Abstract
Observational and genetic studies have reported the relationship between dyslexia and Alzheimer’s disease (AD). Until now, the causal effect of dyslexia on AD risk has remained unclear. We conducted a two-sample univariable Mendelian randomization (MR) analysis to determine the causal association between dyslexia and the risk of AD, vascular dementia (VD), Lewy body dementia (LBD), and frontotemporal dementia (FTD) and its four subtypes. First, we selected 42 dyslexia genetic variants from a large-scale genome-wide association studies (GWAS) dataset and extracted their corresponding GWAS summary statistics from AD, VD, LBD, and FTD. Second, we selected four MR methods, including inverse-variance weighted (IVW), weighted median, MR-Egger, and MR-PRESSO. Heterogeneity, horizontal pleiotropy, and leave-one-out sensitivity analysis were then used to evaluate the reliability of all causal estimates. We also conducted multivariable MR (MVMR) and mediation analysis to assess the potential mediating role of cognitive performance (CP) or educational achievement (EA) on the causal association between dyslexia and AD. Two MVMR methods, including MV IVW and MV-Egger, and two-step MR were used to perform the analysis. Using IVW, we found a significant causal association between increased dyslexia and increased risk of AD (OR = 1.15, 95% CI: 1.04–1.28, P = 0.006), but not VD, LBD, FTD, or its four subtypes. MR-PRESSO further supported the statistically significant association between dyslexia and AD (OR = 1.15, 95% CI: 1.05–1.27, P = 0.006). All sensitivity analyses confirmed the reliability of causal estimates. Using MV IVW and mediation analysis, we found no causal relationship between dyslexia and AD after adjusting for CP but not EA, CP mediated the total effect of dyslexia on AD with a proportion of 46.32%. We provide genetic evidence to support a causal effect of increased dyslexia on increased risk of AD, which was largely mediated by CP. Reading activity may be a potential intervention strategy for AD by improving cognitive function.
Subject terms: Molecular neuroscience, Clinical genetics
Introduction
Alzheimer’s disease (AD), vascular dementia (VD), Lewy body dementia (LBD), and frontotemporal dementia (FTD) are the common types of dementia, which are characterized by the loss of cognitive functioning and behavioral abilities, including thinking, remembering, and reasoning among older adults [1–4]. Language impairments, including dyslexia (reading disability), are usually one of the first cognitive signs of the onset of dementia [5, 6].
Until now, observational studies have evaluated the association of reading activity with general cognitive function in a community-based cohort and AD. Some studies showed that reading is protective of cognitive function in later life [7–11]. A 14-year longitudinal study showed that frequent reading activities (≥1 time a week) were associated with a reduced risk of cognitive decline for community-dwelling older adults at all levels of education in the long term [7]. A prospective community-based cohort study indicated that reading activities contributed to maintaining and improving cognitive function, especially in people with low levels of education [8]. Meanwhile, evidence showed that cognitively stimulating activities, including reading, reduced the risk of AD and delayed the onset of AD by as much as 5 years [9–11].
The large-scale genome-wide association study (GWAS) of dyslexia found a suggestive genetic correlation between increased dyslexia and the risk of clinically diagnosed AD from the International Genomics of Alzheimer’s Project (IGAP) (rg = 0.14, P = 4.22E–02) [12]. This indicated that neurodevelopmental and neurodegenerative disorders may share underlying genetic associations. Evidence from the latest GWAS showed that several dyslexia genetic variants were also associated with educational attainment (EA) [12], and 15 of 42 (36%) dyslexia genetic variants overlapped with the general cognitive ability GWAS [12]. Genetic variance in dyslexia is explained by both general and reading-specific cognitive abilities [13]. Our previous study found that the protective effect of cognitive performance (CP) against AD was independent of EA (OR = 0.74, P = 2.00E−03) [14]. However, the relationships between dyslexia, CP, EA, and AD have remained unclear until now.
These observational and genetic studies have prompted us to investigate the link between dyslexia, CP, EA, and AD, which may further contribute to developing effective therapy or prevention strategies. Until recently, large-scale GWAS have been conducted in dyslexia, CP, EA, and four common types of dementia, including AD, VD, LBD, and FTD [15–18]. These GWAS datasets provide strong data support to evaluate the causal association between dyslexia and dementias using Mendelian randomization (MR) and to assess the potential mediating role of CP or EA on the causal association between dyslexia and AD using multivariable MR (MVMR) and mediation analysis. MR is an alternative method to infer the potential causality of exposures on outcomes using genetic variants as potential instrumental variables (IVs) [19]. Compared with conventional observational studies, MR is less likely to be affected by confounding factors or reverse causation and is widely used to determine the risk factors for complex human diseases and phenotypes [19, 20]. MVMR is an extension of MR for estimating causal effects using genetic variants associated with more than one risk factor [21, 22].
Here, we selected 42 newly identified dyslexia genetic variants from a recent large-scale dyslexia GWAS (N = 1,138,870) as potential IVs and extracted their corresponding summary statistics from the AD (N = 63,926), VD (N = 331,030), LBD (N = 6618), and FTD (N = 6462) GWAS datasets. We then conducted a two-sample MR analysis to determine the causal association between dyslexia and the risk of four main dementias as well as the four subtypes of FTD. Finally, we evaluated the effect of two potential mediators, CP (N = 257,828) and EA (N = 766,345), on the significant causal relationship between dyslexia and the risk of dementia using MVMR and mediation analysis.
Materials and methods
Study design
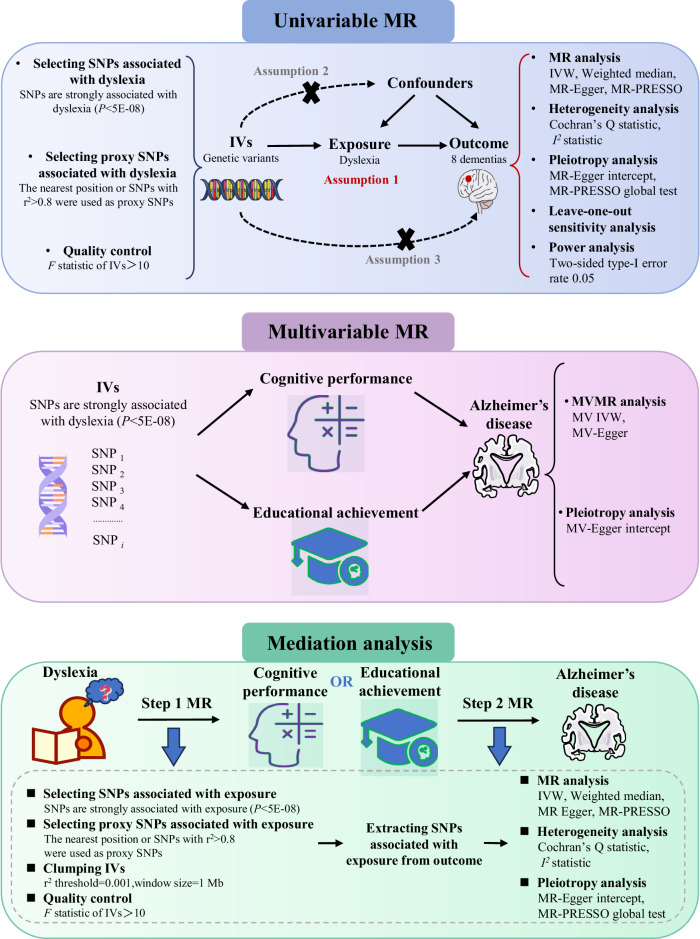
We first used a two-sample MR design to investigate the causal association between the exposure (dyslexia) and the outcomes (eight dementias). In general, MR design only uses the GWAS summary data and has three core assumptions (IV assumptions) [23]. First, IVs (genetic variants, SNPs) should be robustly associated with dyslexia. Second, IVs are not associated with the confounders of dyslexia and eight dementias. Third, IVs influence the risk of eight dementias separately, only through dyslexia and not via alternative pathways. The second and third assumptions are collectively known as “independence from pleiotropy” [24, 25]. We further performed the MVMR and mediation analysis to assess the potential mediating role of CP or EA on the significant causal relationship between dyslexia and dementia. Figure 1 depicts our MR design workflow. Meanwhile, all participants have given their informed consent as provided in these corresponding original studies.
Fig. 1. Study design and workflow.
We first used a two-sample MR design to investigate the causal association between the exposure (dyslexia) and the outcomes (eight dementias). We further performed the MVMR and mediation analysis (two-step MR) to assess the potential mediating role of CP or EA on the significant causal relationship between dyslexia and AD. SNP single-nucleotide polymorphism, MR Mendelian randomization, MVMR multivariable MR, IVW inverse-variance weighted, MR-PRESSO MR pleiotropy residual sum and outlier, AD Alzheimer’s disease.
Dyslexia genetic variants
We selected genetic variants around 42 independent genome-wide significant dyslexia loci with the genome-wide significance P < 5.00E−08 as the potential IVs, as provided in Supplementary Table 1. These 42 dyslexia loci were newly identified by the large-scale dyslexia GWAS, which included 51,800 cases (21,513 males and 30,287 females) and 1,087,070 controls (446,054 males and 641,016 females) [12]. All participants were 18 years of age or older, with a mean age of 49.6 years (s.d. 16.2) and 51.7 years (s.d. 16.6) for the dyslexia cases and controls, respectively [12]. The top 10,000 significant SNPs from the main dyslexia GWAS are publicly available.
In order to select eligible dyslexia genetic variants, we performed a series of quality controls, especially using the top 10,000 significant SNPs. First, we selected the nearest common non-palindromic genetic variants with P < 5.00E−08 as their proxies for indels (insert or deletion) [12]. Second, we used the LDlink database to identify the proxy non-palindromic common genetic variants (R2 > 0.8) for palindromic genetic variants (e.g., A/T or G/C) based on the European 1000 Genomes linkage disequilibrium (LD) information [26]. Third, we extracted the summary data corresponding to these eligible dyslexia genetic variants in AD, VD, LBD, and FTD GWAS datasets and harmonized the effect sizes from dyslexia and dementias. Fourth, we calculated the proportion of dyslexia variance explained by these genetic variants approximation R2
where EAF is the effect allele frequency, and βj is the estimated genetic effect on dyslexia. Fifth, we evaluated the strength of these genetic variants as the IVs using the F statistic, which could be calculated by
where R2 is the proportion of dyslexia variance explained by these genetic variants, N is the sample size for the dyslexia GWAS, and k is the number of IVs. SNP with an F statistic value > 10 was considered to be a “strong” instrument [27, 28].
Dementia GWAS datasets
We selected multiple large-scale dementia GWAS datasets with sample sizes ranging from 6462 to 331,030, as provided in Table 1. The AD GWAS dataset is from the International Genomics of Alzheimer’s Project (IGAP), which includes 21,982 clinically diagnosed late-onset AD patients and 41,944 cognitively normal controls of European ancestry [15]. We selected the VD GWAS dataset from the FinnGen, which contained 2048 cases and 328,982 controls of European ancestry [16]. The LBD GWAS dataset consists of 2591 cases and 4027 neurologically healthy elderly controls of European ancestry [17]. The FTD GWAS dataset is from a meta-analysis of four subtypes, including behavioral variant FTD (bvFTD), SD (semantic dementia), progressive non-fluent aphasia (PNFA), and FTD overlapping with motor neuron disease (MND) [18]. We also selected five GWAS datasets from FTD and its four subtypes, given that different FTD subtypes may be genetically heterogeneous [18].
Table 1.
Summary data of all GWAS used in the current study.
| Disease | Abbr. | Sample size | Cases | Controls | Ethnics | References |
|---|---|---|---|---|---|---|
| Dyslexia | NA | 1,138,870 | 51,800 | 1,087,070 | EUR | [12] |
| Alzheimer’s disease | AD | 63,926 | 21,982 | 41,944 | EUR | [15] |
| Vascular dementia | VD | 331,030 | 2048 | 328,982 | EUR | [16] |
| Lewy body dementia | LBD | 6618 | 2591 | 4027 | EUR | [17] |
| Frontotemporal dementia | FTD | 6462 | 2154 | 4308 | EUR | [18] |
| Behavioral variant FTD | bvFTD | 4131 | 1377 | 2754 | EUR | [18] |
| Semantic dementia | SD | 924 | 308 | 616 | EUR | [18] |
| Progressive non-fluent aphasia | PNFA | 807 | 269 | 538 | EUR | [18] |
| FTD overlapping with motor neuron disease | MND | 600 | 200 | 400 | EUR | [18] |
| Cognitive performance | CP | 257,828 | NA | NA | EUR | [29] |
| Educational attainment | EA | 766,345 | NA | NA | EUR | [29] |
GWAS genome-wide association study, Abbr. abbreviation, NA not applicable, EUR European.
CP and EA GWAS datasets
We selected a large-scale CP GWAS dataset based on a meta-analysis of the Cognitive Genomics Consortium and the UK Biobank, and a large-scale EA GWAS dataset from a meta-analysis of 70 cohort-level results, which could investigate the potential mediating effect of CP or EA on the causal association between dyslexia and AD [29], as provided in Table 1. All these GWAS summary statistics are publicly available, and no additional informed consent or ethical approval is required.
CP and EA genetic variants
For mediation analysis, we used PLINK to obtain the genome-wide significant and independent IVs for CP and EA by clumping SNPs using the European 1000 Genomes LD information (P < 5E−08; r2 < 0.001, a clumping window size of 1 Mb) [30, 31]. We selected 174 independent CP and 423 EA non-palindromic genetic variants to be potential IVs.
Univariable Mendelian randomization
We selected four univariable MR analysis methods, including inverse-variance weighted (IVW), weighted median, MR-Egger, and MR pleiotropy residual sum and outlier (MR-PRESSO) [32, 33]. IVW assumes that all IVs meet the IV assumptions, and is always taken as the main analysis method. However, the IVW estimate may be biased if any of these IVs have horizontal pleiotropy. Weighted median allows the IV assumptions to be violated, and provides an unbiased estimate if up to 50% of the weight comes from valid IVs [34]. MR-Egger tests the directional pleiotropy and estimates the causal effect by correcting for the pleiotropy [35]. MR-PRESSO evaluates the horizontal pleiotropy using the MR-PRESSO global test, adjusts for the horizontal pleiotropy via outlier removal using the MR-PRESSO outlier test, and provides causal estimates before and after outlier removal [36]. Meanwhile, we performed additional heterogeneity and horizontal pleiotropy analysis using Cochran’s Q statistic, I2 statistic, and leave-one-out sensitivity analysis method. The causal estimates were provided using odds ratios (ORs) and 95% confidence intervals (CIs). The Bonferroni corrected P value < 0.00625 (0.05/8) and P value < 0.05 were considered to be statistically significant for MR analysis and heterogeneity or horizontal pleiotropy analysis, respectively.
Multivariable Mendelian randomization
Here, we used an extension of MR that used multiple genetic variants associated with several measured risk factors (e.g., dyslexia, CP, and EA) to simultaneously estimate the causal effect of each risk factor on AD. We selected the MVMR extension of the IVW and MR-Egger methods, including MV IVW and MV-Egger, to perform the analysis. In brief, we combined effect sizes for selected IVs from the relevant GWASs: dyslexia, CP or EA, and AD. The MV-Egger intercept was used to evaluate the horizontal pleiotropy. The causal estimates were provided using ORs and 95% CIs. P values < 0.05 were considered to be statistically significant for the MVMR and pleiotropy tests.
Mediation analysis
To estimate the proportion of the effect of dyslexia on AD that was mediated through CP or EA, we first conducted a two-step MR analysis to assess the mediating effects [37]. In step 1, IVs for dyslexia were used to estimate the causal effect of dyslexia on the potential mediator CP or EA (β1). In step 2, IVs for CP or EA were used to assess the causal effect of potential mediators on the risk of AD (β2). We further estimated the mediating effect using the coefficient product method by multiplying β1 by β2 [38]. The total effect of dyslexia on AD calculated above was labeled β3. Finally, we calculated the proportion of the total effect of dyslexia on AD mediated by CP or EA by dividing the mediating effect (β1 × β2) by the total effect (β3) [39].
Results
Univariable Mendelian randomization results for the causal effect of dyslexia on eight dementias
We first selected at least 39 genome-wide significant independent genetic variants around 42 independent genome-wide significant dyslexia loci as potential IVs. We further extracted their corresponding summary statistics from the AD, VD, LBD, and FTD GWAS datasets. All these genetic variants were strongly associated with dyslexia, with F statistic values > 10, indicating no evidence of weak instrument bias. Here, we provided the summary results about these genetic variants from the dyslexia and dementia GWAS datasets in Supplementary Tables 2–9.
Using IVW, we found a statistically significant causal relationship between increased dyslexia and increased risk of AD (OR = 1.15, 95% CI: 1.04–1.28, P = 0.006). Meanwhile, MR-PRESSO further supported the statistically significant finding (OR = 1.15, 95% CI: 1.05–1.27, P = 0.006). The estimates from the weighted median and MR-Egger were also consistent with the estimates from IVW and MR-PRESSO in terms of direction, although they lacked statistically significant associations, as provided in Table 2. However, we did not identify any significant association of dyslexia with VD, LBD, and FTD and its four subtypes using IVW, weighted median, MR-Egger, or MR-PRESSO. All the MR estimates about the causal effect of dyslexia on AD, VD, LBD, and FTD and its four subtypes were provided in Table 2. MR effects for dyslexia on each dementia using the IVW method were shown as a forest plot in Fig. 2A and scatter plots in Fig. 2B and Supplementary Figs. 1–7.
Table 2.
Univariable MR results for the causal effect of dyslexia on eight dementias.
| Exposure | Outcome | No. of SNPs | Methods | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Dyslexia | AD | 41 | MR-Egger | 1.23 (0.69, 2.19) | 0.479 |
| Weighted median | 1.04 (0.90, 1.21) | 0.580 | |||
| IVW | 1.15 (1.04, 1.28) | 0.006 | |||
| MR-PRESSO | 1.15 (1.05, 1.27) | 0.006 | |||
| Dyslexia | VD | 41 | MR-Egger | 1.94 (0.61, 6.13) | 0.261 |
| Weighted median | 1.12 (0.80, 1.57) | 0.520 | |||
| IVW | 1.05 (0.82, 1.35) | 0.687 | |||
| MR-PRESSO | 1.05 (0.82, 1.35) | 0.689 | |||
| Dyslexia | LBD | 39 | MR-Egger | 0.99 (0.17, 5.61) | 0.987 |
| Weighted median | 0.85 (0.57, 1.27) | 0.437 | |||
| IVW | 0.96 (0.71, 1.29) | 0.780 | |||
| MR-PRESSO | 0.96 (0.71, 1.29) | 0.781 | |||
| Dyslexia | FTD | 40 | MR-Egger | 1.14 (0.16, 8.22) | 0.897 |
| Weighted median | 1.04 (0.67, 1.61) | 0.867 | |||
| IVW | 0.99 (0.72, 1.36) | 0.949 | |||
| MR-PRESSO | 0.99 (0.72, 1.36) | 0.949 | |||
| Dyslexia | bvFTD | 40 | MR-Egger | 0.90 (0.09, 8.53) | 0.927 |
| Weighted median | 0.95 (0.56, 1.60) | 0.840 | |||
| IVW | 1.07 (0.74, 1.55) | 0.698 | |||
| MR-PRESSO | 1.07 (0.75, 1.54) | 0.694 | |||
| Dyslexia | SD | 40 | MR-Egger | 14.53 (0.12, 1772.17) | 0.275 |
| Weighted median | 0.98 (0.33, 2.92) | 0.968 | |||
| IVW | 0.66 (0.30, 1.45) | 0.303 | |||
| MR-PRESSO | 0.66 (0.32, 1.36) | 0.269 | |||
| Dyslexia | PNFA | 40 | MR-Egger | 2.43 (0.01, 477.35) | 0.742 |
| Weighted median | 0.60 (0.18, 2.04) | 0.411 | |||
| IVW | 0.62 (0.26, 1.46) | 0.271 | |||
| MR-PRESSO | 0.62 (0.26, 1.45) | 0.275 | |||
| Dyslexia | MND | 40 | MR-Egger | 0.04 (0.00, 44.98) | 0.368 |
| Weighted median | 2.57 (0.61, 10.82) | 0.199 | |||
| IVW | 1.91 (0.61, 5.96) | 0.265 | |||
| MR-PRESSO | 1.91 (0.61, 5.96) | 0.272 |
A P value below 0.00625 (0.05/8) is considered statistically significant after the Bonferroni correction. The significant P value 0.006 is bold.
AD Alzheimer’s disease, VD vascular dementia, LBD Lewy body dementia, FTD frontotemporal dementia, bvFTD behavioral variant FTD, SD semantic dementia, PNFA progressive non-fluent aphasia, MND FTD overlapping with motor neuron disease, IVW inverse-variance weighted, MR-PRESSO MR pleiotropy residual sum and outlier, OR odds ratio, CI confidence interval.
Fig. 2. Univariable Mendelian randomization analysis results between dyslexia and eight dementias.
A The forest plot of MR effect size for dyslexia on AD, VD, LBD, and FTD and its four subtypes using the IVW method. The error bars represent 95% CIs. A P value below 0.00625 (0.05/8) was considered statistically significant after the Bonferroni correction. B The scatter plot was used to visualize the causal effect of dyslexia on AD using the IVW method. The X-axis showed the effect and standard error on dyslexia for each of the 41 SNPs, and the Y-axis showed the effect and standard error on AD. The regression line for the IVW method was shown. C The forest plot of MR leave-one-out sensitivity analysis for dyslexia on AD, where each SNP in the instrument was iteratively removed from the instrument variables. OR odds ratio, CI confidence interval, MR Mendelian randomization, AD Alzheimer’s disease, VD vascular dementia, LBD Lewy body dementia, FTD frontotemporal dementia, bvFTD behavioral variant FTD, SD semantic dementia, PNFA progressive non-fluent aphasia, MND FTD overlapping with motor neuron disease, SNP single-nucleotide polymorphism, IVW inverse-variance weighted.
Using Cochran’s Q statistics and I2 statistics, we did not observe any evidence of heterogeneity among these genetic variants (Supplementary Table 10). MR-Egger intercepts were close to zero, which suggested no significant pleiotropy effects (Supplementary Table 10). MR-PRESSO did not identify any potential instrumental outliers (Supplementary Table 10). Meanwhile, leave-one-out sensitivity analysis demonstrated that no single genetic variant drove the causal estimates, as provided in Fig. 2C and Supplementary Figs. 8–14. Overall, this additional analysis confirmed the reliability and stability of the MR causal estimates.
Our current univariable MR analysis indicates that these selected genetic variants could explain the 3.75–3.94% variance of dyslexia. Using mRnd, we had 93% power to detect an OR of 1.15 for the risk of AD. Our MR study also had 80% power to detect an OR > 1.32, OR > 1.42, and OR > 1.43 for the risk of VD, LBD, and FTD, respectively.
Multivariable MR results for the causal effect of dyslexia on AD after adjusting for CP and EA
We further performed a multivariable MR to simultaneously estimate the direct effect of dyslexia on AD conditioned by CP or EA. We combined effect sizes for selected IVs from dyslexia, CP or EA, and AD GWAS datasets, as provided in Supplementary Tables 11 and 12. Using MV IVW, we found no statistically significant causal relationship between dyslexia and increased risk of AD (Table 3, OR = 1.12, 95% CI: 0.93–1.35, P = 0.237) after adjustment for CP. However, we found that dyslexia remained significantly and positively associated with AD (Table 3, OR = 1.17, 95% CI: 1.04–1.31, P = 0.011) after adjustment for EA. The results of MV-Egger were also consistent with the estimates from MV IVW in terms of direction. The MVMR-Egger intercept estimates indicated no evidence of horizontal pleiotropy (Supplementary Table 13, P > 0.05).
Table 3.
Multivariable MR results for the causal effect of dyslexia on AD after adjusting for CP and EA.
| Model | Exposure | Outcome | No. of SNPs | Method | OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| MVMR1 | Dyslexia | AD | 38 | MV IVW | 1.12 (0.93, 1.35) | 0.237 |
| MV-Egger | 1.22 (0.65, 2.29) | 0.537 | ||||
| MVMR2 | Dyslexia | AD | 38 | MV IVW | 1.17 (1.04, 1.31) | 0.011 |
| MV-Egger | 1.29 (0.72, 2.33) | 0.395 |
P values below 0.05 were considered statistically significant and were marked in bold.
SNP single nucleotide polymorphism, OR odds ratio, CI confidence interval, MVMR1 multivariable MR adjusting for cognitive performance (CP), MVMR2 multivariable MR adjusting for educational attainment (EA), AD Alzheimer’s disease.
CP and EA partly mediate the causal effect of dyslexia on AD using mediation analysis
We conducted a two-step MR analysis to investigate the mediating pathway from dyslexia to AD via two phenotypes, including CP and EA (Fig. 3A).
Fig. 3. MR mediation analysis of the effect of dyslexia on AD via CP or EA.
A We assessed the mediation proportion of the effect of dyslexia on AD using a two-step MR analysis. Step 1 MR estimated the effect of dyslexia on CP or EA (β1); step 2 MR assessed the effect of CP or EA on AD (β2). The β3 represents the total effect of dyslexia on AD. B The forest plot of MR effect size for dyslexia on CP or EA using the IVW method. C The forest plot of MR effect size for CP or EA on AD using the IVW method. MR Mendelian randomization, CP cognitive performance, EA educational attainment, AD Alzheimer’s disease. The error bars represent 95% CIs. P < 0.05 was considered significant.
In step 1, 41 independent non-palindromic genetic variants for dyslexia were used to estimate the causal effect of dyslexia on the potential mediators (CP and EA). We provided summary results about these genetic variants from the dyslexia, CP, and EA GWAS datasets in Supplementary Tables 14 and 15. Using IVW, we found statistically significant causal relationships that increased dyslexia was associated with decreased CP (beta = −0.213, 95% CI: −0.260 to −0.166, P = 1.46E−18) and EA (beta = −0.061, 95% CI: −0.098 to −0.024, P = 1.08E−03). The estimates from the weighted median and MR-PRESSO were also consistent with the estimates from IVW, as provided in Supplementary Table 16. The MR-Egger intercept test showed no evidence of horizontal pleiotropy (P > 0.05) (Supplementary Table 17). Using Cochran’s Q statistics and I2 statistics, we observed heterogeneity among these genetic variants (P < 0.05) (Supplementary Table 17). Meanwhile, the MR-PRESSO global test detected horizontal pleiotropy (P < 0.05), as provided in Supplementary Table 17. MR analyses were performed by removing outliers using the MR-PRESSO outlier test (Supplementary Tables 16). The effects of increased dyslexia on decreased CP and EA using the IVW method were visualized as a forest plot in Fig. 3B.
In step 2, we selected 174 independent CP and 423 EA non-palindromic genetic variants to be potential IVs, respectively. We estimated the causal effect of potential mediators (CP and EA) on the risk of AD. We provided summary results about these genetic variants from the CP, EA, and AD GWAS datasets in Supplementary Tables 18–19. Using IVW, we found a statistically significant causal relationship between increased CP and decreased risk of AD (beta = −0.311, 95% CI: −0.456 to −0.166, P = 2.65E−05). We also found a statistically significant causal relationship between increased EA and decreased risk of AD (beta = −0.343, 95% CI: −0.474 to −0.211, P = 3.49E−07). The estimates from the weighted median and MR-PRESSO were consistent with the estimates from IVW, as provided in Supplementary Table 20. The MR-Egger intercept test showed no evidence of horizontal pleiotropy (P > 0.05) (Supplementary Table 21). Using Cochran’s Q statistics and I2 statistics, we observed heterogeneity among these genetic variants (P < 0.05) (Supplementary Table 21). Meanwhile, the MR-PRESSO global test detected horizontal pleiotropy (P < 0.05), as provided in Supplementary Table 21. MR analyses were performed by removing outliers in EA but not in CP, as no significant outliers were detected using the MR-PRESSO outlier test (Supplementary Table 20). The effects of increased CP and EA on decreased AD using the IVW method were visualized as a forest plot in Fig. 3C.
Finally, we estimated the indirect effect of dyslexia on AD through CP and found a mediated effect of 0.066 for CP with a mediation proportion of 46.32%. We further estimated the indirect effect of dyslexia on AD through EA and found a mediated effect of 0.021 for EA with a mediation proportion of 14.63%.
Discussion
Until now, both observational and genetic studies have found the link between dyslexia and AD, yet their causal association remains unknown. Here, we aimed to establish the causal link between dyslexia and AD and other different types of dementias by conducting a two-sample univariable MR analysis and assessing the potential mediating role of CP or EA on the causal association between dyslexia and AD by conducting the MVMR analysis and mediation analysis. We found a statistically significant causal effect of dyslexia on increased risk of AD but not on VD, LBD, FTD or its four subtypes in the univariable MR. This significant association between dyslexia and AD was attenuated and no longer statistically significant in the MVMR, adjusting for CP but not EA, implying that CP may largely mediate the causal association between dyslexia on AD. We finally calculated the CP with a mediation ratio of 46.32%, which is much larger than the EA’s mediation ratio of 14.63%.
The first statistically significant finding, we found a causal relationship between increased dyslexia and increased risk of AD. Our MR finding was supported by the recent dyslexia GWAS, where Doust and colleagues found a suggestive genetic association between increased dyslexia and increased risk of AD using linkage disequilibrium score regression (rg = 0.14, P = 4.22E–02) [12]. Although this genetic relationship no longer existed after correcting for multiple testing, this genetic finding was consistent with our univariable MR finding in terms of direction. Meanwhile, an observational study also supported our MR finding. Evidence from the Nun Study, which assessed language ability 50 years prior to the diagnosis of AD, showed that low language ability in early life was a strong predictor of low cognitive function and AD in later life [40].
We did not identify any significant association between dyslexia and VD, LBD, and FTD and its four subtypes, but observational studies have provided evidence that dyslexia was associated with other dementia-related phenotypes. Dyslexia is the most common type of learning disability, a retrospective study including 678 cases showed that patients with probable learning disability were more likely to be diagnosed with atypical dementia (OR = 13.1, 95% CI 1.3–128.4) [41]. Patients with primary progressive aphasia (PPA) generally have a history of dyslexia, indicating an innate vulnerability in their language network [42]. The reason for those null causal relationships between dyslexia and VD, LBD, and FTD may be the small sample size of cases in the corresponding outcome GWAS datasets, especially for the FTD subtype, which led to insufficient statistical power in this study. This may further explain the wide range of estimates for FTD subtypes. Hence, large-scale VD, LBD, and FTD GWAS datasets are required to verify our current findings in the future. In addition to observational studies, genetic association studies also showed that several genetic variants for dyslexia (P < 5.00E−08) were related to VD, LBD, and FTD. POU6F2 rs62453457 (P = 0.045) and GGNBP2 rs12150665 (P = 0.031) were associated with VD, BABAM2 rs1969131 (P = 0.024) was associated with LBD, PMVK rs4845687 (P = 0.005) and ACVR2A rs497418 (P = 0.033) were associated with FTD, as provided in Supplementary Tables 3–5.
Meanwhile, genetic studies have shown that susceptibility genes for dyslexia are linked to brain connectivity. Variant rs17243157 in the dyslexia susceptibility gene KIAA0319, which was associated with atrophy of speech-related brain areas, gray and white matter in the left middle and inferior temporal gyri, in patients with FTD and PPA, led to vulnerability of the speech network [43]. Compared with non-carriers, frontotemporal lobar degeneration patients with language FOXP2 gene polymorphisms had a higher degree of hypoperfusion in the frontal region [44, 45]. The possible mechanism underlying the association between dyslexia and AD may be that the altered pattern of connectivity within the language network in dyslexia interferes with the synaptic transmission of pathological proteins in AD [6]. Neurodevelopmental and AD pathological changes overlapped in the lateral perirhinal area, a region associated with phonological deficits in logopenic variant PPA and dyslexia, and the findings supported that early neurodevelopmental changes such as dyslexia might influence susceptibility to early-onset AD [6]. Thus, poor development of the language network may lead to selective vulnerability of language-related brain regions to neurodegeneration in old age.
The second statistically significant finding we identified was that CP largely mediated the causal association between dyslexia and AD. Given that several dyslexia genetic variants were also associated with CP and EA, we added two exposures to the MVMR model, including the primary relevant exposure (dyslexia) and the secondary relevant exposure (CP or EA). We found that the direct effect of dyslexia on AD was no longer significant after correction for CP (P = 0.237) but not EA (P = 0.011). We then calculated the proportion of CP or EA mediation using mediation analysis [37, 39]. We calculated that CP mediated the total effect of dyslexia on AD, with a mediating proportion of CP of 46.32%. Notably, although EA also had a mediating effect of 14.63%, the causal relationship between dyslexia and AD was significant after controlling for EA in MVMR, suggesting a small mediating role for EA. Interestingly, we found two dyslexia genetic variants (P < 5.00E−08), FSHB rs676217 and SATB2 rs6435017, were also associated with AD and CP (P < 0.05) but not with EA (P > 0.05), as provided in Supplementary Tables 11, 12, which was consistent with our MR results. In addition to reading impairment, dyslexics also have difficulties with cognitive phenotypes, arithmetic calculations, and second language skills [46, 47]. Notably, observational studies have been suggested to reduce the incidence of AD by increasing the frequency of cognitive activities [11, 48]. A learning therapy that improves cognitive function by reading aloud and solving arithmetic problems has been developed in Japan for the treatment of AD, and this therapy also improves cognitive function in normal elderly people [49, 50]. Kawashima and colleagues also found that reading aloud and arithmetic calculations improved frontal lobe function and facilitated cognitive recovery in patients with dementia [51]. In addition, previous studies have shown that bilingualism alters brain structure by increasing white matter density, which protects the aging brain and is associated with enhanced connectivity for cognitive function [52, 53]. Therefore, learning a second language may be neuroprotective against AD, another measure to prevent AD by improving cognition.
Strengths
Our current study may have several strengths. First, the selected genetic variants as instrumental variables could explain 3.75–3.94% of the variance of dyslexia and showed no evidence of weak instrument bias with F statistic values > 10. Importantly, these genetic variants were strongly associated with dyslexia rather than four common dementias, which contributed to avoiding the reverse causality. Second, multiple MR methods, including IVW, weighted median, MR-Egger, MR-PRESSO, and MVMR methods, including MV IVW and MV-Egger, were selected to ensure the reliability of the causal estimates. Third, Cochran’s Q statistics, I2 statistics, MR-Egger intercept test, MR-PRESSO global test, and MV-Egger intercept were used to test the heterogeneity and horizontal pleiotropy and ensure the MR assumptions. Fourth, the leave-one-out sensitivity analysis was used to confirm the stability of the MR causal estimates. Fifth, all dyslexia, AD, VD, LBD, FTD, CP, and EA GWAS datasets are from participants of European descent, which further reduces the potential bias caused by population stratification.
Limitations
Several limitations should also be considered. First, we cannot exclude the possibility of unmeasured pleiotropy, although multiple statistical analyses have been performed. Second, our current results only reflect the findings in participants of European descent, which should be validated in other ancestries.
Conclusions
Collectively, we highlighted a significant causal link between dyslexia and increased risk of AD, which may further promote mechanistic hypotheses, biomarker development, and clinical trials. Reading activities such as reading a newspaper or book, visiting a library, reading aloud, training in arithmetic calculations, and learning a second language, which are cognitively active lifestyles, may improve cognitive function and stave off the cognitive symptoms of AD in older adults.
Supplementary information
Acknowledgements
We thank the 23andMe Research Team for providing dyslexia GWAS summary results data. We thank the FinnGen project for sharing the VD GWAS summary statistics. We thank the NHGRI-EBI GWAS Catalog for sharing the LBD GWAS summary statistics. We thank the International FTD-Genetics Consortium (IFGC) for the summary data. We thank the Social Science Genetic Association Consortium (SSGAC) for sharing the CP and EA GWAS summary statistics. We thank the International Genomics of Alzheimer’s Project (IGAP) for providing summary results data for these analyses. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in the analysis or writing of this report. IGAP was made possible by the generous participation of the control subjects, the patients, and their families. The i-Select chips were funded by the French National Foundation on AD and related disorders. EADI was supported by the LABEX (laboratory of Excellence program investment for the future) DISTALZ grant, Inserm, Institut Pasteur de Lille, Université de Lille 2, and the Lille University Hospital. GERAD was supported by the Medical Research Council (Grant no. 503480), Alzheimer’s Research UK (Grant no. 503176), the Wellcome Trust (Grant no. 082604/2/07/Z), and the German Federal Ministry of Education and Research (BMBF): Competence Network Dementia (CND) grant nos. 01GI0102, 01GI0711, and 01GI0420. CHARGE was partly supported by the NIH/NIA grant R01 AG033193 and the NIA AG081220 and AGES contract N01-AG-12100, the NHLBI grant R01 HL105756, the Icelandic Heart Association, and the Erasmus Medical Center and Erasmus University. ADGC was supported by the NIH/NIA grants: U01 AG032984, U24 AG021886, U01 AG016976, and the Alzheimer’s Association grant ADGC-10-196728. We thank the individual patients who provided the sample that made data available, without them, the study would not have been possible.
Author contributions
GYL and PZ designed the study. PZ, SG, SYW, and XL analyzed the data. CH and YC critically revised the manuscript for important intellectual content. All authors contributed to the interpretation of the results and approved the final version of the manuscript.
Funding
This work was supported by funding from the National Natural Science Foundation of China (Grant Nos. 82071212, and 81901181), the Beijing Natural Science Foundation (Grant No. JQ21022), the Mathematical Tianyuan Fund of the National Natural Science Foundation of China (Grant No. 12026414), and the Beijing Ten Thousand Talents Project (Grant No. 2020A15).
Data availability
All relevant data are within the paper. The authors confirm that all data underlying the findings are either fully available without restriction through consortia websites, or may be made available from consortia upon request. AD GWAS is available at https://www.niagads.org/datasets/ng00075; VD GWAS is available at https://www.finngen.fi; LBD GWAS is available at https://www.ebi.ac.uk/gwas/home; FTD GWAS is available at https://ifgcsite.wordpress.com/data-access/; CP and EA is available at https://www.thessgac.org/data; LDlink: https://ldlink.nci.nih.gov/?tab=ldproxy; mRnd: https://shiny.cnsgenomics.com/mRnd/.
Code availability
The statistical tests were performed using MendelianRandomization (version 0.6.0), TwoSampleMR (version 0.5.6), and MRPRESSO packages in R Software 4.2.1. R. A power analysis was conducted using the online web tool mRnd [54].
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This article contains human participants collected from several studies performed by previous studies. All participants gave informed consent in all the corresponding original studies. Here, our study is based on publicly available, large-scale datasets, and not individual-level data. Hence, ethical approval was not sought.
Consent for publication
All authors give consent for publication.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Chen, Email: bingyan-1209@163.com.
Guiyou Liu, Email: liuguiyou1981@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03082-9.
References
- 1.2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023;19:1598–695. [DOI] [PubMed]
- 2.Mangal R, Ding Y. Mini review: prospective therapeutic targets of Alzheimer’s disease. Brain Circ. 2022;8:1–5. 10.4103/bc.bc_20_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322:1589–99. 10.1001/jama.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakkar N, Martis PB, Kutikuppala LVS, Kuchana SK, Mohapatra RK. Lecanemab: a hope in the management of Alzheimer’s disease. Brain Circ. 2023;9:194–5. 10.4103/bc.bc_10_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passafiume D, Di Giacomo D, Giubilei F. Reading latency of words and nonwords in Alzheimer’s patients. Cortex. 2000;36:293–8. 10.1016/S0010-9452(08)70531-8 [DOI] [PubMed] [Google Scholar]
- 6.Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136:3461–73. 10.1093/brain/awt242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang YH, Wu IC, Hsiung CA. Reading activity prevents long-term decline in cognitive function in older people: evidence from a 14-year longitudinal study. Int Psychogeriatr. 2021;33:63–74. 10.1017/S1041610220000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Wang S, Zhu W, Liang N, Zhang C, Pei Y, et al. Reading activities compensate for low education-related cognitive deficits. Alzheimers Res Ther. 2022;14:156. 10.1186/s13195-022-01098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–8. 10.1001/jama.287.6.742 [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Wang T, Yu L, Grodstein F, Bennett DA, Boyle PA. Cognitive activity and onset age of incident Alzheimer disease dementia. Neurology. 2021;97:e922–e29. 10.1212/WNL.0000000000012388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–4. 10.1212/01.WNL.0000036905.59156.A1 [DOI] [PubMed] [Google Scholar]
- 12.Doust C, Fontanillas P, Eising E, Gordon SD, Wang Z, Alagöz G, et al. Discovery of 42 genome-wide significant loci associated with dyslexia. Nat Genet. 2022;54:1621–9. 10.1038/s41588-022-01192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haworth CMA, Kovas Y, Harlaar N, Hayiou-Thomas ME, Petrill SA, Dale PS, et al. Generalist genes and learning disabilities: a multivariate genetic analysis of low performance in reading, mathematics, language and general cognitive ability in a sample of 8000 12-year-old twins. J Child Psychol Psychiatry. 2009;50:1318–25. 10.1111/j.1469-7610.2009.02114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Zhang Y, Zhang H, Gao S, Wang L, Wang T, et al. Cognitive performance protects against Alzheimer’s disease independently of educational attainment and intelligence. Mol Psychiatry. 2022;27:4297–306. 10.1038/s41380-022-01695-4 [DOI] [PubMed] [Google Scholar]
- 15.Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30. 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18. 10.1038/s41586-022-05473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chia R, Sabir MS, Bandres-Ciga S, Saez-Atienzar S, Reynolds RH, Gustavsson E, et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet. 2021;53:294–303. 10.1038/s41588-021-00785-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari R, Hernandez DG, Nalls MA, Rohrer JD, Ramasamy A, Kwok JBJ, et al. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 2014;13:686–99. 10.1016/S1474-4422(14)70065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey Smith G, Ebrahim S. What can Mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330:1076–9. 10.1136/bmj.330.7499.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–60. 10.1093/aje/kwu283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rees JMB, Wood AM, Burgess S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med. 2017;36:4705–18. 10.1002/sim.7492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan S, Larsson SC. Coffee and caffeine consumption and risk of kidney stones: a Mendelian randomization study. Am J Kidney Dis. 2022;79:9–14.e1. 10.1053/j.ajkd.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 24.Julian TH, Boddy S, Islam M, Kurz J, Whittaker KJ, Moll T, et al. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain. 2022;145:832–42. 10.1093/brain/awab420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao S, Zhang M, Dong S-S, Wang J-H, Zhang K, Guo J, et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat Hum Behav. 2022;6:1569–76. 10.1038/s41562-022-01412-9 [DOI] [PubMed] [Google Scholar]
- 26.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–7. 10.1093/bioinformatics/btv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64. 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 28.Flatby HM, Ravi A, Damås JK, Solligård E, Rogne T. Circulating levels of micronutrients and risk of infections: a Mendelian randomization study. BMC Med. 2023;21:84. 10.1186/s12916-023-02780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21. 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Yu K, Dong S-S, Yao S, Rong Y, Wu H, et al. Mendelian randomization analyses support causal relationships between brain imaging-derived phenotypes and risk of psychiatric disorders. Nat Neurosci. 2022;25:1519–27. 10.1038/s41593-022-01174-7 [DOI] [PubMed] [Google Scholar]
- 32.Jiang L, Li J-C, Tang B-S, Guo J-F, Shen L. Lack of bidirectional association between age-related macular degeneration and Alzheimer’s disease: a Mendelian randomization study. Alzheimers Dement. 2022;18:2725–9. 10.1002/alz.12775 [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Kong J, Pan J, Huang K, Zhou W, Diao X, et al. Kidney damage causally affects the brain cortical structure: a Mendelian randomization study. EBioMedicine. 2021;72:103592. 10.1016/j.ebiom.2021.103592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol. 2016;40:304–14. 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89. 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess S, Daniel RM, Butterworth AS, Thompson SG. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44:484–95. 10.1093/ije/dyu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. 10.1146/annurev-publhealth-032315-021402 [DOI] [PubMed] [Google Scholar]
- 39.Carter AR, Gill D, Davies NM, Taylor AE, Tillmann T, Vaucher J, et al. Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. BMJ. 2019;365:l1855. 10.1136/bmj.l1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–32. 10.1001/jama.1996.03530310034029 [DOI] [PubMed] [Google Scholar]
- 41.Seifan A, Assuras S, Huey ED, Mez J, Tsapanou A, Caccappolo E. Childhood learning disabilities and atypical dementia: a retrospective chart review. PLoS ONE. 2015;10:e0129919. 10.1371/journal.pone.0129919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–92. 10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paternicó D, Premi E, Alberici A, Archetti S, Bonomi E, Gualeni V, et al. Dyslexia susceptibility genes influence brain atrophy in frontotemporal dementia. Neurol Genet. 2015;1:e24. 10.1212/NXG.0000000000000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padovani A, Cosseddu M, Premi E, Archetti S, Papetti A, Agosti C, et al. The speech and language FOXP2 gene modulates the phenotype of frontotemporal lobar degeneration. J Alzheimers Dis. 2010;22:923–31. 10.3233/JAD-2010-101206 [DOI] [PubMed] [Google Scholar]
- 45.Premi E, Pilotto A, Alberici A, Papetti A, Archetti S, Seripa D, et al. FOXP2, APOE, and PRNP: new modulators in primary progressive aphasia. J Alzheimers Dis. 2012;28:941–50. 10.3233/JAD-2011-111541 [DOI] [PubMed] [Google Scholar]
- 46.Colvin MK, Sherman JC. Considering learning disabilities and attention-deficit hyperactivity disorder when assessing for neurodegenerative disease. Neurol Clin Pract. 2020;10:520–6. 10.1212/CPJ.0000000000000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Downey DM, Snyder LE, Hill B. College students with dyslexia: persistent linguistic deficits and foreign language learning. Dyslexia. 2000;6:101–11. [DOI] [PubMed] [Google Scholar]
- 48.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–20. 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- 49.Kulason K, Nouchi R, Hoshikawa Y, Noda M, Okada Y, Kawashima R. The beneficial effects of cognitive training with simple calculation and reading aloud in an elderly postsurgical population: study protocol for a randomized controlled trial. Trials. 2016;17:334. 10.1186/s13063-016-1476-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uchida S, Kawashima R. Reading and solving arithmetic problems improves cognitive functions of normal aged people: a randomized controlled study. Age (Dordrecht). 2008;30:21–9. 10.1007/s11357-007-9044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawashima R, Okita K, Yamazaki R, Tajima N, Yoshida H, Taira M, et al. Reading aloud and arithmetic calculation improve frontal function of people with dementia. J Gerontol A Biol Sci Med Sci. 2005;60:380–4. 10.1093/gerona/60.3.380 [DOI] [PubMed] [Google Scholar]
- 52.Perani D, Farsad M, Ballarini T, Lubian F, Malpetti M, Fracchetti A, et al. The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer’s dementia. Proc Natl Acad Sci USA. 2017;114:1690–5. 10.1073/pnas.1610909114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sala A, Malpetti M, Farsad M, Lubian F, Magnani G, Frasca Polara G, et al. Lifelong bilingualism and mechanisms of neuroprotection in Alzheimer dementia. Hum Brain Mapp. 2022;43:581–92. 10.1002/hbm.25605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brion M-JA, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501. 10.1093/ije/dyt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper. The authors confirm that all data underlying the findings are either fully available without restriction through consortia websites, or may be made available from consortia upon request. AD GWAS is available at https://www.niagads.org/datasets/ng00075; VD GWAS is available at https://www.finngen.fi; LBD GWAS is available at https://www.ebi.ac.uk/gwas/home; FTD GWAS is available at https://ifgcsite.wordpress.com/data-access/; CP and EA is available at https://www.thessgac.org/data; LDlink: https://ldlink.nci.nih.gov/?tab=ldproxy; mRnd: https://shiny.cnsgenomics.com/mRnd/.
The statistical tests were performed using MendelianRandomization (version 0.6.0), TwoSampleMR (version 0.5.6), and MRPRESSO packages in R Software 4.2.1. R. A power analysis was conducted using the online web tool mRnd [54].