Abstract
Case series
Patients: Female, 23-year-old • Female, 56-year-old
Final Diagnosis: Herpesvirus infection
Symptoms: Abdominal pain • cognitive impairment • fever • headache • skin rash
Clinical Procedure: —
Specialty: Infectious Diseases • Rheumatology
Objective:
Unusual clinical course
Background:
Anifrolumab, a monoclonal antibody targeting the type 1 interferon (IFN-I) signaling pathway, holds promise as a therapeutic intervention for systemic lupus erythematosus (SLE). However, its use is associated with an increased risk of infections, particularly viral infections like herpes zoster (HZ). Results from the clinical trials on anifrolumab show yearly rates of upper respiratory tract infections of 34% and HZ of 6.1%. An increased frequency of other specific viral infections, including herpes simplex virus (HSV), was not reported.
Case Reports:
Here, we present 2 cases of patients with SLE treated with anifrolumab, both experiencing severe adverse reactions in the form of disseminated herpesvirus infections, specifically disseminated HSV-2 and varicella zoster virus (VZV, HZ encephalitis). To the best of our knowledge, no previous reports of severe disseminated HSV-2 or HZ have been published in anifrolumab-treated patients. The patient in case 1 experienced a primary HSV-2 infection following anifrolumab treatment, potentially explaining the severity of the infection. The patient in case 2 had a history of previous HZ skin infections, which may have increased her risk of disseminated infection. Both patients recovered from the infections with minor sequelae, but they still require prophylactic antiviral treatment. These cases highlight the critical role of IFN-I immunity in protecting against herpesvirus infections.
Conclusions:
Thorough risk assessment before anifrolumab initiation, considering the patient’s viral infection history, vaccination status, and potential exposure risks, is essential. Administration of recombinant zoster vaccine before anifrolumab therapy may benefit susceptible individuals.
Key words: Anifrolumab; Autoimmunity; Drug-Related Side Effects and Adverse Reactions Interferon-alpha; Lupus Erythematosus, Systemic
Introduction
The type I interferon (IFN-I) is a family of potent antiviral cytokines comprising IFN-α (12 subtypes), IFN-β, IFN-ω, IFN-ε, and IFN-κ, and plays a central role in the control of viral infections. IFN-I also represents a compelling therapeutic target in systemic lupus erythematosus (SLE), which has garnered significant research attention over the past decade [1,2]. Studies have been conducted to explore the selective inhibition of IFN-α inhibition using sifalimumab and rontalizumab, as well as the comprehensive suppression of the IFN-I signaling pathway achieved with anifrolumab, targeting the IFN-α/β receptor subunit 1 (IFNAR1) (Figure 1) [2–7].
Figure 1.
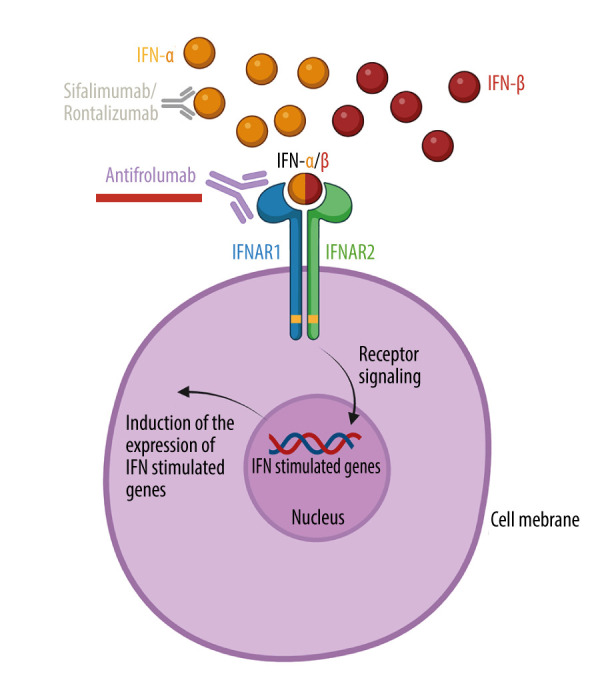
Mechanisms of action of type 1 interferon-linked monoclonal antibodies. The type 1 interferon (IFN-I) receptor (IFNAR) comprises the 2 subunits IFNAR1 and IFNAR2. Upon activation, intracellular receptor-linked kinases initiate phosphorylation and coupling of specific proteins, subsequently translocating to the nucleus. Here, they activate the expression of INF-stimulated genes, which mediate the antiviral effector functions of IFN-I. Anifrolumab (underlined red) targets IFNAR1, thereby preventing IFN-I (here illustrated with IFN-α and IFN-β) from activating IFNAR. Sifalimumab and rontalizumab selectively bind to IFN-α, allowing the other cytokine members of the IFN-I family (such as IFN-β) to still activate IFNAR.
Created with BioRender.com.
In 2022, anifrolumab was approved by the European Medicines Agency (EMA) for patients with SLE as an add-on therapy to the standard of care in moderate-to-severe disease as its exclusive indication [8]. Despite showing efficacy in the 3 existing randomized clinical trials on anifrolumab and SLE [9–11], concerns over serious adverse effects arose due to the broad inhibition of the IFN-I response, which is a critical part of the human antiviral response [12]. Results from the clinical trials and reports from the EMA on anifrolumab show undesirable effects, with yearly rates of upper respiratory tract infections of 34% and herpes zoster (HZ) infections of 6.1% [9–11,13]. The long-term follow-up (feeder trial of TULIP-1 and TULIP-2 trial), encompassing 4 years of treatment, reported similar rates [10,11,13]. HZ infections were predominantly mild and resolved without discontinuing anifrolumab therapy, even though cases of disseminated HZ infection with multi-dermal and central nervous system involvement have been reported to the EMA [13]. An increased frequency of other specific viral infections, including herpes simplex virus (HSV), was not reported.
Here, we present 2 cases of severe disseminated herpesvirus infections observed in patients with SLE undergoing treatment with anifrolumab.
Case Reports
Case 1
The patient was a 32-year-old woman with SLE since the age of 20 years. The diagnosis was established based on a constellation of clinical and immunological manifestations. The clinical features were fever, oral ulcers, serositis, arthritis, alopecia, skin rash, and antiphospholipid syndrome (APS). Her blood samples showed lymphopenia and normocytic anemia of chronic disease, with immunological and inflammatory manifestations such as low complement C3, elevated C-reactive protein, and the following positive autoantibodies: antinuclear antibody with a homogenous nuclear staining pattern, anti-double-stranded deoxyribonucleic acid (dsDNA) antibody, anti-Sjogren syndrome-related antibody, and Coombs test. At the age of 25 years, the diagnosis of APS was established based on persistent positivity in the lupus anticoagulant test (single anti-phospholipid antibody positivity) and the occurrence of a deep vein thrombosis and pulmonary embolism within 6 months from the positive test results. Apart from that, no organ damage had occurred. Other comorbidities included attention-deficit/hyperactivity disorder (AD/HD). Regarding anticoagulation therapy, she was initially treated with warfarin upon the diagnosis of APS, but managing the therapeutic dose proved challenging over the years. Consequently, after 7 years without any recurrence of thrombosis, she was switched to rivaroxaban. Beside her anticoagulant treatment and AD/HD treatment with methylphenidate, her immunosuppressive treatment prior to the present disease course included hydroxychloroquine (HCQ); she had previously been treated with azathioprine and prednisolone. She had no history of severe herpesvirus infections. Medical records from the past 10 years showed several negative vaginal swabs. Previous vaccinations included diphteria-tetanus-pertussis-polio-Haemophilus influenza type-pneumococcal, measles, mumps, and rubella, human papilloma virus, and COVID-19 vaccines.
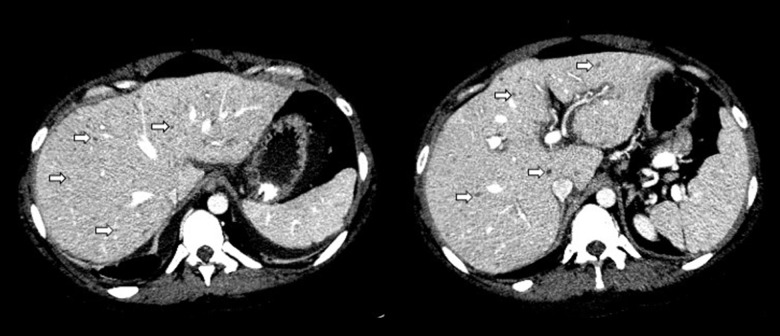
In 2023, she presented with a moderate SLE flare with extreme fatigue, recurrent arthralgia, severe skin rash, persistent mild cognitive impairment (memory and concentration difficulties that was primarily attributed to the AD/HD diagnosis), and reduced quality of life. No sign of serological activity was seen, with normal C3 levels and persistent undetectable levels of anti-dsDNA-antibodies, since 2014. She was prescribed anifrolumab treatment (intravenous 300 mg/every 28 days) as an add-on to HCQ. Six weeks after initiation of anifrolumab treatment (after 2 infusions with no clinical response to treatment yet), she was admitted to the hospital with fever, lower abdominal pain, and severe headache. Laboratory investigations revealed elevated C-reactive protein level of 412 mg/L (reference interval (RI): <8 mg/L), alanine transaminase of 591 U/L (RI: 10–45 U/L), lactate dehydrogenase of 865 U/L (RI: 105–205U/L), and low-level pleocytosis from the cerebrospinal fluid (leucocytes count of 7×106/L [RI: <4×106/L] with no concomitant red blood cells). A computed tomography scan of the thorax, abdomen, and pelvis revealed signs of widespread inflammation, with (1) bilateral pleural and pericardial effusion, (2) peripancreatic fluid and adipose tissue reaction, (3) ascites and periportal edema, (4) numerous hypodense changes in the liver (suggestive of herpes hepatitis) (Figure 2), (5) splenomegaly, and (6) reaction around the vagina and left ovary. Several positive HSV-2 DNA polymerase chain reaction (PCR) tests from the plasma and ascites confirmed the diagnosis of disseminated HSV-2 infection. Expositions preceding admission involved unprotected vaginal intercourse 3 weeks before. A vaginal swab also found a positive PCR test of HSV-2 DNA. Exacerbation of cognitive impairment, headache, photophobia, and low-level pleocytosis strongly suggested HSV-2 central nervous system involvement as well. However, HSV-2 analysis of the cerebrospinal fluid and cerebral magnetic resonance imaging did not confirm this, since the PCR tests from the cerebrospinal fluid was negative, and the scan was normal. The anti-HSV-2 immunoglobin-G (IgG) antibodies were positive 3 weeks after admission, but previous HSV-2-antibody measurements were not available. Other infectious, autoimmune (including SLE disease flair), and malignant differential diagnoses were considered but ultimately excluded. Anti-dsDNA-antibody levels remained undetectable. A whole genome sequencing found no evidence of inborn error of immunity, based on an analysis of 572 associated genes, according to the most recent guidelines [14]. The only other positive microbiological finding was Ureaplasma parvum DNA from a vaginal swab.
Figure 2.
Computed tomography scan of the liver in Case 1. Herpes simplex virus 2 hepatitis with millimetric hypodensities resembling multiple hepatic micro abscesses (arrows) is shown.
The patient was treated with antibiotics, including meropenem and doxycycline, during hospitalization. After the suspicion of HSV-2 infection (later confirmed by PCP from blood and vaginal material), the patient was treated with intravenous acyclovir (30 mg/kg/day) for 3 weeks, followed by oral valaciclovir 2 g/day for 2 weeks. Following the antiviral intervention, clinical and laboratory improvements were observed. No immunosuppressives aside from HCQ were administered during the hospitalization. Following discharge, a month after discontinuation of antiviral therapy, the patient presented with a recurrent HSV-2-positive skin lesion, prompting treatment with valacyclovir followed by prophylactics (acyclovir 800 mg/day). As of today, almost 6 months after the HSV-2 disease, the patient has recovered but continues to require prophylactic acyclovir treatment and treatment for post-herpetic neuralgia. Notably, there has been no sign of SLE disease activity. The patient is currently maintained on HCQ, with no additional immunosuppressive medications administered since the last dose of anifrolumab.
Case 2
The patient was a 56-year-old woman with SLE and mixed connective tissue disease features since the age of 25 years. Clinical characteristics of the disease included malar rash, photosensitivity, arthritis, oral ulcer, alopecia, puffy fingers, and Raynaud’s disease. Immunological characteristics included low complement C3 and C4, lymphopenia, and the following positive autoantibodies: antinuclear antibody with nucleus speckled staining pattern, anti-dsDNA antibody, anti-Smith antibody, and anti-U1 ribonucleoprotein antibody.
The patient had no comorbidities, signs of organ damage, or history of central nervous system or renal involvement. Since the diagnosis of SLE, the patient has been continuously on HCQ. Treatments with azathioprine, methotrexate, and mycophenolate had proven inadequate to treat her severe skin manifestations and persistent arthritis. The patient commenced belimumab (anti-B lymphocyte stimulator monoclonal antibody) in 2020, resulting in near remission of her disease, albeit with ongoing skin manifestations.
In 2022, a significant flare involving the skin, joints, and alopecia, coupled with the necessity for continuous prednisolone doses exceeding 10 mg/day, prompted a transition to anifrolumab treatment (intravenous 300 mg/every 28 days) as an adjunct to HCQ and prednisolone 5 mg/day. The patient reported no initial adverse effects from the anifrolumab treatment. Having been treated for single dermatome skin HZ twice by her general practitioner years prior to the current presentation, she opted not to receive vaccination for HZ before commencing anifrolumab therapy. The patient experienced substantial improvement in clinical symptoms, particularly the resolution of skin manifestations and hand puffiness, which had not previously achieved remission with other treatments. Persistently, anti-dsDNA-antibody levels were in the range of 20–50 × 103 IE/L and C3 levels below 0.9 g/L.
One and a half years after anifrolumab initiation, she was admitted to the hospital due to fever, severe headache, mild cognitive impairment, and a rash and neuropathic pain on the left side of the thorax overlapping several dermatomes. Analysis of the rash vesicles detected VZV DNA by PCR. A cerebrospinal fluid analysis found 489 × 106 leucocytes/L (RI: <4×106/L) and positivity to VZV DNA. The patient received a diagnosis of disseminated VZV, with suspected central nervous system involvement. At the time of admission, her immunosuppressive treatment included HCQ 400 mg/day, prednisolone 10 mg/day, and anifrolumab 300 mg/every 28 days (latest administration 4 weeks before admission). HCQ and prednisolone were continued during hospitalization, and intravenous antiviral treatment with acyclovir (30 mg/kg/day) was given. The patient subsequently experienced slow clinical improvement. No signs of SLE activity were observed. No significant increase in anti-dsDNA-antibody levels were observed during hospitalization. After 2 weeks of intravenous antiviral treatment, the patient was discharged and switched to oral treatment with valacyclovir 6 g/day for another week.
Presently, 1 month after discharge, the patient is in recovery, although she continues to experience intermittent headaches, insomnia, and mild cognitive impairment. Her SLE disease remains in remission on HCQ and prednisolone 10 mg/day. Anifrolumab has not been administered since the hospitalization. The patient has not previously been vaccinated against HZ, but a recombinant zoster vaccine (RZV) is scheduled before any potential further immunosuppression.
Discussion
In the past decades, significant progress has been made in understanding SLE disease and developing novel treatment strategies to control disease activity and prevent organ damage. Despite these advances, infections remain a prominent cause of death in SLE [15]. Bacterial infections constitute a substantial proportion, accounting for up to 75% of all reported infections in SLE [16]. Various opportunistic viral and mycobacterial infections also contribute significantly to morbidity, and the co-occurrence of infections with disease flares further complicates the diagnosis and management of concurrent infections in SLE [17]. Infections in SLE are influenced by various risk factors, including conventional elements such as advanced age and concurrent comorbidities, alongside more specific characteristics, such as recent onset of SLE (<5 years), early disease onset, high disease activity, presence of organ damage, and use of glucocorticoids and other immunosuppressive therapies [17].
Upper respiratory tract infections are the most prevalent type of infections in SLE [18]. Among viral infections in general, HZ stands out as one of the most prevalent [19]. In contrast to other types of infections in SLE, two-thirds of HZ cases occur after 5 years of disease duration, and 80% exhibit no or mild disease activity [19]. HZ in SLE generally carries a favorable prognosis, however, independent risk factors for an unfavorable outcome includes lymphopenia and immunosuppressive drugs [19,20].
In this paper, we highlight 2 cases of patients with SLE who were subjected to anifrolumab therapy and experienced severe adverse reactions manifested as disseminated herpesvirus infections, specifically disseminated HSV-2 and VZV. Regarding viral infections following anifrolumab treatment, HZ infection is well known from previous clinical trials [9–11], and reports of severe COVID-19 infections in 2 anifrolumab-treated SLE patients has previously been published [21]. To the best of our knowledge, no severe disseminated HZ infections or severe HSV-2 infections have previously been reported in anifrolumab-treated patients. Rigorous clinical investigations, including a comprehensive genetic immunodeficiency workup involving whole-genome sequencing in case 1, failed to reveal any alternative explanations for the observed infections, strongly implicating anifrolumab as a potential contributing factor in both cases.
Typically, HSV-2 infections manifest with mild symptoms localized to the skin or mucosa, although disseminated infections are well-documented in immunocompromised individuals, particularly in cases presumed to be primary infections [22–24]. The status of a primary HSV-2 infection in case 1 remains uncertain. Notably, she lacked prior history of HSV symptoms and had a possible exposition to HSV-2 infection 3 weeks before admission.
A comprehensive review in 2020 indicates a pooled mean seroprevalence of HSV-2 antibodies among European women of 14% [25]. Considering the relatively low seroprevalence of anti-HSV-2 antibodies, the practicality of incorporating a screening protocol for HSV-2 antibodies prior to commencing anifrolumab treatment appears to be of limited value. Nonetheless, it stresses the importance of assessing the risks of potential exposition to HSV infections before treatment initiation. Furthermore, exploring the screening of antibodies against other potentially detrimental viruses in scenarios involving primary infections could yield significant benefits. For example, screening for HSV-1 IgG antibodies may be worthwhile, given its reported European mean seroprevalence of 67% [26]. Similarly, assessing IgG antibodies against cytomegalovirus, with a seroprevalence of 70% in European women of reproductive age, can offer valuable insights into the risk evaluation before initiating anifrolumab therapy [27].
It is unknown whether the APS diagnosis in case 1 might have influenced the anifrolumab treatment or susceptibility to infection. In previous trials on anifrolumab and SLE [9–11], APS was not an exclusion criterion if anticoagulant therapy had been stable for 3 months and there was no history of severe or catastrophic APS. However, the number of patients with APS included in these trials, or the results of stratified analysis based on the APS diagnosis, have not been published.
Primary defects in the IFN-I system have been associated with severe viral disease, including COVID-19, herpesvirus disease, and influenza [28,29]. Disseminated HSV infection as well as disseminated VZV infection after living viral VZV vaccination have previously been reported in children with complete deficiency for either of the type I IFN receptor subunits IFNAR1 or IFNAR2, and in indigenous people of Polynesia and Artic ancestry, where loss-of-function alleles of these genes are relatively common [30,31]. Recently, a case of disseminated HSV-2 primary infection was described in a patient harboring high levels of anti-IFN-α autoantibodies, and many years ago, disseminated varicella zoster disease was described in an elderly and otherwise healthy woman with neutralizing anti-IFN antibodies [32,33].
Notably, anti-IFNα autoantibodies have been observed in up to 11% of individuals with SLE, correlating with viral infections and tuberculosis [34,35]. More recently, anti-IFNα antibodies have been linked with reduced disease activity in SLE [36]. Nevertheless, the clinical implications of these autoantibodies remain uncertain. In fact, their presence was also reported in approximately 10% of cases with severe COVID-19 and 5% in cases with severe influenza pneumonia [37,38].
Regarding VZV, the patient in case 2 was not vaccinated against HZ. She had a history of previous HZ but no other HZ risk factors except SLE disease and age > 50 years [39]. The RZV (Shingrix) has recently been granted expanded indications to immunocompromised adults aged >18 years by the Food and Drug Administration [40]. So far, there are no clinical efficacy data on RZV in patients with SLE, but it has been shown to be safe and effective in the general population [41]. The efficacy of the RZV in preventing HZ likely correlates with a comparable reduction in central nervous system manifestations (HZ encephalitis), although this has not been studied so far [42].
The American College of Rheumatology 2022 guideline for vaccination in patients with rheumatic diseases recommends RZV for all patients >18 years taking immunosuppressive medications [43]. The European League Against Rheumatism 2023 guideline for the management of SLE also recommends RZV for all patients [44].
Conclusions
These case presentations highlight the occurrence of severe adverse effects involving disseminated HSV-2 and VZV infections after anifrolumab treatment in 2 patients with SLE. Together with previous publications, it attests to the significant importance of IFN-I immunity in protection against herpesvirus infections in humans. Meticulous and prompt diagnostic workup for viral infection should be done in an affected anifrolumab-treated patient to avoid or reduce potentially irreversible damage. It emphasizes the need for continuous surveillance and comprehensive reporting of adverse events associated with newly approved drugs. Furthermore, this necessitates a meticulous risk assessment before initiating such treatments, explicitly focusing on the patient’s viral infection history, vaccination status, and potential exposure risks. A reasonable strategy to mitigate the escalated risk of severe viral infections, especially in susceptible individuals, is therapeutic education and patient awareness of viral risks and the proactive administration of RZV before initiating anifrolumab. Additionally, screening the antibody status of various viruses can provide valuable insights in select cases.
Acknowledgments
We thank the patients for their consent to publish this case report. We also thank our medical colleagues for their discussion and advice.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Bruera S, Chavula T, Madan R, Agarwal SK. Targeting type I interferons in systemic lupus erythematous. Front Pharmacol. 2022;13:1046687. doi: 10.3389/fphar.2022.1046687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niewold TB. Connective tissue diseases: Targeting type I interferon in systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(7):377–78. doi: 10.1038/nrrheum.2016.83. [DOI] [PubMed] [Google Scholar]
- 3.Merrill JT, Wallace DJ, Petri M, et al. Lupus Interferon Skin Activity (LISA) Study Investigators Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: A phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011;70(11):1905–13. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Wallace DJ, Spindler A, et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: A phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013;65(4):1011–21. doi: 10.1002/art.37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khamashta M, Merrill JT, Werth VP, et al. CD1067 study investigators Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(11):1909–16. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi T, Tanaka Y, Matsumura R, et al. Safety and tolerability of sifalimumab, an anti-interferon-α monoclonal antibody, in Japanese patients with systemic lupus erythematosus: A multicenter, phase 2, open-label study. Mod Rheumatol. 2020;30(1):93–100. doi: 10.1080/14397595.2019.1583832. [DOI] [PubMed] [Google Scholar]
- 7.Kalunian KC, Merrill JT, Maciuca R, et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE) Ann Rheum Dis. 2016;75(1):196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency EMA/6640/2022 – Saphnelo (anifrolumab): An overview of Saphnelo and why it is authorised in the EU. 2022; Available from: https://www.ema.europa.eu/en/documents/overview/saphnelo-epar-medicine-overview_en.pdf.
- 9.Furie R, Khamashta M, Merrill JT, et al. CD1013 Study Investigators Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 2017;69(2):376–86. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furie RA, Morand EF, Bruce IN, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): A randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1(4):e208–e19. doi: 10.1016/S2665-9913(19)30076-1. [DOI] [PubMed] [Google Scholar]
- 11.Morand EF, Furie R, Tanaka Y, et al. TULIP-2 Trial Investigators Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382(3):211–21. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 12.McNab F, Mayer-Barber K, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency Saphnelo (anifrolumab): Product information; Available from: https://www.ema.europa.eu/en/documents/product-information/saphnelo-epar-product-information_en.pdf.
- 14.Bousfiha A, Moundir A, Tangye SG, et al. The 2022 update of IUIS Phenotypical classification for human inborn errors of immunity. J Clin Immunol. 2022;42(7):1508–20. doi: 10.1007/s10875-022-01352-z. [DOI] [PubMed] [Google Scholar]
- 15.Barber MRW, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(9):515–32. doi: 10.1038/s41584-021-00668-1. [Erratum in: Nat Rev Rheumatol. 2021;17(10):642] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walunas TL, Jackson KL, Chung AH, et al. Disease outcomes and care fragmentation among patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017;69(9):1369–76. doi: 10.1002/acr.23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao M, Mikdashi J. A framework to overcome challenges in the management of infections in patients with systemic lupus erythematosus. Open Access Rheumatol. 2023;15:125–37. doi: 10.2147/OARRR.S295036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pego-Reigosa JM, Nicholson L, Pooley N, et al. The risk of infections in adult patients with systemic lupus erythematosus: Systematic review and meta-analysis. Rheumatology (Oxford) 2021;60(1):60–72. doi: 10.1093/rheumatology/keaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome of Herpes zoster in systemic lupus erythematosus. J Clin Rheumatol. 2010;16(3):119–22. doi: 10.1097/RHU.0b013e3181d52ed7. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Li H, Xie J, et al. Herpes zoster in patients with systemic lupus erythematosus: Clinical features, complications and risk factors. Exp Ther Med. 2017;14(6):6222–28. doi: 10.3892/etm.2017.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breillat P, Mathian A, Rozenberg F, et al. Is there an increased risk of severe COVID-19 among patients with systemic lupus erythematosus treated with anifrolumab? Lupus. 2023;32(3):453–55. doi: 10.1177/09612033231153536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momméja-Marin H, Lafaurie M, Scieux C, et al. Herpes simplex virus type 2 as a cause of severe meningitis in immunocompromised adults. Clin Infect Dis. 2003;37(11):1527–33. doi: 10.1086/379520. [DOI] [PubMed] [Google Scholar]
- 23.Brown TS, Callen JP. Atypical presentation of herpes simplex virus in a patient with chronic lymphocytic leukemia. Cutis. 1999;64(2):123–25. [PubMed] [Google Scholar]
- 24.Abbo L, Alcaide ML, Pano JR, Robinson PG, Campo RE. Fulminant hepatitis from herpes simplex virus type 2 in an immunocompetent adult. Transpl Infect Dis. 2007;9(4):323–26. doi: 10.1111/j.1399-3062.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- 25.Alareeki A, Osman AMM, Khandakji MN, et al. Epidemiology of herpes simplex virus type 2 in Europe: Systematic review, meta-analyses, and meta-regressions. Lancet Reg Health Eur. 2023;25:100558. doi: 10.1016/j.lanepe.2022.100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 27.Yousuf W, Ibrahim H, Harfouche M, et al. Herpes simplex virus type 1 in Europe: Systematic review, meta-analyses and meta-regressions. BMJ Glob Health. 2020;5(7):e002388. doi: 10.1136/bmjgh-2020-002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan CJA, Randall RE, Hambleton S. Genetic lesions of type I interferon signalling in human antiviral immunity. Trends Genet. 2021;37(1):46–58. doi: 10.1016/j.tig.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastard P, Hsiao KC, Zhang Q, et al. A loss-of-function IFNAR1 allele in Polynesia underlies severe viral diseases in homozygotes. J Exp Med. 2022;219(6):e20220028. doi: 10.1084/jem.20220028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan CJA, Skouboe MK, Howarth S, et al. Life-threatening viral disease in a novel form of autosomal recessive IFNAR2 deficiency in the Arctic. J Exp Med. 2022;219(6):e20212427. doi: 10.1084/jem.20212427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozzetto B, Mogensen KE, Tovey MG, Gresser I. Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J Infect Dis. 1984;150(5):707–13. doi: 10.1093/infdis/150.5.707. [DOI] [PubMed] [Google Scholar]
- 33.Martinot M, Gravier S, Mohseni-Zadeh M, et al. Severe acute herpes virus type 2 primo-infection and its association with anti-type 1 interferon autoantibodies. Eur J Clin Microbiol Infect Dis. 2023;42(12):1531–35. doi: 10.1007/s10096-023-04688-5. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Tatouli IP, Rosen LB, et al. Distinct functions of autoantibodies against interferon in systemic lupus erythematosus: A comprehensive analysis of anticytokine autoantibodies in common rheumatic diseases. Arthritis Rheumatol. 2016;68(7):1677–87. doi: 10.1002/art.39607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beydon M, Nicaise-Roland P, Mageau A, et al. Autoantibodies against IFNα in patients with systemic lupus erythematosus and susceptibility for infection: A retrospective case-control study. Sci Rep. 2022;12(1):11244. doi: 10.1038/s41598-022-15508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathian A, Breillat P, Dorgham K, et al. Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-α. Ann Rheum Dis. 2022;81(12):1695–703. doi: 10.1136/ard-2022-222549. [DOI] [PubMed] [Google Scholar]
- 37.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Pizzorno A, Miorin L, et al. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J Exp Med. 2022;219(11):e20220514. doi: 10.1084/jem.20220514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curran D, Doherty TM, Lecrenier N, Breuer T. Healthy ageing: Herpes zoster infection and the role of zoster vaccination. NPJ Vaccines. 2023;8(1):184. doi: 10.1038/s41541-023-00757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: Recommendations of the Advisory Committee on Immunization Practices – United States, 2022. Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New Engl J Med. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 42.Matthews E, Beckham JD, Piquet AL, et al. Herpesvirus-associated encephalitis: An update. Curr Trop Med Rep. 2022;9(3):92–100. doi: 10.1007/s40475-022-00255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken) 2023;75(3):449–64. doi: 10.1002/acr.25045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024;83(1):15–29. doi: 10.1136/ard-2023-224762. [DOI] [PubMed] [Google Scholar]