Abstract
Purpose:
The phase II, multiarm, signal-searching BALTIC study (NCT02937818) assessed novel treatment combinations for platinum-refractory/resistant extensive-stage small cell lung cancer (ES-SCLC).
Patients and Methods:
Patients with ES-SCLC with progressive disease during or within 90 days of completing first-line platinum-based chemotherapy received one of three regimens: durvalumab plus tremelimumab followed by durvalumab monotherapy (arm A), adavosertib plus carboplatin (arm B), or ceralasertib plus olaparib (arm C). The primary endpoint was the objective response rate. Prespecified exploratory biomarker analyses were conducted in arms A and C.
Results:
In arm A (n = 41), arm B (n = 10), and arm C (n = 21), the confirmed objective response rates were 7.3%, 0%, and 4.8%, respectively. Safety profiles in all arms were consistent with those of the individual drugs. In arm A, patients with PD-L1 expression (tumor cells or immune cells) ≥1% seemed to have a greater likelihood of achieving disease control with durvalumab plus tremelimumab than those with PD-L1 (tumor cells and immune cells) <1%, and lower baseline ctDNA and reduction in the on-treatment ctDNA level were both associated with longer overall survival. Among patients treated with ceralasertib plus olaparib in arm C, specific immune response-relevant circulating chemokines and cytokines were identified as early biomarkers of survival and pharmacodynamic biomarkers.
Conclusions:
In BALTIC, all combination regimens demonstrated tolerable safety profiles, but antitumor activity was limited in refractory/resistant ES-SCLC. Among patients treated with durvalumab plus tremelimumab, an association of on-treatment reduction in ctDNA with longer overall survival suggests the potential use of ctDNA as a surrogate of treatment response, warranting further investigation.
Translational Relevance.
Refractory/resistant extensive-stage small cell lung cancer (ES-SCLC) is a difficult-to-treat disease with poor prognosis (median overall survival around 6 months) and an unmet need to improve outcomes. The phase II BALTIC study assessed three novel combination regimens involving agents targeting immune checkpoints (durvalumab plus tremelimumab) or components of the DNA damage repair pathway (adavosertib plus carboplatin and ceralasertib plus olaparib) in ES-SCLC. All three combinations demonstrated tolerable safety profiles consistent with those of the individual drugs. However, antitumor activity was limited. Notably, exploratory analyses in patients treated with durvalumab plus tremelimumab revealed that reduction in the ctDNA level on treatment was associated with longer overall survival, supporting existing evidence that early changes in ctDNA may predict response to immune checkpoint inhibitors across different tumor types. Circulating biomarker analyses identified specific immune response-relevant chemokines and cytokines as early biomarkers of survival and pharmacodynamic biomarkers in patients treated with ceralasertib plus olaparib.
Introduction
Platinum-based chemotherapy was the primary treatment modality for patients with extensive-stage small cell lung cancer (ES-SCLC) for many years, with improvements in overall survival (OS) only recently demonstrated by the addition of PD-L1 inhibitors to chemotherapy (1, 2). Nonetheless, most patients relapse within months of completing initial therapy, and platinum-refractory or -resistant ES-SCLC has a particularly poor prognosis (3), highlighting the need for novel treatment options to improve outcomes. The current standard-of-care second-line treatment outside Japan is typically topotecan, associated with an objective response rate (ORR) of 9.4% and median OS of 5.7 months in patients refractory to first-line therapy (4).
The rationale for investigating immunotherapy in SCLC is based on its immunogenic nature (3). However, early successes in treating relapsed ES-SCLC with inhibitors of programmed cell death 1 (PD1) have not yet been confirmed in phase III studies (5, 6). Durvalumab is a selective, high-affinity human IgG1κ mAb, which binds to PD-L1 and blocks the interaction of PD-L1 with PD1 and CD80 (7). First-line durvalumab in combination with platinum–etoposide (EP) is approved in patients with ES-SCLC in multiple countries worldwide based on the results of the phase III CASPIAN study (1). In pretreated ES-SCLC, durvalumab monotherapy exhibited promising clinical activity in a phase I/II study (8).
Combination of PD1/PD-L1 inhibitors with cytotoxic T lymphocyte–associated antigen 4 (CTLA4) inhibitors is of interest because PD-L1 and CTLA4 regulate immune responses by different, nonredundant mechanisms (9). Tremelimumab, a selective human IgG2 monoclonal antibody targeting CTLA4 (10), in combination with durvalumab has demonstrated a manageable safety profile and encouraging antitumor activity phase I studies in tumor types including ES-SCLC and non–small cell lung cancer (NSCLC; refs. 11, 12). In the phase III POSEIDON study, durvalumab plus tremelimumab plus chemotherapy significantly improved OS and progression-free survival (PFS) versus chemotherapy alone in patients with metastatic NSCLC (13). However, outcomes of other phase III studies in metastatic NSCLC have been variable (14, 15). In CASPIAN, addition of tremelimumab to first-line durvalumab plus EP was not associated with a significant improvement in OS versus EP in patients with ES-SCLC (16).
Inhibitors of the DNA damage repair pathway have potential as novel treatment options in patients with platinum-refractory/resistant ES-SCLC given the high incidence of genetic aberrations with associated aggregation of DNA damage and genomic instability in SCLC. Adavosertib (AZD1775) is a small-molecule inhibitor of the DNA damage checkpoint kinase WEE1 that potentiates genotoxic chemotherapy (17, 18). Preclinical studies demonstrated that WEE1 inhibition abrogates G2 checkpoint control and selectively sensitizes TP53-deficient cells to the cytotoxic effects of DNA-damaging chemotherapy (17–19). In early-phase studies, adavosertib plus chemotherapy showed antitumor activity in advanced solid tumors (20) and in platinum-refractory p53-mutated ovarian cancer (21). Since SCLC tumors exhibit a high frequency of TP53 mutations (22), the combination of adavosertib plus carboplatin is of particular interest.
Ataxia telangiectasia and Rad3–related (ATR) kinase is a serine/threonine-specific apical kinase in one of the DNA damage–induced checkpoint pathways that is vital for cell response to replication stress (23). Ceralasertib (AZD6738), a potent, selective inhibitor of ATR (24), demonstrated growth inhibition as monotherapy against multiple cancer cell lines in vitro, with the strongest activity in cell lines with deficiencies in the ataxia telangiectasia mutated (ATM) signaling pathway (25–27). Olaparib (AZD2281) is an orally bioavailable inhibitor of PARP, which traps PARP onto DNA at sites of single-strand DNA breaks, preventing their repair and generating double-strand DNA breaks (DSB) during replication (28). During cell division, tumor cells (TC) with homologous recombination repair deficiencies are unable to accurately repair DSBs, leading to cell death. When PARP inhibition leads to the conversion of single-strand DNA breaks into DSBs during replication, this also causes replication stress, triggering activation and dependence on ATR (29). The combined antitumor effect of ceralasertib plus olaparib may therefore be particularly pronounced in tumors that are aggressively dividing and have underlying DNA repair defects, such as SCLC; preclinical studies have demonstrated the synergy of ceralasertib plus olaparib in ATM-deficient cancers in vitro and in vivo (30, 31).
Currently, there is a lack of evidence supporting the validity of predictive biomarkers in SCLC. In NSCLC and other solid tumors, biomarkers predictive of response to immune checkpoint inhibitors have been evaluated, including PD-L1 and ctDNA dynamics (32, 33). Molecular response based on ctDNA dynamics may be predictive of benefit from immunotherapy in NSCLC, complementing radiologic disease assessment and potentially enabling early clinical decision-making (34). In SCLC, several studies have reported an association between ctDNA levels at baseline or changes in ctDNA levels during treatment and patient outcomes (35), suggesting that ctDNA may be useful in this disease setting.
Here, we report the results of the phase II BALTIC study, which assessed the combinations of durvalumab plus tremelimumab (arm A), adavosertib plus carboplatin (arm B), or ceralasertib plus olaparib (arm C) in patients with ES-SCLC refractory or resistant to first-line platinum-based chemotherapy. We also present the results of exploratory biomarker analyses: In arm A, we report the association of PD-L1 expression, baseline ctDNA level, and ctDNA dynamics with treatment outcomes, and in arm C, we report specific immune response–relevant circulating chemokines and cytokines as early biomarkers of survival and longitudinal pharmacodynamic biomarkers. A plain-language summary of this article can be found in the Supplementary Material.
Patients and Methods
Study design
This phase II, open-label, multidrug, multicenter, multiarm study (NCT02937818) was conducted at 12 sites in Germany, Hungary, Poland, Spain, and Ukraine. Patients were enrolled in three independent experimental arms: arm A (durvalumab plus tremelimumab followed by durvalumab monotherapy), arm B (adavosertib plus carboplatin), and arm C (ceralasertib plus olaparib). There was no randomization in the study; patients who satisfied all the inclusion and exclusion criteria to participate in a particular treatment arm were enrolled in the arm for which they were most suitable. Enrollment into each arm of the study was sequential and followed a staged design. Initially, a minimum of 10 patients were enrolled in each arm in stage 1; after a minimum of 12 weeks, an interim ORR analysis was performed to determine whether recruitment should expand to stage 2 (enrollment of an additional 10 patients per treatment arm for a total of ∼20 eligible patients) or stop. If the results of the primary ORR analysis from the completed second stage were encouraging, the Sponsor Review Committee could permit enrollment of ∼20 additional eligible patients (for a total of ∼40 eligible patients per treatment arm) in an expansion cohort to further explore the findings.
All patients provided written informed consent for participation. The study was performed in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local regulatory requirements. The study protocol and all modifications were approved by relevant ethics committees or institutional review boards.
Eligibility criteria
All three study arms enrolled male or female patients aged ≥18 years at the time of screening, with histologically or cytologically documented American Joint Committee on Cancer stage IV ES-SCLC (T any, N any, and M1 a/b) at initial diagnosis. Patients must have demonstrated progressive disease (PD) during or within 90 days of completing platinum-based chemotherapy and not received further treatment. Patients were also required to have a life expectancy of at least 8 weeks, the World Health Organization (WHO) performance status (PS) of 0 to 1 (or, in arm A only, PS 0–2 after the first 10 patients were enrolled), and adequate organ and marrow function. At least one lesion that could be accurately measured at baseline and had not been irradiated previously was also required. Brain metastases were required to be asymptomatic or treated and stable off steroids and anticonvulsants for at least 1 month prior to study treatment; patients with suspected brain metastases at screening were required to have a scan of the brain using computed tomography or magnetic resonance imaging (preferred) prior to study entry.
Patients enrolled in arm A were required to have a body weight >30 kg and no prior exposure to immune-mediated therapy (including, but not limited to, other anti-CTLA4, anti-PD1, anti-PD-L1, and anti-PD-L2 antibodies; excluding therapeutic anticancer vaccines), and provision of a fresh or archival tumor biopsy was mandatory. Patients in arms B and C were required to be able to swallow oral medication. Full eligibility criteria for each treatment arm can be found in the Supplementary Material.
Procedures
In arm A, patients received durvalumab 1,500 mg plus tremelimumab 75 mg via i.v. infusion every 4 weeks, for up to four doses/cycles, followed by durvalumab 1,500 mg as monotherapy via i.v. infusion every 4 weeks. In arm B, patients received oral adavosertib 225 mg twice daily for 2.5 days from Day 1 plus i.v. carboplatin AUC5 on Day 1 of each 21-day cycle. In arm C, patients received oral ceralasertib 160 mg once daily, administered on days 1 to 7, plus oral olaparib 300 mg twice daily on days 1 to 28 of each 28-day cycle. In all arms, treatment continued until confirmed PD and determination by the investigator that the patient was no longer receiving clinical benefit from treatment, until unacceptable toxicity, or until other discontinuation criteria were met.
Outcomes
The primary endpoint was ORR, defined as the percentage of patients with a confirmed objective response of complete response (CR) or partial response (PR), assessed by the investigator using RECIST v1.1. Secondary endpoints were duration of response (DoR), disease control rate (DCR), time to response (TTR), PFS, and OS. The safety objective was to determine the safety and tolerability profile of each treatment arm in terms of adverse events (AE), deaths, laboratory data, vital signs, electrocardiograms, and exposure. Exploratory objectives included the investigation of potential biomarkers associated with disease progression or biomarkers that may prospectively identify those patients who are likely to respond to novel combination treatments.
Assessments
Tumor assessment was performed by investigators according to RECIST v1.1 using CT or MRI scans. Assessments were carried out at baseline (days −28 to −1), Week 8, and Week 12; then every 8 weeks ±1 week in arms A and C and at baseline; and then every 6 weeks ±1 week in arm B, until PD (or end of clinical benefit for patients treated beyond progression) or discontinuation from the study. Survival assessments were conducted monthly for the first 3 months following treatment discontinuation and then every 2 months thereafter.
Measurement of PD-L1 and ctDNA (arm A)
Where sufficient tissue was available, tumor specimens were assessed for PD-L1 expression on TCs and immune cells (IC) using the VENTANA PD-L1 (SP263) Assay (Roche Diagnostics). Based on findings from the phase III CASPIAN study (36), PD-L1 analyses used a cutoff of 1% of TCs or ICs expressing PD-L1 at any intensity. Mandatory blood samples were collected in Streck DNA blood collection tubes at Week 0 and Week 8 for analysis of ctDNA in arm A. ctDNA was characterized using the GuardantOMNI panel (Guardant Health Inc.). Levels of ctDNA were determined by the maximum variant allele frequency (MaxVAF), defined as the alteration with the highest variant allele frequency (VAF) in a given sample. As the VAF is the number of mutant molecules over the total number of molecules at a specific location in the genome, MaxVAF is often used as a surrogate for tumor fraction in ctDNA. The change in MaxVAF while on treatment was used to assess the impact of therapy, known as the molecular response.
Measurement of circulating chemokines and cytokines (arm C)
In arm C, the measurement of 183 cytokines and chemokines in plasma was conducted to determine the potential biomarkers of survival and pharmacodynamic biomarkers. Plasma samples for the biomarker analyses were collected at four different time points [predose, cycle (C) 1 Day (D) 7, C1D15, and C2D1] and analyzed with the Olink proximity extension assay technology platform using panel IO and cardiovascular disease III panels (183 analytes), including immune response, immuno-oncology disease-relevant, cardiovascular, and inflammatory markers.
Statistical analysis
All patients who received treatment were included in the full analysis set, used for efficacy and safety assessments. The biomarker evaluable populations (BEP) for PD-L1 and ctDNA analyses (in arm A) and circulating biomarkers (in arm C) included all patients with an evaluable sample. All statistical analyses were descriptive and performed using SAS version 9.3. ORR was estimated for each treatment arm with corresponding two-sided exact 95% confidence intervals (CI). The analysis was based on the programmatically derived ORR according to investigator assessments and using all scans, regardless of whether they were scheduled or not. The median time-to-event endpoints, including DoR, PFS, and OS, were estimated by the Kaplan–Meier method with 95% CIs. In arm C, a linear mixed model was used to analyze longitudinal pharmacodynamic changes.
Data availability
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at: www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at: https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.
Results
Patients
In arm A, 49 patients were enrolled, and 41 received treatment with durvalumab plus tremelimumab. In arm B, 13 patients were enrolled, and 10 received adavosertib plus carboplatin. In arm C, 26 patients were enrolled, and 21 received ceralasertib plus olaparib. Baseline patient demographics and disease characteristics are shown in Table 1. The median age was 63.0 years in arm A, 63.5 years in arm B, and 61.0 years in arm C; 75.6%, 80.0%, and 66.7% of patients in arms A, B, and C, respectively, were male, and 75.6%, 90.0%, and 66.7%, respectively, had WHO PS 1. Most patients were current or former smokers (95.1% in arm A, 100% in arm B, and 95.2% in arm C). Representativeness of study participants is described in Supplementary Table S1.
Table 1.
Baseline patient demographics and disease characteristics—full analysis set.
| Arm A durvalumab + tremelimumab (n = 41) | Arm B adavosertib + carboplatin (n = 10) | Arm C ceralasertib + olaparib (n = 21) | |
|---|---|---|---|
| Median age, years (range) | 63.0 (40–76) | 63.5 (48–78) | 61.0 (34–76) |
| Age group, years | |||
| <65 | 27 (65.9) | 6 (60.0) | 14 (66.7) |
| ≥65 | 14 (34.1) | 4 (40.0) | 7 (33.3) |
| Sex, n (%) | |||
| Male | 31 (75.6) | 8 (80.0) | 14 (66.7) |
| Female | 10 (24.4) | 2 (20.0) | 7 (33.3) |
| Race, n (%) | |||
| White | 21 (100.0) | 10 (100.0) | 21 (100.0) |
| WHO PS, n (%) | |||
| 0 | 10 (24.4) | 1 (10.0) | 7 (33.3) |
| 1 | 31 (75.6) | 9 (90.0) | 14 (66.7) |
| Smoking history, n (%) | |||
| Never smoker | 2 (4.9) | 0 | 1 (4.8) |
| Former smoker | 18 (43.9) | 4 (40.0) | 7 (33.3) |
| Current smoker | 21 (51.2) | 6 (60.0) | 13 (61.9) |
| SCLC subtype, n (%) | |||
| Neuroendocrine | 4 (9.8) | 2 (20.0) | 3 (14.3) |
| Oat cell | 1 (2.4) | 2 (20.0) | 0 |
| Other SCLC | 36 (87.8) | 6 (60.0) | 18 (85.7) |
| Disease stage, n (%) | |||
| II | 0 | 0 | 1 (4.8) |
| III | 5 (12.2) | 0 | 0 |
| IV | 36 (87.8) | 10 (100.0) | 20 (95.2) |
| Brain or CNS metastases, n (%) | 4 (9.8) | 2 (20.0) | 2 (9.5) |
| Liver metastases, n (%) | 18 (43.9) | 3 (30.0) | 6 (28.6) |
| Previous treatment modalities, n (%)a | |||
| Immunotherapy | 0 | 0 | 1 (4.8) |
| Cytotoxic chemotherapy | 41 (100.0) | 9 (90.0) | 21 (100.0) |
| Radiotherapy | 10 (24.4) | 4 (40.0) | 6 (28.6) |
| Other | 0 | 2 (20.0) | 0 |
| Best response to previous therapy | |||
| CR | 1 (2.4) | 0 | 1 (4.8) |
| PR | 9 (22.0) | 3 (30.0) | 4 (19.0) |
| SD | 11 (26.8) | 0 | 7 (33.3) |
| PD | 20 (48.8) | 7 (70.0) | 8 (38.1) |
| NE | 0 | 0 | 1 (4.8) |
| Median time from the last dose of previous therapy to the first dose of study treatment, months (range) | 1.61 (0.6–39.8) | 2.15 (1.3–4.1) | 2.17 (1.1–4.0) |
Abbreviations: CNS, central nervous system; NE, not evaluable; SD, stable disease.
Patients could have received more than one previous treatment modality.
Treatment exposure
Full details of treatment exposure and patient disposition are provided in Supplementary Table S2. In arm A, the median total exposure duration was 84 days, and the median number of treatment cycles received was 2.0 with both durvalumab and tremelimumab. At data cutoff (DCO), 7.3% of patients were ongoing durvalumab treatment and 92.7% had discontinued; 39.0% of patients had completed tremelimumab treatment, and 61.0% had discontinued. In arm B, the median total exposure duration was 69 days with adavosertib and 70 days with carboplatin; the median number of treatment cycles received was 2.5 for both adavosertib and carboplatin. At DCO, all 10 patients had discontinued treatment with both adavosertib and carboplatin. In arm C, the median total exposure duration was 84 days, and the median number of treatment cycles received was 3.0 for both ceralasertib and olaparib. At DCO, 1 patient (4.8%) was ongoing treatment, and 95.2% had discontinued treatment with both ceralasertib and olaparib.
Efficacy
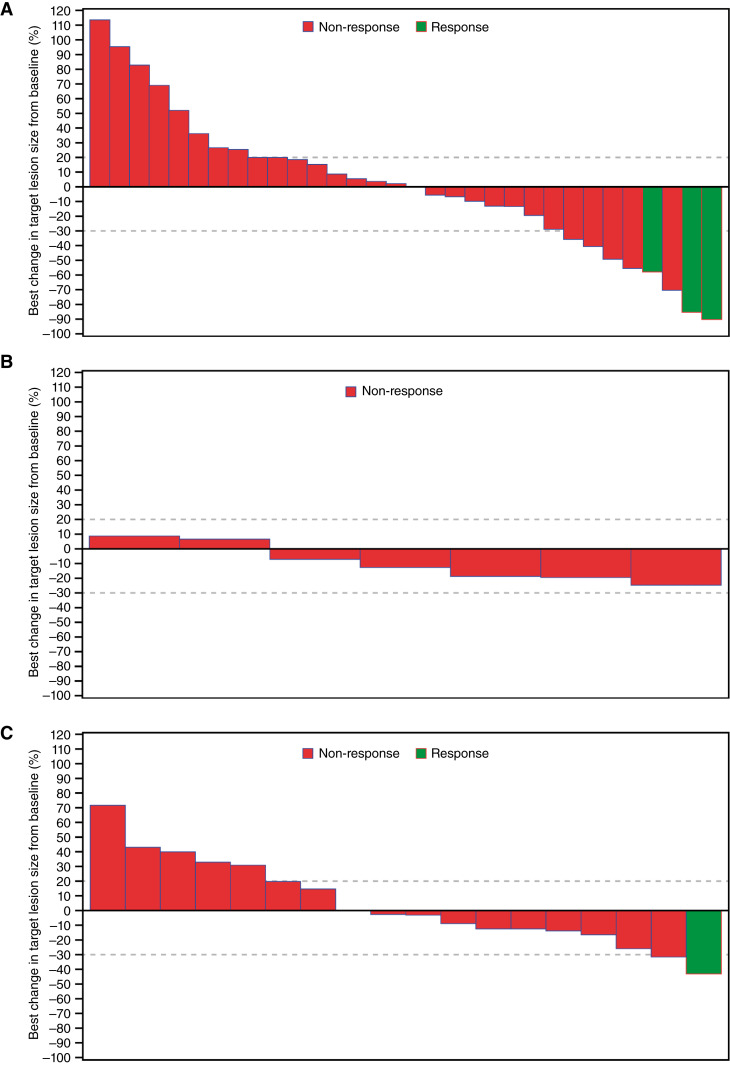
The best response to treatment for all study arms is shown in Table 2. In arm A, the confirmed ORR with durvalumab plus tremelimumab was 7.3% (95% CI, 1.54–19.92); no patients achieved a CR, and three patients achieved a PR (Fig. 1A; Supplementary Fig. S1A). The median DoR among the three patients with confirmed responses was 3.0 months, and the median TTR was 1.8 months. The DCR at 12 weeks was 26.8%. One additional patient achieved a PR after initially having PD; taking this patient into account, the ORR was 9.8%. The median PFS with durvalumab plus tremelimumab was 1.84 months (95% CI, 1.77–1.91; Supplementary Fig. S2A); the PFS rate was 8.9% at 6 months and 5.9% at 12 months. The median OS was 5.36 months (95% CI, 2.89–7.23; Supplementary Fig. S2B); the OS rate was 41.5% at 6 months and 26.8% at 12 months.
Table 2.
Summary of tumor response—full analysis set.
| Arm A durvalumab + tremelimumab (n = 41) | Arm B adavosertib + carboplatin (n = 10) | Arm C ceralasertib + olaparib (n = 21) | |
|---|---|---|---|
| Confirmed objective response, n (%)a | 3 (7.3) | 0 | 1 (4.8) |
| Best objective response, n (%) | |||
| CRa | 0 | 0 | 0 |
| PRa | 3 (7.3) | 0 | 1 (4.8) |
| SD | 9 (22.0) | 6 (60.0) | 10 (47.6) |
| Unconfirmed CR or PRb | 1 (2.4) | 0 | 1 (4.8) |
| PD | 28 (68.3) | 3 (30.0) | 7 (33.3) |
| NE | 0 | 1 (10.0) | 2 (9.5) |
Confirmatory scan required at least 4 weeks after the initial response.
PR or CR achieved but either no confirmation assessment performed or a confirmation assessment performed but response not confirmed.
Figure 1.
Best percentage change from baseline in selected target lesion size per investigator assessment—full analysis set. A, Arm A, durvalumab + tremelimumab. B, Arm B, adavosertib + carboplatin. C, Arm C, ceralasertib + olaparib. In arms A, B, and C, respectively, 10, 3, and 4 patients had no measurements of target lesion size after baseline and were excluded from the plots. Best change in target lesion size is the maximum reduction from baseline or minimum increase from baseline in the absence of a reduction. Response includes confirmed CR or PR. Dotted reference lines at −30% and 20% indicate thresholds for PR and PD, respectively.
In arm B, the ORR with adavosertib plus carboplatin was 0%; no patients achieved a CR or PR (Fig. 1B; Supplementary Fig. S1B). DCR at 12 weeks was 30.0%. The median PFS was 2.60 months (95% CI, 0.56–4.83; Supplementary Fig. S2C); the PFS rate was 11.1% at 6 months. The median OS was 4.67 months (95% CI, 0.56–5.98) (Supplementary Fig. S2D); the OS rate was 20.0% at 6 months and 10.0% at 12 months.
In arm C, the confirmed ORR with ceralasertib plus olaparib was 4.8% (95% CI, 0.12–23.82), with one patient achieving a PR (Fig. 1C; Supplementary Fig. S1C). The duration of PR for this patient was 8.5 months, and the TTR was 1.7 months. The DCR at 12 weeks was 38.1%. The median PFS was 2.92 months (95% CI, 1.81–4.53; Supplementary Fig. S2E); the PFS rate was 15.8% at 6 months and 0% at 12 months. The median OS was 7.56 months (95% CI, 4.21–12.58; Supplementary Fig. S2F); the OS rate was 58.2% at 6 months and 31.8% at 12 months.
Safety
In arm A, treatment-related AEs (TRAE) of any grade occurred in 19 patients (46.3%) and were grade ≥3 in 8 patients (19.5%; Table 3). The most common TRAEs with durvalumab plus tremelimumab were hyperthyroidism (n = 4), diarrhea, fatigue, and hypothyroidism (each n = 3). Eight patients (19.5%) experienced 10 treatment-related serious AEs [SAE; diarrhea (n = 2), and gastroenteritis, pneumonia, myasthenic syndrome, peripheral sensory neuropathy, colitis, hemorrhagic enterocolitis, pancreatitis, and hepatic enzyme increased (n = 1 each)]. Five patients (12.2%) discontinued durvalumab and tremelimumab treatment because of TRAEs [pneumonia, diarrhea, hemorrhagic enterocolitis, pancreatitis, and hepatic enzyme increased (each n = 1); the treatment-related hemorrhagic enterocolitis resulted in death].
Table 3.
Summary of safety profile, and all TRAEs—full analysis set.
| Arm A durvalumab + tremelimumab (n = 41) | Arm B adavosertib + carboplatin (n = 10) | Arm C ceralasertib + olaparib (n = 21) | ||||
|---|---|---|---|---|---|---|
| Any AE (all causality), n (%) | 33 (80.5) | 8 (80.0) | 18 (85.7) | |||
| Any TRAEa, n (%) | 19 (46.3) | 8 (80.0) | 16 (76.2) | |||
| Grade ≥3 TRAEs | 8 (19.5) | 6 (60.0) | 6 (28.6) | |||
| TRAEs leading to death | 1 (2.4) | 1 (10.0) | 0 | |||
| Serious TRAEs | 8 (19.5) | 3 (30.0) | 4 (19.0) | |||
| TRAEs leading to discontinuation of study treatmentb | 6 (14.6) | 1 (10.0) | 1 (4.8) |
| TRAEs, n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
|---|---|---|---|---|---|---|
| Alanine aminotransferase increased | 0 | 0 | 0 | 0 | 1 (4.8) | 0 |
| Anemia | 0 | 0 | 3 (30.0) | 1 (10.0) | 12 (57.1) | 5 (23.8) |
| Asthenia | 0 | 0 | 3 (30.0) | 0 | 1 (4.8) | 0 |
| Axillary vein thrombosis | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Colitis | 1 (2.4) | 1 (2.4) | 0 | 0 | 0 | 0 |
| Constipation | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Creatinine renal clearance decreased | 0 | 0 | 0 | 0 | 1 (4.8) | 0 |
| Decreased appetite | 0 | 0 | 1 (10.0) | 0 | 1 (4.8) | 0 |
| Diarrhea | 3 (7.3) | 3 (7.3) | 6 (60.0) | 1 (10.0) | 1 (4.8) | 0 |
| Enterocolitis, hemorrhagic | 1 (2.4) | 1 (2.4) | 0 | 0 | 0 | 0 |
| Fatigue | 3 (7.3) | 0 | 3 (30.0) | 0 | 1 (4.8) | 0 |
| Gastroenteritis | 1 (2.4) | 1 (2.4) | 0 | 0 | 0 | 0 |
| Hematotoxicity | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Hepatic enzyme increased | 1 (2.4) | 1 (2.4) | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 0 | 0 | 1 (10.0) | 0 | 0 | 0 |
| Hyperchloremia | 0 | 0 | 1 (10.0) | 0 | 0 | 0 |
| Hyperglycemia | 1 (2.4) | 0 | 1 (10.0) | 0 | 0 | 0 |
| Hypersensitivity | 0 | 0 | 0 | 0 | 1 (4.8) | 0 |
| Hyperthyroidism | 4 (9.8) | 0 | 0 | 0 | 0 | 0 |
| Hypomagnesemia | 0 | 0 | 1 (10.0) | 0 | 0 | 0 |
| Hypothyroidism | 3 (7.3) | 0 | 0 | 0 | 0 | 0 |
| Leukopenia | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Muscular weakness | 0 | 0 | 1 (10.0) | 0 | 0 | 0 |
| Myasthenic syndrome | 2 (4.9) | 2 (4.9) | 0 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 6 (60.0) | 0 | 3 (14.3) | 0 |
| Nephritis | 0 | 0 | 1 (10.0) | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 3 (30.0) | 3 (30.0) | 0 | 0 |
| Neutrophil count decreased | 0 | 0 | 0 | 0 | 1 (4.8) | 0 |
| Pancreatitis | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Pancytopenia | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Peripheral motor neuropathy | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Peripheral sensory neuropathy | 1 (2.4) | 1 (2.4) | 1 (10.0) | 0 | 0 | 0 |
| Platelet count decreased | 0 | 0 | 0 | 0 | 2 (9.5) | 1 (4.8) |
| Pleural effusion | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Pneumonia | 1 (2.4) | 1 (2.4) | 0 | 0 | 0 | 0 |
| Pruritus | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Psoriatic arthropathy | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Rash | 2 (4.9) | 0 | 0 | 0 | 0 | 0 |
| Somnolence | 0 | 0 | 1 (10.0) | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 6 (60.0) | 2 (20.0) | 2 (9.5) | 2 (9.5) |
| Type 2 diabetes mellitus | 1 (2.4) | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 3 (30.0) | 0 | 1 (4.8) | 0 |
Any AE assessed by the investigator to be related to either drug in the combination.
Any AE that resulted in discontinuation of either durvalumab or tremelimumab (arm A), adavosertib or carboplatin (arm B), or ceralasertib or olaparib (arm C).
In arm B, any-grade TRAEs occurred in eight patients (80.0%) and were grade ≥3 in six patients (60.0%). The most common TRAEs with adavosertib plus carboplatin (Table 3) were diarrhea, nausea, and thrombocytopenia (each n = 6). Three patients (30.0%) experienced treatment-related SAEs (diarrhea, hematotoxicity, and pancytopenia in one patient each). There was one treatment-related death (pancytopenia), and no other patients discontinued treatment because of a TRAE.
In arm C, any-grade TRAEs occurred in 16 patients (76.2%) and were grade ≥3 in 6 patients (28.6%). The most common TRAEs with ceralasertib plus olaparib (Table 3) were anemia (n = 12), nausea (n = 3), and thrombocytopenia and decreased platelet count (each n = 2). Four patients (19.0%) experienced treatment-related SAEs (anemia and thrombocytopenia in one patient, and anemia alone in three patients). There were no TRAEs leading to treatment discontinuation or death in arm C.
Arm A (durvalumab plus tremelimumab) PD-L1 biomarker analyses
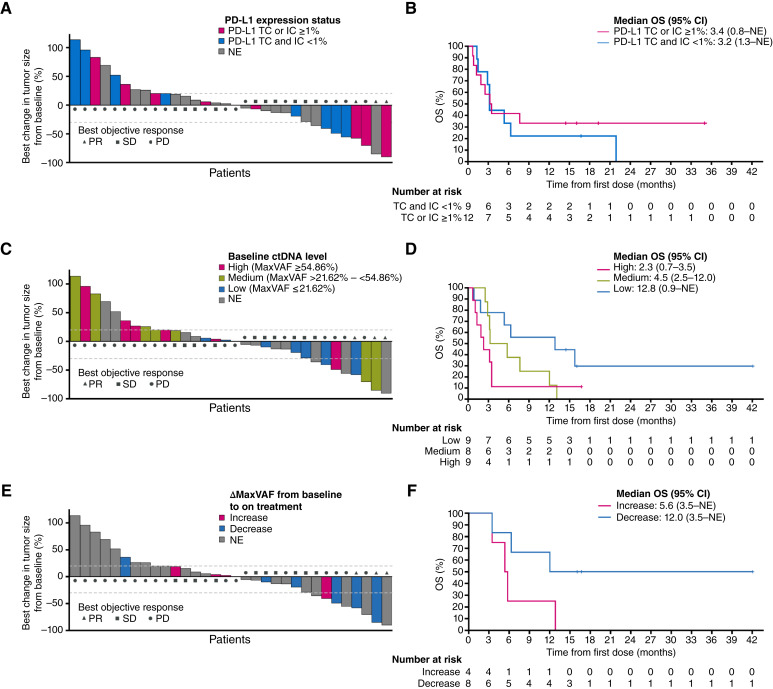
The PD-L1 BEP included 21 patients, comprising 51% of the full analysis set. The prevalence of PD-L1 expression on ≥1% of TCs was 4.8% (1/21 patients) and of PD-L1 expression on ≥1% of ICs was 57.1% (12/21 patients). For our analyses, patients were divided into two subgroups by PD-L1 expression level: (i) PD-L1 TC ≥1% or PD-L1 IC ≥1% and (ii) PD-L1 TC and IC <1%. The best percentage change in tumor size by PD-L1 status is shown in Fig. 2A; 3 (25%) of 12 patients with PD-L1 TC or IC expression ≥1% had a best response of PR or stable disease (SD), compared with 1 (11%) of the remaining 9 patients who had PD-L1 expression on <1% of both TCs and ICs. Among 20 patients who were not evaluable (NE) for PD-L1 status, 9 (45.0%) had a best response of PR or SD. The median OS in patients with PD-L1 expression (TC or IC) ≥1% compared with PD-L1 expression (TC and IC) <1% was 3.4 vs. 3.2 months (HR, 0.77; 95% CI, 0.29–2.07; Fig. 2B).
Figure 2.
Response and OS by PD-L1 expression status, baseline ctDNA level, and by change in ctDNA levels (ΔMaxVAF) from baseline to on treatment—arm A PD-L1 BEP and ctDNA BEP. A, Best percentage change in tumor size from baseline in patients evaluable for response (n = 32)* by PD-L1 expression status. B, Kaplan–Meier curves for OS by PD-L1 expression status. C, Best percentage change in tumor size from baseline in patients evaluable for response (n = 32)* by baseline ctDNA level. D, Kaplan–Meier curves for OS, by tertiles of baseline ctDNA level [low (MaxVAF ≤ 21.62%; n = 9)], medium (MaxVAF > 21.62% to <54.86%; n = 8), or high (MaxVAF ≥ 54.86%; n = 9). E, Best percentage change in tumor size from baseline in patients evaluable for response (n = 32)* by change in the ctDNA level from baseline to on treatment [MaxVAF decrease (ΔMaxVAF < 0) or increase (ΔMaxVAF > 0)]. F, Kaplan–Meier curves for OS, by change in the ctDNA level from baseline to on treatment [decrease (negative ΔMaxVAF) or increase (positive ΔMaxVAF)]. *Nine patients did not have postbaseline tumor size measurements, so are not included in the figure; in A, C, and E, dashed reference lines at −30% and 20% indicate thresholds for PR and PD.
Arm A (durvalumab plus tremelimumab) ctDNA biomarker analyses
The ctDNA BEP comprised 26 patients (63.4% of the full analysis set) with a baseline sample; 10 (24.4%) patients had both a baseline sample and an on-treatment sample taken before the start of cycle 3. The baseline ctDNA level was classified by tertiles as low (MaxVAF ≤21.62%; n = 9), medium (MaxVAF >21.62% to <54.86%; n = 8), or high (MaxVAF ≥54.86%; n = 9). The best percentage change in tumor size by baseline ctDNA level is shown in Fig. 2C. A best response of PR or SD was achieved by four (44.4%) of nine patients with low baseline ctDNA levels, two (25.0%) of eight patients with medium baseline ctDNA levels, and one (11.1%) of nine patients with high baseline ctDNA levels. Among 15 patients who were NE for baseline ctDNA, 6 (40.0%) had a best response of PR or SD. Patients with low baseline ctDNA levels had numerically longer median OS than those with medium or high baseline ctDNA levels (Fig. 2D).
The impact of ctDNA dynamics on patient outcomes was investigated by the change in baseline MaxVAF on treatment (ΔMaxVAF), which was calculated by subtracting the baseline MaxVAF from MaxVAF at cycle 3. Patients were grouped according to whether MaxVAF decreased (ΔMaxVAF < 0) or increased (ΔMaxVAF > 0) on treatment. A best response of PR or SD was achieved by four (66.7%) of six patients who showed a decrease from baseline in on-treatment ctDNA level (negative ΔMaxVAF), and two (50.0%) of four patients with an increase (positive ΔMaxVAF; Fig. 2E). Among 31 patients who were NE for on-treatment ctDNA analysis, 7 (22.6%) had a best response of PR or SD. Patients with a decrease in ctDNA level from baseline to on treatment (negative ΔMaxVAF) had a longer OS than those with an increase (positive ΔMaxVAF), although this difference was not statistically significant (HR, 0.30; 95% CI, 0.06–1.38; Fig. 2F).
Arm C (ceralasertib plus olaparib) circulating baseline and pharmacodynamic biomarker analyses
The circulating biomarkers BEP comprised 21 patients (100% of the complete analysis set) with a baseline sample; 74 plasma samples were collected at various time points (76% of patients had both baseline and on-treatment samples).
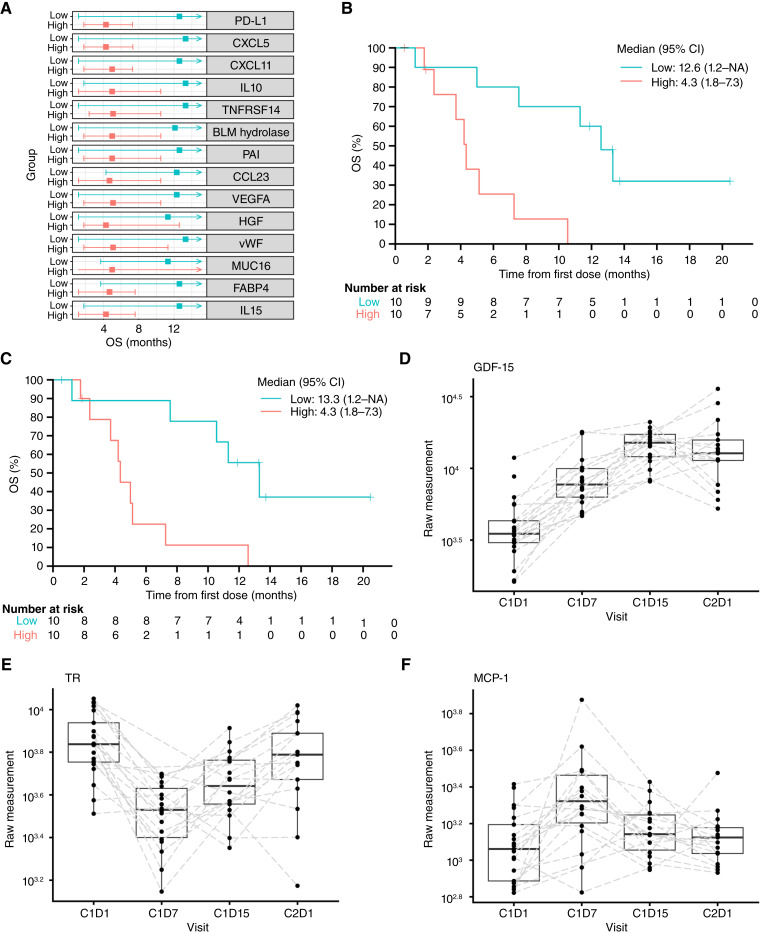
Baseline circulating plasma cytokine and chemokine levels were evaluated in patients receiving ceralasertib plus olaparib to identify biomarkers of survival. Patients were dichotomized into low and high groups based on median OS. Higher baseline plasma concentrations of PD-L1, C–X–C motif chemokine ligand 5 (CXCL5) and CXCL11, IL10, TNF receptor superfamily member 14, bleomycin hydrolase, plasminogen activator inhibitor 1, C–C motif chemokine ligand 23, VEGFA, hepatocyte growth factor, von Willebrand factor, mucin 16, fatty acid–binding protein 4, and IL15 were associated with shorter OS (Fig. 3A). Furthermore, patients with high levels of plasma PD-L1 (Fig. 3B) and CXCL5 (Fig. 3C) displayed a shorter OS than those with lower levels.
Figure 3.
Circulating biomarkers associated with ceralasertib + olaparib treatment response and pharmacodynamics—arm C circulating biomarker BEP. A, Baseline cytokine and chemokine distribution according to OS. B, Kaplan–Meier curves for OS by baseline PD-L1 expression status. C, Kaplan–Meier curves for OS by baseline CXCL5 expression status. D, Change in GDF15 expression over time in cycles 1 and 2. E, Change in TR expression over time in cycles 1 and 2. F, Change in MCP1 expression over time in cycles 1 and 2. BLM, bleomycin; CCL23, C–C motif chemokine ligand 23; CXCL, C–X–C motif chemokine ligand; FABP4, fatty acid–binding protein 4; HGF, hepatocyte growth factor; MUC16, mucin 16; PAI, plasminogen activator inhibitor; TNFRSF14, TNF receptor superfamily member 14; vWF, von Willebrand factor.
Pharmacodynamic assessments of olaparib, ceralasertib, or a combination, based on blood samples collected at C1D1 (predose), C1D7, C1D15, and C2D1, showed that sustained increases in growth/differentiation factor 15 (GDF15) were associated with olaparib treatment and increased during the treatment cycle (Fig. 3D). Furthermore, ceralasertib-related effects were identified in the circulation, such as a decrease in transferrin receptor protein 1 (TR, also known as CD71, which is abnormally expressed in various cancers) and an increase in monocyte chemoattractant protein 1 (MCP1; Fig. 3E and F). After 3 weeks off ceralasertib, both TR and MCP1 levels recovered toward baseline. Similar ceralasertib-driven bounce-back effects were observed in glycoprotein VI platelet, angiopoietin 1, IL7, EGF, platelet-derived growth factor, and latency-associated peptide transforming growth factor β1 (Supplementary Fig. S3).
Discussion
The phase II BALTIC trial enrolled patients with ES-SCLC who had refractory or resistant disease following first-line platinum-based chemotherapy, who received treatment with one of three novel combination regimens: durvalumab plus tremelimumab followed by durvalumab monotherapy (arm A), adavosertib plus carboplatin (arm B), or ceralasertib plus olaparib (arm C). All arms demonstrated a tolerable safety profile, with no new safety signals. Overall, 71 patients were treated across the three regimens, with relatively low numbers recruited in arms B (n = 10) and C (n = 21). These small numbers mean that efficacy estimates in BALTIC are associated with wide CIs, which should be considered when comparing point estimates to those reported in larger studies.
In all arms, response rates were low and did not meet prespecified criteria to warrant development in a randomized setting in patients with refractory/resistant ES-SCLC. Current second-line treatment in ES-SCLC is typically topotecan, which exhibited an ORR of 9.4%, median PFS of 2.6 months (95% CI, 1.8–3.3), and median OS of 5.7 months (95% CI, 4.1–7.0) in a subgroup of patients refractory to first-line therapy (4). Efficacy results from BALTIC were broadly consistent with these data. With durvalumab plus tremelimumab, ORR, median PFS, and median OS were 7.3%, 1.84, and 5.36 months, respectively. With adavosertib plus carboplatin, no objective responses were observed, the median PFS was 2.60 months, and the median OS was 4.67 months. With ceralasertib plus olaparib, ORR was low (4.8%), with a median PFS of 2.92 months and a median OS of 7.56 months.
The addition of the CTLA4 inhibitor tremelimumab to the PD-L1 inhibitor durvalumab provided no apparent improvement in efficacy versus durvalumab alone; the ORR (7.3%) was lower than that reported in a previous study of durvalumab monotherapy in pretreated ES-SCLC (9.5%; ref. 8). Although cross-trial comparisons are unreliable, similar results have been reported in previously for PD1/PD-L1 inhibitor plus CTLA4 inhibitor combinations in ES-SCLC. For example, ORR was lower with ipilimumab plus nivolumab than nivolumab alone (9.1% vs. 11.5%; ref. 5), and durvalumab plus tremelimumab plus chemotherapy than durvalumab plus chemotherapy (58% vs. 68%; ref. 16).
Based on mechanistic and preclinical rationale (17) and early-phase clinical data (20, 21) antitumor activity with the combination of adavosertib plus carboplatin could have been expected in ES-SCLC, but there was a lack of response to the combination in BALTIC. It is possible that limited carboplatin efficacy, which can be associated with various mechanisms of chemoresistance (37), was a contributing factor. Of note, only 3 of 10 patients in arm B had achieved response to first-line platinum-based chemotherapy prior to enrollment. Similarly, the combination of ceralasertib plus olaparib has demonstrated preclinical synergistic activity in ATM-deficient cancer cells (30), but this did not translate into notable clinical antitumor activity for patients in BALTIC. Although dependence on DNA damage repair is generally considered to make SCLC susceptible to DNA damage repair pathway inhibitors (38), some literature suggests that alternative DNA damage repair pathway components may compensate for the comutations of TP53 and RB1 and their impact on genome integrity, and limit their effect on overall cell survival (39–41). This may account for the lack of activity of medications that target DNS repair mechanisms in arms B and C of BALTIC. Targeting the compensatory pathways may provide an alternative future therapeutic approach to sensitize SCLC tumors to DNA-damaging agents.
In all three arms, some patients derived more benefits than others. Understanding the underlying mechanism of response or resistance may help enhance treatment effects in the future. Biomarker findings for both PD-L1 and ctDNA from arm A of BALTIC were exploratory, and limited by both the small sample sizes and the proportion of patients who were biomarker evaluable and should be interpreted with caution. The prevalence of PD-L1 expression on TCs was low (PD-L1 ≥ 1% in 4.8% of patients) and similar to that previously reported in the first-line setting in the phase III CASPIAN (5.4%) and IMpower133 (5.8%) studies; the prevalence of PD-L1 expression on ICs (PD-L1 ≥ 1% in 57% of patients) was somewhat higher than in CASPIAN (25.8%) but similar to that in IMpower133 (50.4%; refs. 36, 42). These data suggest that TC PD-L1 expression may not be notably different in platinum-refractory versus platinum-naïve disease. Although small sample size limits interpretation, patients with PD-L1 expression on ≥1% of TCs or ICs seemed to have a greater likelihood of achieving disease control with durvalumab plus tremelimumab than patients with PD-L1 expression on <1% of TCs and ICs. PD-L1 expression did not seem to be associated with OS.
Identification of predictive biomarkers in SCLC is hindered by the difficulty of obtaining tumor biopsies of adequate size and quality, and the lack of collection beyond the initial diagnostic sample. Liquid biopsies are of interest as a rapidly applicable, noninvasive alternative to tumor biopsies to assess the molecular profile of disease and examine clonal evolution during treatment. However, existing data on ctDNA in SCLC are limited, especially in the context of novel therapies (35, 43). In arm A, analyses of baseline ctDNA measured by MaxVAF suggested a differential OS benefit, with a trend for longer OS with durvalumab plus tremelimumab in patients with lower baseline MaxVAF. Evidence from other studies has been inconsistent; a recent meta-analysis of patients with NSCLC treated with immune checkpoint inhibitors failed to show a significant association between baseline ctDNA and efficacy outcomes (44), but several other reports in a range of solid tumors, including SCLC, support our observation of a prognostic effect of disease burden (33, 35, 45, 46).
A reduction in the ctDNA level on treatment (defined as ΔMaxVAF < 0) was found to be associated with longer OS in arm A of BALTIC, possibly indicating a decrease in disease burden on response to durvalumab plus tremelimumab treatment. However, this finding was based on a small sample size resulting in a wide CI surrounding the HR for risk of death, with the upper boundary >1. Previous analyses in patients treated with immune checkpoint inhibitors have also suggested that ctDNA dynamics are predictive of clinical outcomes and radiologic response. For example, in patients with advanced NSCLC or urothelial cancer who received durvalumab (first- to sixth-line), a reduction in mean VAF after 6 weeks of treatment was associated with greater tumor volume reduction and longer PFS and OS (47). A larger pooled analysis of patients with solid tumors who received durvalumab with or without tremelimumab confirmed the predictive nature of on-treatment ctDNA dynamics based on change in VAF at 6 weeks (33). In the phase III MYSTIC study (durvalumab ± tremelimumab vs. chemotherapy), molecular response based on early ctDNA dynamics was predictive of radiologic response in all treatment arms, although only of OS in the immunotherapy arms (34). The prospective INSPIRE study (pembrolizumab in solid tumors) showed that baseline ctDNA correlated with clinical response and survival outcomes; this correlation was strengthened when ctDNA kinetics during treatment were also considered (45). Data from BALTIC and previous studies suggest that future treatment of tumors including ES-SCLC may be adapted based on early ctDNA kinetics to identify responding or resistant patients in a timely manner. However, studies have used different methods to evaluate ctDNA, and often had small sample sizes, limiting the generalization of results. The optimum threshold to identify clinically significant ctDNA changes and the optimal timepoint for analysis are currently unknown.
Exploratory circulating biomarker analyses in arm C showed that patients treated with ceralasertib plus olaparib produced specific immune response-relevant circulating chemokines and cytokines (including PD-L1, CXCL5, C–X–C motif chemokine ligand-11, IL10, TNF receptor superfamily member 14, bleomycin hydrolase, plasminogen activator inhibitor, CC motif chemokine ligand 23, VEGFA, hepatocyte growth factor, von Willebrand factor, mucin 16, fatty acid binding–protein 4, and IL15) as early biomarkers of survival outcomes; high levels of these cytokines in the circulation were associated with decreased OS. These systemic immune-inflammation cytokines can act directly on carcinoma cells or recruit immunosuppressive and proangiogenic cells (48–50). Furthermore, we observed an on-treatment sustained increase in inflammatory and stress-induced cytokine expression of GDF15, which is often increased upon tissue injury and is implicated in tumor progression, tumor load, tumor-promoting inflammation, and tumor immune evasion in several cancer types (51). Given the exploratory nature of our analyses, further studies are required to evaluate the mechanistic and clinical implications of these findings.
In conclusion, limited antitumor activity was observed with the novel combination regimens of durvalumab plus tremelimumab, adavosertib plus carboplatin, and ceralasertib plus olaparib in patients with platinum-refractory or -resistant ES-SCLC. All three combinations demonstrated tolerable safety profiles, consistent with the known safety signals of the individual drugs. Analyses of ctDNA dynamics in patients treated with durvalumab plus tremelimumab support increasing evidence that early changes in ctDNA may be predictive of response to immune checkpoint inhibitors across different tumor types. The circulating chemokines and cytokines identified in patients treated with ceralasertib plus olaparib as early biomarkers of poor survival and pharmacodynamics warrant additional mechanistic investigation. Recent research has identified four biologically distinct subtypes of SCLC based on the expression of specific transcription factors, with apparently distinct sensitivities to different therapeutic approaches (52). In the future, studies enrolling a selected patient population may therefore prove more successful in identifying effective treatments for platinum-refractory or -resistant ES-SCLC than the all-comer approach used in BALTIC.
Supplementary Material
Supplementary tables and figures
Acknowledgments
The study was funded by AstraZeneca. We thank the patients, their families and caregivers, all investigators involved in this study, and all members of the independent data monitoring committee for their contributions to the study. We thank Paola Marco-Casanova of AstraZeneca for her contributions to the arm C biomarker analysis. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Helen Kitchen of Ashfield MedComms, an Inizio company, and funded by AstraZeneca.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors’ Disclosures
N. Reinmuth reports personal fees from Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, GSK, Hoffmann-La Roche, Janssen, MSD, Pfizer, Asklepios GmbH Gauting, Boehringer Ingelheim, Eli Lilly and Company, Sanofi, and Takeda outside the submitted work. O.J. Juan-Vidal reports personal fees from AstraZeneca, Bristol Myers Squibb, Johnson & Johnson, Eli Lilly and Company, Merck Sharp & Dohme, Roche/Genentech, and Takeda outside the submitted work. M. Bryl reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca, Roche/Genentech, MSD, Bristol Myers Squibb, Boehringer Ingelheim, Takeda, Novartis, Sanofi, and Pfizer outside the submitted work. D. Vicente reports personal fees from AstraZeneca, Roche, Pfizer, Novartis, Bristol Myers Squibb, Takeda, Eli Lilly and Company, and MSD outside the submitted work. J. Armstrong reports personal fees and other support from AstraZeneca during the conduct of the study, as well as personal fees and other support from AstraZeneca outside the submitted work. T. Dalvi reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work; and employment with AstraZeneca and ownership of stock. M. Xie reports personal fees from AstraZeneca during the conduct of the study. S. Iyer reports other support from AstraZeneca during the conduct of the study, as well as other support from AstraZeneca outside the submitted work. Y. Shrestha reports other support from AstraZeneca outside the submitted work. H. Jiang reports employment with AstraZeneca, which is the sponsor of the trial. No disclosures were reported by the other authors.
Authors’ Contributions
N. Reinmuth: Resources, writing–review and editing. O. Juan-Vidal: Data curation, investigation, writing–review and editing. D. Kowalski: Data curation, supervision, investigation, writing–review and editing. M. Bryl: Investigation, writing–review and editing. A. Kryzhanivska: Investigation. D. Vicente: Investigation, writing–review and editing. Z. Horváth: Investigation, writing–review and editing. G. Gálffy: Resources, supervision, investigation, writing–original draft, writing–review and editing. E. Csánky: Investigation, project administration. Z. Pápai Székely: Supervision, investigation, writing–review and editing. I. Vynnychenko: Investigation, writing–review and editing. J. Armstrong: Formal analysis, writing–review and editing. T. Dalvi: Conceptualization, data curation, formal analysis, visualization, writing–original draft, writing–review and editing. M. Xie: Conceptualization, data curation, formal analysis, visualization, writing–original draft, writing–review and editing. S. Iyer: Conceptualization, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. Y. Shrestha: Conceptualization, data curation, formal analysis, supervision, funding acquisition, writing–review and editing. H. Jiang: Conceptualization, data curation, supervision, writing–review and editing. I. Bondarenko: Investigation.
References
- 1. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39. [DOI] [PubMed] [Google Scholar]
- 2. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 3. Das M, Padda SK, Weiss J, Owonikoko TK. Advances in treatment of recurrent small cell lung cancer (SCLC): insights for optimizing patient outcomes from an expert roundtable discussion. Adv Ther 2021;38:5431–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Pawel J, Jotte R, Spigel DR, O’Brien MER, Socinski MA, Mezger J, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012–9. [DOI] [PubMed] [Google Scholar]
- 5. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol 2021;39:1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 2020;38:2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015;3:1052–62. [DOI] [PubMed] [Google Scholar]
- 8. Goldman JW, Dowlati A, Antonia SJ, Nemunaitis JJ, Butler MO, Segal NH, et al. Safety and antitumor activity of durvalumab monotherapy in patients with pretreated extensive disease small-cell lung cancer (ED-SCLC). J Clin Oncol 2018;36(Suppl 15):8518. [Google Scholar]
- 9. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol 2005;23:8968–77. [DOI] [PubMed] [Google Scholar]
- 11. Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho DC, Mahipal A, Dowlati A, Chow WA, Segal NH, Chung KY, et al. Safety and clinical activity of durvalumab in combination with tremelimumab in extensive disease small-cell lung cancer (ED-SCLC). J Clin Oncol 2018;36(Suppl 15):8517. [Google Scholar]
- 13. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol 2023;41:1213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020;6:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Castro G Jr, Rizvi NA, Schmid P, Syrigos K, Martin C, Yamamoto N, et al. NEPTUNE: phase 3 study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J Thorac Oncol 2023;18:106–19. [DOI] [PubMed] [Google Scholar]
- 16. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51–65. [DOI] [PubMed] [Google Scholar]
- 17. Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther 2009;8:2992–3000. [DOI] [PubMed] [Google Scholar]
- 18. Mizuarai S, Yamanaka K, Itadani H, Arai T, Nishibata T, Hirai H, et al. Discovery of gene expression-based pharmacodynamic biomarker for a p53 context-specific anti-tumor drug Wee1 inhibitor. Mol Cancer 2009;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther 2010;9:514–22. [DOI] [PubMed] [Google Scholar]
- 20. Leijen S, van Geel RMJM, Pavlick AC, Tibes R, Rosen L, Razak ARA, et al. Phase I study evaluating WEE1 inhibitor AZD1775 as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J Clin Oncol 2016;34:4371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leijen S, van Geel RMJM, Sonke GS, de Jong D, Rosenberg EH, Marchetti S, et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol 2016;34:4354–61. [DOI] [PubMed] [Google Scholar]
- 22. George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yazinski SA, Zou L. Functions, regulation, and therapeutic implications of the ATR checkpoint pathway. Annu Rev Genet 2016;50:155–73. [DOI] [PubMed] [Google Scholar]
- 24. Foote KM, Nissink JWM, McGuire T, Turner P, Guichard S, Yates JWT, et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and RAD3 related (ATR) kinase with application as an anticancer agent. J Med Chem 2018;61:9889–907. [DOI] [PubMed] [Google Scholar]
- 25. Vendetti FP, Lau A, Schamus S, Conrads TP, O’Connor MJ, Bakkenist CJ. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget 2015;6:44289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwok M, Davies N, Agathanggelou A, Smith E, Oldreive C, Petermann E, et al. ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells. Blood 2016;127:582–95. [DOI] [PubMed] [Google Scholar]
- 27. Jin J, Fang H, Yang F, Ji W, Guan N, Sun Z, et al. Combined inhibition of ATR and WEE1 as a novel therapeutic strategy in triple-negative breast cancer. Neoplasia 2018;20:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murai J, Huang S-yn, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012;72:5588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med 2016;8:362ps17. [DOI] [PubMed] [Google Scholar]
- 30. Lloyd RL, Wijnhoven PWG, Ramos-Montoya A, Wilson Z, Illuzzi G, Falenta K, et al. Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene 2020;39:4869–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson Z, Odedra R, Wallez Y, Wijnhoven PWG, Hughes AM, Gerrard J, et al. ATR inhibitor AZD6738 (ceralasertib) exerts antitumor activity as a monotherapy and in combination with chemotherapy and the PARP inhibitor olaparib. Cancer Res 2022;82:1140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Q, Luo J, Wu S, Si H, Gao C, Xu W, et al. Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov 2020;10:1842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peters S, Rizvi NA, Kuziora M, Lai Z, Shrestha Y, Dey A, et al. Early circulating tumour DNA (ctDNA) dynamics for predicting and monitoring response to immunotherapy (IO) vs chemotherapy (CT) in patients with 1L metastatic (m) NSCLC: analyses from the phase III MYSTIC trial. Ann Oncol 2021;32(Suppl 5):S949–1039. [Google Scholar]
- 35. Pizzutilo EG, Pedrani M, Amatu A, Ruggieri L, Lauricella C, Veronese SM, et al. Liquid biopsy for small cell lung cancer either de novo or transformed: systematic review of different applications and meta-analysis. Cancers (Basel) 2021;13:2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paz-Ares L, Garassino MC, Chen Y, Reinmuth N, Hotta K, Poltoratskiy A, et al. Durvalumab ± tremelimumab + platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): outcomes by PD-L1 expression and tissue tumor mutational burden. Clin Cancer Res 2024;30:824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashrafi A, Akter Z, Modareszadeh P, Modareszadeh P, Berisha E, Alemi PS, et al. Current landscape of therapeutic resistance in lung cancer and promising strategies to overcome resistance. Cancers (Basel) 2022;14:4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sen T, Gay CM, Byers LA. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res 2018;7:50–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nyquist MD, Corella A, Coleman I, De Sarkar N, Kaipainen A, Ha G, et al. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep 2020;31:107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caracciolo D, Riillo C, Di Martino MT, Tagliaferri P, Tassone P. Alternative non-homologous end-joining: error-prone DNA repair as cancer’s Achilles’ heel. Cancers (Basel) 2021;13:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pannunzio NR, Li S, Watanabe G, Lieber MR. Non-homologous end joining often uses microhomology: implications for alternative end joining. DNA Repair (Amst) 2014;17:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021;39:619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Devarakonda S, Sankararaman S, Herzog BH, Gold KA, Waqar SN, Ward JP, et al. Circulating tumor DNA profiling in small-cell lung cancer identifies potentially targetable alterations. Clin Cancer Res 2019;25:6119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang H, Zhou F, Qiao M, Li X, Zhao C, Cheng L, et al. The role of circulating tumor DNA in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol 2021;11:671874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bratman SV, Yang SYC, Iafolla MAJ, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer 2020;1:873–81. [DOI] [PubMed] [Google Scholar]
- 46. Herbreteau G, Langlais A, Greillier L, Audigier-Valette C, Uwer L, Hureaux J, et al. Circulating tumor DNA as a prognostic determinant in small cell lung cancer patients receiving atezolizumab. J Clin Med 2020;9:3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raja R, Kuziora M, Brohawn PZ, Higgs BW, Gupta A, Dennis PA, et al. Early reduction in ctDNA predicts survival in patients with lung and bladder cancer treated with durvalumab. Clin Cancer Res 2018;24:6212–22. [DOI] [PubMed] [Google Scholar]
- 48. Deng J, Jiang R, Meng E, Wu H. CXCL5: a coachman to drive cancer progression. Front Oncol 2022;12:944494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang C, Li Z, Xu L, Che X, Wen T, Fan Y, et al. CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer. BMC Cancer 2018;18:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhong C-Q, Zhang X-P, Ma N, Zhang E-B, Li J-J, Jiang Y-B, et al. FABP4 suppresses proliferation and invasion of hepatocellular carcinoma cells and predicts a poor prognosis for hepatocellular carcinoma. Cancer Med 2018;7:2629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wischhusen J, Melero I, Fridman WH. Growth/differentiation factor-15 (GDF-15): from biomarker to novel targetable immune checkpoint. Front Immunol 2020;11:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021;39:346–60.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figures
Data Availability Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at: www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at: https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.