotes skin clearance and is associated with improvements in health-related quality of life (HRQoL), anxiety, and depression. It is unclear whether improvements in patient-reported outcomes are due to resolution of skin symptoms or the direct result of GUS treatment.
Methods
Two phase 3, placebo- and active-comparator-controlled studies randomized patients with moderate-to-severe PsO to GUS, placebo (crossing over to GUS at week 16), or adalimumab. Post hoc mediation analyses examined direct and indirect effects of GUS, versus adalimumab, on Dermatology Life Quality Index (DLQI) or Hospital Anxiety and Depression Scale (HADS) after adjusting for indirect effects mediated by skin clearance, evaluated via Psoriasis Area and Severity Index (PASI), to determine the direct effect of GUS on dermatology HRQoL, depression, and anxiety.
Results
Compared with adalimumab, the natural direct effect (NDE) of GUS on change in DLQI from baseline was − 2.04 (P < 0.001), using PASI improvement as a mediator, indicating 89.2% of the total treatment effect was due to direct effects of GUS; using PASI 90 as a mediator, NDE of GUS was − 1.43 (P < 0.001), with 62.2% of the total treatment effect attributed to direct effects of GUS. Compared with adalimumab, 25.5% of change in HADS anxiety score was mediated through PASI improvement (NDE − 0.74; P = 0.002), indicating 74.5% of the total effect was independent of PASI improvement. Similarly, 24% of treatment effect was mediated through PASI 90 (NDE − 0.76; P = 0.002). Comparable proportions of the total improvement in HADS depression scores were due to direct and indirect effects of GUS mediated through PASI improvement (direct, 50.2%; indirect, 49.8%) or PASI 90 (direct, 59.5%; indirect, 40.5%).
Conclusions
GUS-mediated improvements in anxiety, depression, and overall HRQoL are not solely mediated by resolution of PsO signs, suggesting GUS use has a potential direct effect on anxiety and depression.
Keywords: Anxiety, Depression, Guselkumab, Health-related quality of life, Mediation analysis, Psoriasis
Key Summary Points
| Why carry out this study? |
| Treatment of plaque psoriasis with guselkumab promotes skin clearance and is associated with improvements in health-related quality of life, anxiety, and depression. |
| This ad hoc analysis sought to determine the direct effect of guselkumab on dermatology health-related quality of life, depression, and anxiety, after adjusting for indirect effects mediated by skin clearance. |
| What was learned from the study? |
| Compared with adalimumab, < 40% of change in Dermatology Life Quality Index (DLQI) was mediated through skin clearance (as measured by Psoriasis Area and Severity Index [PASI]), indicating that ≥ 60% of the total effect of guselkumab was independent of PASI improvement; approximately 50% of the effect of guselkumab on change in Hospital Anxiety and Depression Scale anxiety/depression scores was independent of PASI improvement. |
| Guselkumab-mediated improvements in anxiety, depression, and overall health-related quality of life are not solely mediated by resolution of psoriasis signs, suggesting guselkumab has a potential direct effect on anxiety and depression. |
Introduction
Plaque psoriasis (PsO) is a chronic, immune-mediated inflammatory condition that manifests in skin-related signs (e.g., scaling, redness, and cracking) and symptoms (e.g., itch and pain) of disease. Patients with PsO also demonstrate impaired health-related quality of life (HRQoL), which may include feelings of anxiety or depression that, in turn, may negatively impact social interactions or self-esteem [1–4]. The prevalence of anxiety and depression is typically higher in patients with PsO than among the general population or among patients with other dermatologic disorders [5–7].
The impact of PsO on HRQoL may be influenced by a number of factors. Predictably, severity of PsO can contribute to impact on HRQoL [8]; for example, depression is more common in patients with more severe disease [5, 9, 10]. Severity of disease had traditionally been defined by the percentage of affected body surface area (BSA), but the location of psoriatic plaques on the body can also have a substantial effect on HRQoL [11]. For instance, PsO in certain locations, such as the face, genitals, or hands and/or feet, has a disproportionately negative impact on social interactions or self-esteem [11]. Further, the relationship between PsO and anxiety/depression may be reciprocal: while the signs and symptoms of PsO may contribute to feelings of depression and anxiety, stress may also exacerbate PsO [5]. Given the multiple, potentially interacting, factors that can contribute to impaired HRQoL in patients with PsO, it is important to determine how and to what extent treatments directly or indirectly improve HRQoL. In other words, do treatments improve overall HRQoL through the indirect effects of improving skin or can treatments directly affect mental health and other aspects of HRQoL through direct action?
Treatment of PsO focuses on mitigating skin-related manifestations of disease, with the expectation that alleviating those signs and symptoms will improve HRQoL [11]. This idea is supported by the observed correlation between improvement in clinical endpoints, such as Psoriasis Area and Severity Index (PASI), and patient-reported outcomes (PRO), such as the Dermatology Life Quality Index (DLQI) and Hospital Anxiety and Depression Scale (HADS) scores [4, 12–16]. However, studies have shown that even complete clearance of PsO does not necessarily eliminate decrements in skin-related HRQoL. For example, in a pooled analysis of two phase 3 studies, a notable proportion of patients who achieved clear skin (PASI 100) still reported an impact of disease on HRQoL, as measured by a PsO-specific PRO [4]. Taken together, these observations suggest that, while skin-related signs and symptoms of PsO have a marked effect on daily life, other factors may also play a role in overall HRQoL.
Proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-12, and IL-23 are present in psoriatic lesions and play a key role in the pathogenesis of PsO [17, 18]. There is also growing evidence for a link between inflammatory pathways and mood disorders [19–22]. Biologics targeting inflammatory cytokines have demonstrated efficacy in evoking a clear skin response and have also been associated with benefits to HRQoL, including decreased anxiety and depression [12, 13, 23, 24].
Guselkumab (GUS), a monoclonal antibody that targets the p19 subunit of IL-23, is approved for the treatment of moderate to severe plaque PsO [25]. Two phase 3 studies, VOYAGE 1 and VOYAGE 2, confirmed the efficacy and safety of GUS in patients with moderate to severe PsO [23, 26]. In both studies, significantly greater proportions of GUS-treated patients achieved clear or nearly clear skin (an Investigator’s Global Assessment [IGA] score of 0/1 or PASI 90 response) versus placebo or adalimumab. In VOYAGE 2, which evaluated PRO using HADS, a greater proportion of patients treated with GUS had significantly improved HADS-anxiety and HADS-depression scores versus patients treated with placebo or adalimumab [13]. Whether improvements in anxiety and depression are due to resolution of skin-related signs and symptoms of disease or the direct result of GUS treatment deserves further examination.
This study sought to determine the direct effect of GUS treatment on dermatology HRQoL, depression, and anxiety after adjusting for the indirect effect mediated by skin clearance (evaluated via PASI) to better understand the direct treatment effects of GUS on HRQoL in patients with PsO.
Methods
VOYAGE 1 and VOYAGE 2 Study Designs
VOYAGE 1 and VOYAGE 2 were phase 3, randomized, double-blinded, placebo- and active-comparator-controlled studies of GUS; study designs for both trials have been reported in detail, elsewhere [23, 26]. Both studies enrolled adults (≥ 18 years of age) with moderate-to-severe plaque PsO for at least 6 months (defined as IGA score ≥ 3, PASI score ≥ 12, and BSA involvement ≥ 10%) and who were candidates for phototherapy or systemic therapy. In VOYAGE 1 (N = 837) and VOYAGE 2 (N = 992), patients were randomized to GUS 100 mg administered via subcutaneous injection (SC) at weeks 0 and 4, then every 8 weeks (q8w); or placebo at weeks 0, 4, and 12, followed by GUS 100 mg SC at weeks 16 and 20, then q8w; or adalimumab 80 mg SC at week 0, 40 mg at week 1, then 40 mg every 2 weeks. Through week 24, the study designs for VOYAGE 1 and VOYAGE 2 were identical. In VOYAGE 1, patients randomized to adalimumab continued to receive adalimumab every 2 weeks through week 47, then crossed over, at week 52, to received open-label GUS 100 mg q8w through week 252. In VOYAGE 2, PASI 90 responders at week 28 entered a randomized withdrawal and GUS re-treatment period (weeks 28–72), and then received open-label GUS (weeks 76–252).
In both VOYAGE 1 and VOYAGE 2, the coprimary endpoints were the proportion of patients reaching a ≥ 90% improvement in PASI (PASI 90) and the proportion of patients achieving an IGA score of 0/1; primary results have been reported elsewhere [23, 26]. Impact of PsO on HRQoL, evaluated using DLQI, was a secondary endpoint in both studies [23, 26]. DLQI is a dermatology-specific instrument designed to assess the impact of the disease on a patient’s HRQoL; scores range from 0 to 30, with higher scores indicating more impact of disease [27]. In addition, in VOYAGE 2, the emotional impact of PsO was measured using HADS to assess symptoms of anxiety and depression. HADS consists of two subscales, one for anxiety and one for depression, with scores ranging from 0 to 21. Higher scores indicate more severe symptoms, with scores ≥ 8 indicating anxiety or depression [28].
Ethical Approval
This article is based on data from previously conducted studies, VOYAGE 1 (NCT02207231) and VOYAGE 2 (NCT 02207244), which were conducted in accordance with the Declaration of Helsinki and were consistent with good clinical practices and regulatory requirements. Patients provided written informed consent prior to enrollment in either trial.
Mediation Analysis Scenarios
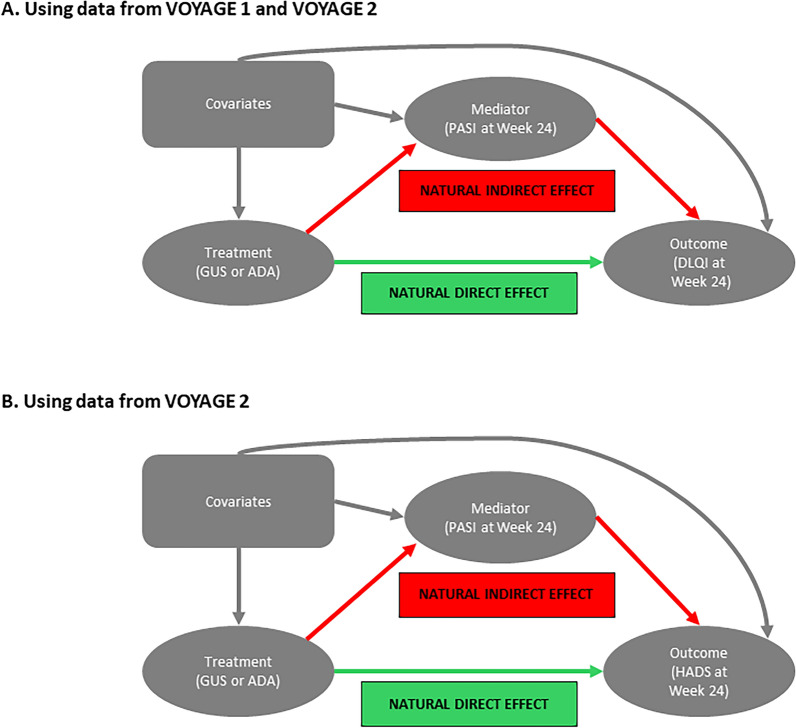
Post hoc mediation analyses to examine the direct and indirect effects of GUS treatment on DLQI were performed after adjusting for the indirect effects mediated by PASI, a clinician-reported measure of skin-related signs of PsO, using pooled data from VOYAGE 1 and VOYAGE 2 (Fig. 1a). The mediation analysis involved fitting two models: (1) a mediation model for modeling the mediator variable, given the treatment and covariates, and (2) an outcomes model for modeling the outcome of interest (e.g., DLQI), given the treatment, mediator variable, and covariates. Variables used in these mediation analyses included covariates (age, gender, body mass index, PsO duration, comorbidity of known psoriatic arthritis with joint pain, DLQI score at baseline, and IGA score at baseline), outcome (change from baseline in absolute DLQI score at week 24), mediator variables (absolute PASI improvement versus baseline or PASI 90 response at week 24), and treatment (GUS versus adalimumab).
Fig. 1.
Causal diagram of the mediation analyses. These models allow for exposure–mediator interactions. The natural direct effect is independent of the treatment effect on the outcome that is beyond its effect on the mediator; natural indirect effect is the treatment effect on the outcome that is mediated (explained) by its effect on the mediator. Models evaluated treatment effect on DLQI adjusting for PASI (a) and treatment effect on HADS (anxiety and depression) adjusting for PASI (b). GUS guselkumab, ADA adalimumab, PASI Psoriasis Area and Severity Index, DLQI Dermatology Life Quality Index, HADS Hospital Anxiety and Depression Scale
In addition, mediation analyses were performed to examine the direct and indirect effects of GUS treatment on HADS after adjusting for the indirect effects mediated by PASI using data from VOYAGE 2 (Fig. 1b). The mediation analysis involved fitting two models: (1) a mediation model for modeling the mediator variable, given the treatment and covariates, and (2) an outcomes model for modeling the outcome of interest (e.g., HADS score), given the treatment, mediator variable, and covariates. Variables used in these mediation analyses included covariates (age, gender, body mass index, PsO duration, HADS scores at baseline, PASI at baseline, and race), outcome (change from baseline in absolute HADS score at week 24), mediator variables (absolute PASI improvement versus baseline at week 24 or PASI 90 response at week 24), and treatment (GUS versus adalimumab).
In both scenarios, estimates from the mediation analysis included control direct effect (independent treatment effect on the outcome when the mediator is set to a fixed level), natural direct effect (NDE; effect on the outcome after adjusting for the indirect effect exerted by the mediator), natural indirect effect (NIE; treatment effect on the outcome that is influenced by the mediator variable), and total effect (total treatment effect on the outcome = NDE + NIE).
Results
Direct and Indirect Effects on DLQI
Compared with adalimumab, the NDE of GUS on change in DLQI score from baseline was − 2.04 (95% CI − 2.55, − 1.53; P < 0.001) using PASI improvement as the mediator; 89.2% of the total effect was attributed to a direct effect of GUS treatment. When PASI 90 response was used as the mediator, the NDE of GUS on change in DLQI was − 1.43 (95% CI − 1.95, − 0.91; P < 0.001), with 62.2% of the total effect attributed to a direct effect of GUS treatment (Table 1).
Table 1.
Mediation analysis of change from baseline in DLQI score at week 24 in patients from VOYAGE 1 and VOYAGE 2
| Effect | GUS versus ADA: estimated (95% CI) | P valuea |
|---|---|---|
| With PASI improvement as a mediator | ||
| Control direct effectb | − 2.08 (− 2.58, − 1.59) | < 0.001 |
| Natural direct effect | − 2.04 (− 2.55, − 1.53) | < 0.001 |
| Natural indirect effect | − 0.25 (− 0.38, − 0.12) | < 0.001 |
| Total effect | − 2.29 (− 2.81, − 1.76) | < 0.001 |
| Percent indirect effectc | 10.8% | |
| Percent direct effectd | 89.2% | |
| With PASI 90 response as a mediator | ||
| Control direct effect (PASI 90: non-responder) | − 2.44 (− 3.34, − 1.55) | < 0.001 |
| Control direct effect (PASI 90: responder) | − 0.66 (− 1.24, − 0.07) | 0.029 |
| Natural direct effect | − 1.43 (− 1.95, − 0.91) | < 0.001 |
| Natural indirect effect | − 0.87 (− 1.14, − 0.61) | < 0.001 |
| Total effect | − 2.30 (− 4.65, 0.04) | 0.054 |
| Percent indirect effectc | 37.8% | |
| Percent direct effectd | 62.2% | |
The higher the DLQI score, the more health-related QoL was impaired
DLQI Dermatology Life Quality Index, GUS guselkumab, ADA adalimumab, CI confidence interval, PASI Psoriasis Area and Severity Index, QoL quality of life
aP value compares GUS versus ADA
bLevel of mediator at which the controlled direct effect was estimated was 17.60 (median PASI improvement at week 24)
cPercent indirect effect = natural indirect effect/total effect
dPercent direct effect = natural direct effect/total effect
Direct and Indirect Effects on Anxiety
The NDE of GUS on change in HADS anxiety score mediated through PASI improvement was − 0.74 (95% CI − 1.22, − 0.27; P = 0.002), compared with adalimumab; 74.5% of the total effect at week 24 was independent of PASI improvement and 25.5% of the change in anxiety was mediated through PASI improvement (Table 2). Similarly, when PASI 90 was used as the mediator, the NDE of GUS (versus adalimumab) on change in HADS anxiety score was − 0.76 (95% CI − 1.24, − 0.28; P = 0.002), with 76.0% of the total effect on anxiety due to a direct effect of GUS (Table 2).
Table 2.
Mediation analysis of change from baseline in HADS anxiety score at week 24 in VOYAGE 2 patients
| Effect | GUS vs ADA: estimate (95% CI) | P valuea |
|---|---|---|
| With PASI improvement as the mediatorb,c,d | ||
| Control direct effecte | − 0.75 (− 1.23, − 0.27) | 0.002 |
| Natural direct effect | − 0.74 (− 1.22, − 0.27) | 0.002 |
| Natural indirect effect | − 0.25 (− 0.40, − 0.11) | 0.0005 |
| Total effect | − 1.00 (− 1.47, − 0.52) | 0.00004 |
| Percent indirect effectf | 25.5% | |
| Percent direct effectg | 74.5% | |
| With PASI 90 response as the mediatorb,c | ||
| Control direct effect (PASI 90: non-responder)h | − 0.70 (− 1.49, 0.08) | 0.078 |
| Control direct effect (PASI 90: responder)i | − 0.80 (− 1.40, − 0.20) | 0.009 |
| Natural direct effect | − 0.76 (− 1.24, − 0.28) | 0.002 |
| Natural indirect effect | − 0.24 (− 0.39, − 0.09) | 0.002 |
| Total effect | − 1.00 (− 1.47, − 0.53) | 0.000033 |
| Percent indirect effectf | 24.0% | |
| Percent direct effectg | 76.0% | |
The higher the HADS score, the worse the anxiety
HADS Hospital Anxiety and Depression Scale, GUS guselkumab, ADA adalimumab, CI confidence interval, PASI Psoriasis Area and Severity Index, DLQI Dermatology Life Quality Index, QoL quality of life
aP value compares GUS versus ADA
bThe higher the PASI score, the worse the health condition
cThe delta method was used to estimate standard error and confidence intervals
dPASI improvement at week 24 = PASI total score at baseline − PASI total score at week 24
eThe level of mediator at which the controlled direct effect was estimated was 17.10 (median PASI improvement at week 24)
fPercent indirect effect = natural indirect effects/total effect
gPercent direct effect = natural direct effect/total effect
hPASI 90 non-responders had < 90% improvement in PASI from baseline
iPASI 90 responder had ≥ 90% improvement in PASI from baseline
Direct and Indirect Effects on Depression
The direct effect of GUS on change in HADS depression score was more modest than the direct effect of GUS on HADS anxiety score (Table 3). Compared with adalimumab, the NDE of GUS on change in HADS depression score, as mediated through PASI improvement, was − 0.29 (95% CI − 0.76, 0.19; P = 0.24); similar proportions of the total effect on depression at week 24 could be attributed to a direct treatment effect of GUS (50.2%) and an effect mediated through PASI improvement (49.8%). The NDE of GUS, mediated through PASI 90 response, was − 0.34 (95% CI − 0.82, 0.15; P = 0.17), with 59.5% of the total effect on HADS depression score resulting from a direct effect of GUS.
Table 3.
Mediation analysis of change from baseline in HADS depression score at week 24 in VOYAGE 2 patients
| Effect | GUS vs ADA: Estimate (95% CI) | P valuea |
|---|---|---|
| With PASI improvement as the mediatorb,c,d | ||
| Control Direct Effecte | − 0.29 (− 0.77, 0.20) | 0.24 |
| Natural Direct Effect | − 0.29 (− 0.76, 0.19) | 0.24 |
| Natural Indirect Effect | − 0.28 (− 0.43, − 0.13) | 0.00022 |
| Total Effect | − 0.57 (− 1.04, − 0.09) | 0.019 |
| Percent indirect effectf | 49.8% | |
| Percent direct effectg | 50.2% | |
| With PASI 90 response as the mediatorb,c | ||
| Control direct effect (PASI 90: non-responder)h | − 0.33 (− 1.12, 0.46) | 0.41 |
| Control direct effect (PASI 90: responder)i | − 0.35 (− 0.95, 0.26) | 0.26 |
| Natural direct effect | − 0.34 (− 0.82, 0.15) | 0.17 |
| Natural indirect effect | − 0.23 (− 0.38, − 0.08) | 0.0024 |
| Total effect | − 0.57 (− 1.04, − 0.09) | 0.019 |
| Percent indirect effectf | 40.5% | |
| Percent direct effectg | 59.5% | |
HADS Hospital Anxiety and Depression Scale, GUS guselkumab, ADA adalimumab, CI confidence interval, PASI Psoriasis Area and Severity Index, DLQI Dermatology Life Quality Index, QoL quality of life
The higher the HADS score, the worse the depression
aP value compares GUS versus ADA
bThe higher the PASI score, the worse the health condition
cDelta method was used to estimate the standard error and confidence interval
dPASI improvement at week 24 = PASI total score at baseline–PASI total score at week 24
eThe level of mediator at which the controlled direct effect is estimated is 17.10 (median PASI improvement at week 24)
fPercent indirect effect = natural indirect effects/total effect
gPercent direct effect = natural direct effect/total effect
hPASI 90 non-responders had < 90% improvement in PASI from baseline
iPASI 90 responder had ≥ 90% improvement in PASI from baseline
Discussions
This study sought to determine the direct effect of GUS on HRQoL, independent of any benefits mediated through skin clearance using mediation analysis. Here, mediation analysis of pooled data from VOYAGE 1 and VOYAGE 2 showed a significant, direct effect of GUS treatment versus adalimumab, on dermatology-specific HRQoL (measure by DLQI) after adjusting for the indirect effect mediated through PASI improvement or PASI 90 response. This significant independent treatment effect of GUS indicates that GUS confers additional benefits to HRQoL, beyond those derived through improvement in skin appearance, as measured by PASI. Indeed, significant improvements in fatigue have been reported in GUS-treated patients, as measured by a short-form survey (SF-36), compared with patients who received adalimumab [29]. A direct effect of GUS on HRQoL is supported by results from a previous mediation analysis that demonstrated an effect of GUS on fatigue that was independent of improvement in clinical outcomes measures in patients with psoriatic arthritis [30].
Determining the direct effect of GUS on depression, beyond that associated with skin improvement, is important. Specifically, VOYAGE 2 collected PRO data using HADS, a measure of general depression and anxiety. In VOYAGE 2, the observed decrease in anxiety was not related to PASI 90 response alone, whereas the observed decrease in depression was more strongly influenced by PASI improvement. After adjustment for the indirect effect mediated through PASI improvement or PASI 90 response, the NDE of GUS compared to adalimumab on anxiety was substantial (approximately 75%). The direct treatment effect of GUS on depression was not as robust as its effect on anxiety, with a moderate effect (approximately 50%) when adjusted for the indirect effect of change in PASI or PASI 90 response. Thus, GUS exerted an effect on anxiety and depression beyond its impact on skin clearance, although its direct effect on anxiety was more marked than its direct effect on depression.
There is growing evidence for a link between mood disorders, such as depression and anxiety, and inflammatory cytokines; these pathways present potential mechanisms through which GUS might exert a direct effect on mood-related QoL. High proportions of inflammatory molecules are reported in patients with major depressive disorder [19, 20] and meta-analyses of clinically depressed patients have identified significantly elevated levels of TNFα, C-reactive protein (CRP), IL-1, and IL-6 compared to controls [21, 22]. Similarly, increased levels of TNFα, CRP, IL-2, and IL-6 were observed in meta-analyses of patients with panic disorders versus healthy controls [31]. Conversely, reduced depression and anxiety symptoms have been observed in patients treated with drugs/antibodies that inhibit inflammatory cytokines (e.g., IL-1, IL-6, and TNFα) [19, 32]. Further, inflammatory cytokines may affect neuropsychiatric function by activating the enzymes involved in the synthesis or metabolism of serotonin or dopamine or by modulating the receptors for these neurotransmitters, by activating pathways that promote excitotoxicity or oxidative stress, or by impairing neuroplasticity and antidepressant response via reduction of brain-derived neurotrophic factor (BDNF) [19, 20]. Thus, it is possible that, by targeting IL-23, GUS may also affect the pathways that contribute to mood disorders, such as anxiety and depression, and hence impact HRQoL in patients with PsO through a mechanism that is unrelated to skin clearance.
Mediation analysis can be used to estimate the direct and indirect treatment effects on an outcome of interest while adjusting for other mediators in the causal pathway of the treatment effect. This mediation analysis used the methodology from Valeri and VanderWeele, which allows for interaction between the treatment and mediator (i.e., exposure–mediator interaction) [33]. Mediation analysis clarifies the relationship between treatment and PRO and, by characterizing the extent to which a mediator contributes to overall treatment effect, may suggest the contribution of pathways not captured by other clinical assessments [33]. Mediation analyses are well established in psychology but their use in the clinical dermatology setting is more limited [34]. A mediation analysis in patients with atopic dermatitis concluded that the direct effect of treatment with a topical phosphodiesterase 4 inhibitor on QoL was largely mediated through its alleviation of pruritus [35]. In patients with PsO, a mediation analysis identified stigmatization and negative self-image as key drivers in the relationship between skin lesions and depression [36]. The mediation analysis described here suggests that GUS treatment impacts HRQoL in patients with PsO, in part, through a mechanism that is independent of skin clearance. Taken together, these studies suggest that further application of mediation analyses in dermatology will broaden the field’s understanding of complex, multifactorial diseases.
Using combined data from two phase 3 studies provided a large data set from which to build the models used in these analyses. The similarities between the study designs in VOYAGE 1 and VOYAGE 2 facilitated pooling these data. However, the results from these analyses are limited by the patient populations enrolled in the VOYAGE studies and, therefore, extrapolation to other patient populations (e.g., those with mild PsO) may not be appropriate. This analysis was possible because the VOYAGE studies collected both clinical and PRO data. The findings from these analyses justify the importance of including PRO in future study designs and protocols. While several PRO tools exist, HADS is a widely available and well-validated measure of anxiety and depression in patients with PsO, which makes it a useful endpoint for future studies. Of note, these analyses focused on evaluation of HRQoL measures at a particular time point, week 28. To understand the cumulative direct and indirect effects of GUS on patient outcomes, the longitudinal impact of PsO on physiological, psychological, and social factors will need to be clarified. The VOYAGE studies did not include evaluation of alexithymia, which has been associated with PsO, particularly in those with anxiety and depression [37]. Further examination of alexithymia and neuroinflammation may be helpful in clarifying the interaction between depression, anxiety, and alexithymia in PsO. Additionally, while the focus of this analysis was to evaluate treatment effects of GUS on HADS after adjusting for clinician-reported outcomes based on PASI, other factors that could be related to improvement in HADS, such as improvements in sexual or social life, should be investigated further.
Conclusions
These mediation analyses reveal that GUS-mediated improvements in anxiety, depression, and overall HRQoL cannot be explained solely by the resolution of PsO signs, as assessed by PASI, suggesting a direct, physiological effect of GUS on anxiety and depression. The mechanism of the direct effect of GUS on anxiety and depression, beyond its role in skin clearance, warrants additional research.
Acknowledgements
The authors thank the patients and the trial personnel who made the VOYAGE 1 and VOYAGE 2 studies, which provided the data for this analysis, successful.
Medical Writing and Editorial Assistance
Medical writing support was provided by Erica Chevalier-Larsen, Ph.D., of Janssen Scientific Affairs, under the direction of the authors and in accordance with Good Publication Practice guidelines (Ann Intern Med. 2022; 10.7326/M22-1460). Janssen Research & Development, LLC, also funded the medical writing and editorial assistance.
Author Contributions
April W. Armstrong, Peter Foley, Yan Liu, Megan Miller, Rachel E. Teneralli, Anthony Bewley, Kenneth B. Gordon, Kim A. Papp and Chenglong Han contributed to the study conception and design. Data analyses were performed by Yan Liu and Chenglong Han and all authors participated in interpretation of the data. All authors contributed to the manuscript draft, provided critical review of the content, and approved the final manuscript for publication.
Funding
This analysis was supported by Janssen Research & Development, LLC. Publication and the Rapid Service Fee were provided by Janssen Research & Development, LLC.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the trial data can be submitted through Yale Open Data Access (YODA). Project site at http://yoda.yale.edu.
Declarations
Conflict of Interest
April W. Armstrong has served as a research investigator, scientific advisor, and/or speaker for AbbVie, Almirall, Arcutis, ASLAN, Beiersdoft, BI, BMS, EPI, Incyte, LEO, UCB, Janssen, Lilly, Mindera, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, and Pfizer. Peter Foley has received grant support and/or travel grants and/or honoraria from and/or served as an investigator and/or advisory board member and/or consultant and/or speaker for AbbVie, Akaal, Amgen, Arcutis, Argenx, Aslan, AstraZeneca, Boehringer Ingelheim, Botanix, Bristol-Myers Squibb, Celgene, Celtaxsys, CSL, CSL Seqirus, Cutanea, Dermira, Eli Lilly, Evelo Biosciences, Galderma, Genentech, GenesisCare, GlaxoSmithKline, Hexima, Incyte, Janssen, Kymab, LEO Pharma, Mayne Pharma, MedImmune, Melaseq/Geneseq, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., Reistone, Roche, Sanofi, Sun Pharma, Teva, UCB Pharma, and Valeant. Anthony Bewley has received ad hoc consultancy and/or travel bursaries from Abbvie, Almirall, Galderma, Leo Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi, and UCB. Kenneth B. Gordon has received research/grant support and/or honoraria for consultation from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, Janssen, Novartis, and UCB Pharma. Kim A. Papp has received clinical research grants and/or honoraria as a consultant, and/or speaker, and/or investigator, and/or scientific officer, and/or advisory board member, and/or Steering Committee member for AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol-Myers Squibb, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly, Evelo, Galapagos, Galderma, Genentech, Gilead, GSK, Incyte, Janssen, Kyowa Hakko Kirin, Leo, Medimmune, Meiji Seika Pharma, Merck (MSD), Merck-Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda and UCB. Rachel E. Teneralli, and Chenglong Han are employees of Janssen Global Services, LLC, Ya-Wen Yang is an employee of Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, and Megan Miller is an employee of Janssen Research & Development, LLC; employees may own stock in Johnson & Johnson, of which Janssen is a subsidiary. Yan Liu was an employee of Janssen Global Services, LLC, at the time this study was conducted.
Ethical Approval
This article is based on data from previously conducted studies, VOYAGE 1 (NCT02207231) and VOYAGE 2 (NCT 02207244), which were conducted in accordance with the Declaration of Helsinki and were consistent with good clinical practices and regulatory requirements. Patients provided written informed consent prior to enrollment in either trial.
Footnotes
Prior Presentation: These data were previously presented, in part, at the American Academy of Dermatology (AAD) Virtual Meeting Experience (April 23–25, 2021) and the AAD Annual Meeting (March 25–29, 2022; Boston MA; Poster 33028).
References
- 1.Hong J, Koo B, Koo J. The psychosocial and occupational impact of chronic skin disease. Dermatol Ther. 2008;21(1):54–9. 10.1111/j.1529-8019.2008.00170.x [DOI] [PubMed] [Google Scholar]
- 2.Pithadia DJ, Reynolds KA, Lee EB, Wu JJ. Psoriasis-associated cutaneous pain: etiology, assessment, impact, and management. J Dermatolog Treat. 2019;30(5):435–40. 10.1080/09546634.2018.1528330 [DOI] [PubMed] [Google Scholar]
- 3.Pithadia DJ, Reynolds KA, Lee EB, Wu JJ. Psoriasis-associated itch: etiology, assessment, impact, and management. J Dermatolog Treat. 2020;31(1):18–26. 10.1080/09546634.2019.1572865 [DOI] [PubMed] [Google Scholar]
- 4.Gordon KB, Han C, Li S, et al. Correlation of physician-assessed psoriasis area and severity index score with patient-reported psoriais symptoms and signs diary scores among patients with moderate-to-severe psoriasis: results From VOYAGE 1 and VOYAGE 2 studies. J Psorias Psoriat Arthritis. 2019;4(3):147–52. 10.1177/2475530319854781 [DOI] [Google Scholar]
- 5.Devrimci-Ozguven H, Kundakci TN, Kumbasar H, Boyvat A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2000;14(4):267–71. 10.1046/j.1468-3083.2000.00085.x [DOI] [PubMed] [Google Scholar]
- 6.Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–91. 10.1038/jid.2014.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134(6):1542–51. 10.1038/jid.2013.508 [DOI] [PubMed] [Google Scholar]
- 8.Zachariae R, Zachariae H, Blomqvist K, et al. Quality of life in 6497 Nordic patients with psoriasis. Br J Dermatol. 2002;146(6):1006–16. 10.1046/j.1365-2133.2002.04742.x [DOI] [PubMed] [Google Scholar]
- 9.Kimball AB, Gieler U, Linder D, Sampogna F, Warren RB, Augustin M. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24(9):989–1004. 10.1111/j.1468-3083.2010.03705.x [DOI] [PubMed] [Google Scholar]
- 10.Korman NJ, Zhao Y, Pike J, Roberts J. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41(5):514–21. 10.1111/ced.12841 [DOI] [PubMed] [Google Scholar]
- 11.Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6(6):383–92. 10.2165/00128071-200506060-00005 [DOI] [PubMed] [Google Scholar]
- 12.Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63(3):457–65. 10.1016/j.jaad.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Gordon KB, Armstrong AW, Han C, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol. 2018;32(11):1940–9. 10.1111/jdv.15012 [DOI] [PubMed] [Google Scholar]
- 14.Hesselvig JH, Egeberg A, Loft ND, Zachariae C, Kofoed K, Skov L. Correlation between Dermatology Life Quality Index and Psoriasis Area and Severity Index in patients with psoriasis treated with ustekinumab. Acta Derm Venereol. 2018;98(3):335–9. 10.2340/00015555-2833 [DOI] [PubMed] [Google Scholar]
- 15.Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–7. 10.1111/jdv.12106 [DOI] [PubMed] [Google Scholar]
- 16.Revicki DA, Willian MK, Menter A, Saurat JH, Harnam N, Kaul M. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology. 2008;216(3):260–70. 10.1159/000113150 [DOI] [PubMed] [Google Scholar]
- 17.Elloso MM, Gomez-Angelats M, Fourie AM. Targeting the Th17 pathway in psoriasis. J Leukoc Biol. 2012;92(6):1187–97. 10.1189/jlb.0212101 [DOI] [PubMed] [Google Scholar]
- 18.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397(10281):1301–15. 10.1016/S0140-6736(20)32549-6 [DOI] [PubMed] [Google Scholar]
- 19.Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. 2019;1437(1):57–67. 10.1111/nyas.13712 [DOI] [PubMed] [Google Scholar]
- 20.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–38. 10.1016/j.pharmthera.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 22.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–86. 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- 23.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17. 10.1016/j.jaad.2016.11.041 [DOI] [PubMed] [Google Scholar]
- 24.Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62(5):812–8. 10.1016/j.jaad.2009.07.022 [DOI] [PubMed] [Google Scholar]
- 25.Janssen Biotech. TREMFYA (guselkumab) injection, for subcutaneous use. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TREMFYA-pi.pdf. Accessed 1 Dec 2022.
- 26.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31. 10.1016/j.jaad.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 27.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 29.Strober B, Liu Y-H, Yang Y-W, et al. Correlation of fatigue severity and psoriasis severity, and the treatment effect of guselkumab: a detailed analysis of SF-36 vitality scores from the VOYAGE 2 study. J Am Acad Dermatol. 2022;87(3(Suppl)):153.34252469 10.1016/j.jaad.2022.06.642 [DOI] [Google Scholar]
- 30.Rahman P, Mease PJ, Helliwell PS, et al. Guselkumab demonstrated an independent treatment effect in reducing fatigue after adjustment for clinical response-results from two phase 3 clinical trials of 1120 patients with active psoriatic arthritis. Arthritis Res Ther. 2021;23(1):190. 10.1186/s13075-021-02554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CH, Hua N, Yang HY. Alterations in peripheral C-reactive protein and inflammatory cytokine levels in patients with panic disorder: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2021;17:3539–58. 10.2147/NDT.S340388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306. 10.1002/da.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–50. 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson EL, Yosipovitch G, Bushmakin AG, et al. Direct and indirect effects of crisaborole ointment on quality of life in patients with atopic dermatitis: a mediation analysis. Acta Derm Venereol. 2019;99(9):756–61. 10.2340/00015555-3181 [DOI] [PubMed] [Google Scholar]
- 36.Lakuta P, Marcinkiewicz K, Bergler-Czop B, Brzezinska-Wcislo L. The relationship between psoriasis and depression: a multiple mediation model. Body Image. 2016;19:126–32. 10.1016/j.bodyim.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Innamorati M, Quinto RM, Imperatori C, et al. Health-related quality of life and its association with alexithymia and difficulties in emotion regulation in patients with psoriasis. Compr Psychiatry. 2016;70:200–8. 10.1016/j.comppsych.2016.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the trial data can be submitted through Yale Open Data Access (YODA). Project site at http://yoda.yale.edu.